Summary
The mucosal immune system is governed by a unique set of rules and regulations. The local microenvironment dictates the necessity for these differences. The intestinal epithelial cell (IEC) sits at the interface between an antigen-rich lumen and a lymphocyte-rich lamina propria (LP). The cross talk that occurs between these compartments serves to maintain intestinal homeostasis. IECs have the capacity to talk to LP lymphocytes, activating populations of unique regulatory T cells. These cells have the capacity to talk back to the epithelium, influencing epithelial cell growth and differentiation. This review looks at this cross talk and places it in the context of mucosal immunoregulation.
Keywords: epithelial cells, mucosal immunity, lamina propria lymphocytes
Introduction
For many years, intestinal epithelial cells (IECs) were believed to be exclusively involved in the absorptive process of digestion. However, based on their location between the lumen and a wide array of mucosal lymphocytes, the concept that the IECs played a role in immune regulation evolved. The IEC lies at the border between an enormous number of dietary antigens and commensal bacteria present in the intestinal lumen and the most abundant accumulation of lymphocytes in the body, the mucosa-associated lymphoid tissue (MALT). As a physical barrier, the intestinal epithelium represents a potential entry site for both dietary and non-dietary luminal antigens. Far from being a passive barrier between these different compartments, evidence from a number of laboratories suggests that IECs can receive signals and interpret and transmit this information to the diverse populations of lymphocytes in the underlying lamina propria (LP). Considering the tremendous load of foreign antigen ingested daily, combined with the large number of lymphocytes resident in the mucosa, the intestine seems primed for an inevitable accident. Yet, despite these conditions, the normal state of the gut is one characterized by immune hyporesponsiveness. This suppressed state has implications for both normal and abnormal mucosal immune regulation, such as that seen in intestinal inflammatory disorders. With this in mind, the central issue in mucosal immunity relates to the mechanism by which the immune response in the gut is maintained and regulated. One possibility is that a key interaction occurs between the T cells that either regulate or promote inflammation and the IECs that control access to the mucosal compartments. Under normal circumstances, the presentation of antigen by IECs appears to lead to inactivation or suppression of the immune response. Several groups have proposed that IECs may also receive signals from underlying lymphocytes that might alter their barrier function or differentiation state. In this model, IECs are a central and critical component in a lively dialogue between bacteria or dietary antigen and underlying lymphocytes. In the intestine, two main lymphocyte populations exist: the intraepithelial lymphocytes (IELs), which remain associated with the basolateral membrane of the IECs, and the lamina propria lymphocytes (LPLs), which can localize to the subepithelial LP and are in contact with IECs via basolateral projections through the semiporous basement membrane (1).
IECs as sentinels
The human intestinal tract is continuously exposed to a wide range of food and other antigens from the external environment. It has the formidable task of discriminating between antigens that are harmless and those that are pathogenic. Unlike other epithelial surfaces such as the bladder or lung, which are sterile under non-pathological conditions, the epithelium of the colon must maintain a physical and immunological barrier in the presence of enormous quantities of luminal bacteria (2, 3). Commensal bacteria and food proteins must be distinguished from billions and trillions of potentially harmful organisms. The intestinal immune system has developed to simultaneously protect the host from pathogenic infections, while maintaining a quiet coexistence with the commensal flora.
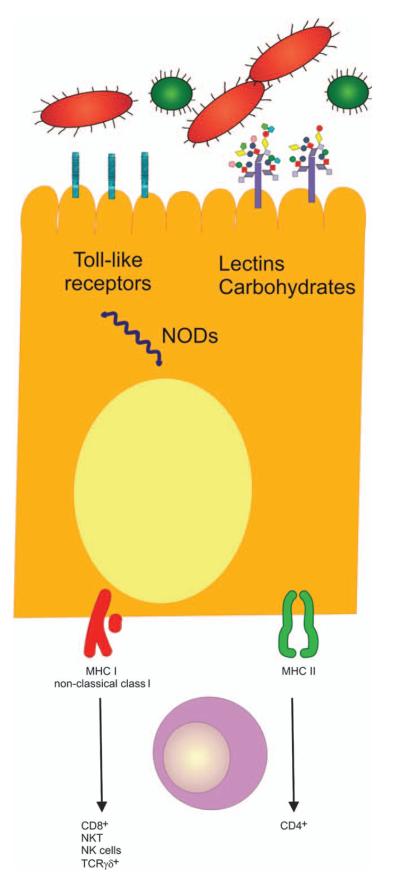
The ability of IECs to recognize and respond to this enormous diversity of microorganisms relies upon a set of receptors that recognize conserved bacterial and viral motifs. These include lectins and adhesins, the nucleotide-binding oligomerization domain (NOD) family, and the Toll-like receptors (TLRs) (Fig. 1) (4).
Fig. 1. IEC-mediated regulation of the immune response.
Resident and invasive bacteria and their molecules are released into the intestinal lumen and can be recognized by IECs. Sensing of bacteria and their products are mediated by pattern recognition molecules (TLRs and cytosolic NODs). As a result, non-classical class I molecules (CD1d, HLA-E, MICA/B) are expressed. Resident lymphocytes underlying the epithelial cells can bind to these non-classical MHC-I molecules leading to a diverse array of responses. Adapted from Shao et al.(4). NKT, natural killer T cells.
The innate immune system recognizes the presence of an infection and provides the necessary signals to elicit an adaptive immune response to the pathogen. In particular, TLR signaling is important for the maturation of antigen-presenting cells (APCs) that is necessary for the activation of T cells.
