Abstract
Objective
To assess patients' preferences of the timing of referral for genetic counseling and testing in relation to the diagnosis, treatment and recurrence of ovarian, tubal, or primary peritoneal cancers.
Methods/Materials
Ninety-two patients who underwent counseling and testing by one certified genetic counselor were identified. Introductory letter, consent form and questionnaire were mailed to gather information regarding factors influencing the decision to undergo genetic counseling and testing and opinions regarding optimal timing. Medical records were reviewed for demographic and clinical data.
Results
Of 47 consenting women, 45 underwent testing. Eight (18%) were found to have a genetic mutation. Women lacked consensus about the optimal time for referral for and to undergo genetic testing, though women with stage I disease preferred testing after completion of chemotherapy. Most women were comfortable receiving the results by phone, but 1/3 preferred an office visit.
Conclusions
Patients' views regarding the best time to be referred for and undergo counseling and testing varied greatly. Due to the high mortality of this disease, clinicians should discuss referral early and personalize the timing to each patient. The subset of patients who prefer results disclosure during an office visit should be identified at the time of their initial counseling.
Keywords: genetic counseling, genetic testing, BRCA mutation, timing, ovarian/fallopian tube/primary peritoneal cancer
Introduction
Ovarian, fallopian tube and primary peritoneal cancers are the most lethal gynecologic malignancies, causing an estimated 15,500 deaths in the United States in 2012.(1) It is believed that 12-16% of these cancers are due to inherited susceptibility.(2-5) Most of these result from a mutation in the BRCA1 or BRCA2 genes.(6-8) While BRCA mutations were initially identified to have a high prevalence in Ashkenazi Jewish women, mutations in these genes have been identified in multiple ethnic and racial groups.(5, 9, 10) Recent data suggested that 23% of women with high-grade serous ovarian cancers harbor a germline mutation in one of the BRCA genes(11) with the rate of BRCA mutations in women with fallopian tube cancer being even higher, ranging from 20-43%.(11-13) Genetic testing in women with ovarian cancer has significant implications. BRCA mutations have been associated with improved survival(14-16) and increased responsiveness to chemotherapy regimens.(17, 18) Recently, BRCA-mutated ovarian cancers have been shown to be particularly sensitive to poly ADP ribose polymerase (PARP)-inhibitors.(17, 19, 20) Additionally, identification of a BRCA mutation allows for counseling and testing of other family members with the potential to intervene with risk reduction strategies before oncogenesis occurs. Negative results can reduce anxiety and allow for more informed counseling of women from high-risk families. Genetic testing of all women with a history of epithelial ovarian cancer is recommended as per NCCN guidelines(21) and Medicare guidelines cover genetic testing for women with a personal history of these cancers.(22)
The optimal time to provide genetic counseling and perform genetic testing for women diagnosed with ovarian cancer is unclear. Early disclosure may reduce uncertainty, but women coping with a new cancer diagnosis may have difficulty processing the risks and benefits of genetic testing. A study evaluating women newly diagnosed with breast cancer found no difference in patient satisfaction between counseling prior to or after definitive surgery.(23) In breast cancer, this is of particular importance as knowledge of mutation status may influence immediate treatment recommendations including the performance of concomitant contralateral risk-reducing mastectomy and risk-reducing bilateral salpingo-oophorectomy.(24)
Ovarian, fallopian tube and primary peritoneal cancers differ from breast cancer as most are diagnosed in advanced stages when patients present with symptoms. While the diagnosis may be suspected prior to surgery, definitive diagnosis can only be made after pathological examination of the surgical specimen, limiting the feasibility of preoperative testing. Thus, the timing of genetic counseling and testing must balance the necessity for immediate testing with the stress patients feel as they cope with the diagnosis of ovarian cancer, treatment plans and prognosis.
A prior study reported a single institution's experience in the timing of genetic counseling in ovarian cancer, demonstrating that a third of patients underwent genetic testing after documented recurrence with some undergoing testing during end of life care.(25) However, patients' preferences were not assessed. In this study, we evaluated patients' preferences for the timing of genetic counseling and testing in relation to the diagnosis, treatment and recurrence of ovarian, tubal, or primary peritoneal cancer.
Materials and Methods
Study Design
We identified for inclusion all patients 18 years of age or older who were treated for epithelial ovarian, fallopian tube or primary peritoneal cancer at Washington University School of Medicine/Barnes-Jewish Hospital and who underwent genetic counseling between January 1, 2010 and December 31, 2011. All genetic counseling was performed by one certified genetic counselor (S.A.B.). The study protocol was approved by the Washington University School of Medicine Human Research Protection Office (HRPO# 201111163).
