Abstract
Mytilus foot protein type 6 (mfp-6) is crucial for maintaining the reducing conditions needed for optimal wet adhesion in marine mussels. In this report we describe the expression and production of a recombinant Mytilus californianus foot protein type 6 variant 1 (rmfp-6.1) fused with a hexa-histidine affinity tag in Escherichia coli and its purification by affinity chromatography. Recombinant mfp-6 showed high purification yields of 5–6 mg/L cell culture and excellent solubility in low pH buffers that retard oxidation of its many thiol groups. Purified rmfp-6.1 protein showed high DPPH radical scavenging activity as compared to Vitamin C. Using the highly sensitive surface force apparatus (SFA) technique to measure interfacial surface forces in the nanoNewton range we show that rmfp-6.1 is also able to rescue the oxidation-dependent adhesion loss of mussel foot protein 3 (mfp-3) at pH 3. The adhesion rescue is related to a reduction of dopaquinone back to DOPA in mfp-3 which is the reverse reaction observed during the detrimental enzymatic browning process in fruits and vegetables. Broadly viewed, rmfp-6.1 has potential as a versatile antioxidant for applications ranging from personal products to anti-spoilants for perishable foods during processing and storage.
Keywords: Adhesion rescue, antioxidant, DOPA oxidation, recombinant mussel protein, thiol
1 Introduction
Strong underwater adhesion is an evolutionary adaptation that developed in parallel among many different sessile organisms, yet only scarce information is available regarding the underlying molecular mechanisms involved. Recent investigations of the adhesive plaques of Mytilus californianus have provided deeper insights into the reactivity and organization of some of these proteins [1]. Among these proteins, five are unique to the plaque (mfp-2, -3, -4, -5, and -6) and all contain the tyrosine derivative 3,4-dihydroxyphenyl-L-alanine (DOPA) that by itself is known to mediate strong adhesion on polar surfaces [2]. However, at the high pH of seawater (~ 8.2) DOPA containing protein readily undergoes auto-oxidation to dopaquinone (DQ) resulting in a considerable loss of adhesion [3]. In contrast to the other four proteins, mfp-6 shows weak adhesion and contains small amounts of DOPA (< 5 mol %) but significant levels of cysteine (11 mol %, [4]). Interestingly, recent biochemical studies on mfp-6 supported the hypothesis of mfp-6 as a highly potent proteinogenic antioxidant for the DOPA-containing adhesives [3]. The five mfp-6 isoforms annotated to date have a highly conserved amino acid composition and sequence homology (Table S1) assuming that their respective antioxidant activities are similar. Here we report the construction and characterization of Mytilus californianus recombinant foot protein type 6 variant 1 (rmfp-6.1) fused with a hexahistidine affinity tag in Escherichia coli. The His6-tagged rmfp-6.1 protein showed high antioxidant activity that was determined using an optimized 1,1-diphenyl-2-picryl-hydrazyl (DPPH) assay as described previously [5]. Rescue of oxidation-dependent adhesion loss due to rmfp-6.1 mediated reduction of dopaquinone back to DOPA in the mussel foot protein mfp-3 was tested using a Surface Forces Apparatus [6].
2 Materials and Methods
Chemicals
2,2-Diphenyl-1-picrylhydrazyl (DPPH•), L-Ascorbic acid (> 99 % purity), and Lysozyme (Chicken Egg White) were obtained from Sigma Aldrich (St. Louis, USA). Spectrophotometric grade of methanol and the nonionic detergent Triton X-100 were purchased from Fisher Scientific (Pittsburgh, USA). Citric acid monohydrate (> 99 % purity) and sodium phosphate monobasic (> 98 % purity) were purchased from EMD Millipore (San Diego, USA). Double distilled water (EMD Millipore, San Diego, USA) was used throughout the experiments.