TLRs function as pattern recognition receptors (PRRs) in the innate immune system (5). They protect against dysregulated inflammation in the presence of commensal bacteria while defending the host against pathogens. In response to their different and specific ligands, they activate signal transduction pathways, usually through the nuclear factor-κB family of transcription factors, that induce the expression of a variety of immune/proinflammatory responses. The expression of TLRs is variable depending on the region within the intestinal tract (TLRs differ in the colon versus the small intestine where there is a lower bacterial load) (6, 7), the types of cells within the mucosa that express TLRs, and the regulation of TLR expression to specific compartments within cells (8, 9) (Table 1). In general, TLR expression is carefully regulated to shut down a proinflammatory response to commensal organisms. Some groups have reported that IECs do express several members of the TLR family, although this finding has been controversial. TLRs have been found to be associated with the Golgi apparatus, resulting in intracellular recognition of lipopolysaccharide. By controlling which cells express which TLRs and in which cellular compartment, the small intestine maintains tissue-specific homeostasis. This mechanism is underscored by several recent papers assessing the effects of TLR expression and function in mucosal inflammation models. The laboratories of Medhitov and Abreu both reported that dextran sodium sulfate (DSS)–induced colitis is more severe (greater bleeding and weight loss) in TLR4−/− and myeloid differentiation factor 88 (MyD88)−/− mice. This phenotype appears to segregate with the source of the IEC and stroma. From these data, one could hypothesize that TLR signals help to maintain the epithelial barrier and promote normal growth and differentiation of IECs. Others have brought this concept into the human model of inflammatory bowel disease (IBD), Crohn’s disease (CD). NOD2, an intracellular PRR recognizing muramyl dipeptide, has been implicated in the pathogenesis of CD. Between 19% and 37% of patients with CD carry one of three mutations in NOD2. The leucine-rich repeat region of NOD2 confers binding to MDP, and the three common mutations occur in this region, resulting in a failure to bind MDP. In Paneth cells, this result translates into a failure to secrete defensins. In the IECs, it may correlate with a failure to clear intracellular bacteria resulting in IEC dysfunction.
Table 1.
| TLR |
Ligands |
|---|---|
| TLR1 | Triacyl lipopeptides |
| TLR2 | Lipoprotein, HSP60, HSP70, PGN, LTA, porins, lipopolysaccharide, Pam3Cys, lipoarabinomannan, zymosan, trypansomal phospholipids, defensins |
| TLR3 | dsRNA, poly (I:C), endogenous mRNA |
| TLR4 | Taxol, lipopolysaccharide, Pseudomonas aeruginosa exoenzymes S, HSP60, trepansomal lipids, HSP70, HSP90, hyaluronic acid, fibrinogen, fibronectin, β-defensin, heparin sulphate, RSV F protein, MMTV envelope protein |
| TLR5 | Flagellin |
| TLR6 | Diacyl lipopeptides |
| TLR7 | Imiquimod, ssRNA |
| TLR8 | Resquimod, ssRNA |
| TLR9 | Bacterial/viral DNA, CpG DNA |
| TLR10 | Unidentified |
| TLR11 | Toxoplama gondii profilin-like protein |
PGN, peptidoglycan; LTA, lipoteichoic acid; RSV, respiratory syncytial virus; MMTV, murine mammary tumor virus; ssRNA, single stranded RNA.
There are other mechanisms whereby IECs act as a sentinel for mucosal immune responses. Both cytokine and chemokine production occurs within the epithelium. This production was initially documented in systems where bacterial invasion triggered a cascade of chemokines [e.g. interleukin-8 (IL-8), epithelial neutrophil-activating protein 78, monocyte chemotactic protein 1, macrophage inflammatory protein 3α (MIP-3α)]. Upregulation of epithelial-derived chemokines was also shown in IBD specimens. Similarly, a wide array of cytokines has been shown to be secreted by IECs, including IL-18, IL-7, IL-15, granulocyte-macrophage colony-stimulating factor, IL-6, and transforming growth factor-β (TGF-β). These cytokines can play inflammatory or regulatory roles, depending upon the local conditions. For example, recent data suggest that the ‘toxic’ portion of the gliadin peptide is a potent stimulus of IL-15 secretion. The aberrant production of this cytokine may play a key role in the pathogenesis of celiac disease.
IECs and their role as non-professional APCs
Antigen uptake
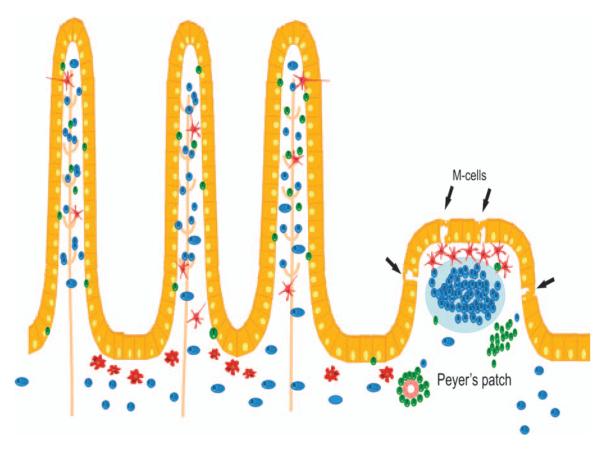
In order for IECs to act as APCs, they must be able to internalize and process antigen. The apical mucous layer and glycocalyx in the intestine can act as the initial barrier and restrict the size of particles capable of being internalized by IECs; this restriction would favor antigen uptake by the modified ‘dome’ epithelium (M cells), which overlie Peyer’s patches (PPs) (10). The highly specialized M cells facilitate transport of macromolecules, without notable or significant ‘cellular processing’, to the underlying lymphoid tissue, which contains several different types of professional APCs, including macrophages, dendritic cells (DCs), and B cells (11) (Fig. 2). However, the surface area of the villous epithelium is extraordinary, and IECs have a well-documented role in nutrient and soluble antigen uptake. IECs are exposed to a wide variety of antigens of diverse sizes and varying biochemical properties, including some antigens, such as gliadin, with known pathological significance in celiac disease. Collectively, these observations highlight a physiological role for IECs in the uptake and processing of luminal antigens and pathogens. Studies by So et al. (114) documented the ability of IEC lines as well as primary cells to take up soluble but not particulate antigens with kinetics that were slower than those seen with conventional APCs. IECs are also able to take up antigen by receptor-mediated endocytosis (e.g. CD23, asialo-GM1), although these antigens may traffic via different intracellular pathways. Indeed, the literature is replete with data from a variety of in vivo models showing uptake and endolysosomal delivery of macromolecules by IECs from the lumen of the gastrointestinal (GI) tract (12).
Fig. 2. The epithelial barrier.
The physical barrier that separates the large number of commensal bacteria and potential pathogens from the underlying tissues is only a simple single-cell epithelial layer. This monolayer predominantly consists of enterocyte interspersed by mucus-secreting goblet cells. Antigen might enter through special ‘gateways’, which comprise membranous cells (M cells) located in the follicles of the MALT in PPs. Here antigen is subsequently transferred to underlying DCs. Antigen sampling has also been reported by DCs reaching to the lumen between enterocytes. Uptake of antigens may also occur through the absorptive enterocytes. Here, in contrast, antigens are further processed before being presented to the immune system. T, T cells; B, B cells; MΘ, macrophages.