We were unable to find a previously validated questionnaire that addressed the topics relevant to our research question. Therefore, the authors developed questionnaire items related to the timing and decision process of undergoing genetic counseling and testing. Questions included: 1) “When do you think the best time is for a healthcare provider to discuss the option of an appointment with a genetic counselor to review the possible genetic component to your ovarian cancer?” 2) “When is the BEST time for an ovarian cancer patient to actually meet with the genetic counselor?” Options for these questions were: a) Before your first cancer surgery, b) After your staging surgery, before starting chemotherapy, c) During chemotherapy, d) After completion of chemotherapy, at time of remission, e) When diagnosed with recurrence or f) Other. Other questions included: 3) “Which factors influenced your decision to schedule an appointment with the genetic counselor the most?” 4) “If you decided to actually have genetic testing, what factors influenced your decision?” Options for these questions were: a) Your doctor's suggestion, b) Thought it may influence your treatment, c) Thought it may influence your medical follow-up, d) Thought it may decrease my risk of developing other cancers, e) To benefit other family members, f) Encouraged by family members, g) other. Additional questions included: 5) “What do you think is the best way for healthcare providers to notify patients of genetic test results?” a) Phone call, b) In person in a provider's office, c) Written via e-mail or U.S. Postal Service or d) Other. 6) “If you decided not to have blood drawn for DNA banking, what factors influenced your decision?” a) I was still trying to cope with new cancer diagnosis/treatment, b) I did not feel that DNA banking was important/valuable, c) I feared/knew insurance would not cover the cost of DNA banking, d) Other. Patients were allowed to choose multiple answers for each question.
Data Collection
In January 2012 an introductory letter, consent form and questionnaire were mailed to all eligible participants to gather information regarding the factors influencing the timing of genetic counseling and testing, as well as each patient's opinion regarding optimal timing to offer genetic evaluation, schedule genetic counseling and undergo genetic testing. Two weeks after the initial mailing, non-responders were contacted by phone to assess their interest in participation. Those who declined participation were not further contacted. A second survey was sent to those who had still not declined participation one month after the initial invitation. Those who did not return this second survey were considered to have declined entry in to the study.
The medical records of consenting patients were reviewed to collect relevant demographic and clinical data, including ovarian cancer stage and histology, recurrence, other primary cancer diagnoses, date of surgery, date of genetic counseling, date and uptake of genetic testing, genetic test result, and uptake of DNA banking.
Genetic Counseling and Testing
Patients undergoing treatment for ovarian, fallopian tube or primary peritoneal carcinomas are referred by their gynecologic oncologist for genetic counseling for hereditary cancer risk assessment and genetic testing. Patients are provided a brochure describing the genetic counseling process and a family history worksheet to assist them in obtaining detailed cancer/medical family history information and are scheduled for an appointment. The genetic counseling appointment includes: review of the probands personal medical history; review of 3-generation cancer family history; discussion of the genetic testing being recommended including specific genes being tested. Patients are counseled about possible results, psychosocial impacts of genetic testing and the availability of free psychological counseling services for all patients of our cancer center. These genetic counseling appointments typically last 45-60 minutes, and allow for ample time to answer all of the patient's and her family's questions.
Telephone disclosure of BRCA genetic testing results is the norm in our office. The majority of our ovarian cancer patients will be returning to our office for follow-up within 3-6 months or sooner if they are in active cancer treatment thus providing access to the gynecologic oncologist and/or genetic counselor for unscheduled follow-up conversation regarding the genetic testing. Occasionally, in-person disclosure is recommended by the genetic counselor for those patients who appear to be overwhelmed by the information presented during the genetic counseling appointment or if requested by the patient.
Data Analysis
Since this was a cross-sectional, pilot descriptive study, we reported the frequencies (%) of categorical measures (ex: stage, site of disease and histology) and median (interquartile range [IQR]) of continuous measures (age at diagnosis and genetic testing and time from diagnosis to counseling). Patients' opinions about the best timing for genetic counseling/testing were summarized using counts and percentages. Patients' preferences as to the timing of genetic counseling were dichotomized into prior to the completion versus after the completion of chemotherapy. Chi-square tests, Fisher's exact tests, and Student's t-tests were used to evaluate for differences in patients' preferences as to the timing of genetic testing. A p-value of <0.05 was considered statistically significant.
