Abstract
The 18 glycosyl hydrolase family of chitinases is an ancient gene family that is widely expressed from prokaryotes to eukaryotes. In mammals, despite the absence of endogenous chitin, a number of chitinases and chitinase-like proteins (C/CLPs) have been identified. However, their roles have only recently begun to be elucidated. Acidic mammalian chitinase (AMCase) inhibits chitin-induced innate inflammation; augments chitin-free, allergen-induced Th2 inflammation; and mediates effector functions of IL-13. The CLPs BRP-39/YKL-40 (also termed chitinase 3-like 1) inhibit oxidant-induced lung injury, augments adaptive Th2 immunity, regulates apoptosis, stimulates alternative macrophage activation, and contributes to fibrosis and wound healing. In accord with these findings, levels of YKL-40 in the lung and serum are increased in asthma and other inflammatory and remodeling disorders and often correlate with disease severity. Our understanding of the roles of C/CLPs in inflammation, tissue remodeling, and tissue injury in health and disease is reviewed below.
Keywords: asthma, fibrosis, BRP-39/YKL-40, AMCase, chitotriosidase
INTRODUCTION
The production of type 2 cytokines, including interleukin (IL)-4, IL-5, and IL-13 by CD4 T helper (Th)2 and other immune cells, plays a critical role in the pathogenesis of asthma and allergic responses. Studies over the past two decades have given us a glimpse of the complexity of these responses, highlighting the importance of other inflammatory cytokines [e.g., IL-17, IL-18, IL-33, transforming growth factor (TGF)-β, vascular endothelial growth factor (VEGF), thymic stromal lymphopoietin (TSLP)] and chemokines [chemokine (C-C-motif) (CCL)17, CCL22)] and the complex cell-cell interactions involving dendritic cells, basophils, eosinophils,mast cells, and the newly described nuocytes, multipotent progenitor type 2 (MPPtype2) cells, and natural helper cells that orchestrate these reactions (for reviews, see References 1–3). However, the molecular and cellular mechanisms of these responses are still incompletely understood, and the pathways connecting innate immunity and adaptive immunity during Th2 responses have not been adequately defined. In an attempt to define the mechanisms that are involved in type 2 responses, antigen-driven and antigen-free experimental systems have been employed. Evaluations of these systems highlighted impressive alterations in the expression of 18 glycosyl hydrolases, including the true chitinase acidic mammalian chitinase (AMCase) and the chitinase-like protein (CLP) breast regression protein (BRP)-39/YKL-40. These abnormalities were readily appreciated in the aeroallergen models of Th2 inflammation and transgenic mice in which Th2 cytokines are overexpressed in a lung-specific manner (4, 5). This prompted investigations to define the roles of these molecules in these responses and prompted studies of chitin, which is believed to be a major target of some of these moieties. These studies demonstrated that chitinases/chitinase-like proteins (C/CLPs) play a pivotal role in both innate and adaptive type 2 immune responses (5–9). Although nonspecific immune stimulatory functions of chitin and chitin derivatives were reported three decades ago, these studies also prompted the appreciation that appropriately sized chitin is a potent stimulator of type 1 and type 2 innate immune responses (7, 10, 11) and a potent immune adjuvant that enhances viral or type 2 adaptive immune responses (12, 13). In mammals, although endogenous chitin or chitin synthases do not exist, C/CLPs are endogenously expressed in the lung and other organs and are impressively dysregulated in allergic disorders and a variety of diseases and disease models characterized by chronic inflammation and tissue remodeling (4, 5). Together these findings strongly support an evolving concept regarding the roles of C/CLPs in mammals. In this conceptualization, C/CLPs are induced at sites of infection as part of an innate antipathogen response. Selected moieties, in turn, feed back to control tissue injury by minimizing oxidant damage and specific innate immune responses such as those induced by chitin fragments. Simultaneously, they augment adaptive immune responses to chitin-free antigens, ensuring pathogen eradication and the development of immunity. Many of the C/CLPs are further induced during these adaptive responses and contribute to local tissue healing and fibrosis (Figure 1). This overall concept is the result of studies of C/CLPs and chitin in lower and higher life forms and mammals, including recent evaluations of null mutant and transgenic mice. Such studies are summarized below.
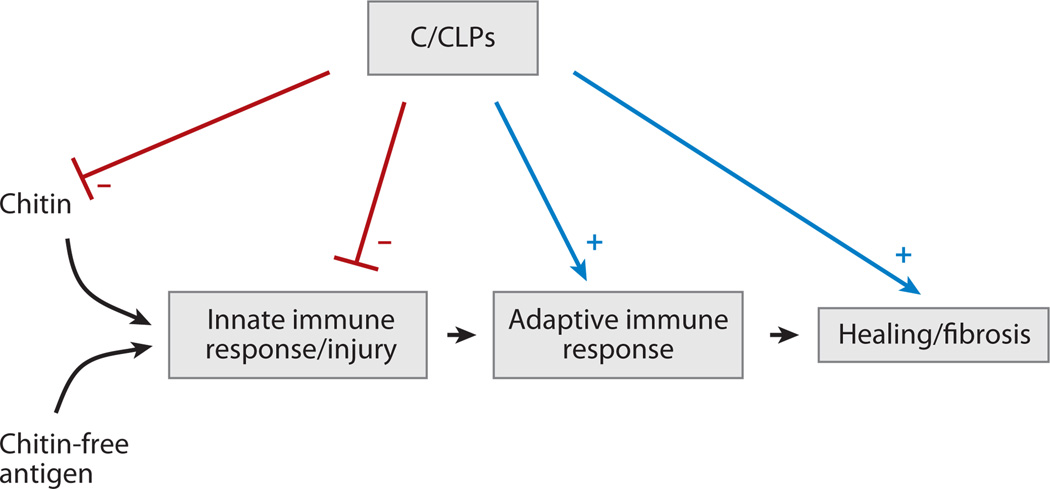
Figure 1.
Immune and regulatory effects of chitin, chitinases (C), and chitinase-like proteins (CLP). Chitin is a potent stimulator of innate immune responses and subsequent tissue injury. Chitin-free antigens are also able to induce the innate reactions that, in turn, lead to adaptive immune responses. When appropriately managed, these responses lead to tissue healing. When excessive injury or repair occurs, tissue fibrosis can ensue. C/CLP can inhibit (−) chitin-induced innate immune and injury responses. The ability of AMCase to directly degrade chitin via its chitinase activity and to inhibit epithelial cell apoptosis via a chitinolytic-independent mechanism and the ability of BRP-39/YKL-40 to inhibit oxidant-induced injury are examples of this regulation. Simultaneously, C/CLP-like acidic mammalian chitinase (AMCase) and BRP-39/YKL-40 enhance adaptive immune responses to chitin-free moieties, thereby ensuring the development of selective antigen-specific immunity. This has been studied most intensively with BRP-39/YKL-40, which enhance antigen sensitization, augment the survival of inflammatory cells, and induce alternative (M2) macrophage differentiation. C/CLP are further induced during the type 2 immune response, and their ability to inhibit structural cell apoptosis, to induce macrophage differentiation, and to aid in the production of TGF-β1 may also contribute to healing and fibrosis.
CHITIN AND CHITINASES IN NATURE AND LOWER ORGANISMS
Chitin, a polymer made up of repeating units of β-(1–4)-poly-N-acetyl-d-glucosamine, is the second-most-abundant polysaccharide in nature after cellulose. Chitin is found in the cell walls of bacteria and fungi, mushrooms, the exoskeleton of crustaceans (crabs, shrimp, etc.) and insects, the microfilarial sheath of parasitic nematodes, and the lining of the digestive tracts of many insects (14–18). In these locations, chitin is used by chitin-containing organisms for protection against the harsh conditions in their environment and host antiparasite/pathogen immune responses. Thus, the absence of chitin can lead to the death of the pathogen. Despite its ubiquity, chitin does not accumulate in the environment because chitinolytic bacteria or saprophytes efficiently recycle most of the chitin in nature (19). In lower life forms, chitin deposition is regulated by the balance of biosynthesis and degradation. The chitinases, which are endo-β-1,4-N-acetylglucosamidases, are the key degrading enzymes in this setting. They are produced in significant quantities by hosts as a defense against infection with chitin-containing organisms. This attempt to damage the chitin coat of the infecting organism is part of the innate immune response (19). This process also produces differentially sized chitin fragments that can trigger innate immunity pattern recognition receptors (PRRs) to induce tumor necrosis factor (TNF)-α, IL-17, and/or IL-10 elaboration (10, 20). Chitinases also contribute to the life cycle of chitin-containing fungi and parasites, in which they control growth and molting. Chitinases are also used by pathogens to invade or exploit chitin-containing structures in the host and thus play a critical role(s) in the transmission of infection from one vertebrate host to another by insect vectors (18, 21, 22). This is nicely illustrated in plasmodia, which use chitinases to penetrate the chitin surrounding the blood meal in the mosquito (23), and in fungi, which produce chitinases to penetrate the cuticle (24). As a result of the importance of chitin in the protection of pathogens and the importance of appropriately regulated chitinase production in the life cycle of pathogens, chitin synthesis inhibitors and chitinase inhibitors have received significant attention as potential biopesticides to eradicate insects, fungi, and helminthic parasites (24, 25).
CHITIN AS AN IMMUNE MODULATOR
Early studies of selected high-molecular-weight chitin derivatives demonstrated that chitin is nontoxic, nonallergenic, biodegradable, and biocompatible. As a result, chitin has been used in the manufacture of a number of medical devices such as artificial skin, contact lenses, prosthetics, and surgical stitches (26). Chitin preparations can also have powerful immune effects. A group of Japanese investigators identified the nonspecific immune-stimulatory function of chitin or chitin derivatives more than three decades ago. This group showed that chitin or chitin derivatives have the ability to stimulate macrophages to produce H2O2, thereby conferring nonspecific host resistance to bacterial or viral infections and antitumor activity (27–29). Chitin also stimulates inflammatory cytokines, including IL-1β, granulocyte-macrophage colony–stimulating factor (GM-CSF), and interferon-γ (IFN-γ), in vivo, and an adjuvant activity of chitin has been identified. Shibata et al. (11) subsequently reevaluated the immunological effects of chitin in vivo and in vitro using fractionated, phagocytosable, small-sized chitin particles. Intravenous administration of fractionated chitin particles (1 to 10 µm) induced alveolar macrophage production of IL-12, TNF-α, and IL-18 and natural killer (NK) cell production of IFN-γ (11). These macrophage effects were at least partially mediated by the macrophage mannose receptor (30). In keeping with this type 1 biased immune response, the studies of Shibata et al. (10) have demonstrated that chitin inhibits Th2 immunity, including IgE production and lung eosinophilia. Similarly, intranasal application of chitin microparticles downregulated the symptoms of allergic hypersensitivity to antigens from Dermatophagoides pteronyssinus or Aspergillus furmigatus (31), and water-soluble chitosan (deacetylated chitin) reduced allergen-induced inflammation via TSLP and arginase I (32).
In seeming contrast to these studies, Reese et al. (7) recently reported that direct intrapulmonary administration of chitin beads coated with reacetylated chitosan stimulated the recruitment of IL-4-positive cells, in particular eosinophils and basophils, into the lung via a signal transducer and activator of transcription-6 (STAT-6)-independent, Rag-independent, and leukotriene B4–dependent mechanism(s). Reese et al. also demonstrated that alternative activation of macrophages was critically involved in chitin-mediated inflammatory responses. These investigators speculated that these studies provide mechanistic clues relevant to the high prevalence of asthma in workers who have high environmental exposure to chitin (33, 34).