In addition to the transfer of information by IECs to the MALT, indirectly via the secretion of chemokines, IECs can transmit information from luminal microorganisms and antigens by directly processing and presenting them to antigen-specific lymphocytes. IECs throughout the bowel constitutively express major histocompatibility complex (MHC) class I, but they also express MHC class II constitutively, predominantly in the small bowel. The expression of these molecules can be upregulated in response to proinflammatory signals, such as interferon-γ (IFN-γ), throughout the intestine. IFN-γ has also been shown to cause antigens to be sorted into MHC II–positive late endosomes in epithelial cells (13). Epithelial antigen processing occurs in a highly polarized fashion with apical antigens being sorted, processed, and presented exclusively basolaterally (14, 15). Hershberg’s group further documented that IEC lines expressed cathepsins and were able to process intact antigen into appropriate immunogenic peptides for presentation to CD4+ T-cell hybridomas.
Like other non-professional APCs, IECs lack expression of the classical costimulatory molecules (CD80, CD86) needed to activate naive T-cell responses. However, IECs do express novel members of the B7 family, including inducible costimulatory ligand (ICOS/B7h) and programmed death-1 ligand (PD1-L/B7-H1) (16). Additionally, IECs express a number of non-classical MHC class I molecules that enable them to transmit luminal signals to LPLs and make them a unique APC in the gut. These molecules include CD1d, MHC class I chain–related gene A/B (MICA/B), human leukocyte antigen-E (HLA-E), FcRn, MR1, and UL16-binding protein. The expression, constitutive or induced, of these molecules plays a key role in activating populations of T cells in the LP that can either regulate or promote inflammation.
Various phenotypically distinct subpopulations of CD4+ and CD8+ T cells interact with IECs within the GI mucosa. CD4+ T-cell responses are critical both for the establishment of oral tolerance (17, 18) and, in several experimental models, IBD (19). Two distinct populations of CD4+ T cells are present within the intestinal mucosa and are in intimate contact with IECs: a limited but significant number of IELs express CD4 (most notably in the colon) (20, 21), and approximately two thirds of LPLs express CD4. IECs are highly polarized cells, with distinct apical and basolateral domains with very different physicochemical properties. The highly polarized morphology of IECs highlights several important distinctions in MHC class II processing between IECs and other more conventional APCs. Classical MHC class II pathways have been described in IECs for over a decade (22, 23). The expression of HLA class II antigens in vivo and in cell culture models of polarized IECs is mostly restricted to the basolateral membrane, a situation that implies a possible in vivo interaction with mucosal lymphocytes (15, 24, 25). Class II molecules have been shown to be functional as well. Indeed, IECs have the machinery required for appropriate antigen processing; whole antigen can be processed into immunogenic fragments for presentation. Because of the lack of classical costimulatory molecules on the IEC surface, the interaction between IECs and naive CD4+ T cells could result in the induction of anergy.
In the earliest studies assessing the capacity of IECs to act as non-professional APCs, the T cells activated were predominantly CD8+, which functionally suppressed immune responses in an antigen–non-specific fashion (23). Consistent with the somewhat unusual features of these CD8+ T cells stimulated by IECs (the lack of cytotoxicity and the ability of these cells to suppress a variety of T-cell functions), these findings supported the expression of class I–like molecules IECs.
IECs and their suppressive effect
The LP in the normal state and in patients with CD is replete with macrophages and DCs, which play an essential role in antigen presentation and shaping an immune response to particular antigens, including bacterial antigens. DCs contribute to the decision by naive T cells to drive polarized differentiation into T-helper 1 (Th1) or Th2 cytokine-producing lymphocytes (26). Intestine-specific DCs and macrophages use signals from luminal bacteria to generate tolerance/hyporesponsiveness through Tregs (27–30). A number of experimental systems show that altered regulation of intestinal T-cell function can result in chronic intestinal inflammation (19, 31–35). We know from these models that antigenic stimuli from the gut lumen may drive the expansion of both effector cells (potentially pathogenic) and regulatory T cells (Tregs), the latter being there to control the immune response (36). A lack of activation and/or expansion of regulatory cells could play a role in the uncontrolled inflammation seen in IBD. Distinct Tregs have been described, including CD4+ and CD8+ T-cell populations, many of which reside in the intestinal mucosa (37).
Tregs can be functionally defined as T cells that suppress immune responses either by producing IL-10 or TGF-β, resulting in antigen–non-specific decreases in T-effector responses or by cell–cell contact. The potential importance of CD4+ Tregs in the gut comes from numerous observations made in mouse models of IBD and oral tolerance. Initial studies using a cell transfer model of colitis, where disease is induced simply by the adoptive transfer of naive CD45RBhi cells into severe combined immunodeficiency (SCID) or recombination-activating gene (RAG)–deficient mice, have shown that cotransfer of CD4+ T cells with a ‘memory’ phenotype (CD45RBlo) prevents the development of disease (35). Since these early studies, several subpopulations of CD4+ Tregs have been described in mice. Tr1 cells, initially described by Groux and associates (115), produce high levels of IL-10 with little TGF-β and no IL-4. These cells have been found in the intestine of normal mice, were shown to be capable of inhibiting the induction of experimental colitis and murine allergic asthma, and may be involved in limiting immune responses to intestinal pathogens (38–41). Th3 cells, first described by Weiner and colleagues, were shown to be generated following the induction of oral tolerance to MBP and were shown to suppress the development of experimental autoimmune encephalitis through the production of TGF-β along with varying amounts of IL-4 and IL-10 (39, 40, 42). While both of these cells types have yet to be identified in humans, a third class of CD4+ Tregs has been described in both species. First identified by Sakaguchi et al., CD4+CD25+ forkhead box protein 3 (Foxp3)+ Tregs comprise 5–10% of total circulating CD4+ T cells, and these cells are believed to be critical for the regulation of autoreactive T cells that escape negative selection in the thymus (40, 43–46). Though initially believed to be generated only in the thymus, recent studies in mice have shown that these cells can be generated in the periphery from CD4+CD25− precursors (43). They primarily function through an unknown mechanism requiring direct cell–cell contact that may or may not involve the expression of cytotoxic T-lymphocyte antigen-4 (CTLA-4) and/or membrane-bound TGF-β, although they have also been shown to produce variable amounts of TGF-β and IL-10 (39, 40, 42). CD4+CD25+ Tregs constitutively express the T-cell-inhibitory receptor CTLA-4 and the glucocorticoid-inducible TNF receptor (47–50). These cells also express the transcription factor Foxp3, which serves as a marker of these cells. In murine models of IBD, the protective role of a variety of Tregs has been definitively shown (38, 51–53). Investigators have used a variety of technical approaches to identify regulatory cell defects in CD. Some of the limitations of these studies have to do with identifying reliable markers of Tregs in vivo.
