Abstract
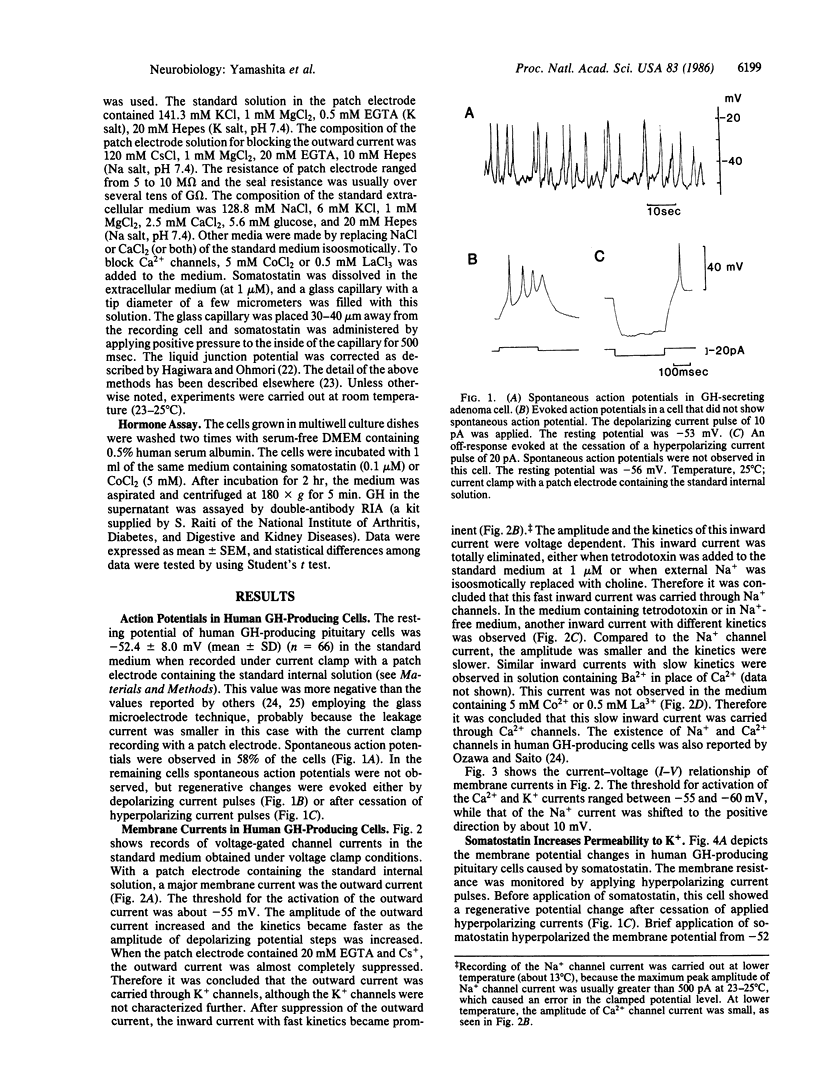
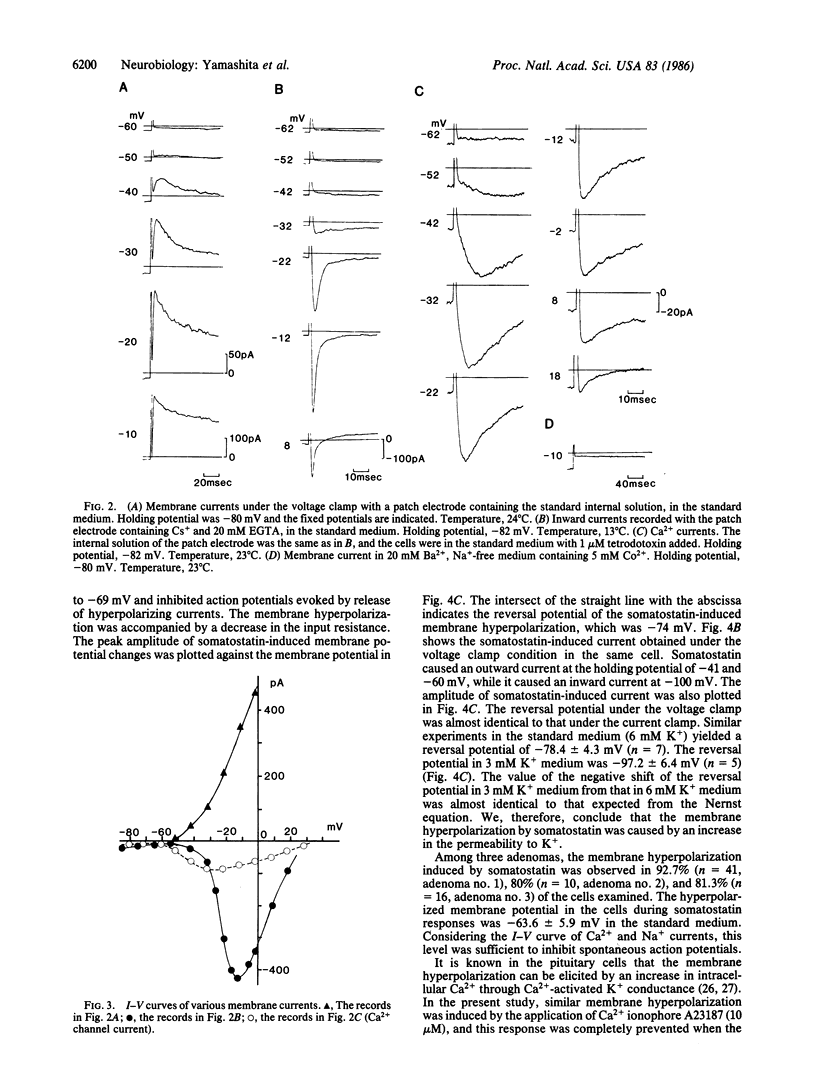
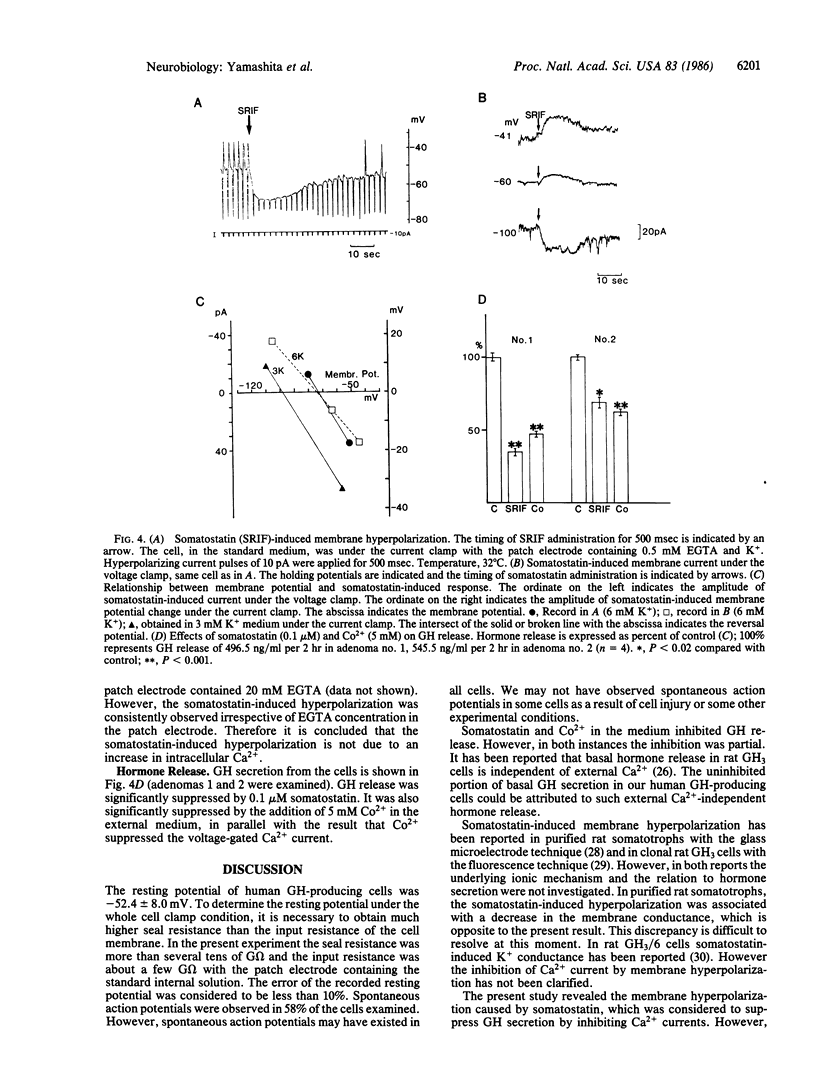
Membrane electrical properties and the response to somatostatin were examined in dissociated human pituitary adenoma cells that secrete growth hormone (GH). Under current clamp condition with a patch electrode, the resting potential was -52.4 +/- 8.0 mV, and spontaneous action potentials were observed in 58% of the cells. Under voltage clamp condition an outward K+ current, a tetrodotoxin-sensitive Na+ current, and a Ca2+ current were observed. Cobalt ions suppressed the Ca2+ current. The threshold of Ca2+ current activation was about -60 mV. Somatostatin elicited a membrane hyperpolarization associated with increased membrane permeability in these cells. The reversal potential of somatostatin-induced hyperpolarization was -78.4 +/- 4.3 mV in 6 mM K+ medium and -97.2 +/- 6.4 mV in 3 mM K+ medium. These reversal potential values and a shift with the external K+ concentration indicated that membrane hyperpolarization was caused by increased permeability to K+. The hyperpolarized membrane potential induced by somatostatin was -63.6 +/- 5.9 mV in the standard medium. This level was subthreshold for Ca2+ and Na+ currents and was sufficient to inhibit spontaneous action potentials. Hormone secretion was significantly suppressed by somatostatin and cobalt ions. Therefore, we suggest that Ca2+ entering the cell through voltage-dependent channels are playing an important role for GH secretion and that somatostatin suppresses GH secretion by blocking Ca2+ currents. Finally, we discuss other possibilities for the inhibitory effect of somatostatin on GH secretion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. F., Brajkovich I. E., Mashiter K. Hormone secretion by dispersed cell cultures of human pituitary adenomas: effects of theophylline, thyrotropin-releasing hormone, somatostatin, and 2-bromo-alpha-ergocryptine. J Clin Endocrinol Metab. 1979 Jul;49(1):120–126. doi: 10.1210/jcem-49-1-120. [DOI] [PubMed] [Google Scholar]
- Bilezikjian L. M., Vale W. W. Stimulation of adenosine 3',5'-monophosphate production by growth hormone-releasing factor and its inhibition by somatostatin in anterior pituitary cells in vitro. Endocrinology. 1983 Nov;113(5):1726–1731. doi: 10.1210/endo-113-5-1726. [DOI] [PubMed] [Google Scholar]