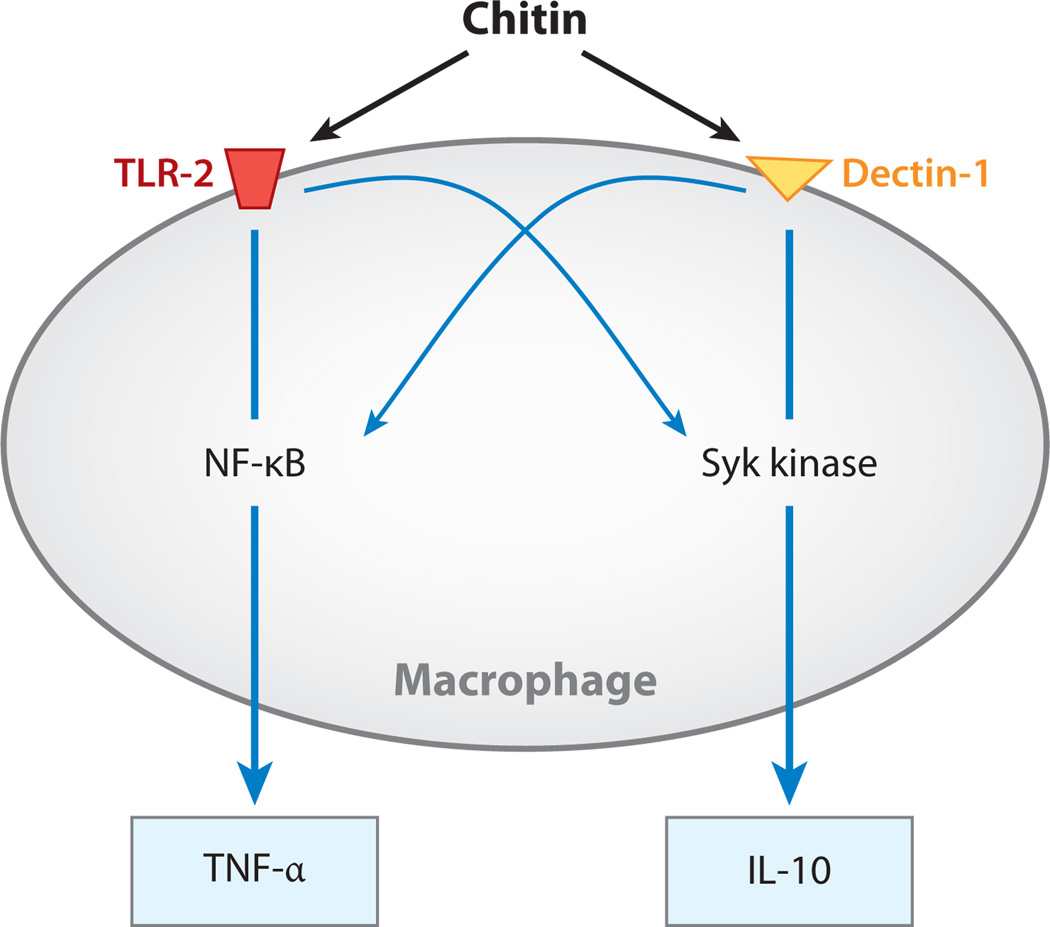
On superficial analysis, the studies noted above report seemingly contradictory findings, with chitin inducing type 1 and type 2 innate responses and acting as an adjuvant while inhibiting adaptiveTh2 reactions. Recent studies from our laboratory have, however, provided insights into at least one of the likely explanations for these findings by demonstrating that different chitin preparations, particularly preparations of different sizes, have different effects and activate inflammation via different mechanisms. In these in vivo and macrophage-based in vitro studies, large chitin fragments were inert. In contrast, intermediate-sized chitin (40–70 µm) stimulated macrophage IL-17A production and IL-17A receptor expression via a pathway(s) that involved Toll-like receptor (TLR)-2 and MyD88. We further demonstrated that chitin uses a TLR-2-, MyD88-, and IL-17A-dependent mechanism(s) to induce acute inflammation (35). Interestingly, when even smaller chitin fragments were utilized, additional insights were obtained. In these studies, both intermediate-sized chitin (40–70 µm) and small chitin (<40 µm, usually 2–10 µm) stimulated TNF-α elaboration. In contrast, only the small chitin induced IL-10 elaboration. Pathways that involved TLR-2, dectin-1, and nuclear factor-κB (NF-κB) mediated the effects of intermediate-sized chitin. In contrast, the effects of small chitin were mediated by TLR-2-dependent/TLR-2-independent, dectin-1-dependent pathways that involved the mannose receptor and spleen tyrosine kinase (Syk). When viewed in combination, these studies demonstrate that chitin contains size-dependent pathogen-associated molecular patterns that stimulate TLR-2, dectin-1, and the mannose receptor; that differentially activate NF-κB and Syk; and that stimulate the production of pro- and anti-inflammatory cytokines (20) (Figure 2). Most recently, studies from our laboratory demonstrated that 40–70-µm chitin is a multifaceted adjuvant for Th2, Th1, and Th17 responses (13). When the entire body of literature is viewed in combination, it is clear that chitin induces variable host responses depending on its size and possibly the nature of the chitin employed and on the methods of preparation, timing, and route of administration.
Figure 2.
Chitin regulation of macrophage proinflammatory [tumor necrosis factor (TNF)-α] and anti-inflammatory [interleukin (IL)-10] cytokine production. Chitin stimulates the expression of TNF-α and IL-10 in a size-dependent manner. Large chitin is inert, and intermediate-sized chitin (40–70 µm) is a powerful stimulator of TNF. This activation is mediated mainly via a Toll-like receptor-2–nuclear factor-κB (TLR-2-NF-κB) pathway, with lesser contributions from spleen tyrosine kinase (Syk). In contrast, small chitin (<40 µm, usually 2–10 µm) is a potent stimulator of IL-10 production. This inductive event is mediated largely via a dectin-1-Syk pathway, with lesser contributions from TLR-2 and NF-κB.
THE 18 GLYCOSYL HYDROLASE FAMILY IN MAMMALS
Both chitinases and C/CLPs contain a glyco-18 domain, the characteristic feature of evolutionarily conserved 18 glycosyl hydrolase family. Family 18 chitinases are expressed in a wide range of organisms from prokaryotes to eukaryotes, including mammals (for evolutionary insight of this family, see References 36 and 37 for reviews). Chitin is the only documented substrate for these enzymes and is readily appreciated in lower life forms. Interestingly, although true chitinases are found, chitin and chitin synthases have not been detected in mammals. As a result of this conundrum, endogenous carbohydrates that have structural similarities with chitin, such as heparan sulfate and hyaluronic acid, have been proposed to be substrates of mammalian chitinases. Direct proof of this interaction, however, has not been demonstrated. Currently, more than seven family 18 chitinases and CLPs have been identified in mice and humans (Table 1). AMCase, chitotriosidase (chitinase 1), oviductin, YKL-40/HcGP-39 (chitinase 3-like 1), YKL-39, Stabilin-1-interacting chitinase-like protein (SI-CLP), and chitinase domain–containing 1 (CHID1) have been described in humans. AMCase, chitotriosidase, oviductin, BRP-39, SI-CLP, and Ym1/Ym2 have been described in mice (38–44). Ym1 and Ym2, which are produced by macrophages after parasitic (41) or fungal (45) infection, are mouse specific because comparable genes have yet to be described in humans.
Table 1.
Representative members of the 18 glycosyl hydrolase family in humans and micea
| Name | Alias(es) | Humans | Mice |
|---|---|---|---|
| Acidic mammalian chitinase (AMCase) | CHIA, eosinophil chemotactic cytokine | + | + |
| Chitotriosidase | Chitinase 1 | + | + |
| YKL-40/BRP-39 | CHI3L1, cartilage glycoprotein 1, GP-39, HcGP-39 | + | + |
| Oviductin | Oviductal glycoprotein 1 | + | + |
| Chondrocyte protein 39 | CHI3L2, YKL-39 | + | |
| Stabilin-1-interacting chitinase-like protein (SI-CLP) | + | + | |
| Ym1 | Chi3l3 | + | |
| Ym2 | Chi3l4 | + |
Additional sequences belonging to the 18 glycosyl hydrolase family, such as Ym3, Bclp2, and BYm, are described in mice.
An overview of these moieties reveals two big categories. The first category comprises the true chitinases, of which there are only two: AMCase and chitotriosidase. All the rest are CLPs (also termed chitolectins) that lack chitinase activity as a result of mutations in their highly conserved putative enzyme sites (40, 41). BRP-39/YKL-40, Ym1, and Ym2 are the prototypes of this subfamily. As a result, their roles in biology are particularly enigmatic. A complete understanding of the biology of the C/CLPs requires the elucidation of the roles of true chitinases and the CLPs. Insights into the roles of these moieties have only recently begun to be elucidated. AMCase regulates innate (7) and adaptive (5) Th2 inflammatory and remodeling responses. Studies from our laboratory and others have demonstrated that YKL-40 is dysregulated in a variety of human disorders and that BRP-39/YKL-40 contribute to adaptive Th2 inflammation and fibrosis and inhibit oxidant-induced lung injury (4, 46, 47). Additional details are provided below.
True Chitinases (Chitinases with Chitolytic Enzyme Activity)
AMCase and chitotriosidase contain both a chitin-binding domain and an enzymatically active domain that catalyze hydrolysis of β(1 → 4) glycosidic bonds in carbohydrate. Although no endogenous substrate for these true chitinases has been identified in mammals, recent studies suggest that they have significant roles in inflammation, tissue injury responses, and pathogenesis of human diseases.
AMCase
AMCase is produced by lung epithelial cells, macrophages, and eosinophils at sites of Th2 inflammation (5). In the IL-13-exposed murine airway, AMCase is expressed mainly in distal non-mucous-producing epithelial cells (48). Interestingly, IL-13 is necessary and sufficient for the induction of this chitinase (5). This induction appears to involve the epidermal growth factor receptor (EGFR) because in adaptive inflammation and IL-13 transgenic mice, epithelial AMCase expression was paralleled by and colocalized with increased expression of EGFR and its activator, TNF-α-converting enzyme (TACE). In airway epithelial cells (A549), cotransfection of AMCase with EGFR enhanced, whereas blocking of EGFR function using small-molecule kinase inhibitors impaired, AMCase secretion. Blocking the EGFR upstream transactivator TACE or the EGFR downstream molecule Ras further inhibited the secretion of AMCase. These studies demonstrate that AMCase secretion is regulated via a TACE/EGFR/Ras-dependent pathway (49). AMCase inhibits the innate type 2 inflammation induced by reacetylated chitosan beads (7). AMCase also plays a critical role in adaptive Th2 inflammation and remodeling in chitin-free situations (5). The former is mediated by the chitinase activity of this enzyme. However, the chitinase activity of AMCase may not mediate all the biological effects of AMCase. For instance, point mutations that abrogate the chitinase activity of AMCase do not eliminate its antiapoptotic effect on the epithelium (50).
Chitotriosidase (chitinase 1)
Chitotriosidase is the best-characterized true chitinase from a clinical and biological perspective. Mature monocyte-derived macrophages, Gaucher’s cells, and lung macrophages express this chitinase. Proinflammatory cytokines (e.g., GM-CSF, TNF-α) and lipopolysaccharide (LPS) stimulate the expression of chitotriosidase in monocyte-derived macrophages, whereas IFN-γ and IL-4 inhibit chitotriosidase expression (51–53). Two enzymatically active isoforms of chitinase 1 (of 50 kDa and 39 kDa) and an alternatively spliced 40-kDa variant have been identified in splenic or Gaucher’s cells. The 50-kDa moiety is the predominantly secreted isoform, whereas the 39-kDa and 40-kDa isoforms are expressed and stored in intracellular lysosomes and lysosome-related organelles (LROs) (54, 55). The biological significance of this chitinase as a component of lysosomes and LROs in disease pathogenesis is not fully understood. However, elevated levels of chitinase 1 expression have been described in lysosomal storage diseases (e.g., Gaucher diseases, Niemann-Pick syndrome, Fabry disease). Elevated levels are also seen in a variety of other diseases, including infections (fungal and bacterial infection, malaria), chronic inflammation (atherosclerosis, sarcoidosis), liver diseases [nonalcoholic fatty liver disease–steatohepatitis (NASH)], and neurodegenerative diseases (Alzheimer’s disease, ischemic cerebrovascular dementia) (for reviews, see References 56 and 57).