Strains and Plasmid Construction
E. coli One Shot TOP10 chemically competent cells [F- mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 Δlacχ74 recA1 araD139 Δ(ara-leu) 7697 galU galK rpsL (StrR) endA1 nupG λ-] (Life Technologies Grand Island, NY) were used for recombinant plasmid construction. E. coli Rosetta 2 (DE3) cells [F− ompT hsdSB(rB− mB−) gal dcm (DE3) pRARE (CamR)] were used as a host strain for expressing recombinant rmfp-6.1. We used the Rosetta 2 (DE3) cells to compensate for rarely used tRNAs in E. coli and thus enhance the heterologous expression of the eukaryotic mussel protein. Previous studies on the heterologous expression of recombinant mussel adhesive proteins frequently led to low yields or failed to express functional protein in E. coli BL21 or JM109 strains (personal communication, Dr. Dong-Soo Hwang). Gene sequence information for native mfp6-v1 was obtained from GenBank (DQ351537.1; http://www.ncbi.nlm.nih.gov/genbank/). The mfp6-v1 sequence (without the signal sequence) was cloned out of a mixture of Mytilus californianus foot cDNA library ([4], Table S1) using combined gradient and touchdown polymerase chain reaction (PCR). Specific codon-optimized primers for PCR amplification were synthesized (Table S2). The forward primer was: v1-NdeI-FW, 5′-GGG CAT ATG GGT GGG GGA AAC TAC AGA GG – 3′ (29 nt, containing a NdeI recognition site), and reverse primer was: v1-HindIII-Rev, 5′-GGG AAG CTT AGT AAC CAC TAC GAA GAC AAC - 3′ (30 nt, containing a HindIII recognition site). The bolded parts of primer sequences are complementary to the nucleotide sequences of the mfp6-v1 gene, whereas 5′ overhanging ends of primers contain recognition sites for restriction endonucleases (underlined), and are designed to facilitate cloning. Designed primers and 2.5 units of Taq DNA polymerase (Fisher Scientific, Pittsburgh, PA) were used in a touchdown PCR reaction for 10 cycles with a temperature profile of 30s at 95°C, 45s at 70°C, and 1min at 72°C as well as another 20 cycles with a temperature profile of 30s at 95°C, 45s at 60°C, and 1min at 72°C in an Eppendorf Mastercycler Gradient (Eppendorf, Hauppauge, NY). The amplification products were analyzed by electrophoresis on a 1% agarose gels stained with ethidium bromide. An approximately 300bp-specific rmfp-6.1 PCR product was inserted into a pCR 2.1-TOPO vector for sequencing using the TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA). E. coli One Shot TOP10 cells were transformed with the vector construct and three independently derived clones containing the rmfp-6.1 gene were selected and sequenced using a commercial service (Genewiz, South Plainfield, NJ). The sequenced gene construct was digested with NdeI and HindIII, purified and ligated into the Nde I and Hind III sites of the pET-28a(+) vector (EMD Chemicals, Philadelphia, PA). The vector contains a hexahistidine (His6) tag at the N-terminus to simplify protein purification and a T7 promoter that is inducible by isopropyl-β-D-1-thiogalactopyranoside (IPTG). E. coli Rosetta 2 (DE3) cells were transformed with the ligation mixture and used for the expression and purification procedure.
Media and Cell Culture
For strain construction and protein expression, E. coli cells were grown in enriched 2x TY medium containing 16 g/L tryptone, 10 g/L yeast extract, and 5 g/L NaCl. The constructed transformant harboring the plasmid was stored at −80 °C. Culture experiments were performed with 2L of 2xTY medium supplemented with 50 μg/mL kanamycin and 50 μg/mL chloramphenicol (Sigma-Aldrich, St. Louis, MO) at 37 °C and shaking at 250 rpm. Cell growth was monitored by measuring the optical density at 600 nm (OD600) using a Nanodrop 2000c UV-vis spectrophotometer (Thermo Scientific, Wilmington, DE). When cultures reached an OD600 of 0.7–0.8, 10 μM (final concentration) IPTG was added to the culture broth for induction of recombinant mfp-6.1. It has to be noted that commonly used IPTG concentrations to initiate protein expression range from 0.5 – 4 mM final concentration. However, inducing the plasmid containing E. coli cells with IPTG concentrations higher than 50 μM led to cell death (Figure 1A). The induced cells were grown for 5h, harvested and centrifuged at a 5,000 g for 20 min at 4 °C, and the pellets were stored at −80 °C for further analyses.
Figure 1. Expression of Recombinant mfp-6.1.
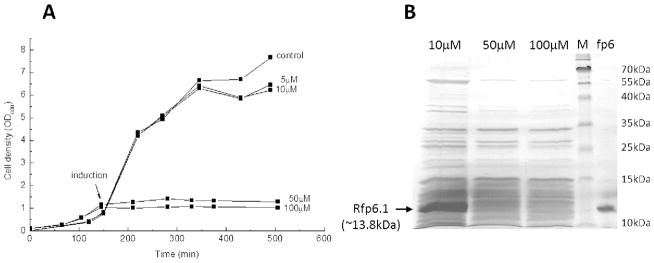
(A) Growth profiles of E. coli Rosetta 2 cells expressing rmfp-6.1 with and N-terminal His6-tag (V1DH). Cells were cultured in 2xTY medium at 37 °C and 250 rpm. The OD600 was determined in duplicate with two independent samples out of each culture and the mean value was plotted against the cultivation time. Induction with different amounts of IPTG was carried out when cells reached an OD600 of 0.7-0.8. Un-induced cells were grown as a control. Protein yields were 5-6 mg/L cell culture when induced with 10μM IPTG. (B) Effect of different inductor concentrations (IPTG) on the yield of rmfp-6.1 as shown by PAGE. 15% SDS-PAGE with Coomassie blue staining of the insoluble cell debris fraction with rmfp-6.1 expressed in inclusion bodies. Recombinant cells were cultured in LB medium at 37 °C and 250 rpm. Protein expression was induced at an OD600 of 0.7-0.8 using different amounts of IPTG (10, 50, 100μM). Lanes: M, protein molecular weight marker; mfp-6, native mfp-6 (11.6-12.2kDa) from M. californianus feet.