- Cronin M. J., Rogol A. D., Myers G. A., Hewlett E. L. Pertussis toxin blocks the somatostatin-induced inhibition of growth hormone release and adenosine 3',5'-monophosphate accumulation. Endocrinology. 1983 Jul;113(1):209–215. doi: 10.1210/endo-113-1-209. [DOI] [PubMed] [Google Scholar]
- Dorflinger L. J., Schonbrunn A. Somatostatin inhibits basal and vasoactive intestinal peptide-stimulated hormone release by different mechanisms in GH pituitary cells. Endocrinology. 1983 Nov;113(5):1551–1558. doi: 10.1210/endo-113-5-1551. [DOI] [PubMed] [Google Scholar]
- Dorflinger L. J., Schonbrunn A. Somatostatin inhibits vasoactive intestinal peptide-stimulated cyclic adenosine monophosphate accumulation in GH pituitary cells. Endocrinology. 1983 Nov;113(5):1541–1550. doi: 10.1210/endo-113-5-1541. [DOI] [PubMed] [Google Scholar]
- Dufy B., Israel J. M., Zyzek E., Dufy-Barbe L., Guerin J., Fleury H., Vincent J. D. An electrophysiological study of cultured human pituitary cells. Mol Cell Endocrinol. 1982 Jul;27(2):179–190. doi: 10.1016/0303-7207(82)90107-1. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heisler S., Reisine T. D., Hook V. Y., Axelrod J. Somatostatin inhibits multireceptor stimulation of cyclic AMP formation and corticotropin secretion in mouse pituitary tumor cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6502–6506. doi: 10.1073/pnas.79.21.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuyama S., Nawata H., Kato K., Karashima T., Ibayashi H., Nakagaki H. Specific somatostatin receptors on human pituitary adenoma cell membranes. J Clin Endocrinol Metab. 1985 Oct;61(4):666–671. doi: 10.1210/jcem-61-4-666. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Yamaji T. Direct effects of catecholamines, thyrotropin-releasing hormone, and somatostatin on growth hormone and prolactin secretion from adenomatous and nonadenomatous human pituitary cells in culture. J Clin Invest. 1984 Jan;73(1):66–78. doi: 10.1172/JCI111208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M., Yamaji T. Effects of hypophysiotropic factors on growth hormone and prolactin secretion from somatotroph adenomas in culture. J Clin Endocrinol Metab. 1985 May;60(5):985–993. doi: 10.1210/jcem-60-5-985. [DOI] [PubMed] [Google Scholar]