Results
Ninety-two women met the inclusion criteria, and 47 (51%) consented to the study and returned their questionnaires. The median age at the time of diagnosis and at the time of genetic counseling was 57.3 (interquartile range [IQR]: 46.8-61.2) and 58.8 (IQR: 50.9-63.9) years. Most women were diagnosed with Stage III/IV disease (77%) and had ovarian cancer (81%). All women but one described themselves as non-Hispanic Caucasian, and 29 (62%) had obtained a college or more advanced degree. Demographic and clinical characteristics of these patients are reported in Table 1.
Table 1. Patient Demographic and Clinical Characteristics (N = 47).
| Age at diagnosis (years) | Median (IQR) | 57.3 | 46.8-61.2 |
| Age at genetic counseling (years) | Median (IQR) | 58.8 | 50.9-63.9 |
| Time from diagnosis to counseling (months) | Median (IQR) | 11.5 | 3.6-48.6 |
| N = 47 | % | ||
| Race | |||
| Caucasian | 46 | 97.9 | |
| Black | 1 | 2.1 | |
| Tumor site | |||
| Ovary | 38 | 80.9 | |
| Fallopian Tube | 2 | 4.3 | |
| Primary Peritoneal | 7 | 14.9 | |
| Stage | |||
| I | 7 | 14.9 | |
| II | 3 | 6.4 | |
| III | 32 | 68.1 | |
| IV | 4 | 8.5 | |
| Unstaged | 1 | 2.2 | |
| Histology | |||
| High-grade serous | 23 | 48.9 | |
| Mixed tumor | 9 | 19.2 | |
| Low-grade/borderline | 7 | 14.9 | |
| Endometrioid | 3 | 6.4 | |
| Clear cell | 3 | 6.4 | |
| Other | 2 | 4.3 | |
| Highest Education Level | |||
| High School | 18 | 38.3 | |
| College | 18 | 38.3 | |
| Post-college degree | 11 | 23.4 | |
| Disease status at the time of genetic counseling | |||
| No evidence of disease | 23 | 48.9 | |
| Recurrence | 21 | 44.7 | |
| Progressive | 3 | 6.4 | |
| Family History of Breast Cancer | |||
| Yes | 20 | 42.6 | |
| No | 27 | 57.5 | |
| Family History of Ovarian, Tubal or Peritoneal Cancer | |||
| Yes | 10 | 21.3 | |
| No | 37 | 78.7 |
IQR – Interquartile Range
Two women (4%) who underwent genetic counseling did not undergo genetic testing. These women stated that their reasons for not undergoing genetic testing included fear that insurance would not cover the test, coping with new cancer diagnosis/treatment and feeling that the testing was not important. Of the 45 women who underwent genetic testing, eight (17%) were diagnosed with a genetic mutation: 4 (9%) with BRCA1, 3 (6%) with BRCA2 and 1 (2%) with MSH2 mutations.
The median time from diagnosis to genetic counseling was 11.5 months (IQR: 3.6-48.6 months). Fifteen (32%) women were counseled after the diagnosis of a recurrence or progressive disease while nine (19%) other women who were eventually diagnosed with recurrent disease received counseling prior to the diagnosis of recurrence. The remaining women (49%) remained without evidence of disease recurrence at the time of this analysis.
The 45 women who underwent genetic testing were asked when they felt the best time was for a healthcare provider to discuss meeting with a genetic counselor. There was considerable variation in responses regarding patients' preferences for timing of genetic counseling: prior to surgery (15%), after surgery but before chemotherapy (22%), during chemotherapy (22%) and after chemotherapy (28%). Only one woman felt that this conversation should take place at the time of recurrence.