Purification of Recombinant mfp-6-v1
The cell pellets were dissolved in lysis buffer (1x PBS (137mM NaCl, 2.7mM KCl, 10mM Na2HPO4, 2mM KH2PO4) pH 7.4, 1 μg/ml pepstatin, 1 μg/ml leupeptin, 5 mg/ml lysozyme, 1 U/ml DNAse) and disrupted using a Q700 sonicator at 4°C (Qsonica, Newtown, CT) and in pulse mode (5 s pulse, 10 s break, 10 min total, 40 % amplitude). Cell debris and inclusion bodies (IB) were spun down at 10,000 g and 4°C for 30min and washed twice with washing buffer (1x PBS pH 7.4, 1 μg/μl Pepstatin, 1 μg/μl Leupeptin, and 1 % v/v Triton X-100). Recombinant mfp-6.1 was extracted from the pellet with extraction buffer (5% v/v acetic acid, 50mM TCEP, 8M urea) and the supernatant was dialyzed overnight against 5% v/v acetic acid in a total volume ratio of 1:1200 and in 1 kDa-cut off dialysis tubing (Spectrum Laboratories, Rancho Dominguez, CA). Immobilized metal affinity chromatography (IMAC) purification was performed using the Akta MFPLC Purification System at room temperature with 1 mL/min and a pre-packed 1ml HisTrap FF Crude column (GE Healthcare, Pittsburgh, PA) as affinity purification resin. Affinity purification was performed under denaturing conditions. Dialyzed samples were freeze-dried and resuspended in washing buffer (50 mM Na2HPO4, 8 M Urea, 500 mM NaCl, 10 mM Imidazole, pH 7.4). The column was equilibrated with 5 resin volumes of washing buffer and then loaded with 2 ml of the resuspended denatured samples. Target recombinant mpf6.1 was eluted with elution buffer (50 mM Na2HPO4, 8 M Urea, 100 mM NaCl, 250 mM Imidazole, pH 7.4). Eluted rmfp-6.1 was dialyzed in 5 % v/v acetic acid overnight at 4 °C, concentrated by freeze-drying, and finally resolved in 5 % v/v acetic acid. A final purification was performed using reverse phase C8 HPLC with a 260×7 mm RP-300 Aquapore (Applied Biosystems, San Jose, CA) column and a linear gradient of aqueous acetonitrile acidified with 0.1 % v/v TFA at 1 ml/min. Acid-urea or SDS polyacrylamide gel electrophoresis (PAGE) with Coomassie blue staining were performed to assess the purity of protein samples. To calculate the purified rmfp-6.1 concentration the protein’s absorbance at 280 nm was measured on a Nanodrop 2000C spectrophotometer (Thermo Scientific, Wilmington, DE) or quantified by a standard Bradford assay (Life Science Research, Hercules, CA). We used GelQuant.NET software provided by biochemlabsolutions.com to quantify relative protein yields in the PAGE gels.
MALDI-TOF Mass Spectrometry Analysis
Matrix-assisted laser desorption ionization (MALDI) mass spectrometry analysis with time-of-flight (TOF) was performed on a Voyager Mass Spectrometer LBT2 (Applied Biosystems, San Jose, CA) with 1.2 meter ion path in the positive ion linear mode. As matrix solution α-cyano-4-hydroxy-cinnamic acid (CHCA) in 50 % acetonitrile and 0.1 % trifluoroacetic acid (TFA) was used. Samples were diluted 1:20 with matrix solution and 1 μL was spotted onto the MALDI sample target plates and evaporated using a vacuum pump. Spectra were obtained in the mass range between 500 and 30,000 Da with 300 laser shots per spectrum. Accelerating voltage was 25.000 V with a grid voltage of 93 % and a guide wire 0 percentage of 0.3 %. Spectra were recorded in delayed extraction with a delay time of 300 ns. Internal calibration was performed using chicken egg white lysozyme (Sigma-Aldrich, St. Louis, MO) with a calculated molecular mass of 14.3 kDa. All data was analyzed using Voyager Data Explorer 4.0.0.0 (Applied Biosystems) and plotted using Origin 7.0 (Originlab).
DPPH coupled antioxidant assay
A well-established protocol for the determination of antioxidant activity in food and other biological samples is the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay [7]. In its radical form DPPH absorbs at 515nm (ε = 11,240 M−1 cm−1 in MeOH), but upon reduction by an antioxidant or radical species, the absorption disappears and the color of the solution changes from violet to pale yellow [8]. We used an optimized protocol as described previously [5] to test for the antioxidant activity of a protein like rmfp-6.1. Briefly, a mild non-ionic detergent (0.3 % v/v Triton X-100) is used to keep both the hydrophobic hydrazyl radical and the basic recombinant rmfp-6.1 protein soluble. To start the reaction, 5 μM of purified rmfp-6.1 or L-ascorbic acid (control antioxidant) was added to 100 μM freshly prepared DPPH in 0.1 M citrate phosphate buffer, pH 3.0 [9]. The decrease in absorbance at 515 nm is followed over 60 min to monitor the reduction of free radical DPPH to DPPH2. The absorbance reduction at 515 nm was normalized against 100 mM DPPH without the addition of antioxidant as control. Antioxidant activity is described as relative DPPH reduction in percentage. The absorbance measurements were carried out in standard 1.5 ml micro-cuvettes on a Nanodrop 2000C Dual-mode UV-Vis Spectrophotometer at RT (Thermo Fisher Scientific Inc., Waltham, USA). When needed, the buffer pH was readjusted using glacial acetic acid (17.4 N) or sodium hydroxide solution (10 N) purchased from Fisher Scientific (Pittsburgh, USA) and followed with a digital pH meter (Radiometer model PHM 210, Radiometer Analytical SAS, France). The data was plotted using Origin 7.0 (Originlab) and fitted to a biphasic exponential decay function (y = y0 + A1*exp(-x/t1) + A2*exp(-x/t2)).