- Israel J. M., Denef C., Vincent J. D. Electrophysiological properties of normal somatotrophs in culture. An intracellular study. Neuroendocrinology. 1983 Sep;37(3):193–199. doi: 10.1159/000123542. [DOI] [PubMed] [Google Scholar]
- Kraicer J., Spence J. W. Release of growth hormone from purified somatotrophs: use of high K+ and the ionophore A23187 to elucidate interrelations among Ca++, adenosine 3',5'-monophosphate, and somatostatin. Endocrinology. 1981 Feb;108(2):651–657. doi: 10.1210/endo-108-2-651. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., Verleun T., Oosterom R. The interrelationship between the effects of somatostatin and human pancreatic growth hormone-releasing factor on growth hormone release by cultured pituitary tumor cells from patients with acromegaly. J Clin Endocrinol Metab. 1984 Feb;58(2):250–254. doi: 10.1210/jcem-58-2-250. [DOI] [PubMed] [Google Scholar]
- Moyse E., Le Dafniet M., Epelbaum J., Pagesy P., Peillon F., Kordon C., Enjalbert A. Somatostatin receptors in human growth hormone and prolactin-secreting pituitary adenomas. J Clin Endocrinol Metab. 1985 Jul;61(1):98–103. doi: 10.1210/jcem-61-1-98. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Kimura N. Membrane potential changes caused by thyrotropin-releasing hormone in the clonal GH3 cell and their relationship to secretion of pituitary hormone. Proc Natl Acad Sci U S A. 1979 Nov;76(11):6017–6020. doi: 10.1073/pnas.76.11.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S., Saito T. Sodium and calcium action potentials in human anterior pituitary cells. Experientia. 1980 Oct 15;36(10):1235–1236. doi: 10.1007/BF01976149. [DOI] [PubMed] [Google Scholar]
- Ozawa S. TRH-induced membrane hyperpolarization in rat clonal anterior pituitary cells. Am J Physiol. 1985 Jan;248(1 Pt 1):E64–E69. doi: 10.1152/ajpendo.1985.248.1.E64. [DOI] [PubMed] [Google Scholar]
- Pace C. S., Tarvin J. T. Somatostatin: mechanism of action in pancreatic islet beta-cells. Diabetes. 1981 Oct;30(10):836–842. doi: 10.2337/diab.30.10.836. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Landolt A. M. High density of somatostatin receptors in pituitary tumors from acromegalic patients. J Clin Endocrinol Metab. 1984 Dec;59(6):1148–1151. doi: 10.1210/jcem-59-6-1148. [DOI] [PubMed] [Google Scholar]