Adoptive transfer of CD4+ T cells depleted of CD25+ T cells into immune-deficient mice (SCID or RAG−/−) results in the development of severe colitis, which is only prevented by cotransfer of CD4+CD25+ T cells (54). Furthermore, Foxp3 deficiency in both mice (scurfy mouse) and humans [immune dysfunction, polyendocrinopathy, enteropathy, X-linked inheritance (IPEX syndrome)] results in fatal multiorgan autoimmunity that includes involvement of the small bowel, indicating that these cells may play a role in normal gut immune homeostasis (55–58). In contrast, CD4+CD25+FoxP3+ Tregs have been cultured from the inflamed mucosa of patients with CD, indicating that the presence of these regulatory cells is not enough to inhibit disease (59). Thus, while numerous mouse models have described the importance of CD4+ Tregs in immunity in the gut, their functional role in mucosal immune responses in humans is poorly understood.
Our laboratory has examined the in vitro and in vivo potential to generate Tregs in the human intestine (60, 61). We have shown that IECs may play a role in the regulation of immune responses toward luminal antigen. We showed that a subset of CD8+ T cells undergoes oligoclonal expansion in the intestinal mucosa, through the interaction of these T cells with a unique complex expressed on IECs, formed by a CEA subfamily member (gp180) and CD1d. This subset, which is regulatory in vitro, may play a role in the control of intestinal immune responses toward luminal antigens. A lack of expansion of these CD8+ Tregs, probably related to the defective expression of the gp180/CD1d complex, is observed in IBD (62). Notably, we have also shown that patients with IBD are deficient in the expression of CEA subfamily members and that this lack correlates with an inability of these patients to be tolerized to orally administered antigens (63, 64). Together, these findings suggest that CD8+ T cells with regulatory activity present in the LP of healthy individuals, but not in the LP of patients with IBD activated by IECs through a CD1d/gp180 complex, play a central role in maintaining controlled inflammation in the intestinal mucosa.
Using another epithelial cell–T-cell interaction model, we confirmed our findings regarding the importance of distinct Treg populations regulated by unique restriction elements, costimulatory signals, and antigens (65). Mucosal surfaces such as the intestine are subject to constant antigenic challenge and have developed specific mechanisms for controlling immune responses, including the activation of Tregs. Similarly, the uterus may face a persistent, albeit temporary, antigenic challenge in the form of a semiallogeneic fetus (66, 67). Trophoblasts are anatomically poised to serve as modulators of the maternal immune system during pregnancy. Indeed, a recent study in our laboratory showed that trophoblasts could modulate the immune system through an interaction, via a member of the CEA family, with CD8+ T cells. The specificity of trophoblast-activated CD8+ T cells for regulating T-cell-dependent B-cell responses and the efficiency at which these cells are able to suppress immunoglobulin secretion suggests that these cells may occupy a regulatory niche during pregnancy.
The expansion of Tregs may have therapeutic efficacy in the treatment of IBD, especially in maintaining remission. Several Treg subsets produce high levels of IL-10. However, IL-10 has not been proven to be effective in the treatment of human IBD. A better understanding of the pathway of activation of Tregs may help to expand them in vivo.
IEC–T-cell interactions from the IEC’s point of view
For a very long time, the intestinal epithelial barrier was believed to exclusively serve an absorptive cell function in the digestive process. Later, the concept of IECs playing a role in mucosal immunity emerged. More recently, others have focused on the effect of IEC–T-cell interactions from the standpoint of the effect on IECs, and in particular on the role of LP T cells or IELs in IEC proliferation, differentiation, and homeostasis.
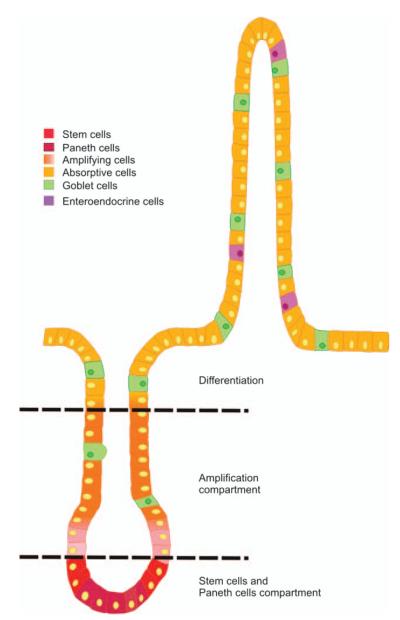
Cell proliferation, lineage-specific differentiation, migration, and finally apoptosis and/or cell shedding are tightly regulated processes that are spatially and temporally regulated along the crypt/surface axis in the colon. The epithelium is characterized by its rapid and continuous renewal. This process involves cell generation and migration from the stem cell population located at the bottom of the crypt to the extrusion of terminally differentiated cells at the tip of the villus (68, 69). Thus, the crypt is mainly composed of proliferative and poorly differentiated cells, whereas the villus is lined with functional absorptive, goblet, and endocrine cells (70). The molecular and cellular mechanisms responsible for the fine coordination between proliferation, migration, and differentiation along the crypt–villus axis are still largely unknown (Fig. 3).
Fig. 3. Crypt–villus axis.
Schematic representation of the crypt–villus. Stem cells are located near the crypt bottom. They divide bidirectionally and slowly differentiate into Paneth cells at the bottom or into enterocytes, absorptive cells, and enteroendocrine cells along the villus axis.