Chitinase-Like Proteins
BRP-40/YKL-40, YKL-39, SI-CLP, and Ym1/Ym2 are CLPs (56). Oviductin/MUC9 also contains an additional long stretch of O-glycosylation at its carboxyl terminal, characteristic of mucins. These CLPs can bind chitin by inducing conformational changes that depend on the length of the oligosaccharides in a similar fashion as catalytically active family 18 chitinases (58). However, due to mutations in their active domains, CLPs do not have chitinolytic enzyme activity (for a review, see Reference 56).
BRP-39/YKL-40 (Chi3l1)
BRP-39 was originally discovered in mouse breast cancer cells (59). Subsequently, researchers described a variety of homologs with different names, including human HcGP-39, human YKL-40, porcine 38-kDa heparin-binding glycoprotein (GP38K), bovine 39-kDa whey protein, and Drosophila imaginal disc growth factors (60–63). BRP-39, HcGP-39, and YKL-40 are on chromosome 1 in mice and humans and are synthesized as 39-kDa proteins that lack chitinase activity. BRP-39/YKL-40 are expressed in a variety of cells, including macrophages; neutrophils; chondrocytes; fibroblasts; vascular smooth muscle cells; endothelial cells; hepatic stellate cells; and colonic, ductal, and airway epithelial cells (4, 61). YKL-40 is not expressed in monocytes and is marginally expressed in monocyte-derived dendritic cells but is strongly induced at late stages of human macrophage differentiation. Thus, YKL-40 is regarded as a macrophage differentiation marker (64, 65). The transcriptional factor Sp1 regulates the expression of BRP-39/YKL-40. The YKL-40 promoter sequence also contains consensus binding sites for several known transcription factors and can support the specific binding of nuclear PU.1, Sp1, Sp3, upstream stimulatory factor (USF), acute myeloid leukemia (AML)-1, and CCAAT/enhancer-binding protein (C/EBP) (65). The expression of BRP-39/YKL-40 is regulated by various cytokines and hormones, including IL-6, IL-13, IFN-γ, vasopressin, and parathyroid hormone–related protein (66). The inflammatory cytokines TNF-α and IL-1β also stimulate the expression of YKL-40 in articular chondrocytes (67). In contrast, YKL-40 inhibits cellular responses induced by IL-1 and TNF-α (68), suggesting that the induction of BRP-39/YKL-40 feeds back to control local tissue responses. Selective repression of YKL-40 by NF-κB activation through recruitment of histone deacetylases was also demonstrated in glioma cells (69). These mechanisms may contribute to the dysregulation of YKL-40 seen in a number of human diseases characterized by acute or chronic inflammation and tissue remodeling (see below). YKL-40 levels are a predictor of all-cause mortality in the elderly (70) and have a significant association with rates of overall and cardiovascular mortality (71). However, the biological roles of YKL-40 in these events have only recently begun to be defined.
YKL-39 (Chi3l2)
YKL-39 is expressed in chondrocytes and synoviocytes (72). It is also expressed in an exaggerated manner, with YKL-40, in the microglia of patients with Alzheimer’s disease (73). The biological and clinical significance of YKL-39 has, however, not been appropriately defined.
Ym1/Ym2 (Chi3l3/Chi3l4)
Ym1 and Ym2, which are rodent-specific CLPs, are expressed in macrophages after stimulation with IL-4 or IL-13 and in response to parasitic infection or allergic stimulation (41, 74, 75). Substantial evidence suggests that they may play an important role(s) in the development of Th2 inflammation.
Oviductin (mucin 9, oviduct-specific glycoprotein)
Oviductin is exclusively expressed and secreted by oviductal epithelium (76). Its expression is regulated by hormones such as estradiol and luteinizing hormone. The biology of oviductin has not been adequately defined.
SI-CLP
SI-CPL is produced by a variety of cells, including primary macrophages, Jurkat cells, tumor cell lines, and sinusoidal endothelial cells in liver, spleen, lymph node, and bone marrow (43). The expression of SI-CLP was strongly induced by the Th2 cytokine IL-4 and by the glucocorticoid dexamethasone. Exaggerated SI-CLP expression has been noted in chronic airway inflammation and in patients with sarcoidosis. Because SI-CLP is the only CLP that is upregulated by glucocorticoids, the level of macrophage steroid-induced SI-CLP may be used as a marker for individual responsiveness to corticosteroid treatment as well as a predictor of side effects. This CLP is also targeted to lysosomes through its interaction with stabilin-1 (77).
C/CLPS IN Th2 INFLAMMATION, OXIDANT-INDUCED LUNG INJURY, AND HUMAN DISEASES AND DISORDERS
As noted above, our understanding of the roles of C/CLPs in human disease is still quite limited. However, several studies have demonstrated the importance of C/CLPs in murine models of Th2 and oxidant-induced lung injury, and several research groups have reported significant dysregulation of these genes in asthma and bronchopulmonary dysplasia (BPD). Our present concepts of asthma pathogenesis, the roles of chitinases and CLPs in Th2 inflammation and oxidant-induced lung injury, and the alterations in YKL-40 in asthma, BPD, and other human diseases are summarized below.
Concepts of Asthma Pathogenesis
Asthma is one of the most common chronic airway disorders, with a current global prevalence of more than 300 million and a projected global prevalence of 400 million by 2025 (78, 79). Accordingly, asthma imposes an enormous economic burden; the annual cost of asthma management is approximately $20 billion per year in the United States alone (80).The clinical significance and the economic burden of asthma have fueled intense research on its pathogenesis. In contrast to earlier concepts that focused on bronchospasm, inflammation is now believed to play a central role in the disorder (81–85). The analysis of bronchoalveolar lavage (BAL) and bronchial biopsy in patients with asthma has provided a glimpse of the complexity of this response. A variety of cells and mediators are involved in aeroallergen-induced asthma; immunoglobin (Ig)E triggers mast cells in the acute response; eosinophils may be important effectors in the late response; and T cells (particularly Th2 cells) play key roles in the recognition of antigen and orchestration of airway events via their production of IL-4, IL-5, IL-9, IL-13, and other cytokines (82–84, 86–90). Type 2 cytokine–producing cells are now believed to be major contributors to the initiation and maintenance of airway inflammation, the regulation of B cell and eosinophil function, the induction of mucus, and the stimulation of remodeling (91, 92).
As a result, the critical genes that are downstream of Th2 cytokines and that mediate their asthma-relevant effects are being investigated. IL-13 is a product of a gene on chromosome 5 at q31 that is produced by stimulated CD4+ Th2 cells (93, 94) and possibly by the recently described nuocytes, MPPtype2 cells, and natural helper cells (3). IL-13 has a variety of effects that are relevant to asthma and Th2 inflammation, including the ability to induce IgE production (95), CD23 expression (96), mucus hypersecretion and goblet cell hyperplasia (85, 87, 90, 97), and endothelial P-selectin and vascular cell adhesion molecule-1 (VCAM-1) expression (98, 99) and to inhibit eosinophil apoptosis (100). Studies from our laboratory demonstrated that the overexpression of IL-13 in the murine lung causes eosinophil-, lymphocyte-, and macrophage-rich inflammation; mucus metaplasia; airway hyperresponsiveness (AHR) on methacholine challenge; and airway fibrosis (97). The exaggerated production of IL-13 is well documented in atopic and nonatopic asthma (89, 101, 102), and polymorphisms in the IL-13 promoter and coding region have been associated with asthma in study populations (103). As a result of these observations, deregulated IL-13 production is now felt to be a cornerstone in the pathogenesis of Th2 inflammation and remodeling, the pathological hallmarks of asthmatic airways. However, the cellular and molecular events that are responsible for these IL-13-induced tissue alterations have not been fully defined.
Chitinases in Th2 Inflammation
Host immune responses to parasites include an early innate component designed to control or eliminate parasitic infestation and, over time, an adaptive response that controls the parasite in an antigen-specific fashion. The latter is frequently Th2 dominated and shares key features with tissue allergy, including the increased production of IL-4, IL-5, IL-13, and IgE and prominent tissue eosinophilia. As a result, it is believed that Th2 inflammatory responses originally evolved to deal with parasites and other pathogens and that allergy, atopic asthma, and their associated inflammatory and remodeling responses occur when these Th2 responses are poorly controlled and/or are elicited in a parasite-independent fashion (104, 105). An ideal type 2 response would control infection, minimize local tissue injury, foster protective immunity, and augment healing. The innate response is a major controller of the invading pathogen. Regulatory mechanisms that inhibit and fine-tune the innate response and augment adaptive immunity would minimize collateral tissue injury, ensure lasting control of the infectious agent, and contribute to long-term immunity.
AMCase has a role in many of these events. In keeping with the ability of chitin in parasites and some antigens to stimulate type 2 innate immune responses, AMCase ameliorates chitin-induced innate type 2 inflammation in the lung (7). In adaptive Th2 responses induced by chitin-free aeroallergens, AMCase is dramatically upregulated in airway epithelial cells and macrophages (5). Importantly, when the binding capacity and enzymatic activity of AMCase were simultaneously abrogated with C/CLP inhibitors (allosamidin or dimethylallosamidin) or neutralizing antiserum, allergen-induced airway inflammation and AHR were significantly diminished (5, 106). Knocking down AMCase also abrogated aeroallergen-induced AHR, eosinophil infiltration, and eotaxin and IL-13 secretion and decreased levels of serum antigen-specific IgE (107). Similarly, AMCase expression was induced in the lungs of IL-13-overexpressing transgenic mice, and interventions with allosamidin or neutralizing antibody also significantly reduced IL-13-induced eosinophilic inflammation, AHR, and chemokine production (5). In vitro studies further demonstrated the ability of AMCase to stimulate chemokine production directly from epithelial cells (5, 49).
Some microbial and plant chitinases are also potent allergens that contribute to avocado, latex, and house dust mite (Def f 15) allergies (40, 108, 109). These studies suggest that AMCase plays a different role, depending on the type of stimulant and the stage of the immune response in question. Specifically, these studies demonstrate that AMCase inhibits innate type 2 reactions while augmenting adaptive responses and contributing to the effector responses induced by IL-13. When such studies are viewed in combination, one can hypothesize that AMCase, in addition to protecting the host by directly injuring chitin-containing pathogens, also contributes to optimal type 2 responses by controlling local innate responses and augmenting the evolution of adaptive immunity. Additional investigations will be required to define the relevance of this conceptualization to chitin-free stimulators of innate immunity and chitin-containing antigens that stimulate adaptive immune responses. The relative contributions of ligand binding and enzymatic activity will also need to be elucidated. Lastly, caution must also be employed in attempts to extrapolate studies of AMCase from mice to humans because AMCase is the major chitinase in lungs from mice, whereas chitotriosidase is the major chitinase in human respiratory tissues (110, 111).
AMCase in Asthma
Recent studies described the exaggerated expression of AMCase in lung tissues from patients with asthma (5). Associations between AMCase polymorphisms and asthma have also been described (112, 113). However, other investigations did not detect a significant correlation between AMCase activity or AMCase polymorphisms and the disorder (111, 114). These differences may be technical because the severity of asthma in the studied populations was not the same and environmental differences were not adequately addressed. Additional investigation will be required to address this issue in more detail.
