Abstract
The role/s of T lymphocytes in the foreign body response has not been thoroughly elucidated. Lymphocytes are known to augment macrophage adhesion and fusion in vitro. Furthermore T lymphocytes are a possible source of the cytokines, IL-4 and IL-13 which induce macrophage fusion. In this study we used BALB/c mice and BALB/c (nu/nu) nude mice to investgate foreign body giant cell (FBGC) formation in a T cell deficient setting. Mice were implanted with Elasthane 80A (PEU), silicone rubber (SR) or poly(ethylene terephthalate) (PET) for 7, 14, or 21 days using the cage implant system. Exudate cells and IL-4 and IL-13 levels in exudate supernatants were analyzed by flow cytometry and a multiplex immunoassay, respectively, at days 7, 14, and 21. Macrophage adhesion and fusion on material surfaces were analyzed using optical microscopy. T cell deficient mice had lower total leukocyte concentrations at the biomaterial implant site at all time points. Adherent cell density was comparable between normal and T cell deficient mice except in the PEU group at day 21. However, percent fusion, average nuclei per FBGC, and FBGC morphology was comparable between normal and T cell deficient mice. IL-4 was not detected in any samples, but IL-13 levels were also comparable between normal and T cell deficient mice indicating Th2 polarized T cells are not the sole source of this cytokine. We have shown that there are pathways that do not require thymus-matured T lymphocytes which lead to a normal foreign body response to biomaterials in a murine model.
Keywords: foreign body response, IL-4, IL-13, T lymphocyte, biomaterials
Introduction
The cellular components of the foreign body reaction (FBR) are characterized by macrophages and/or foreign body giant cells (FBGCs) that persist at the biomaterial interface. The role of lymphocytes, specifically T lymphocytes, in the foreign body reaction is still under investigation. Following biomaterial implantation, lymphocytes are transiently present during the chronic inflammatory stage1. In vitro, lymphocytes enhance macrophage adhesion and fusion on material surfaces2. Furthermore, lymphocyte effects on macrophage adhesion, fusion, and cytokine secretion are material dependent3, 4.
IL-4 and IL-13 are cytokines that induce macrophages to have an alternatively activated phenotype and are necessary for macrophages to undergo foreign body giant cell formation on biomaterial surfaces5-7. The source of these cytokines has been hypothesized to be Th2 polarized T lymphocytes1. However these cells are not the only source of IL-4 and IL-13 since NK cells, NKT cells, mast cells, eosinophils, and basophils are also capable of producing these cytokines8, 9. Al-Saffar et al. have identified mast cells containing IL-4 at the bone-material interface in samples from patients undergoing revision surgery. An increase in mast cell quantity correlated with an increase in foreign body giant cells10. Mast cells have been found to have an important role in the foreign body response because they are critical for leukocyte recruitment to the biomaterial implant site during acute inflammation in a murine model11.
A common pathway leading to mast cell production of IL-4 and IL-13 is via IgE crosslinking by antigen. The production of IgE by B cells and subsequent mast cell proliferation and effector functions are dependent on Th2 lymphocytes12. Even though mast cells are one of the likely sources of IL-4 and/or IL-13 at the biomaterial implant site, this does not exclude the possibility that T cells are playing critical roles in inducing mast cell IL-4/IL-13 production. Also it is likely that there are multiple cellular sources, inclusive of T cells, of IL-4 and/or IL-13 at biomaterial implant sites.
In vivo the induction of alternatively activated macrophages is mediated by innate immune responses whereas the maintenance of the alternatively activated phenotype in macrophages requires CD4+ T cells. This implicates mast cells and/or eosinophils in providing the necessary IL-4/IL-13 stimulus to alternatively activate macrophages during acute inflammation whereas maintenance of this phenotype during chronic inflammation requires CD4+ T cells 13. Studies on foreign body giant cell formation have shown that an alternatively activated phenotype in macrophages is necessary for these cells to undergo fusion. Most notably mannose receptor and dendritic cell specific transmembrane protein (DC-STAMP) are upregulated on alternatively activated macrophages and inhibition of these receptors prevents macrophage fusion into FBGCs7, 14, 15. It not known whether T cells are critical contributors to the complex phenotypic changes required in macrophages to undergo fusion into FBGCs.