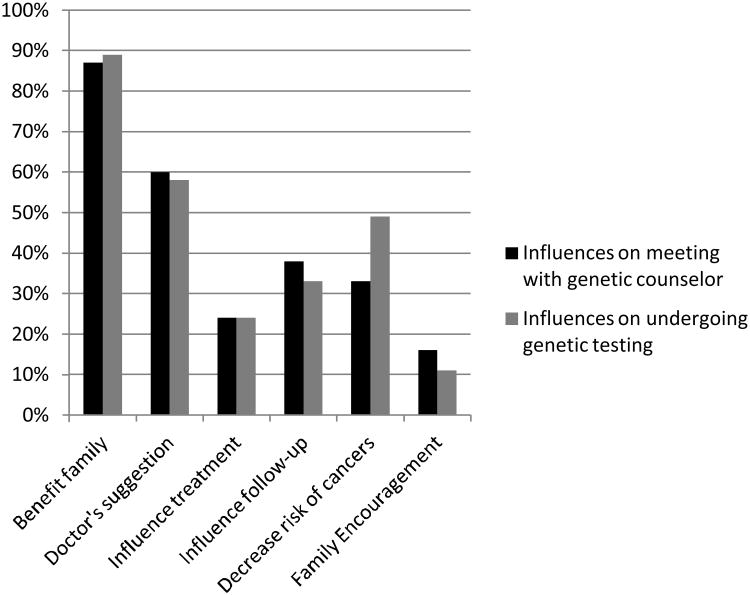
Participants were asked to choose factors that influenced their decision to meet with a genetic counselor. The strongest influence on women's decision was a desire to benefit their family members (71%), and 87% reported this benefit as an influential factor. Other reasons included: following their doctor's suggestion (60%), beliefs that it might influence their treatment (24%) and medical follow-up (38%), decreased risk of developing other cancers (33%) as well as encouragement from family members (16%).
Similar results were obtained when these women were queried about factors that influenced their decision to undergo genetic testing. Most women (73%) reported that a desire to benefit their family members was the strongest influence on this decision, with 89% overall reporting this as an influential factor. Other reasons given included: following their doctor's suggestion (58%), belief that it might influence their treatment (24%), belief that it might influence medical follow-up (33%), to decrease their risk of developing other cancers (49%) and encouragement from family members (11%). (Figure 1)
Figure 1.
Factors influencing patients' decisions to meet with a genetic counselor and to undergo genetic counseling testing (patients were allowed to select more than one choice).
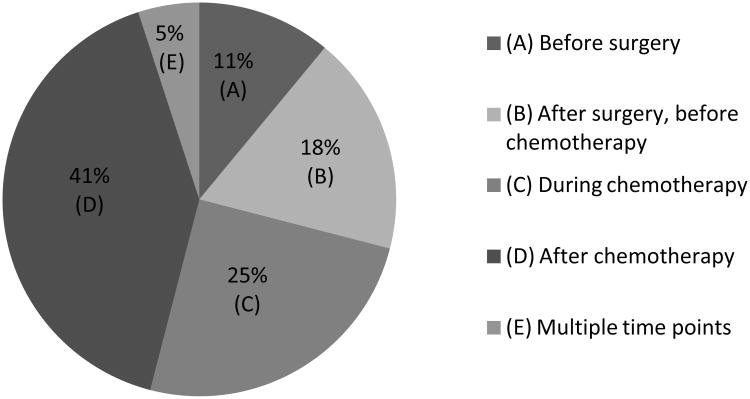
These women also expressed varied opinions as to the best time to meet with a genetic counselor and undergo genetic testing. The largest percentage of women (41%) felt that the most appropriate time was after completion of chemotherapy. The remaining women expressed interest in meeting with the genetic counselor before surgery (11%), after surgery but before chemotherapy (18%) or during chemotherapy (25%). (Figure 2) Participants were asked what the best way was to inform patients of genetic test results. Most women (64%) felt that it was appropriate to receive results either over the phone or via mail, but 33% felt that results should be given in person in the provider's office.
Figure 2.
Patients' opinion of the optimal time to meet with a genetic counselor.
Women with at least stage II disease were significantly more likely to prefer to undergo genetic testing prior to the completion of chemotherapy as compared to women with stage I disease (64% vs 14%, p=0.03). No significant difference in age, family history of either breast or ovarian cancer, family history of BRCA mutation or disease status was seen in women who desired genetic testing before as compared to after the completion of chemotherapy.
DNA banking was offered to 36 of 37 (97%) of women who underwent genetic testing and were not found to have a genetic mutation. Three of these 36 women (8%) elected to have DNA banking. Only 86% of those who were counseled for DNA banking remembered having been offered this option. The most common reason for not undergoing DNA banking was the lack of insurance coverage (50%). Only 31% planned on having DNA banked in the future.
Discussion
While women varied with regard to their views on the optimal time to undergo genetic counseling and testing, women with Stage I disease preferred to undergo genetic testing after completion of chemotherapy. The reason for this is unclear but may be related to the shock of an unexpected diagnosis and need for treatment, which seemed to be of greater concern. Patients may also desire to delay as they feel that they have too much to handle until they complete chemotherapy. Because women varied in their preference for timing of genetic counseling, early exploration of individual wishes for initiation of counseling might allow tailored counseling, with greater acceptance of testing and better outcomes for patients and family members.