CLP in Th2 Inflammation
Although the exact biological functions of CLP are still largely elusive, the regulation and roles of Ym1/Ym2 and BRP-39/YKL-40 have been investigated. Ym1 and Ym2 are highly homologous with each other and are rodent specific. Ym1 is induced by Th2 cytokines and allergen challenge via a STAT-6-dependent mechanism (75). Ym1 expression is also induced in lungs of mice infected with the parasite Nocardia brasiliensis (115), and Ym2 expression is upregulated in the lungs of aeroallergen-immunized and challenged mice in a IL-4 signaling– and IL-13 signaling–dependent manner (74). The Ym1 and Ym2 proteins are also commonly encountered as crystals at sites of pulmonary type 2 inflammation, as seen in moth-eaten mice (45), IL-13-overexpressing transgenic mice (97), and aeroallergen-sensitized and aeroallergen-challenged mice. Additionally, Ym1 and Ym2 are highly expressed in macrophages, dendritic cells, and mast cells in proliferative dermatitis, which is characterized by the accumulation of eosinophils and neutrophils (116). How Ym1 and Ym2 contribute to rodent biology is not clearly understood. However, in vitro assays using purified proteins demonstrated that the Ym proteins are chemotactic for eosinophils, T lymphocytes, and polymorphonuclear leukocytes (117).
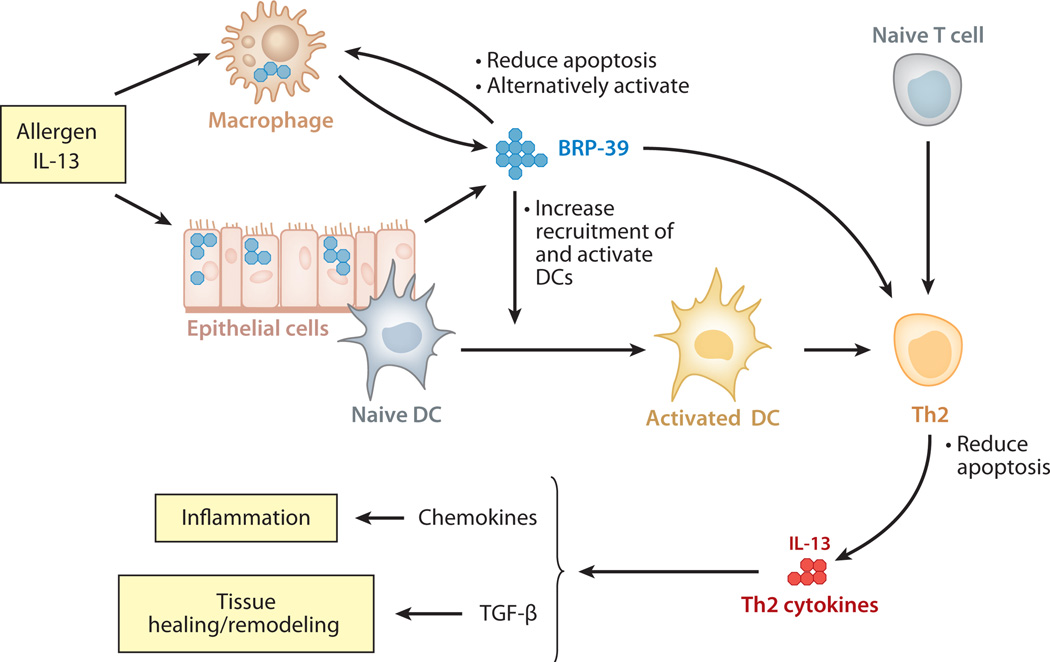
These findings suggest that these moieties may play important roles in Th2 responses in the proper setting. In keeping with YKL-40 levels in the serum of normals and its impressive dysregulation in human diseases and disease models, recent studies have attempted to define the role(s) of BRP-39/YKL-40 in biology. To accomplish this, BRP-39 null mutant mice and lung-specific YKL-40–overexpressing transgenic mice were generated and characterized (4). The null mutant mice had a significant defect in antigen- or IL-13-induced Th2 inflammation and remodeling, and transgenic expression of YKL-40 in the epithelium was sufficient to rescue this defect (4). These studies further demonstrated that BRP-39 and YKL-40 accomplish their proinflammatory effect at least in part by inhibiting inflammatory cell (T cell, macrophage, and eosinophil) apoptosis/cell death through inhibition of Fas expression and by inducing the phosphorylation of protein kinase B (PKB)/Akt. BRP-39/YKL-40 also stimulate dendritic cell accumulation and activation and induces alternative macrophage activation (Figure 3). In vitro assays with connective tissue cells demonstrated the ability of YKL-40 to activate the mitogen-activated protein (MAP) kinase and PKB/Akt pathways (118).
Figure 3.
Contributions of BRP-39/YKL-40 to allergen-induced Th2 inflammation and tissue remodeling. At sites of adaptive Th2 inflammation, cytokines such as interleukin (IL)-13 stimulate BRP-39/YKL-40 production in macrophages and epithelial cells. BRP-39/YKL-40, in turn, contribute to this response by preventing macrophage apoptosis and inducing alternative (M2) macrophage differentiation, by increasing the number of and activating local dendritic cells (DC), and by inhibiting Th2 cell apoptosis. The Th2 cytokines that are then produced induce chemokines and transforming growth factor (TGF)-β1, which contribute to inflammation, remodeling, and healing.
Studies with genetically manipulated mice also identified a novel role of BRP-39 in IL-13-induced tissue fibrosis. Previous studies from our laboratory showed that the fibrogenic effects of IL-13 are mediated, at least in part, by the ability of IL-13 to induce and activate TGF-β1 (119). Intriguingly, TGF-β1 induction in the lungs of IL-13 transgenic mice was significantly decreased in mice deficient in BRP-39. The cellular and molecular mechanism by which BRP-39 links IL-13 and TGF-β1 remains to be determined. The observation that BRP-39/YKL-40 contribute to tissue fibrosis, however, provides interesting insights into the potential roles of YKL-40 in fibrotic human disorders. The studies noted above suggest that BRP-39 plays an important role both in antigen sensitization and in the effector phases of allergic inflammation and remodeling (Figure 3). However, when viewed in combination with the recent observation that BRP-39/YKL-40 also inhibit oxidant injury (see below), one can speculate that BRP-39 can contribute to optimal type 2 responses by inhibiting innate immune–induced tissue injury, by augmenting the transition to adaptive immunity, and by fostering tissue healing. The mechanisms underlying these effects and the receptors and binding partners that mediate them still need to be defined.
BRP-39/YKL-40 in Human Asthma and Atopy
The exaggerated expression of CLPs in animal models of asthma prompted human studies to define the relationship between YKL-40 and this disorder. Studies of three cohorts of patients and controls (two from the United States and one from France) demonstrated that levels of serum YKL-40 are significantly elevated in patients with asthma compared with controls (120).These studies also demonstrated that levels of YKL-40 expression in the lung correlate with levels in the circulation and that levels of circulating YKL-40 are higher in patients with severe disease and airway remodeling. In these experiments, levels of serum YKL-40 positively correlated with asthma severity and correlated inversely with the forced expiratory volume in 1 s (FEV1) (120). Subsequent genetic studies in a founder population of European descent, the Hutterites, identified a promoter single-nucleotide polymorphism (SNP) in the CHI3L1 gene encoding YKL-40; this SNP was associated with elevated levels of serum YKL-40 and the prevalence of asthma, AHR, and abnormal pulmonary function. These studies identified CHI3L1 as a susceptibility gene for asthma, bronchial hyperresponsiveness, and reduced lung function (121). They also provided support for the concept that YKL-40 plays a significant role in the development and progression of asthma and established YKL-40 as a biomarker of disease severity and a therapeutic target in this disorder. A separate genetic study in a Korean cohort identified genetic variations in CHI3L1 in patients with atopy (122). In that study, SNPs in the promoter region and intron 7 were associated with the development of atopy. In vitro assays also demonstrated that the genetic variation in the promoter region of CHI3L1 (g.-237T risk allele) functionally regulates the expression of YKL-40 via increased affinity for the C/EBP. Subjects with this variant allele had significantly higher levels of circulating YKL-40 and IgE. These studies provide additional evidence supporting the concept that YKL-40 plays an important role in allergic diseases.
BRP-39/YKL-40 in Oxidant Injury and Tissue Remodeling
Oxidant-induced lung injury is a major cause of respiratory dysfunction. This can be readily appreciated in its most severe forms, acute respiratory distress syndrome (ARDS) in adults (123) and BPD in premature infants (124). Currently, no specific or effective interventions prevent BPD or ameliorate established BPD, and no established biomarkers predict its occurrence in premature newborns (124). In addition, although significant efforts have been directed at identifying the mechanisms underlying these disorders, our understanding of the pathogenesis of these adverse events is very primitive. Studies have been undertaken to determine if BRP-39/YKL-40 play a role in pulmonary hyperoxic acute lung injury (HALI). These studies demonstrate that exposure to 100% O2 significantly reduces the expression and secretion of BRP-39 in the murine lung (46). They also demonstrate that mice that lack BRP-39 have exaggerated susceptibility to 100% O2 manifesting as augmented alveolar-capillary permeability and protein leak, tissue oxidation, neutrophil and macrophage-rich inflammation, chemokine elaboration, epithelial apoptosis, and premature death. The protective role of BRP-39/YKL-40 was further supported by the observation that transgenic YKL-40 ameliorates HALI, prolongs survival after 100% O2 exposure, and rescues the exaggerated injury in BRP-39 null mice. These studies highlight a novel relationship between BRP-39/YKL-40 and oxidant injury: Oxidant injury decreases the expression and production of BRP-39, and BRP-39 and YKL-40 inhibit oxidant-induced lung injury, vascular permeability, and structural cell apoptosis.
Similarly, in a murine cigarette smoke exposure model in which oxidants play a major role in tissue injury, a null mutation of BRP-39 enhanced alveolar destruction and augmented structural cell apoptosis (47). These studies suggest that endogenous BRP-39 is required for the maintenance of cellular integrity after oxidant injury. Such studies also allow for the intriguing hypothesis that levels of circulating and/or pulmonary BRP-39/YKL-40 are under tight control and any alteration of these levels have significant pathological consequences. Physiological levels of BRP-39/YKL-40 ameliorate the oxidant injury that is caused by day-to-day exposures, whereas decreased levels enhance oxidant injury, as occurs in BPD. In contrast, when levels are too high, inflammation, remodeling, and malignant transformation may occur (see below).
BRP-39/YKL-40 IN OTHER HUMAN DISEASES
During the past decade or so, dysregulation of YKL-40 has been noted in a wide variety of human disease and disorders characterized by acute inflammation, chronic inflammation, and/or tissue remodeling (Table 2). Selected associations are highlighted below.
Table 2.
Human diseases associated with dysregulated YKL-40 expression
| Acute infections |
| Purulent meningitis |
| Pneumonia |
| Chronic inflammatory conditions |
| Asthma/allergy |
| Atherosclerosis |
| Types 1 and 2 diabetes mellitus |
| Rheumatoid arthritis/osteoarthritis |
| Systemic lupus erythematosus |
| Inflammatory bowel disease |
| Chronic obstructive lung disease |
| Cancers (primary and/or metastatic) |
| Osteosarcoma, glioblastoma, breast, colon/rectum, ovary, lung, prostate, kidney, malignant melanoma |
| Others |
| Alcoholic liver cirrhosis |
| Liver fibrosis |
| Schizophrenia |
| Giant-cell arteritis |
Infectious Diseases
Levels of circulating YKL-40 are augmented in patients with infections, for example, in patients with purulent meningitis (125), pneumonia (126), and cystic fibrosis with acute lung infection (127). This pattern is especially significant in patients with streptococcal pneumonia, in whom levels correlate positively with mortality. Because YKL-40 is expressed mainly by activated macrophages and neutrophils and is released by exocytosis from specific granules at the site of inflammation, it can serve as a diagnostic and prognostic serologic marker of specific bacterial infections (128). The mechanism of secretion of YKL-40 and its in vivo role in antibacterial responses, however, have not been defined.