The Surface Forces Apparatus (SFA)
The normal force, F, normalized by the surface radius of curvature, R, was measured as a function of the mica-mica separation distance, D, using a SFA and has been described elsewhere [6]. Freshly cleaved mica surfaces glued onto a silica disc were incubated in a solution of 20 μL mfp-3 or rmfp-6.1 (20 μg/mL) for 15 minutes. The surfaces were then rinsed with the pH 3.0 buffer and always kept wet under the buffer solution without exposure to the atmosphere. The surfaces were then mounted in a sealed SFA for measuring the interfacial energy between the surfaces. The interaction (adhesion) energy of mica coated with a protein film (mfp-3 or rmfp-6.1) against an opposing mica surface (asymmetric) was measured with the SFA. In another experiment, both mica surfaces were coated with the protein (mfp-3 or rmfp-6.1) to measure the cohesive forces (symmetric) of interaction between the respective protein surfaces.
To test adhesion loss recovery about 100 pmol rmfp-6.1 were injected into the gap solution between the two mfp-3 (symmetric configuration) coated mica surfaces. In a different experiment, adhesion loss recovery was gauged by measuring the adhesive force of interaction between mfp-3 and the opposing mica surface (asymmetric configuration) after incubating the mfp-3 covered mica surface in 100 pmol rmfp-6.1, pH 3.0 buffer solution. The pH of the solution was adjusted using the following buffer solutions: 5 % acetic acid (EMD Chemicals, Gibbstown, NJ) (pH 3.0) and 0.016 M potassium phosphate monobasic (Mallinckrodt, Hazelwood, MO) and 0.084 M potassium phosphate dibasic (EMD Chemicals, Gibbstown, NJ) (pH 7.5). All glassware was cleaned in piranha solution and rinsed thoroughly in Milli-Q water (Millipore, Bedford, MA). All the solutions were prepared in Milli-Q water as well.
3 Results and Discussion
Expression of Recombinant mfp-6.1
In this work, the cDNA for M. californianus foot protein-6 variant-1 (mfp-6.1) was successfully amplified from a cDNA library kindly obtained by Dr. Dong-Soo Hwang and then inserted into a cloning vector. The nucleotide sequence was determined by DNA sequencing analysis and found to be identical to that in the GenBank database (DQ351537.1). We cloned the rmfp-6.1 cDNA into an expression vector containing an N-terminal His6-tag sequence and an IPTG-inducible T7 promoter to express recombinant mfp-6.1 in an E. coli expression system. Using this recombinant vector, we were able to successfully express recombinant rmfp-6.1 in E. coli cultures. SDS-gel densitometry using GelQuant.NET confirmed that about 86 % of the overexpressed rmfp-6.1 was found in the insoluble fraction of the cell lysate (Figure 1B and Figure S7). The apparent molecular mass of the recombinant mfp-6.1 according to SDS-PAGE analysis was about the same as for native mcmfp6 that we used as an internal control (Figure 1B). This was unexpected since the mix of native mfp-6 variants extracted from mussel feet has a calculated mass of 11.6 – 12.1 kDa [4] whereas the calculated mass for rmfp-6.1 fused to the N-terminal His6-tag and Thrombin cleavage site is about 13.6 kDa (Table S1). The mismatch between the apparent and actual masses of native and recombinant mfp-6.1 might result from the proteins being both basic but to a different extent (Table S1). For example, the calculated pI value for rmfp-6.1 is 9.0 whereas the calculated pI for the native mfp-6 variants is about 9.3. Proteins with higher pI values tend to bind more SDS molecules, thus increasing their electrophoretic mobility or even precipitating (e.g. mfp-1 in [10]) due to the high proportion of basic amino acid that can bind more SDS relative to the increase in overall charge [11]. Most of the expressed rmfp-6.1 protein was found in the insoluble fraction of the disrupted E. coli cells, indicating the formation of inclusion bodies typical for cysteine-rich proteins (Figure 1B and Figure S7, [12, 13]). Interestingly, cells induced with commonly used concentrations of IPTG (50–100 μM final concentration) failed to express the protein and exhibited a decreased cell growth upon induction. Only at very low concentrations of IPTG (≤ 10 μM), did the cells show growth profiles similar to the un-induced controls thereby expressing rmfp-6.1 in inclusion bodies (Figure 1A and B). This indicates that a slow expression of the thiol-rich protein might favor the formation of inclusion bodies that are more compatible with the cell metabolism of E. coli [14].
In addition, we constructed all three mfp6 variants with and without His6-tags using the codon-optimized primers listed in table S2. The expression and growth profile of the untagged rmfp-6.3 was similar to rmfp-6.1 (Figure S8) with a slightly lower yield of 4–5 mg purified protein per liter of cell culture. Purification of this untagged protein was more challenging and had to go through additional steps including dialysis, desalting, RP-HPLC, and SEC to reach the level of purity of His-tagged rmfp-6.1 after affinity purification (Figure S10). The thrombin cleavage efficiency of purified His6-rmfp-6.1 was explored to insure that His6-tag could be removed for future studies requiring a non-tagged recombinant antioxidant protein (Figure S11). Because the presence of the His6-tag in rmfp-6.1 did not alter its high antioxidant activity, this variant was used for all studies undertaken here.