In one murine model of IBD, the IL-10–deficient mouse, lymphocyte development and antibody responses are normal, but most animals are growth retarded and anemic. The IL-10–deficient mice raised under conventional conditions develop a disease that becomes apparent by weight loss and anemia at the age of 4–8 weeks and is lethal for most animals. Profound alterations are present in the intestinal tract of these animals, such as a chronic enterocolitis that can involve the entire intestinal tract, with the duodenum, proximal jejunum, and the proximal colon being most severely affected. The mucosal inflammation is associated with either hyperregenerative or degenerative lesions of the intestinal epithelia. Some histopathological features of the intestine in these animals are reminiscent of human intestinal disorders with immunopathogenetic underpinnings of ulcerative colitis and celiac disease, the enterocolitis yet that develops cannot directly be equated with any human disease. The authors suggested that the primary defect in IL-10–deficient mice is a failure to control normal intestinal immune responses against enteric antigens, leading to chronic inflammation by unopposed production of TNF-α, IL-1, and IFN-γ. In a second step, enhanced epithelial MHC class II expression and antigen presentation and epithelial lesions may lead to a greater exposure of lymphoid cells in the mucosa to luminal antigens and bacterial cell wall components, reinforcing the inflammatory process. Moreover, the typical architecture of the mucosa is disturbed by the formation of abnormal crypt and villus structures consisting of branched and fused villi, enlarged and branched crypts, and labyrinthine sheets of enterocytes on the surface of the mucosa (33). These findings suggest that IL-10 and probably other factors involved in regulating inflammation may have an essential role in controlling intestinal epithelial homeostasis: proliferation versus differentiation.
The fact that lymphocytes could regulate IEC differentiation has been seen in several models. Kerneis et al. (71) documented the ability of PP lymphocytes to induce the differentiation of Caco-2 cells into M cells and showed that this was cell contact dependent. This observation supports the hypothesis that lymphocytes affect epithelial cell differentiation. Passage of antigens and microorganisms though M cells is an essential step for the development of mucosal immune responses and the pathophysiology of many infectious diseases. A consequence of the increase in follicle-associated epithelium would likely lead to an increase in antigen sampling, possibly promoting chronic inflammation.
Intestinal γδ IELs represent another important subset of mucosal T cells, especially within the epithelium (72). The role of IELs under normal and disease conditions remains controversial. However, IELs are involved in the maintenance of epithelial integrity through the production of cytokines and growth factors (73–76). Activated but not resting intestinal γδ IELs express the epithelial growth factor keratinocyte growth factor (KGF) (74). Administration of recombinant KGF to animals promotes the growth and differentiation of epithelial cells in various organs. In particular, epithelial cells lining the GI tract are highly responsive to KGF (77–81). Supporting a role for KGF in tissue repair, both KGF and its receptor (KGFR) are overexpressed in intestinal tissues obtained from patients with IBD (82, 83). Chen et al. evaluated the role of γδ intraepithelial T cells in the DSS-induced mouse colitis model system. γδ IELs in DSS-treated mice expressed KGF, a potent IEC mitogen. In this study, the authors showed that γδ T cell–deficient mice (TCRδ−/−) and KGF-deficient mice (KGF−/−) but not αβ T cell–deficient mice (TCRα−/−) were more prone than wildtype (WT) mice to DSS-induced mucosal injury and showed delayed tissue repair after termination of DSS treatment. These results suggest that γδ IELs help preserve the integrity of damaged epithelial surfaces by providing the localized delivery of an epithelial cell growth factor. Moreover, γδ IELs residing in skin, another epithelial tissue, were recently shown (84) to perform a role similar to that described by Chen et al. in the intestine. Thus, all these events may be part of a general immune mechanism involving γδ IELs that serves to monitor and maintain the integrity of epithelial tissues.
T cells activated by IECs can feed back on the epithelial cell to upregulate expression of non-classical MHC class I molecules. As seen in Fig. 4, T cells activated by IECs but not by professional APCs (macrophages) induce the expression of CD1d by the IECs. This occurs through contact-dependent mechanisms at the basolateral membrane, not apically. These data provide strong evidence that LPL–IEC interactions go both ways.
Fig. 4.
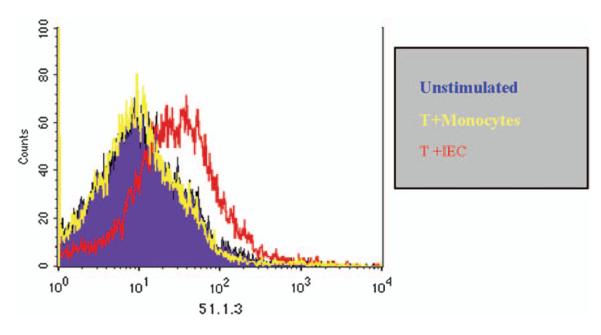
T84 cells were cultured on collagen coated inverted transwell filters until confluence was achieved. Peripheral blood T cells, T cells co-cultured with either allogeneic monocytes for 5 days or allogeneic intestinal epithelial cells for 5 days were added to the basolateral side of the membrane for 48h. CD1d expression on T84 cells was then determined by staining with the monoclonal antibody 51.1.3. CD1 expression was enhanced following co-culture with IED activated T cells.
A study by Pull et al. (85) further underscores the fact that the communication between IECs and mononuclear cells is not only directed toward the mononuclear cell but also toward the IEC. This group (85) described that intestinal macrophages, a crucial mobile cellular element that shapes the crypt progenitor cell niche, allow colonic epithelial progenitors to mount an appropriate regenerative response to injury. A key initial phase of epithelial regeneration involves the expansion of epithelial progenitors in areas immediately adjacent to areas of damage. Colonic epithelial progenitors (CoIEPs) reside in the lower half of mucosal invaginations known as crypts (86). The cellular and molecular factors that determine CoIEP responses to wound repair are unclear, although recent reports have emphasized the importance of the gut microbiota and TLR-mediated pathways (87). After short-term oral administration of DSS to C57BL/6 (B6) mice, there is a pronounced increase in CoIEP proliferation activity in distal colonic crypts located next to areas of exfoliated epithelium. This group clarified a previously unappreciated function of macrophages using germ-free and conventionally raised WT and various knockout mice. Macrophages do not seem to be necessary for normal maintenance of crypts (88), but, when recruited to a site of injury and activated by the microbiota, the macrophages support and promote proliferation of CoIEPs.