Chronic Inflammatory and Remodeling Diseases
Plasma levels of YKL-40 are increased in chronic inflammatory and remodeling disorders including rheumatoid arthritis (RA), osteoarthritis (OA), systemic lupus erythematosus (129), inflammatory bowel disease (129–131), sarcoidosis (132), and chronic obstructive lung disease (47, 133). Liver diseases characterized by hepatic inflammation and fibrosis such as chronic alcoholic hepatitis or cirrhosis also showed elevated levels of circulating YKL-40 (134, 135), which correlated with the degree of liver fibrosis (136). Elevated serum YKL-40 levels are a serologic marker of hepatitis C virus–mediated fibrosis, autoimmune hepatitis–induced cirrhosis, and primary biliary cirrhosis (135, 137).The role of YKL-40 in RA deserves specific mention. In RA, YKL-40 is an autoantigen (138, 139), approximately 50% of RA patients have YKL-40 peptide complexed with major histocompatibility complex (MHC), and dendritic cells presenting YKL-40 peptide have been detected in synovial tissues from RA patients (140). Importantly, levels of YKL-40 in serum and synovial fluid of RA and OA patients reflect the severity of synovial inflammation and articular cartilage degradation and correlate positively with disease activity and progression (141).
Atherosclerosis and Giant-Cell Arteritis
The pathological lesion of atherosclerosis is characterized by the infiltration of monocytes into the walls of blood vessels and subsequent macrophage activation and lipid accumulation. Interestingly, the expression of YKL-40 and chitotriosidase is impressively augmented in a distinct subset of macrophages in the atherosclerotic plaque. This prominent expression of YKL-40 is seen in deeply infiltrated macrophages and in macrophages in early stages of atherosclerotic lesion development. In addition, macrophages stimulated with oxidized low-density lipoprotein, a process that mimics the formation of foam cells, secreted high levels of YKL-40 (142). Similarly, the expression of YKL-40 was also detected in CD68+ giant cells and circulating mononuclear cells in giant-cell arteritis, another inflammatory vessel disorder (143).These studies suggest that YKL-40 plays a role in the development of vascular disorders that are characterized by macrophage/monocyte accumulation and activation. The mechanism(s) of this contribution, however, has not been defined.
Cancer
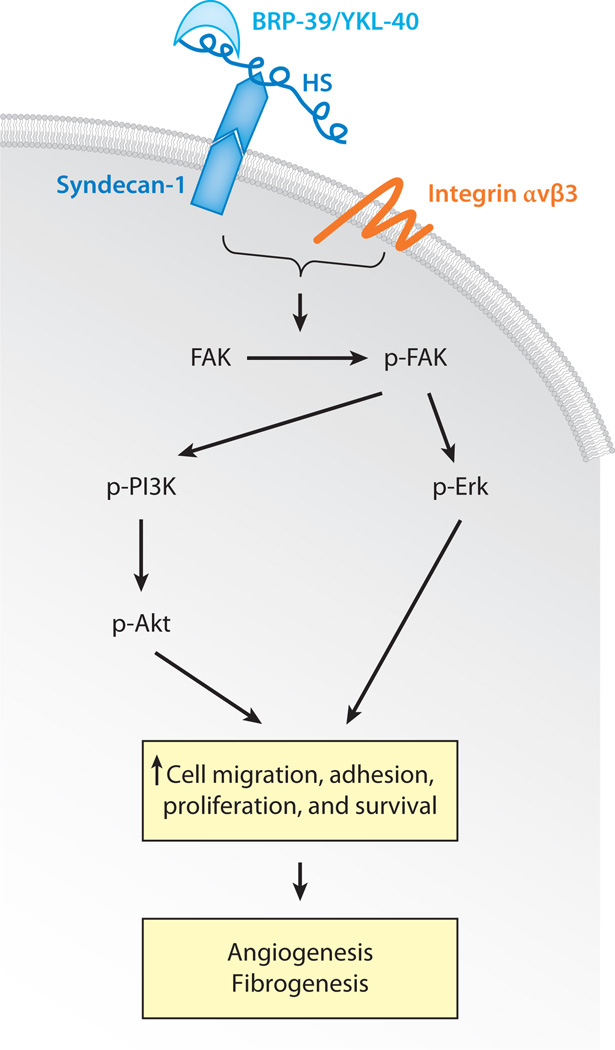
A number of studies have demonstrated strong correlations between YKL-40 expression and the development of primary and metastatic tumors. The types of tumors include tumors of the breast, colon/rectum, ovary, lung, prostate, kidney, brain (glioblastomas), and bone (osteosarcomas) and melanomas (for details, see reviews in References 128, and 144). Levels of YKL-40 frequently correlate inversely with disease-free interval and survival. These studies support the contention that YKL-40 plays a role in the development and progression of a variety of malignancies. However, the mechanisms underlying these YKL-40-cancer associations have not been defined. In vitro studies have demonstrated that YKL-40 is secreted by osteosarcoma (145), glioblastoma (146), and myeloid leukemia cell lines (65) and is strongly expressed by tumor-associated macrophages in small-cell lung cancer biopsies (147). This is potentially important because YKL-40 can contribute to tumor invasion and metastasis through the regulation of cancer cell proliferation and differentiation. In addition, the inhibition of YKL-40 of cancer cell apoptosis in a manner similar to its effects on the survival of inflammatory cells can also contribute to tumorigenesis. Finally, YKL-40 can also modulate certain aspects of the host microenvironment such as angiogenesis (148). Recent studies of YKL-40 and tumor angiogenesis have provided novel mechanistic insights into the regulatory pathways that may mediate these vascular effects (148) (Figure 4). Such studies demonstrated that YKL-40 binds the heparan sulfates of Syndecan-1, a major proteoglycan on the epithelial cell surface, and activates (phosphorylates) focal adhesion kinase (FAK) together with integrin αvβ3. This induces angiogenesis through the subsequent activation of MAP kinase/Erk (148). FAK can also activate phosphatidylinositol 3 kinase (PI3K)/Akt signaling pathways that are critical for cell survival, differentiation, and fibrogenesis (149). Whether Syndecan-1 and its signaling pathways, as shown in angiogenesis, are equally important for the inflammatory, proliferative, and fibrogenic effects of BRP-39/YKL-40 remains to be determined. Of note, serum levels of YKL-40 are being recognized as a promising diagnostic and prognostic biomarker for malignancy, and YKL-40 is increasingly appreciated as a therapeutic target in cancer (144).
Figure 4.
The proposed signaling pathways of YKL-40 in regulating angiogenesis and cellular proliferation. BRP-39 binds to the heparan sulfate (HS) of Syndecan-1, a major cell surface proteoglycan. Along with integrin αvβ3, such binding activates focal adhesion kinase (FAK). Phosphorylated FAK (p-FAK) activates downstream signaling molecules, including mitogen-activated protein (MAP) kinase Erk (p-Erk), phosphatidylinositol 3 kinase (p-PI3K), and Akt (p-Akt). Such activation induces cellular proliferation, adhesion, and survival, all of which contribute to the development and progression of angiogenesis. Similar pathways may contribute to tissue-remodeling responses such as those seen in fibrosis.
Diabetes Mellitus
Levels of circulating YKL-40 are increased in patients with type 1 (150) and type 2 (151, 152) diabetes. In the latter group of patients, YKL-40 levels correlate positively with insulin resistance and features of dyslipidemia (151, 152) but do not correlate with body mass index or other indicators of glucose metabolism (151). Patients with type 2 diabetes, especially those with concurrent obesity, commonly display increased macrophage infiltration in adipose tissue. YKL-40 stimulates macrophage production of monocyte chemoattractant protein-1 (MCP-1; also known as CCL2) and other inflammatory chemokines (133), which in turn can alter adipocyte function and augment monocyte infiltration into the subendothelial space (153). Thus, YKL-40 may affect insulin sensitivity through regulation of macrophage infiltration and subsequent activation in adipose tissue.
Bronchopulmonary Dysplasia
Respiratory distress syndrome (RDS) and respiratory failure are problematic consequences of premature birth. These patients are commonly treated with mechanical ventilation, supplemental oxygen, and surfactant preparations (46, 154) and in many cases rapidly recover. However, in a subset of patients, oxidant injury contributes to the development of BPD with chronic respiratory failure, and death can ensue (124). To determine if there is a relationship between CLPs and BPD, recent studies from our laboratory compared YKL-40 levels in the tracheal aspirate from a cohort of premature babies with RDS who developed BPD or died with YKL-40 levels in premature infants with milder disease who did not experience these adverse consequences. In this cohort the premature infants with the milder disease had higher levels of YKL-40. These observations are in accord with mice studies that demonstrated that levels of pulmonary BRP-39 are decreased after exposure to hyperoxia and that YKL-40 diminishes the adverse effects of 100% O2 (46). Such findings also show for the first time that decreases in the levels of a CLP may be important in the initiation and/or pathogenesis of a disease process. If confirmed in larger studies, these results raise the possibilities that elevated levels of YKL-40 cause the milder disease and that investigators could manipulate BRP-39/YKL-40 to control oxidant-induced pulmonary responses.
SUMMARY POINTS.
Chitin contains size-dependent pathogen-associated molecular patterns that stimulate types 1 and 2 innate immune responses while differentially stimulating TLR-2, dectin-1, and the mannose receptor and differentially activating NF-κB and spleen tyrosine kinase.
The 18 glycosyl hydrolase family contains true chitinases (AMCase and chitotriosidase) and CLPs such as BRP-39 and its human homolog YKL-40, which lack chitinase activity.
AMCase ameliorates chitin-induced type 2 inflammatory responses in innate immune cells via its chitinolytic activity while augmenting the adaptive immune responses and physiological dysregulation induced by chitin-free antigens.
BRP-39/YKL-40 play a critical role in the development of aeroallergen-induced adaptive Th2 inflammation and mediates IL-13-induced pulmonary inflammation and fibrosis.
Oxidant injury decreases the expression and production of BRP-39, and BRP-39 and YKL-40 are important inhibitors of oxidant-induced lung injury, vascular permeability, and structural cell apoptosis.
BRP-39/YKL-40, via interactions with binding partners such as Syndecan-1 and integrin αvβ3, can activate the PI3K/Akt and the MAP kinase/Erk signaling pathways. These activation events can promote cell migration, proliferation, and survival; tumor angiogenesis; and other tissue-remodeling responses.