Disclosure of genetic testing results in our practice is typically provided via telephone as most of the patients are being actively treated in our office and have an established relationship with the physicians. Our study found that one third of patients preferred to receive their genetic testing results in person. This highlights the need for personalization of the genetic testing process and offering patients a choice of how to receive their results.
While DNA banking was offered to all patients who underwent genetic testing with no identified mutation, 15% of the patients who underwent genetic testing did not remember being offered this option for DNA banking. Patients may be relieved to hear that their testing was negative and may not see the need at that time for DNA banking. Despite this, approximately 30% of those queried planned on undergoing DNA banking in the future. These data highlight the role for future follow-up with these patients and counseling on DNA banking. Given the high mortality rate of ovarian cancer and the rapid rate of genetic discoveries of new predisposition genes and as well as BRCA modifier genes, the option of DNA banking is an option for all ovarian cancer patients, regardless of BRCA mutation status.
Our study has a number of limitations. We limited our study to women who underwent genetic counseling over a recent two year period to minimize recall bias, which resulted in a small sample size. Just over half of eligible patients consented and returned their survey, which could introduce selection bias. All participants had been treated and received genetic counseling at one National Cancer Institute-designated comprehensive cancer center; therefore our findings might not be generalizable to patients receiving care at other hospitals. Similarly, we surveyed only a small number of patients, who also were mostly non-Hispanic Caucasian with at least a college degree, which further limits the generalizability of these results.
Prior studies have looked at patients' interest in genetic testing. One study found that the majority of women had never heard of BRCA testing and nearly 90% would be interested in testing if it directly affected their treatment or to benefit their family.(26) Other studies have looked at the utility of decision aids in the genetic counseling process.(27,28) Patients' opinions of treatment focused genetic testing have also previously been assessed.(29)
To our knowledge, this is the first study to prospectively describe ovarian cancer patients' preferences regarding the timing of genetic counseling and testing. We found that patients differed in their reported preferences for the optimal time of genetic counseling and testing and for the best method of relaying testing results. As such, the timing for genetic counseling and testing should be individualized to each patient. Since this was a cross-sectional pilot study describing patients' preferences for timing of genetic counseling and testing, further longitudinal studies might serve to validate the questionnaire we developed in relation to patient outcomes, such as emotional distress or agreement to bank DNA for future research.
Over half of the patients felt that genetic counseling should take place prior to completion of chemotherapy. As such we recommend that providers discuss preferred timing of genetic counseling early in the treatment process to maximize patients' satisfaction, and ensure that patients and their families can avail themselves of genetic testing opportunities and the information it provides for future medical care.
Acknowledgments
We acknowledge services provided by the Health Behavior, Communication, and Outreach Core of the Siteman Cancer Center. The Core is supported in part by the National Cancer Institute Cancer Center Support Grant (P30 CA091842) to the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–16. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 3.Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. Journal of the National Cancer Institute. 2006;98:1694–706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 4.Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. American journal of human genetics. 2001;68:700–10. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez AO, Llacuachaqui M, Pardo GG, et al. BRCA1 and BRCA2 mutations among ovarian cancer patients from Colombia. Gynecologic oncology. 2012;124:236–43. doi: 10.1016/j.ygyno.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 6.Easton DF, Steele L, Fields P, et al. Cancer risks in two large breast cancer families linked to BRCA2 on chromosome 13q12-13. American journal of human genetics. 1997;61:120–8. doi: 10.1086/513891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith SA, Easton DF, Ford D, et al. Genetic heterogeneity and localization of a familial breast-ovarian cancer gene on chromosome 17q12-q21. American journal of human genetics. 1993;52:767–76. [PMC free article] [PubMed] [Google Scholar]