The relationship between epithelial repair and inflammation is complex. An important mediator of both inflammation and repair in the intestine is cyclooxygenase-2 (Cox-2). Cox-1 and Cox-2 regulate prostaglandin (PG) synthesis from arachidonic acid (89). Although IECs express Cox-1 constitutively, Cox-2 is induced by inflammatory mediators. Cox-2–dependent PGE2 production is critical for epithelial repair in the intestine in a variety of contexts. In the setting of IBD, increased Cox-2 and PGE2 have been implicated in the development of colitis-associated cancers (90, 91). It was recently shown that microsomal PGE synthase-1, the enzyme that catalyzes the conversion of PDH2 to PGE2, is increased in IBD mucosa (90), whereas 15-hydroxyprostaglandin dehydrogenase, the enzyme responsible for catabolism of PGE2, is reduced in the inflamed mucosa of IBD (91). This combination results in overall increases in mucosal PGE2 and the potential for enhanced carcinogenesis in the setting of inflammation. Fukata et al. (92) showed that Cox-2-derived PGE2 is important in TLR4-dependent mucosal homeostasis. TLR4-deficient mice fail to upregulate Cox-2 expression in response to epithelial injury. Both IECs and LP macrophages express Cox-2 in a TLR4- and MyD88-dependent fashion. PGE2 is decreased in the mucosa of TLR4−/− mice after DSS injury. Oral supplementation with PGE2 results in increased IEC proliferation and decreased apoptosis in DSS-treated TLR4−/− mice. At least part of the mechanism for TLR4-dependent mucosal healing involves the activation of epidermal growth factor receptor signaling.
Our recent efforts have turned to the study of the effect of LPLs on IEC differentiation. Based on the epithelial dysfunction seen in IL-10–deficient mice and previous results from our group, we hypothesized a putative role for LPL in IEC differentiation. Our findings suggest that there is cross talk between LPL and IECs, which leads to IEC differentiation. This cross talk involves different signaling pathways and transcriptional factors. The control of immune responses in the gut is critical for normal immune homeostasis in the host, and based on our recent studies, unbalanced IEC homeostasis can be proposed as one mechanism for the development of IBD (1).
Conclusions
Accumulating evidence suggests that lymphoepithelial interactions in the intestine help to promote mucosal homeostasis. In the normal state, the epithelial cell serves as a barrier for antigen entry (preventing activation of mucosal lymphocytes), secretes factors that modulate lymphocyte activation and may contribute to the generally immunosuppressed tone of the GI tract (e.g. TGF-β), and interacts with lymphocytes that control inflammation locally (CD8+CD28− TrE cells). Mucosal lymphocytes return the favor by secreting factors (IELs secreting KGFs) or mediating cell–cell interactions that promote IEC growth and differentiation as well as the expression of molecules involved in mucosal immuno-regulation (e.g. CD1d). This dialogue is disrupted in patients with chronic inflammatory diseases (IBD, celiac disease), where alterations in cytokine secretion and Treg interactions promote rather than inhibit inflammation. Understanding the nature of these interactions and the defects present in disease states will aid in the development of novel strategies for therapy.
References
- 1.Hershberg RM, Mayer LF. Antigen processing and presentation by intestinal epithelial cells – polarity and complexity. Immunol Today. 2000;21:123–128. doi: 10.1016/s0167-5699(99)01575-3. [DOI] [PubMed] [Google Scholar]
- 2.Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med. 2002;8:567–573. doi: 10.1038/nm0602-567. [DOI] [PubMed] [Google Scholar]
- 3.Lin AL, Mann BA, Torres-Oviedo G, Lincoln B, Kas J, Swinney HL. Localization and extinction of bacterial populations under inhomogeneous growth conditions. Biophys J. 2004;87:75–80. doi: 10.1529/biophysj.103.034041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shao L, Kamalu O, Mayer L. Non-classical MHC class I molecules on intestinal epithelial cells: mediators of mucosal crosstalk. Immunol Rev. 2005;206:160–176. doi: 10.1111/j.0105-2896.2005.00295.x. [DOI] [PubMed] [Google Scholar]
- 5.Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174:4453–4460. doi: 10.4049/jimmunol.174.8.4453. [DOI] [PubMed] [Google Scholar]
- 6.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 8.Hornef MW, Normark BH, Vandewalle A, Normark S. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J Exp Med. 2003;198:1225–1235. doi: 10.1084/jem.20022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559–570. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frey A, et al. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med. 1996;184:1045–1059. doi: 10.1084/jem.184.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neutra MR. Current concepts in mucosal immunity. V Role of M cells in transepithelial transport of antigens and pathogens to the mucosal immune system. Am J Physiol. 1998;274:G785–G791. doi: 10.1152/ajpgi.1998.274.5.G785. [DOI] [PubMed] [Google Scholar]
- 12.Gonnella PA, Wilmore DW. Co-localization of class II antigen and exogenous antigen in the rat enterocyte. J Cell Sci. 1993;106:937–940. doi: 10.1242/jcs.106.3.937. [DOI] [PubMed] [Google Scholar]
- 13.Buning J, et al. Interferon-gamma mediates antigen trafficking to MHC class II-positive late endosomes of enterocytes. Eur J Immunol. 2005;35:831–842. doi: 10.1002/eji.200425286. [DOI] [PubMed] [Google Scholar]
- 14.Hershberg RM, et al. Intestinal epithelial cells use two distinct pathways for HLA class II antigen processing. J Clin Invest. 1997;100:204–215. doi: 10.1172/JCI119514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hershberg RM, et al. Highly polarized HLA class II antigen processing and presentation by human intestinal epithelial cells. J Clin Invest. 1998;102:792–803. doi: 10.1172/JCI3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakazawa A, et al. The expression and function of costimulatory molecules B7H and B7-H1 on colonic epithelial cells. Gastroenterology. 2004;126:1347–1357. doi: 10.1053/j.gastro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Barone KS, Jain SL, Michael JG. Effect of in vivo depletion of CD4+ and CD8+ cells on the induction and maintenance of oral tolerance. Cell Immunol. 1995;163:19–29. doi: 10.1006/cimm.1995.1094. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Inobe J, Weiner HL. Induction of oral tolerance to myelin basic protein in CD8-depleted mice: both CD4+ and CD8+ cells mediate active suppression. J Immunol. 1995;155:910–916. [PubMed] [Google Scholar]
- 19.Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995;3:171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 20.Beagley KW, et al. Differences in intraepithelial lymphocyte T cell subsets isolated from murine small versus large intestine. J Immunol. 1995;154:5611–5619. [PubMed] [Google Scholar]