Levels of circulating and/or tissue YKL-40 are increased in a variety of inflammatory, remodeling, and neoplastic disorders such as asthma and various primary and metastatic cancers and are decreased in the BAL fluid of children with BPD compared with newborns with RDS who do not present this complication.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Hartl D, Lee CG, Da Silva CA, Chupp GL, Elias JA. Novel biomarkers in asthma: chemokines and chitinase-like proteins. Curr. Opin. Allergy Clin. Immunol. 2009;9:60–96. doi: 10.1097/ACI.0b013e32831f8ee0. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Investig. 2008;118:3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffman RL. Immunology. The origin of TH2 responses. Science. 2010;328:1116–1117. doi: 10.1126/science.1192009. [DOI] [PubMed] [Google Scholar]
- 4.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J. Exp. Med. 2009;206:1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 6.Lee CG, Elias JA. Role of breast regression protein-39/YKL-40 in asthma and allergic responses. Allergy Asthma Immunol. Res. 2010;2:20–27. doi: 10.4168/aair.2010.2.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CG, Da Silva CA, Lee JY, Hartl D, Elias JA. Chitin regulation of immune responses: an old molecule with new roles. Curr. Opin. Immunol. 2008;20:684–689. doi: 10.1016/j.coi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CG. Chitin, chitinases and chitinase-like proteins in allergic inflammation and tissue remodeling. Yonsei Med. J. 2009;50:22–30. doi: 10.3349/ymj.2009.50.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibata Y, Foster LA, Bradfield JF, Myrvik QN. Oral administration of chitin down-regulates serum IgE levels and lung eosinophilia in the allergic mouse. J. Immunol. 2000;164:1314–1321. doi: 10.4049/jimmunol.164.3.1314. [DOI] [PubMed] [Google Scholar]
- 11.Shibata Y, Metzger WJ, Myrvik QN. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: Mannose receptor-mediated phagocytosis initiates IL-12 production. J. Immunol. 1997;159:2462–2467. [PubMed] [Google Scholar]
- 12.Hamajima K, Kojima Y, Matsui K, Toda Y, Jounai N, et al. Chitin micro-particles (CMP): a useful adjuvant for inducing viral specific immunity when delivered intranasally with an HIV-DNA vaccine. Viral Immunol. 2003;16:541–547. doi: 10.1089/088282403771926355. [DOI] [PubMed] [Google Scholar]
- 13.Da Silva CA, Pochard P, Lee CG, Elias JA. Chitin particles are multifaceted immune adjuvants. Am. J. Respir. Crit. Care Med. 2010 doi: 10.1164/rccm.200912-1877OC. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araujo AC, Souto-Padron T, de Souza W. Cytochemical localization of carbohydrate residues in microfilariae of Wuchereria bancrofti and Brugia malayi. J. Histochem. Cytochem. 1993;41:571–578. doi: 10.1177/41.4.8450196. [DOI] [PubMed] [Google Scholar]
- 15.Debono M, Gordee RS. Antibiotics that inhibit fungal cell wall development. Annu. Rev. Microbiol. 1994;48:471–497. doi: 10.1146/annurev.mi.48.100194.002351. [DOI] [PubMed] [Google Scholar]
- 16.Fuhrman JA, Piessens WF. Chitin synthesis and sheath morphogenesis in Brugia malayi microlariae. Mol. Biochem. Parasitol. 1985;17:93–104. doi: 10.1016/0166-6851(85)90130-6. [DOI] [PubMed] [Google Scholar]
- 17.Neville AC, Parry DA, Woodhead-Galloway J. The chitin crystallite in arthropod cuticle. J. Cell Sci. 1976;21:73–82. doi: 10.1242/jcs.21.1.73. [DOI] [PubMed] [Google Scholar]
- 18.Shahabuddin M, Kaslow DC. Plasmodium: parasite chitinase and its role in malaria transmission. Exp. Parasitol. 1994;79:85–88. doi: 10.1006/expr.1994.1066. [DOI] [PubMed] [Google Scholar]
- 19.Burton OT, Zaccone P. The potential role of chitin in allergic reactions. Trends Immunol. 2007;28:419–422. doi: 10.1016/j.it.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Da Silva CA, Chalouni C, Williams A, Hartl D, Lee CG, Elias JA. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J. Immunol. 2009;182:3573–3582. doi: 10.4049/jimmunol.0802113. [DOI] [PubMed] [Google Scholar]
- 21.Shahabuddin M, Vinetz JM. Chitinases of human parasites and their implications as antiparasitic targets. In: Jolles P, Muzzarelli RAA, editors. Chitin and Chitinases. Basel: Birkhauser; 1999. pp. 223–234. [DOI] [PubMed] [Google Scholar]
- 22.Shahabuddin M, Toyoshima T, Aikawa M, Kaslow DC. Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc. Natl. Acad. Sci. USA. 1993;90:4266–4270. doi: 10.1073/pnas.90.9.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dessens JT, Mendoza J, Claudianos C, Vinetz JM, Khater E, et al. Knockout of the rodent malaria parasite chitinase pbCHT1 reduces infectivity to mosquitoes. Infect. Immun. 2001;69:4041–4047. doi: 10.1128/IAI.69.6.4041-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera-Estrella A, Chet I. Chitinases in biological control. Exs. 1999;87:171–184. doi: 10.1007/978-3-0348-8757-1_12. [DOI] [PubMed] [Google Scholar]
- 25.Palli SR, Retnakaran A. Molecular and biochemical aspects of chitin synthesis inhibition. EXS. 1999;87:85–98. doi: 10.1007/978-3-0348-8757-1_6. [DOI] [PubMed] [Google Scholar]
- 26.Muzzarelli RA. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell Mol. Life Sci. 1997;53:131–140. doi: 10.1007/PL00000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura K, Nishimura S, Nishi N, Saiki I, Tokura S, Azuma I. Immunological activity of chitin and its derivatives. Vaccine. 1984;2:93–99. doi: 10.1016/s0264-410x(98)90039-1. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura S, Nishi N, Tokura S, Nishimura K, Azuma I. Bioactive chitin derivatives. Activation of mouse-peritoneal macrophages by O-(carboxymethyl)chitins. Carbohydr. Res. 1986;146:251–258. doi: 10.1016/0008-6215(86)85044-3. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K, Okawa Y, Hashimoto K, Suzuki S, Suzuki M. Protecting effect of chitin and chitosan on experimentally induced murine candidiasis. Microbiol. Immunol. 1984;28:903–912. doi: 10.1111/j.1348-0421.1984.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 30.Shibata Y, Foster LA, Metzger WJ, Myrvik QN. Alveolar macrophage priming by intravenous administration of chitin particles, polymers of N-acetyl-D-glucosamine, in mice. Infect. Immun. 1997;65:1734–1741. doi: 10.1128/iai.65.5.1734-1741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strong P, Clark H, Reid K. Intranasal application of chitin microparticles down-regulates symptoms of allergic hypersensitivity to Dermatophagoides pteronyssinus and Aspergillus fumigatus in murine models of allergy. Clin. Exp. Allergy. 2002;32:1794–1800. doi: 10.1046/j.1365-2222.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen CL, Wang YM, Liu CF, Wang JY. The effect of water-soluble chitosan on macrophage activation and the attenuation of mite allergen-induced airway inflammation. Biomaterials. 2008;29:2173–2182. doi: 10.1016/j.biomaterials.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Matsuo H, Morita E. Cross-reactivity among shrimp, crab and scallops in a patient with a seafood allergy. J. Dermatol. 2006;33:174–177. doi: 10.1111/j.1346-8138.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- 34.Desjardins A, Malo JL, L’Archevêque J, Cartier A, McCants M, Lehrer SB. Occupational IgE-mediated sensitization and asthma caused by clam and shrimp. J. Allergy Clin. Immunol. 1995;96:608–617. doi: 10.1016/s0091-6749(95)70259-8. [DOI] [PubMed] [Google Scholar]
- 35.Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J. Immunol. 2008;181:4279–4286. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funkhouser JD, Aronson NN., Jr Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol. Biol. 2007;7:96. doi: 10.1186/1471-2148-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bussink AP, Speijer D, Aerts JM, Boot RG. Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases. Genetics. 2007;177:959–970. doi: 10.1534/genetics.107.075846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boot RG, Blommaart EF, Swart E, Ghauharali-van der Vlugt K, Bijl N, et al. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J. Biol. Chem. 2001;276:6770–6778. doi: 10.1074/jbc.M009886200. [DOI] [PubMed] [Google Scholar]
- 39.Boot RG, Renkema GH, Verhoek M, Strijland A, Bliek J, et al. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J. Biol. Chem. 1998;273:25680–25685. doi: 10.1074/jbc.273.40.25680. [DOI] [PubMed] [Google Scholar]
- 40.Bleau G, Massicotte F, Merlen Y, Boisvert C. Mammalian chitinase-like proteins. EXS. 1999;87:211–221. doi: 10.1007/978-3-0348-8757-1_15. [DOI] [PubMed] [Google Scholar]
- 41.Chang NC, Hung SI, Hwa KY, Kato I, Chen JE, et al. A macrophage protein, Ym1, transiently expressed during inflammation is a novel mammalian lectin. J. Biol. Chem. 2001;276:17497–17506. doi: 10.1074/jbc.M010417200. [DOI] [PubMed] [Google Scholar]
- 42.Ward JM, Yoon M, Anver MR, Haines DC, Kudo G, et al. Hyalinosis and Ym1/Ym2 gene expression in the stomach and respiratory tract of 129S4/SvJae and wild-type and CYP1A2-null B6, 129 mice. Am. J. Pathol. 2001;158:323–332. doi: 10.1016/S0002-9440(10)63972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kzhyshkowska J, Mamidi S, Gratchev A, Kremmer E, Schmuttermaier C, et al. Novel stabilin-1 interacting chitinase-like protein (SI-CLP) is up-regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood. 2006;107:3221–3228. doi: 10.1182/blood-2005-07-2843. [DOI] [PubMed] [Google Scholar]
- 44.Zheng T, Rabach M, Chen NY, Rabach L, Hu X, et al. Molecular cloning and functional characterization of mouse chitotriosidase. Gene. 2005;357:37–46. doi: 10.1016/j.gene.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Guo L, Johnson RS, Schuh JC. Biochemical characterization of endogenously formed eosinophilic crystals in the lungs of mice. J. Biol. Chem. 2000;275:8032–8037. doi: 10.1074/jbc.275.11.8032. [DOI] [PubMed] [Google Scholar]
- 46.Sohn MH, Kang MJ, Matsuura H, Bhandari V, Yang Z, et al. The chitinase-like proteins breast regression protein-39 and YKL-40 regulate hyperoxia-induced acute lung injury. Am. J. Respir. Crit. Care Med. 2010;182(7):918–928. doi: 10.1164/rccm.200912-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuura H, Hartl D, Kang MJ, Dela Cruz CS, Koller B, et al. Role of breast regression protein (BRP)-39 in the pathogenesis of cigarette smoke-induced inflammation and emphysema. Am. J. Respir. Cell Mol. Biol. 2010 doi: 10.1165/rcmb.2010-0081OC. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Homer RJ, Zhu Z, Cohn L, Lee CG, White WI, et al. Differential expression of chitinases identify subsets of murine airway epithelial cells in allergic inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291:502–511. doi: 10.1152/ajplung.00364.2005. [DOI] [PubMed] [Google Scholar]
- 49.Hartl D, He CH, Koller B, Da Silva CA, Homer R, et al. Acidic mammalian chitinase is secreted via an ADAM17/epidermal growth factor receptor-dependent pathway and stimulates chemokine production by pulmonary epithelial cells. J. Biol. Chem. 2008;283:33472–33482. doi: 10.1074/jbc.M805574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartl D, He CH, Koller B, Da Silva CA, Kobayashi Y, et al. Acidic mammalian chitinase regulates epithelial cell apoptosis via a chitinolytic-independent mechanism. J. Immunol. 2009;182:5098–5106. doi: 10.4049/jimmunol.0803446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Rosa M, Musumeci M, Scuto A, Musumeci S, Malaguarnera L. Effect of interferon-γ, interleukin-10, lipopolysaccharide and tumor necrosis factor-α on chitotriosidase synthesis in human macrophages. Clin. Chem. Lab. Med. 2005;43:499–502. doi: 10.1515/CCLM.2005.088. [DOI] [PubMed] [Google Scholar]