Purification of Recombinant mfp-6.1
The expressed recombinant rmfp-6.1 had a His6 tag, which enabled affinity purification. Protein purity was assessed using SDS- (Figure 2A) or AU-PAGE (Figure S5, lower right panel). Denaturing conditions were used during purification to keep the basic and thiol-rich rmfp-6.1 protein soluble at pH 7.4 in the washing and elution buffers. Cells were lysed with 8M urea yielding 30 mL of cell lysate per liter of culture volume containing approximately 5–6 mg rmfp-6.1. Lysates were loaded into IMAC columns and purified by affinity interaction between nickel and the N-terminal His6 residues (Figure 2A). For further purification we first dialyzed out the urea from the samples (1:1200) and freeze-dried the soluble fraction prior to reconstitution in 5 % acetic acid buffer and application to a RP-HPLC column. This further increased the purity of the samples and resulted in monomeric rmfp-6.1 as shown by MALDI mass spectrometry (Figure 2B). Occasionally, the thiol-rich rmfp-6.1 became oxidized to dimers or higher oligomers during the purification procedure and size exclusion chromatography (SEC) were used to separate the monomeric rmfp-6.1 form for all further experiments. MALDI-TOF MS analysis the purified sample showed that the actual molecular mass of recombinant rmfp-6.1 was almost identical to the calculated mass of 13.6 kDa and confirmed that recombinant rmfp-6.1 had been successfully purified to a high level (Figure 2B).
Figure 2. Purification of Recombinant mfp-6.1.
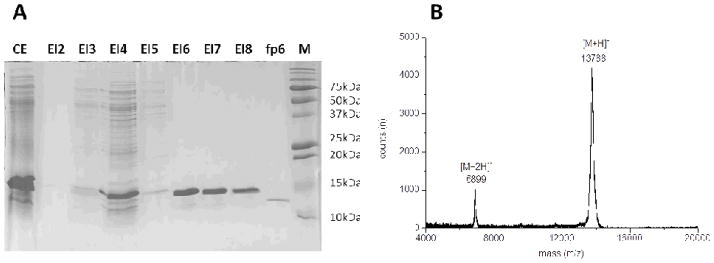
Purification of recombinant mfp-6.1. (A) 15% SDS-PAGE with Coomassie blue staining of the His6-tag purification of rmfp-6.1 using fast protein liquid chromatography and (B) MALDI TOF mass spectrum of the MFPLC-purified rmfp-6.1. Matrix=α-cyano-4-hydroxycinnamic acid, Accelerating Voltage=25000 V, Grid Voltage=93%, Guide Wire Voltage = 0.3, Delay Time=300ms, CE = crude extract, El2-4 = flow through, El5-8 = elution, M = marker. Please refer to the FPLC chromatogram in Figure S9 for more details.
Antioxidant activity of purified rmfp-6.1
We used an optimized DPPH radical quenching assay to determine the relative antioxidant activity of purified rmfp-6.1 as described previously [5]. In short, the DPPH radical has an absorption maximum at 515–520 nm. Upon addition of antioxidants to the reaction buffer the DPPH radical gets reduced to DPPH2, which can be followed by a decrease in absorbance at λ515.
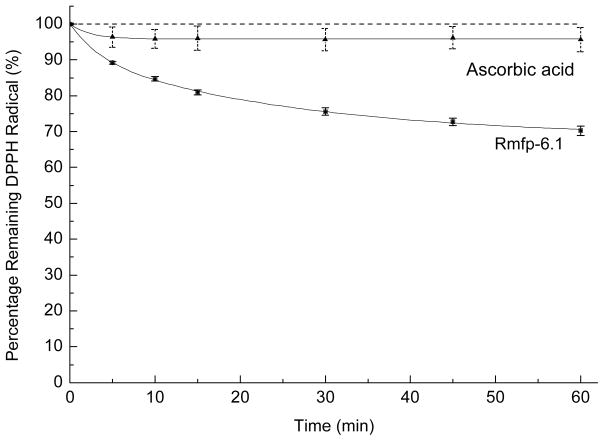
Like the native mfp-6, rmfp-6.1 shows highest solubility at pH 3 and starts precipitating above pH 5. Thus, we tested the antioxidant activity of rmfp-6.1 and ascorbic acid as control antioxidant at pH 3. Adding 5 μM of rmfp-6.1 to 100 μM DPPH in the reaction buffer shows a continuous exponential decay of the relative DPPH absorbance over the time course with a maximum reduction down to 70 % after 60 min (Figure 3). The addition of the molar equivalent of ascorbic acid (5 μM) as control antioxidant decreases the initial DPPH radical concentration by about 5 % after 5 min and goes into equilibrium over the whole time course of 60 min. This shows an antioxidant activity of rmfp-6.1 that is 6 times higher than that of ascorbic acid at a low pH of 3. The curves were fitted to a biphasic exponential decay function using the equation: y = y0 + A1*exp(-x/t1) + A2*exp(-x/t2) and with regression coefficient, r2 values of 0.994 for ascorbic acid and 0.999 for rmfp-6.1. The analysis of the respective rate kinetics reveals that for ascorbic acid we can calculate only one fast time constant with t1 = t2 = 2.4 ± 1.2 s−1 indicative of a single population of electron donating groups involved in the reduction of DPPH. With pKas of 4.2 and 11.6 for the two enolic OH groups of ascorbic acid we expect both reactive groups of ascorbic acid to be essentially protonated at pH 3 and responsible for the DPPH reduction. For rmfp-6.1 we get two time constants, a fast t1 = 3.4 ± 1.4 s−1 and as slow t2 = 26.1 ± 9.7 s−1. Two different electron donating populations exist in this protein and are responsible for DPPH reduction. Interestingly, the fast contribution (t1) is