- 8.Weberpals JI, Clark-Knowles KV, Vanderhyden BC. Sporadic epithelial ovarian cancer: clinical relevance of BRCA1 inhibition in the DNA damage and repair pathway. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:3259–67. doi: 10.1200/JCO.2007.11.3902. [DOI] [PubMed] [Google Scholar]
- 9.Koumpis C, Dimitrakakis C, Antsaklis A, et al. Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from Greece. Hereditary cancer in clinical practice. 2011;9:10. doi: 10.1186/1897-4287-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DS, Yoon SY, Looi LM, et al. Comparable frequency of BRCA1, BRCA2 and TP53 germline mutations in a multi-ethnic Asian cohort suggests TP53 screening should be offered together with BRCA1/2 screening to early-onset breast cancer patients. Breast cancer research: BCR. 2012;14:R66. doi: 10.1186/bcr3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:2654–63. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cass I, Holschneider C, Datta N, et al. BRCA-mutation-associated fallopian tube carcinoma: a distinct clinical phenotype? Obstetrics and gynecology. 2005;106:1327–34. doi: 10.1097/01.AOG.0000187892.78392.3f. [DOI] [PubMed] [Google Scholar]
- 13.Vicus D, Finch A, Cass I, et al. Prevalence of BRCA1 and BRCA2 germ line mutations among women with carcinoma of the fallopian tube. Gynecologic oncology. 2010;118:299–302. doi: 10.1016/j.ygyno.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Boyd J, Sonoda Y, Federici MG, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA: the journal of the American Medical Association. 2000;283:2260–5. doi: 10.1001/jama.283.17.2260. [DOI] [PubMed] [Google Scholar]
- 15.Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187–95. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 16.Quinn JE, Carser JE, James CR, et al. BRCA1 and implications for response to chemotherapy in ovarian cancer. Gynecologic oncology. 2009;113:134–42. doi: 10.1016/j.ygyno.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Konstantinopoulos PA, Spentzos D, Karlan BY, et al. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:3555–61. doi: 10.1200/JCO.2009.27.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safra T, Borgato L, Nicoletto MO, et al. BRCA mutation status and determinant of outcome in women with recurrent epithelial ovarian cancer treated with pegylated liposomal doxorubicin. Molecular cancer therapeutics. 2011;10:2000–7. doi: 10.1158/1535-7163.MCT-11-0272. [DOI] [PubMed] [Google Scholar]
- 19.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 20.Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:2512–9. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 21.Ramsey PS, White AM, Guinn DA, et al. Subcutaneous tissue reapproximation, alone or in combination with drain, in obese women undergoing cesarean delivery. Obstetrics and gynecology. 2005;105:967–73. doi: 10.1097/01.AOG.0000158866.68311.d1. [DOI] [PubMed] [Google Scholar]
- 22.Soisson AP, Olt G, Soper JT, et al. Prevention of superficial wound separation with subcutaneous retention sutures. Gynecologic oncology. 1993;51:330–4. doi: 10.1006/gyno.1993.1299. [DOI] [PubMed] [Google Scholar]
- 23.Vadaparampil ST, Quinn GP, Lee JH, et al. Satisfaction with physician recommendation for and information about genetic counseling among breast cancer patients. The breast journal. 2011;17:79–82. doi: 10.1111/j.1524-4741.2010.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wevers MR, Hahn DE, Verhoef S, et al. Breast cancer genetic counseling after diagnosis but before treatment: a pilot study on treatment consequences and psychological impact. Patient education and counseling. 2012;89:89–95. doi: 10.1016/j.pec.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Daniels MS, Urbauer DL, Stanley JL, et al. Timing of BRCA1/BRCA2 genetic testing in women with ovarian cancer. Genetics in medicine official journal of the American College of Medical Genetics. 2009;11:624–8. doi: 10.1097/GIM.0b013e3181ab2295. [DOI] [PubMed] [Google Scholar]
- 26.Lacour RA, Daniels MS, Westin SN, et al. What women with ovarian cancer think and know about genetic testing. Gynecologic oncology. 2008;111(1):132–6. doi: 10.1016/j.ygyno.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakefield CE, Meiser B, Homewood J, et al. A randomized trial of a breast/ovarian cancer genetic testing decision aid used as a communication aid during genetic counseling. Psycho-oncology. 2008;17:844–54. doi: 10.1002/pon.1353. [DOI] [PubMed] [Google Scholar]
- 28.Wakefield CE, Meiser B, Homewood J, et al. A randomized controlled trial of a decision aid for women considering genetic testing for breast and ovarian cancer risk. Breast cancer research and treatment. 2008;107:289–301. doi: 10.1007/s10549-007-9539-2. [DOI] [PubMed] [Google Scholar]
- 29.Meiser B, Gleeson M, Kasparian N, et al. There is no decision to make: experiences and attitudes toward treatment-focused genetic testing among women diagnosed with ovarian cancer. Gynecologic oncology. 2012;124:153–7. doi: 10.1016/j.ygyno.2011.09.040. [DOI] [PubMed] [Google Scholar]