- 52.Malaguarnera L, Musumeci M, Di Rosa M, Scuto A, Musumeci S. Interferon-γ, tumor necrosis factor-α, and lipopolysaccharide promote chitotriosidase gene expression in human macrophages. J. Clin. Lab. Anal. 2005;19:128–132. doi: 10.1002/jcla.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Eijk M, van Roomen CP, Renkema GH, Bussink AP, Andrews L, et al. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int. Immunol. 2005;17:1505–1512. doi: 10.1093/intimm/dxh328. [DOI] [PubMed] [Google Scholar]
- 54.Renkema GH, Boot RG, Muijsers AO, Donker-Koopman WE, Aerts JM. Purification and characterization of human chitotriosidase, a novel member of the chitinase family of proteins. J. Biol. Chem. 1995;270:2198–2202. doi: 10.1074/jbc.270.5.2198. [DOI] [PubMed] [Google Scholar]
- 55.Renkema GH, Boot RG, Strijland A, Donker-Koopman WE, van den Berg M, et al. Synthesis, sorting, and processing into distinct isoforms of human macrophage chitotriosidase. Eur. J. Biochem. 1997;244:279–285. doi: 10.1111/j.1432-1033.1997.00279.x. [DOI] [PubMed] [Google Scholar]
- 56.Kzhyshkowska J, Gratchev A, Goerdt S. Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights. 2007;2:128–146. [PMC free article] [PubMed] [Google Scholar]
- 57.Malaguarnera L, Di Rosa M, Zambito AM, dell’Ombra N, Nicoletti F, Malaguarnera M. Chitotriosidase gene expression in Kupffer cells from patients with nonalcoholic fatty liver disease. Gut. 2006;55:1313–1320. doi: 10.1136/gut.2005.075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fusetti F, Pijning T, Kalk KH, Bos E, Dijkstra BW. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J. Biol. Chem. 2003;278:37753–37760. doi: 10.1074/jbc.M303137200. [DOI] [PubMed] [Google Scholar]
- 59.Morrison BW, Leder P. neu and ras initiate murine mammary tumors that share genetic markers generally absent in c-myc and int-2-initiated tumors. Oncogene. 1994;9:3417–3426. [PubMed] [Google Scholar]
- 60.Rejman JJ, Hurley WL. Isolation and characterization of a novel 39 kDa whey protein from bovine mammary secretions collected during the nonlactating period. Biochem. Biophys. Res. Commun. 1988;150:329–334. doi: 10.1016/0006-291x(88)90524-4. [DOI] [PubMed] [Google Scholar]
- 61.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J. Biol. Chem. 1993;268:25803–25810. [PubMed] [Google Scholar]
- 62.Shackleton LM, Mann DM, Millis JT. Identification of a 38-kDa heparin-binding glycoprotein (gp39k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J. Biol. Chem. 1995;270:13076–13083. doi: 10.1074/jbc.270.22.13076. [DOI] [PubMed] [Google Scholar]
- 63.Kawamura K, Shibata T, Saget O, Peel D, Bryant PJ. A new family of growth factors produced by the fat body and active on Drosophila imaginal disc cells. Development. 1999;126:211–219. doi: 10.1242/dev.126.2.211. [DOI] [PubMed] [Google Scholar]
- 64.Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 1997;43:221–225. doi: 10.1006/geno.1997.4778. [DOI] [PubMed] [Google Scholar]
- 65.Rehli M, Niller HH, Ammon C, Langmann S, Schwarzfischer L, et al. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J. Biol. Chem. 2003;278:44058–44067. doi: 10.1074/jbc.M306792200. [DOI] [PubMed] [Google Scholar]
- 66.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan. Med. Bull. 2006;53:172–209. [PubMed] [Google Scholar]
- 67.Recklies AD, Ling H, White C, Bernier SM. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J. Biol. Chem. 2005;280:41213–41221. doi: 10.1074/jbc.M510146200. [DOI] [PubMed] [Google Scholar]
- 68.Ling H, Recklies AD. The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumor necrosis factor-α. Biochem. J. 2004;380:651–659. doi: 10.1042/BJ20040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhat KP, Pelloski CE, Zhang Y, Kim SH, deLaCruz C, et al. Selective repression of YKL-40 by NF-κB in glioma cell lines involves recruitment of histone deacetylase-1 and-2. FEBS Lett. 2008;582:3193–3200. doi: 10.1016/j.febslet.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 70.Johansen JS, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK, Bruunsgaard H. High serum YKL-40 level in a cohort of octogenarians is associated with increased risk of all-cause mortality. Clin. Exp. Immunol. 2008;151:260–266. doi: 10.1111/j.1365-2249.2007.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rathcke CN, Raymond I, Kistorp C, Hildebrandt P, Faber J, Vestergaard H. Low grade inflammation as measured by levels of YKL-40: association with an increased overall and cardiovascular mortality rate in an elderly population. Int. J. Cardiol. 2009;143:35–42. doi: 10.1016/j.ijcard.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 72.Hu B, Trinh K, Figueira WF, Price PA. Isolation and sequence of a novel human chondrocyte protein related to mammalian members of the chitinase protein family. J. Biol. Chem. 1996;271:19415–19420. doi: 10.1074/jbc.271.32.19415. [DOI] [PubMed] [Google Scholar]
- 73.Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J. Neuroinflamm. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Webb DC, McKenzie AN, Foster PS. Expression of the Ym2 lectin-binding protein is dependent on interleukin (IL)-4 and IL-13 signal transduction: identification of a novel allergy-associated protein. J. Biol. Chem. 2001;276:41969–41976. doi: 10.1074/jbc.M106223200. [DOI] [PubMed] [Google Scholar]
- 75.Welch JS, Escoubet-Lozach L, Sykes DB, Liddiard K, Greaves DR, Glass CK. TH2 cytokines and allergic challenge induce Ym1 expression in macrophages by a STAT6-dependent mechanism. J. Biol. Chem. 2002;277:42821–42829. doi: 10.1074/jbc.M205873200. [DOI] [PubMed] [Google Scholar]
- 76.Lok IH, Briton-Jones CM, Yuen PM, Haines CJ. Variable expression of oviductin mRNA at different stages of human reproductive cycle. J. Assist. Reprod. Genet. 2002;19:569–576. doi: 10.1023/A:1021263132176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J, Gratchev A, Riabov V, Mamidi S, Schmuttermaier C, et al. A novel GGA-binding site is required for intracellular sorting mediated by stabilin-1. Mol. Cell. Biol. 2009;29:6097–6105. doi: 10.1128/MCB.00505-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beasley R, Crane J, Lai CK, Pearce N. Prevalence and etiology of asthma. J. Allergy Clin. Immunol. 2000;105:S466–S472. doi: 10.1016/s0091-6749(00)90044-7. [DOI] [PubMed] [Google Scholar]
- 79.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 80.Braman SS. The global burden of asthma. Chest. 2006;130:S4–S12. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 81.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 82.Bradding P, Redington AE, Holgate ST. Airway wall remodelling in the pathogenesis of asthma: cytokine expression in the airways. In: Stewart AG, editor. Airway Wall Remodelling in Asthma. Boca Raton: CRC; 1997. pp. 29–63. [Google Scholar]
- 83.Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, et al. Interleukin-4, -5, and -6 and tumor necrosis factor-α in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am. J. Respir. Cell Mol. Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 84.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J. Clin. Investig. 2003;111:291–297. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Curr. Allergy. Asthma Rep. 2004;4:123–131. doi: 10.1007/s11882-004-0057-6. [DOI] [PubMed] [Google Scholar]
- 86.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, et al. CD4+ invariant T-cell–receptor+ natural killer T cells in bronchial asthma. N. Engl. J. Med. 2006;354:1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 87.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamid Q, Azzawi M, Ying S, Moqbel R, Wardlaw AJ, et al. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J. Clin. Investig. 1991;87:1541–1546. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kotsimbos TC, Ernst P, Hamid QA. Interleukin-13 and interleukin-4 are coexpressed in atopic asthma. Proc. Assoc. Am. Physicians. 1996;108:368–373. [PubMed] [Google Scholar]
- 90.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 91.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J. Clin. Investig. 1999;104:1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ray A, Cohn L. Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J. Clin. Investig. 1999;104:985–993. doi: 10.1172/JCI8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Vries JE. The role of IL-13 and its receptor in allergy and inflammatory responses. J. Allergy Clin. Immunol. 1998;102:165–169. doi: 10.1016/s0091-6749(98)70080-6. [DOI] [PubMed] [Google Scholar]
- 94.Minty A, Asselin S, Bensussan A, Shire D, Vita N, et al. The related cytokines interleukin-13 and interleukin-4 are distinguished by differential production and differential effects on T lymphocytes. Eur. Cytokine Netw. 1997;8:203–213. [PubMed] [Google Scholar]
- 95.Emson CL, Bell SE, Jones A, Wisden W, McKenzie AN. Interleukin (IL)-4-independent induction of immunoglobulin (Ig)E, and perturbation of T cell development in transgenic mice expressing IL-13. J. Exp. Med. 1998;188:399–404. doi: 10.1084/jem.188.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zurawski G, de Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol. Today. 1994;15:19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 97.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, et al. Pulmonary expression of interleukin-13 causes inflammation,mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Investig. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bochner BS, Klunk DA, Sterbinsky SA, Coffman RL, Schleimer RP. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J. Immunol. 1995;154:799–803. [PubMed] [Google Scholar]
- 99.Woltmann G, McNulty CA, Dewson G, Symon FA, Wardlaw AJ. Interleukin-13 induces PSGL-1/P-selectin-dependent adhesion of eosinophils, but not neutrophils, to human umbilical vein endothelial cells under flow. Blood. 2000;95:3146–3152. [PubMed] [Google Scholar]
- 100.Horie S, Okubo Y, Hossain M, Sato E, Nomura H, et al. Interleukin-13 but not interleukin-4 prolongs eosinophil survival and induces eosinophil chemotaxis. Intern. Med. 1997;36:179–185. doi: 10.2169/internalmedicine.36.179. [DOI] [PubMed] [Google Scholar]
- 101.Huang SK, Xiao HQ, Kleine-Tebbe J, Paciotti G, Marsh DG, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J. Immunol. 1995;155:2688–2694. [PubMed] [Google Scholar]
- 102.HumbertM,Durham SR, Kimmitt P, Powell N, Assoufi B, et al. Elevated expression of messenger ribonucleic acid encoding IL-13 in the bronchial mucosa of atopic and nonatopic subjects with asthma. J. Allergy Clin. Immunol. 1997;99:657–665. doi: 10.1016/s0091-6749(97)70028-9. [DOI] [PubMed] [Google Scholar]
- 103.Heinzmann A, Mao XQ, Akaiwa M, Kreomer RT, Gao PS, et al. Genetic variants of IL-13 signalling and human asthma and atopy. Hum. Mol. Genet. 2000;9:549–559. doi: 10.1093/hmg/9.4.549. [DOI] [PubMed] [Google Scholar]
- 104.Holt PG. Infections and the development of allergy. Toxicol. Lett. 1996;86:205–210. doi: 10.1016/0378-4274(96)03692-2. [DOI] [PubMed] [Google Scholar]
- 105.Holt PG. Parasites, atopy, and the hygiene hypothesis: resolution of a paradox? Lancet. 2000;356:1699–1701. doi: 10.1016/S0140-6736(00)03198-6. [DOI] [PubMed] [Google Scholar]