similar to that of ascorbic acid and might indicate that mainly protonated enol or phenol groups in rmfp-6.1 are reducing the DPPH radical. With respect to this, the phenol groups of the 20 tyrosines (pKa ~ 10) could serve as proton and electron reservoirs for DPPH reduction by rmfp-6.1. However, we recently showed that also the cysteine thiol moiety (pKa ~ 8–9) in native mfp-6 is contributing to the proteins antioxidant activity [3, 5]. In future experiments it needs to be determined which chemical moiety in the fp-6 proteins is the major contributor to the overall antioxidant activity. For this, a direct comparison of rmfp-6.1 and the DOPA-containing native mfp-6 antioxidant activity as well as a comparison to other antioxidant compounds could further elucidate the comparative antioxidant capacity of this recombinant antioxidant protein.
Figure 3. DPPH coupled assay: Antioxidant activity of rmfp-6.1.
Free radical reporter of antioxidant activity 1,1-diphenyl-2-picrylhydrazyl (DPPH). Time course of DPPH reduction using the antioxidant L-ascorbic acid and rmfp-6.1 protein at pH 3. 1 ml of a 0.1 M citrate phosphate reaction buffer contained 0.3% (v/v) Triton X-100 and 100 μM DPPH alone or supplemented with 5 μM of L-ascorbic acid or rmfp-6.1. The reaction was started by the addition of the antioxidant and the decrease in absorbance was monitored at 515 nm. The absorbance of DPPH without antioxidant addition was stable over 60 min (dashed line). For clarity, the control absorbance of 100 mM DPPH is displayed as 100% DPPH radical.
The Surface Forces Apparatus (SFA)
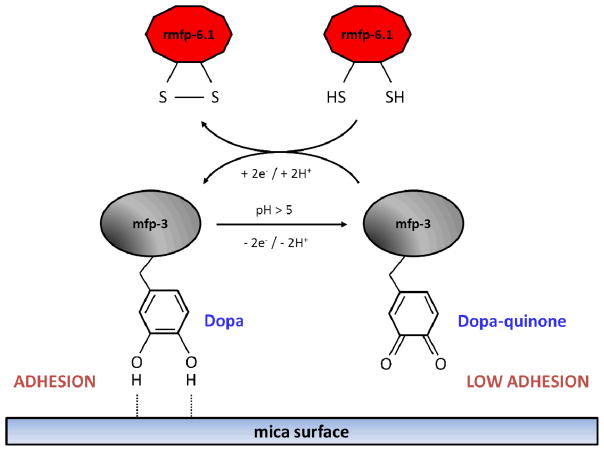
A critical test of function in rmfp-6.1 is to demonstrate that the protein is capable of maintaining the adhesion of Mfps from the mussel plaque of Mytilus californianus by keeping them in the reduced form ([3], Figure 5). Please refer to the supplemental “Materials and Methods” part in [1] for a more detailed description and illustration of using the SFA technique to identify mussel foot protein interactions.
Figure 5. SFA discussion.
Thiol-mediated adhesion loss recovery of rmfp-6.1. In the reduced state, the DOPA side chains of mfp-3 contribute to H-bonding on mica surface (H-bond acceptor). At high pH (>5) auto-oxidation of DOPA to dopaquinone (DQ) prevents H-bonding and thus adhesion to mica. Oxidation of the thiol groups of rmfp-6.1 provides electrons to fully reduce DQ back to DOPA.
The normalized force vs distance (F/R vs. D) profile of rmfp-6.1 coated mica surface against the opposing mica surface (with no protein on it) did not show any adhesion at pH 3.0 or 7.5, and the measured forces were purely repulsive during both approach and separation (Figure 4A). Mfp-3 coated on only one mica sheet (asymmetric configuration) showed a strong adhesion with an adhesion energy of Wad = 3.2±0.4 mJ/m2 at pH 3.0 (Figure 4B). This is a combined effect of hydrogen bonding and hydrophobic interactions. The –OH group in DOPA forms hydrogen bonds with the O atoms of the polysiloxane groups in mica. In addition, the polysiloxane surface of mica exhibits some hydrophobic tendencies at low pH (unpublished data, J. Israelachvili), hence the high adhesion energy could also be attributed to hydrophobic interactions between the mica surface and DOPA or other hydrophobic residues in mfp-3 (i.e. tryptophan and tyrosine). Increasing the pH to 7.5 oxidizes DOPA to dopaquinone but also makes mica more negatively charged and hydrophilic, resulting in an adhesion loss between the surfaces. The increase in hardwall thickness from ~ 2 nm to 10 nm was already reported by [15] and indicates reversible tautomerization of DOPA to Δ-DOPA which stiffens the backbone thereby expanding mfp-3 at higher pHs. Lowering the pH back to 3.0 did not recover the adhesion by substantial amount (Wad = 0.6 mJ/m2). That dopaquinone is not reversibly reduced back to DOPA on pH reversal reflects a kinetic rather than thermodynamic barrier. However, injecting rmfp-6.1 between the mica surfaces recovered about 72 % of the initial adhesion energy (Initial Wad = 3.2±0.4 mJ/m2) of mfp-3 (Figure 4B) with a measured adhesion energy of Wad = 2.3±0.2 mJ/m2.