- 21.Camerini V, Panwala C, Kronenberg M. Regional specialization of the mucosal immune system. Intraepithelial lymphocytes of the large intestine have a different phenotype and function than those of the small intestine. J Immunol. 1993;151:1765–1776. [PubMed] [Google Scholar]
- 22.Geboes K, Rutgeerts P, Penninckx F, Desmet V, Vantrappen G. Changes in small intestinal epithelial expression of MHC class II antigen after terminal ileal resection for Crohn’s disease. Int J Colorectal Dis. 1988;3:102–108. doi: 10.1007/BF01645314. [DOI] [PubMed] [Google Scholar]
- 23.Mayer L, Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987;166:1471–1483. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirata I, Austin LL, Blackwell WH, Weber JR, Dobbins WO., 3rd Immunoelectron microscopic localization of HLA-DR antigen in control small intestine and colon and in inflammatory bowel disease. Dig Dis Sci. 1986;31:1317–1330. doi: 10.1007/BF01299810. [DOI] [PubMed] [Google Scholar]
- 25.Mayrhofer G, Spargo LD. Distribution of class II major histocompatibility antigens in enterocytes of the rat jejunum and their association with organelles of the endocytic pathway. Immunology. 1990;70:11–19. [PMC free article] [PubMed] [Google Scholar]
- 26.Cobrin GM, Abreu MT. Defects in mucosal immunity leading to Crohn’s disease. Immunol Rev. 2005;206:277–295. doi: 10.1111/j.0105-2896.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 27.Pulendran B, Banchereau J, Maraskovsky E, Maliszewski C. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 2001;22:41–47. doi: 10.1016/s1471-4906(00)01794-4. [DOI] [PubMed] [Google Scholar]
- 28.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 29.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 30.Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 2003;51:59–60. doi: 10.1016/s0369-8114(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 31.Mizoguchi A, Mizoguchi E, Bhan AK. Immune networks in animal models of inflammatory bowel disease. Inflamm Bowel Dis. 2003;9:246–259. doi: 10.1097/00054725-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 33.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 34.Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 36.Mudter J, Neurath MF. Mucosal T cells: mediators or guardians of inflammatory bowel disease? Curr Opin Gastroenterol. 2003;19:343–349. doi: 10.1097/00001574-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Allez M, Mayer L. Regulatory T cells: peace keepers in the gut. Inflamm Bowel Dis. 2004;10:666–676. doi: 10.1097/00054725-200409000-00027. [DOI] [PubMed] [Google Scholar]
- 38.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 39.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 40.Akbari O, Stock P, DeKruyff RH, Umetsu DT. Role of regulatory T cells in allergy and asthma. Curr Opin Immunol. 2003;15:627–633. doi: 10.1016/j.coi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Powrie F. Immune regulation in the intestine: a balancing act between effector and regulatory T cell responses. Ann NY Acad Sci. 2004;1029:132–141. doi: 10.1196/annals.1309.030. [DOI] [PubMed] [Google Scholar]
- 42.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 44.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubois B, Chapat L, Goubier A, Papiernik M, Nicolas JF, Kaiserlian D. Innate CD4+CD25+ regulatory T cells are required for oral tolerance and inhibition of CD8+ T cells mediating skin inflammation. Blood. 2003;102:3295–3301. doi: 10.1182/blood-2003-03-0727. [DOI] [PubMed] [Google Scholar]
- 46.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 47.Schwartz RH. Natural regulatory T cells and self-tolerance. Nat Immunol. 2005;6:327–330. doi: 10.1038/ni1184. [DOI] [PubMed] [Google Scholar]
- 48.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 49.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 50.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 51.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 52.Nielsen J, Holm TL, Claesson MH. CD4+CD25+ regulatory T cells: II. Origin, disease models and clinical aspects. APMIS. 2004;112:642–650. doi: 10.1111/j.1600-0463.2004.apm1121002.x. [DOI] [PubMed] [Google Scholar]
- 53.Powrie F, Uhlig H. Animal models of intestinal inflammation: clues to the pathogenesis of inflammatory bowel disease. Novartis Found Symp. 2004;263:164–178. [PubMed] [Google Scholar]
- 54.Veltkamp C, et al. CD4+CD25+ cell depletion from the normal CD4+ T cell pool prevents tolerance toward the intestinal flora and leads to chronic colitis in immunodeficient mice. Inflamm Bowel Dis. 2006;12:437–446. doi: 10.1097/00054725-200606000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Bennett CL, Ochs HD. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr. 2001;13:533–538. doi: 10.1097/00008480-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Kasprowicz DJ, Droin N, Soper DM, Ramsdell F, Green DR, Ziegler SF. Dynamic regulation of FoxP3 expression controls the balance between CD4+ T cell activation and cell death. Eur J Immunol. 2005;35:3424–3432. doi: 10.1002/eji.200526339. [DOI] [PubMed] [Google Scholar]
- 57.Allan SE, et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest. 2005;115:3276–3284. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25− cells. Proc Natl Acad Sci U S A. 2005;102:4103–4108. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelsen J, Agnholt J, Hoffmann HJ, Romer JL, Hvas CL, Dahlerup JF. FoxP3(+)CD4(+)CD25(+) T cells with regulatory properties can be cultured from colonic mucosa of patients with Crohn’s disease. Clin Exp Immunol. 2005;141:549–557. doi: 10.1111/j.1365-2249.2005.02876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allez M, Brimnes J, Dotan I, Mayer L. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology. 2002;123:1516–1526. doi: 10.1053/gast.2002.36588. [DOI] [PubMed] [Google Scholar]
- 61.Allez M, Brimnes J, Shao L, Dotan I, Nakazawa A, Mayer L. Activation of a unique population of CD8(+) T cells by intestinal epithelial cells. Ann NY Acad Sci. 2004;1029:22–35. doi: 10.1196/annals.1309.004. [DOI] [PubMed] [Google Scholar]
- 62.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814–582. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 63.Kraus TA, Toy L, Chan L, Childs J, Cheifetz A, Mayer L. Failure to induce oral tolerance in Crohn’s and ulcerative colitis patients: possible genetic risk. Ann NY Acad Sci. 2004;1029:225–238. doi: 10.1196/annals.1309.054. [DOI] [PubMed] [Google Scholar]
- 64.Kraus TA, Toy L, Chan L, Childs J, Mayer L. Failure to induce oral tolerance to a soluble protein in patients with inflammatory bowel disease. Gastroenterology. 2004;126:1771–1778. doi: 10.1053/j.gastro.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 65.Shao L, Jacobs AR, Johnson VV, Mayer L. Activation of CD8+ regulatory T cells by human placental trophoblasts. J Immunol. 2005;174:7539–7547. doi: 10.4049/jimmunol.174.12.7539. [DOI] [PubMed] [Google Scholar]