- Richardson U. I., Schonbrunn A. Inhibition of adrenocorticotropin secretion by somatostatin in pituitary cells in culture. Endocrinology. 1981 Jan;108(1):281–290. doi: 10.1210/endo-108-1-281. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Wuarin F., Wollheim C. B., Zahnd G. R. Somatostatin lowers the cytosolic free Ca2+ concentration in clonal rat pituitary cells (GH3 cells). Cell Calcium. 1984 Jun;5(3):223–236. doi: 10.1016/0143-4160(84)90038-1. [DOI] [PubMed] [Google Scholar]
- Schofield J. G., Bicknell R. J. Effects of somatostatin and verapamil on growth hormone release and 45Ca fluxes. Mol Cell Endocrinol. 1978 Jan;9(3):255–268. doi: 10.1016/0303-7207(78)90068-0. [DOI] [PubMed] [Google Scholar]
- Spada A., Sartorio A., Bassetti M., Pezzo G., Giannattasio G. In vitro effect of dopamine on growth hormone (GH) release from human GH-secreting pituitary adenomas. J Clin Endocrinol Metab. 1982 Oct;55(4):734–740. doi: 10.1210/jcem-55-4-734. [DOI] [PubMed] [Google Scholar]
- Spada A., Vallar L., Giannattasio G. Presence of an adenylate cyclase dually regulated by somatostatin and human pancreatic growth hormone (GH)-releasing factor in GH-secreting cells. Endocrinology. 1984 Sep;115(3):1203–1209. doi: 10.1210/endo-115-3-1203. [DOI] [PubMed] [Google Scholar]
- Tan K. N., Tashjian A. H., Jr Voltage-dependent calcium channels in pituitary cells in culture. II. Participation in thyrotropin-releasing hormone action on prolactin release. J Biol Chem. 1984 Jan 10;259(1):427–434. [PubMed] [Google Scholar]
- Vale W., Rivier C., Brazeau P., Guillemin R. Effects of somatostatin on the secretion of thyrotropin and prolactin. Endocrinology. 1974 Oct;95(4):968–977. doi: 10.1210/endo-95-4-968. [DOI] [PubMed] [Google Scholar]
- Webb C. B., Thominet J. L., Frohman L. A. Ectopic growth hormone releasing factor stimulates growth hormone release from human somatotroph adenomas in vitro. J Clin Endocrinol Metab. 1983 Feb;56(2):417–419. doi: 10.1210/jcem-56-2-417. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Takuwa Y., Ogata E. Growth hormone-releasing factor reduces voltage-gated Ca2+ channel current in rat GH3 cells. J Membr Biol. 1985;87(3):241–247. doi: 10.1007/BF01871224. [DOI] [PubMed] [Google Scholar]