Figure 4. SFA: mfp-3 adhesion recovery with rmfp-6.1.
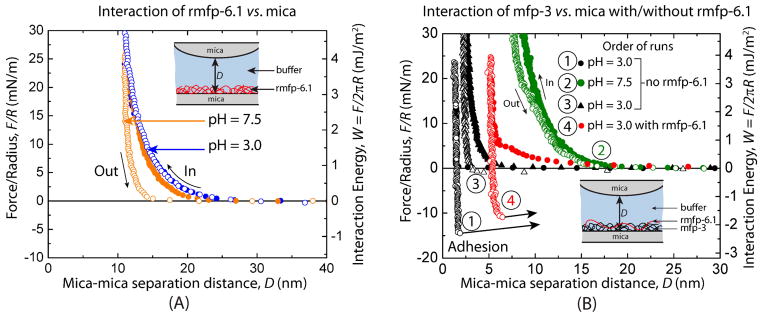
(A) Interaction of rmfp-6.1 deposited on one of the mica surfaces with the opposing mica surface at pH 3.0 and 7.5 showing no adhesion between rmfp-6.1 and the mica surface. (B) Interaction of mfp-3 deposited on one of the mica surfaces with the opposing mica surface at pH 3.0 and 7.5 is shown. Strong adhesion (Wad = 3.2±0.4 mJ/m2) between mfp-3 and the mica surface was observed at pH 3.0 (black circles). There was a significant loss in the adhesion on increasing the pH to 7.5 (green circles). Decreasing the pH back to 3.0 did not recover the loss adhesion (black triangles). Incubating the mfp-3 surface in 100 pmol rmfp-6.1 recovered almost 72 % of the initial adhesion (Wad = 2.3±0.2 mJ/m2) and is shown in red circles. It should be noted that we reported the adhesion energy using the JKR theory (Wad = Fad/1.5πR) and not Derjaguin approximation (Wad = Fad/2πR) since the surfaces go to a flat contact. Forces measured on approach are shown by solid circles and on separation by open circles.
It is noteworthy that rmfp-6.1 shows the same hardwall thickness of about 10 nm at pH 3.0 and at pH 7.5 where it undergoes precipitation (Figure 4A). Once rmfp-6.1 is injected into the gap solution it recovers the adhesion of mfp-3 and only increases the hardwall thickness from about 2 nm up to 5 nm. This slight increase in hardwall thickness upon adhesion loss recovery was previously observed by [3] in the symmetrical setup. This might indicate that upon oxidation both mfp-6 and rmfp-6.1 adapt a more compact structure with a smaller hydrodynamic radius and may partially intercalate into the mfp-3 layer instead of topping off the mfp-3 protein. A decrease in the hydrodynamic radius upon cysteine oxidation is known to occur in human serum albumin [16]. Similar adhesion loss recovery of mfp-3, but to a lower extent, was also observed when the same experiment was repeated with both mica surfaces coated in a symmetric configuration (Figure S7).
4 Concluding remarks
Recombinant mfp-6.1 with a molecular mass of 13.8 kDa was successfully expressed in an E. coli expression system. Affinity purification of recombinant mfp-6 variant under denaturing conditions resulted in a total yield of 5–6 mg purified protein per liter culture medium. Recombinant mfp-6.1 was expressed in inclusion bodies in E. coli. Since inclusion bodies are formed from partially folded protein intermediates and usually composed of aggregates of mostly a single type of polypeptide, rmfp-6.1 would be highly amenable to large-scale expression and purification. The purified protein shows excellent solubility in 5 % acetic acid buffer (pH 3.0), which also retards undesired oxidation product formation during storage and usage. Radical quenching assays and SFA measurements revealed that the purified protein has high antioxidant activity even at low pH of 3 which is in the range of stomach and skin pH [17]. These findings collectively suggest that recombinant rmfp-6.1 not only scavenges radical compounds but can also reduce oxidized quinones back to o-diphenols. Apparently, post-translational modifications in native mfp-6 such as DOPA and phosphoserine are not essential for antioxidant activity. Future experiments to support this hypothesis will include eukaryotic expression systems like Sf9 insect cells that showed in vivo posttranslational modifications of mussel adhesive proteins including DOPA conversion [18]. Hence, the heterologously expressed and purified rmfp-6.1 in this work has promise as an antioxidant additive in industrial and personal products and, in the food industry, as a nutritive antioxidant to prevent enzymatic browning during the processing and storage of perishables [19].