- 66.Zhou M, Mellor AL. Expanded cohorts of maternal CD8+ T-cells specific for paternal MHC class I accumulate during pregnancy. J Reprod Immunol. 1998;40:47–62. doi: 10.1016/s0165-0378(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 67.Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–633. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 68.Grossmann J, Mohr S, Lapentina EG, Fiocchi C, Levine AD. Sequential and rapid activation of select caspases during apoptosis of normal intestinal epithelial cells. Am J Physiol. 1998;274:G1117–G1124. doi: 10.1152/ajpgi.1998.274.6.G1117. [DOI] [PubMed] [Google Scholar]
- 69.Mariadason JM, et al. Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology. 2005;128:1081–1088. doi: 10.1053/j.gastro.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 70.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 71.Kerneis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- 72.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 73.Boismenu R. Function of intestinal gamma-delta T cells. Immunol Res. 2000;21:123–127. doi: 10.1385/IR:21:2-3:123. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Komano H, et al. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA. 1995;92:6147–6151. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mak TW, Ferrick DA. The gammadelta T-cell bridge: linking innate and acquired immunity. Nat Med. 1998;4:764–765. doi: 10.1038/nm0798-764. [DOI] [PubMed] [Google Scholar]
- 77.Ulich TR, et al. Keratinocyte growth factor is a growth factor for mammary epithelium in vivo. The mammary epithelium of lactating rats is resistant to the proliferative action of keratinocyte growth factor. Am J Pathol. 1994;144:862–868. [PMC free article] [PubMed] [Google Scholar]
- 78.Ulich TR, et al. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest. 1994;93:1298–1306. doi: 10.1172/JCI117086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Staiano-Coico L, et al. Human keratinocyte growth factor effects in a porcine model of epidermal wound healing. J Exp Med. 1993;178:865–878. doi: 10.1084/jem.178.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pierce GF, et al. Stimulation of all epithelial elements during skin regeneration by keratinocyte growth factor. J Exp Med. 1994;179:831–840. doi: 10.1084/jem.179.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Housley RM, et al. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest. 1994;94:1764–1777. doi: 10.1172/JCI117524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Finch PW, Pricolo V, Wu A, Finkelstein SD. Increased expression of keratinocyte growth factor messenger RNA associated with inflammatory bowel disease. Gastroenterology. 1996;110:441–451. doi: 10.1053/gast.1996.v110.pm8566591. [DOI] [PubMed] [Google Scholar]
- 83.Brauchle M, et al. Keratinocyte growth factor is highly overexpressed in inflammatory bowel disease. Am J Pathol. 1996;149:521–529. [PMC free article] [PubMed] [Google Scholar]
- 84.Jameson J, et al. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 85.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang WW, Leblond CP. Renewal of the epithelium in the descending colon of the mouse. I. Presence of three cell populations: vacuolated-columnar, mucous and argentaffin. Am J Anat. 1971;131:73–99. doi: 10.1002/aja.1001310105. [DOI] [PubMed] [Google Scholar]
- 87.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 88.Cecchini MG, et al. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 89.Backlund MG, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005;280:3217–3223. doi: 10.1074/jbc.M411221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Subbaramaiah K, et al. Microsomal prostaglandin E synthase-1 is overexpressed in inflammatory bowel disease. Evidence for involvement of the transcription factor Egr-1. J Biol Chem. 2004;279:12647–12658. doi: 10.1074/jbc.M312972200. [DOI] [PubMed] [Google Scholar]
- 91.Otani T, et al. Levels of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase are reduced in inflammatory bowel disease: evidence for involvement of TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2006;290:G361–G368. doi: 10.1152/ajpgi.00348.2005. [DOI] [PubMed] [Google Scholar]
- 92.Fukata M, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ozinsky A, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nilsen N, et al. Lipopolysaccharide and double-stranded RNA up-regulate toll-like receptor 2 independently of myeloid differentiation factor 88. J Biol Chem. 2004;279:39727–39735. doi: 10.1074/jbc.M405027200. [DOI] [PubMed] [Google Scholar]
- 95.Werts C, et al. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol. 2001;2:346–352. doi: 10.1038/86354. [DOI] [PubMed] [Google Scholar]
- 96.Iwaki D, et al. The extracellular toll-like receptor 2 domain directly binds peptidoglycan derived from Staphylococcus aureus. J Biol Chem. 2002;277:24315–24320. doi: 10.1074/jbc.M107057200. [DOI] [PubMed] [Google Scholar]
- 97.Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 98.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 99.Termeer C, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 101.Okamura Y, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 102.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 103.Biragyn A, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 104.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 105.Monick MM, et al. Respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells to endotoxin. J Biol Chem. 2003;278:53035–53044. doi: 10.1074/jbc.M308093200. [DOI] [PubMed] [Google Scholar]
- 106.Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M. Mouse toll-like receptor 4.MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J Biol Chem. 2000;275:2251–2254. doi: 10.1074/jbc.275.4.2251. [DOI] [PubMed] [Google Scholar]
- 107.Hayashi F, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 108.Heil F, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 109.Lund JM, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 204(101):5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zuber AK, et al. Topical delivery of imiquimod to a mouse model as a novel adjuvant for human immunodeficiency virus (HIV) DNA. Vaccine. 2004;22:1791–1798. doi: 10.1016/j.vaccine.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 111.Otero M, et al. Resiquimod is a modest adjuvant for HIV-1 gag-based genetic immunization in a mouse model. Vaccine. 2004;22:1782–1790. doi: 10.1016/j.vaccine.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 112.Bauer S, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yarovinsky F, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 114.So LP, et al. Antigen uptake and trafficking in human intestinal epithelial cells. Dig Dis and Sci. 2000;45:1451–1461. doi: 10.1023/a:1005536927137. [DOI] [PubMed] [Google Scholar]
- 115.Groux H, et al. A CD4+ T cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]