- 106.Matsumoto T, Inoue H, Sato Y, Kita Y, Nakano T, et al. Demethylallosamidin, a chitinase inhibitor, suppresses airway inflammation and hyperresponsiveness. Biochem. Biophys. Res. Commun. 2009;390:103–108. doi: 10.1016/j.bbrc.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 107.Yang CJ, Liu YK, Liu CL, Shen CN, Kuo ML, et al. Inhibition of acidic mammalian chitinase by RNA interference suppresses ovalbumin-sensitized allergic asthma. Hum. Gene Ther. 2009;20:1597–1606. doi: 10.1089/hum.2008.092. [DOI] [PubMed] [Google Scholar]
- 108.McCall C, Hunter S, Stedman K, Weber E, Hillier A, et al. Characterization and cloning of a major high molecular weight house dust mite allergen (Der f 15) for dogs. Vet. Immunol. Immunopathol. 2001;78:231–247. doi: 10.1016/s0165-2427(00)00258-0. [DOI] [PubMed] [Google Scholar]
- 109.Perkin JE. The latex and food allergy connection. J. Am. Diet. Assoc. 2000;100:1381–1384. doi: 10.1016/s0002-8223(00)00384-9. [DOI] [PubMed] [Google Scholar]
- 110.Boot RG, Bussink AP, Verhoek M, de Boer PA, Moorman AF, Aerts JM. Marked differences in tissue-specific expression of chitinases in mouse and man. J. Histochem. Cytochem. 2005;53:1283–1292. doi: 10.1369/jhc.4A6547.2005. [DOI] [PubMed] [Google Scholar]
- 111.Seibold MA, Donnelly S, Solon M, Innes A, Woodruff PG, et al. Chitotriosidase is the primary active chitinase in the human lung and is modulated by genotype and smoking habit. J. Allergy Clin. Immunol. 2008;122:944–950. doi: 10.1016/j.jaci.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bierbaum S, Nickel R, Koch A, Lau S, Deichmann KA, et al. Polymorphisms and haplotypes of acid mammalian chitinase are associated with bronchial asthma. Am. J. Respir. Crit. Care Med. 2005;172:1505–1509. doi: 10.1164/rccm.200506-890OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chatterjee R, Batra J, Das S, Sharma SK, Ghosh B. Genetic association of acidic mammalian chitinase with atopic asthma and serum total IgE levels. J. Allergy Clin. Immunol. 2008;122:202–208. doi: 10.1016/j.jaci.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 114.Wu AC, Lasky-Su J, Rogers CA, Klanderman BJ, Litonjua A. Polymorphisms of chitinases are not associated with asthma. J. Allergy Clin. Immunol. 125:754–757. doi: 10.1016/j.jaci.2009.12.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nair MG, Gallagher IJ, Taylor MD, Loke P, Coulson PS, et al. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigenpresenting cells. Infect. Immun. 2005;73:385–394. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.HogenEsch H, Dunham A, Seymour R, Renninger M, Sundberg JP. Expression of chitinase-like proteins in the skin of chronic proliferative dermatitis (cpdm/cpdm) mice. Exp. Dermatol. 2006;15:808–814. doi: 10.1111/j.1600-0625.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 117.Nio J, Fujimoto W, Konno A, Kon Y, Owhashi M, Iwanaga T. Cellular expression of murine Ym1 and Ym2, chitinase family proteins, as revealed by in situ hybridization and immunohistochemistry. Histochem. Cell Biol. 2004;121:473–482. doi: 10.1007/s00418-004-0654-4. [DOI] [PubMed] [Google Scholar]
- 118.Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signaling pathways. Biochem. J. 2002;365:119–126. doi: 10.1042/BJ20020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J. Exp. Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N. Engl. J. Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 121.Ober C, Tan Z, Sun Y, Possick JD, Pan L, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N. Engl. J. Med. 2008;358:1682–1691. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sohn MH, Lee JH, Kim KW, Kim SW, Lee SH, et al. Genetic variation in the promoter region of chitinase 3-like 1 is associated with atopy. Am. J. Respir. Crit. Care Med. 2009;179:449–456. doi: 10.1164/rccm.200809-1422OC. [DOI] [PubMed] [Google Scholar]
- 123.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, et al. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 124.Bhandari A, Bhandari V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics. 2009;123:1562–1573. doi: 10.1542/peds.2008-1962. [DOI] [PubMed] [Google Scholar]
- 125.Ostergaard C, Johansen JS, Benfield T, Price PA, Lundgren JD. YKL-40 is elevated in cerebrospinal fluid from patients with purulent meningitis. Clin. Diagn. Lab. Immunol. 2002;9:598–604. doi: 10.1128/CDLI.9.3.598-604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nordenbaek C, Johansen JS, Junker P, Borregaard N, Sorensen O, Price PA. YKL-40, a matrix protein of specific granules in neutrophils, is elevated in serum of patients with community-acquired pneumonia requiring hospitalization. J. Infect. Dis. 1999;180:1722–1726. doi: 10.1086/315050. [DOI] [PubMed] [Google Scholar]
- 127.Aris RM, Stephens AR, Ontjes DA, Denene Blackwood A, Lark RK, et al. Adverse alterations in bone metabolism are associated with lung infection in adults with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2000;162:1674–1678. doi: 10.1164/ajrccm.162.5.2002100. [DOI] [PubMed] [Google Scholar]
- 128.Coffman FD. Chitinase 3-Like-1 (CHI3L1): a putative disease marker at the interface of proteomics and glycomics. Crit. Rev. Clin. Lab. Sci. 2008;45:531–562. doi: 10.1080/10408360802334743. [DOI] [PubMed] [Google Scholar]
- 129.Vos K, Steenbakkers P, Miltenburg AM, Bos E, van DenHeuvel MW, et al. Raised human cartilage glycoprotein-39 plasma levels in patients with rheumatoid arthritis and other inflammatory conditions. Ann. Rheum. Dis. 2000;59:544–548. doi: 10.1136/ard.59.7.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kawada M, Chen CC, Arihiro A, Nagatani K, Watanabe T, Mizoguchi E. Chitinase 3-like-1 enhances bacterial adhesion to colonic epithelial cells through the interaction with bacterial chitin-binding protein. Lab. Investig. 2008;88:883–895. doi: 10.1038/labinvest.2008.47. [DOI] [PubMed] [Google Scholar]
- 131.Vind I, Johansen JS, Price PA, Munkholm P. Serum YKL-40, a potential new marker of disease activity in patients with inflammatory bowel disease. Scand. J. Gastroenterol. 2003;38:599–605. doi: 10.1080/00365520310000537. [DOI] [PubMed] [Google Scholar]
- 132.Johansen JS, Milman N, Hansen M, Garbarsch C, Price PA, Graudal N. Increased serum YKL-40 in patients with pulmonary sarcoidosis—a potential marker of disease activity? Respir. Med. 2005;99:396–402. doi: 10.1016/j.rmed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 133.Letuve S, Kozhich A, Arouche N, Grandsaigne M, Reed J, et al. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J. Immunol. 2008;181:5167–5173. doi: 10.4049/jimmunol.181.7.5167. [DOI] [PubMed] [Google Scholar]
- 134.Johansen JS, Moller S, Price PA, Bendtsen F, Junge J, et al. Plasma YKL-40: a new potential marker of fibrosis in patients with alcoholic cirrhosis? Scand. J. Gastroenterol. 1997;32:582–590. doi: 10.3109/00365529709025104. [DOI] [PubMed] [Google Scholar]
- 135.Johansen JS, Christoffersen P, Moller S, Price PA, Henriksen JH, et al. Serum YKL-40 is increased in patients with hepatic fibrosis. J. Hepatol. 2000;32:911–920. doi: 10.1016/s0168-8278(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 136.Nojgaard C, Johansen JS, Christensen E, Skovgaard LT, Price PA, Becker U. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J. Hepatol. 2003;39:179–186. doi: 10.1016/s0168-8278(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 137.Saitou Y, Shiraki K, Yamanaka Y, Yamaguchi Y, Kawakita T, et al. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J. Gastroenterol. 2005;11:476–481. doi: 10.3748/wjg.v11.i4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kirkpatrick RB, Matico RE, McNulty DE, Strickler JE, Rosenberg M. An abundantly secreted glycoprotein from Drosophila melanogaster is related to mammalian secretory proteins produced in rheumatoid tissues and by activated macrophages. Gene. 1995;153:147–154. doi: 10.1016/0378-1119(94)00756-i. [DOI] [PubMed] [Google Scholar]
- 139.Volck B, Johansen JS, Stoltenberg M, Garbarsch C, Price PA, et al. Studies on YKL-40 in knee joints of patients with rheumatoid arthritis and osteoarthritis. Involvement of YKL-40 in the joint pathology. Osteoarthr. Cartil. 2001;9:203–214. doi: 10.1053/joca.2000.0377. [DOI] [PubMed] [Google Scholar]
- 140.Verheijden GF, Rijnders AW, Bos E, Coenen-de Roo CJ, van Staveren CJ, et al. Human cartilage glycoprotein-39 as a candidate autoantigen in rheumatoid arthritis. Arthritis Rheum. 1997;40:1115–1125. doi: 10.1002/art.1780400616. [DOI] [PubMed] [Google Scholar]
- 141.Garnero P, Piperno M, Gineyts E, Christgau S, Delmas PD, Vignon E. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann. Rheum. Dis. 2001;60:619–626. doi: 10.1136/ard.60.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fach EM, Garulacan LA, Gao J, Xiao Q, Storm SM, et al. In vitro biomarker discovery for atherosclerosis by proteomics. Mol. Cell Proteomics. 2004;3:1200–1210. doi: 10.1074/mcp.M400160-MCP200. [DOI] [PubMed] [Google Scholar]
- 143.Johansen JS, Baslund B, Garbarsch C, Hansen M, Stoltenberg M, et al. YKL-40 in giant cells and macrophages from patients with giant cell arteritis. Arthritis Rheum. 1999;42:2624–2630. doi: 10.1002/1529-0131(199912)42:12<2624::AID-ANR17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 144.Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol. Biomarkers Prev. 2006;15:194–202. doi: 10.1158/1055-9965.EPI-05-0011. [DOI] [PubMed] [Google Scholar]
- 145.Johansen JS, Williamson MK, Rice JS, Price PA. Identification of proteins secreted by human osteoblastic cells in culture. J. Bone Miner. Res. 1992;7:501–512. doi: 10.1002/jbmr.5650070506. [DOI] [PubMed] [Google Scholar]
- 146.Junker N, Johansen JS, Hansen LT, Lund EL, Kristjansen PE. Regulation of YKL-40 expression during genotoxic or microenvironmental stress in human glioblastoma cells. Cancer Sci. 2005;96:183–190. doi: 10.1111/j.1349-7006.2005.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Junker N, Johansen JS, Andersen CB, Kristjansen PE. Expression of YKL-40 by peritumoral macrophages in human small cell lung cancer. Lung Cancer. 2005;48:223–231. doi: 10.1016/j.lungcan.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 148.Shao R, Hamel K, Petersen L, Cao QJ, Arenas RB, et al. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene. 2009;28:4456–4468. doi: 10.1038/onc.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, et al. Combinatorial activation of FAK and AKT by transforming growth factor-β1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007;19:761–771. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rathcke CN, Persson F, Tarnow L, Rossing P, Vestergaard H. YKL-40, amarker of inflammation and endothelial dysfunction, is elevated in patients with type 1 diabetes and increases with levels of albuminuria. Diabetes Care. 2009;32:323–328. doi: 10.2337/dc08-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nielsen AR, Erikstrup C, Johansen JS, Fischer CP, Plomgaard P, et al. Plasma YKL-40: a BMI-independent marker of type 2 diabetes. Diabetes. 2008;57:3078–3082. doi: 10.2337/db08-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rathcke CN, Johansen JS, Vestergaard H. YKL-40, a biomarker of inflammation, is elevated in patients with type 2 diabetes and is related to insulin resistance. Inflamm. Res. 2006;55:53–59. doi: 10.1007/s00011-005-0010-8. [DOI] [PubMed] [Google Scholar]
- 153.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ramanathan R. Optimal ventilatory strategies and surfactant to protect the preterm lungs. Neonatology. 2008;93:302–308. doi: 10.1159/000121456. [DOI] [PubMed] [Google Scholar]