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01 DE 018468) and the Materials Research Science and Engineering Centers Program of the National Science Foundation under Award No. DMR 1121053. We thank Garrett Tom who assisted with early protein purification. Dr. Wei Wei kindly provided Mfp-3 protein in the final stage of the project. We thank Dr. Dong-Soo Hwang for generously providing the mussel foot cDNA library. The authors have declared no conflict of interest.
Abbreviations
- DOPA
3,4-dihydroxyphenylalanine
- DQ
dopaquinone
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- Mfps
Mytilus foot proteins
- SFA
surface forces apparatus
Footnotes
Contributions: S.C.T.N constructed overexpressed and purified rmfp-6 proteins, designed and performed the DPPH assay and performed MALDI mass spectrometry and thrombin cleavage experiments; S.D. designed and performed the SFA experiments; N.R.M.R performed the SFA experiments; J.H.W. designed and co-supervised the whole project with J.N.I., who helped analyze the results.
References
- 1.Hwang DS, Zeng H, Masic A, Harrington MJ, Israelachvili J, Waite JH. Protein- and metal-dependent interactions of a prominent protein in mussel adhesive plaques. J Biol Chem. 2010;285:25850–25858. doi: 10.1074/jbc.M110.133157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee HS, Scherer NF, Messersmith PB. Single-molecule mechanics of mussel adhesion. P Natl Acad Sci. 2006;103:12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J, Wei W, Danner E, Ashley RK, Israelachvili JN, Waite JH. Mussel protein adhesion depends on interprotein thiol-mediated redox modulation. Nature Chem Biol. 2011b;7:588–590. doi: 10.1038/nchembio.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao H, Waite JH. Linking adhesive and structural proteins in the attachment plaque of Mytilus californianus. J Biol Chem. 2006;281:26150–26158. doi: 10.1074/jbc.M604357200. [DOI] [PubMed] [Google Scholar]
- 5.Nicklisch SCT, Waite JH. Mini-review: The role of redox in DOPA-mediated marine adhesion. Biofouling. 2012;28(8):865–877. doi: 10.1080/08927014.2012.719023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Israelachvil JN, Min Y, Akbulut YM, Alig A, et al. Recent advances in the surface forces apparatus (SFA) technique. Rep Prog Phys. 2010;73:036601. [Google Scholar]
- 7.Blois MS. Antioxidant Determinations by the Use of a Stable Free Radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 8.Brand-Williams W, Cuvelier M, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food. 1995;8:25–30. [Google Scholar]
- 9.McIlvaine TC. A buffer solution for colorimetric comparison. J Biol Chem. 1921;49:183–186. [Google Scholar]
- 10.Waite JH, Tanzer ML. Polyphenolic Substance of Mytilus edulis: Novel Adhesive Containing L-Dopa and Hydroxyproline. Science. 1981;212:1038–1040. doi: 10.1126/science.212.4498.1038. [DOI] [PubMed] [Google Scholar]
- 11.Shi Q, Jackowski G. In: Gel electrophoresis of proteins: A practical approach. 3. Hames BD, editor. Oxford University Press; New York: 1998. p. 33. [Google Scholar]
- 12.DeCollo TV, Lees WJ. Effects of aromatic thiols on thiol-disulfide interchange reactions that occur during protein folding. J Org Chem. 2001;66:4244–4249. doi: 10.1021/jo015600a. [DOI] [PubMed] [Google Scholar]
- 13.Vallejo LF, Rinas U. Strategies for the recovery of active proteins through refolding of bacterial inclusion body proteins. Microb Cell Fact. 2004;3:11. doi: 10.1186/1475-2859-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sriubolmas N, Panbangred W, Sriurairatana S, Meevootisom V. Localization and characterization of inclusion bodies in recombinant Escherichia coli cells overproducing penicillin G acylase. Appl Microbiol Biotechnol. 1997;47(4):373–8. doi: 10.1007/s002530050943. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Wei W, Danner E, Israelachvili JN, Waite JH. Effects of interfacial redox in mussel adhesive protein films on mica. Adv Mater. 2011a;23:2362–2366. doi: 10.1002/adma.201003580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JY, Hirose M. Partially Folded State of the Disulfide-reduced Form of Human Serum Albumin as an Intermediate for Reversible Denaturation. J Biol Chem. 1992;267(21):14753–14758. [PubMed] [Google Scholar]
- 17.Lambers H, Piessens S, Bloem A, Pronk H, Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmetic Sci. 2006;28:359–370. doi: 10.1111/j.1467-2494.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 18.Lim S, Kim KR, Choi YS, Kim DK, Hwang D, Cha HJ. In vivo post-translational modifications of recombinant mussel adhesive protein in insect cells. Biotechnol Prog. 2011;27(5):1390–6. doi: 10.1002/btpr.662. [DOI] [PubMed] [Google Scholar]
- 19.Whitaker JR, Lee CY. Enzymatic browning and its prevention, Recent advances in chemistry of enzymatic browning. In: Lee CY, Whitaker JR, editors. Amer Chem Soc Symp Ser. Vol. 600. Washington DC: 1995. pp. 2–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.