Previously, a T cell deficient rat model has been used to study the foreign body response to sheep collagen. T cells were found to be important in macrophage recruitment and macrophage foreign body giant cell formation. However, T cells are likely to participate in the host response to a xenogeneic graft which is immunogeneic16. We have previously shown that components of an adaptive immune response, specifically memory, are not present in response to synthetic biomaterials17. In this study we use a T cell deficient model to investigate the role of T cells in foreign body giant cell formation on synthetic non-immunogeneic biomaterials. T cell deficient mice have a mutation in the foxn1 gene that causes the animals to be congenitally athymic and hairless, hence the mice are referred to as nude. The thymus is crucial for the maturation, differentiation and specificity of T cells18. We implanted BALB/c mice and nude BALB/c mice with three different clinically relevant synthetic biomaterials using the cage implant system. We assessed macrophage adhesion and fusion on these surfaces along with measuring IL-4 and IL-13 levels at the implant site.
Methods
Materials
Elasthane 80A, a polyether urethane (PEU), was synthesized by Polymer Technology Group (Berkeley, CA, USA) and extruded by Medtronic (Minneapolis, MN, USA). Polyethylene terephthalate (PET, Mylar ®) (Toray Co., Japan) and a silicate resin filled, cross-linked polydimethylsiloxane (SR) (Dow Corning, Midland, MI) were also used. The polymers were cut into 1.0×0.5 cm rectangular sections, wiped with 100% ethanol, sonicated in 100% ethanol for 10 min, rinsed in distilled water and allowed to air dry.
Surfaces were placed inside cylindrical stainless steel wire mesh cages measuring approximately 1.5cm long and 0.7cm in diameter. The cages were prepared as previously described. All cages, with or without polymers, were sterilized with ethylene oxide by sterilization services at University Hospitals of Cleveland.
Material Implantation
Female 9 week-old BALB/c mice or 9 week old BALB/c nude (nu/nu) mice (Charles River Laboratories), weighing 17-19g, fed ad libitum on standard pellets and water were used (Animal Resource Center, Case Western Reserve University). Nude mice were housed in an athymic facility with 24 hour temperature and humidity monitor and greater than 15 air changes per hour. Animals were kept in autoclaved microisolator caging units with sterilized corncob bedding were used. Nude mice were fed sterilized P3000 autoclavable high-protein diet and autoclaved tap water. All surgical procedures were conducted in under sterile conditions in a laminar flow work station. NIH guidelines for the care and use of laboratory animals were observed. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University.
Cage implantations were performed as previously described19. Mice were anesthetized with an intra-peritoneal injection of anesthetic (0.1-0.2ml/25g, ketamine ® (Henry Schein), xylazine ® (Henry Schein), and acepromazine ® (Henry Schein)). Briefly, 2 cages, each containing the same biomaterial, were implanted subcutaneously in the upper back. Control mice were implanted with empty cages. Each timepoint is representative of data obtained from five mice (n=5).
On days 7, 14, and 21, exudate was collected from the implanted cages as previously described20. Briefly, after anesthetizing the mouse with Aerrane® (Baxter Healthcare Coporation), a heparinized tuberculin syringe (27.5 gauge needle) was inserted through the skin and into the stainless steel cage. 100-400μl of exudate was collected from both cages of each animal at each timepoint. Total leukocyte concentrations were obtained after staining 5μl of exudate with Wright's stain and using a hemacytometer.
Analysis of Exudate Cells by Flow Cytometry
Cells from day 7 and day 14 exudate samples were stained for flow cytometry as previously described17. Day 21 samples were not stained due to extremely low leukocyte concentrations. Exudate samples were spun down at 300g and the supernatant collected and stored at −80°C. Cell pellets were resuspended in stain buffer (BD Pharmingen, Franklin Lakes, USA) and stained with the following directly conjugated rat anti-mouse antibodies: CD3-FITC (clone 17A2), CD19-APC (Clone 1D3), and CD14-PE (clone rmC5-3) and the appropriate isotype controls (BD Biosciences, Franklin Lakes, USA). Samples were analyzed on a Becton Dickinson FACSCalibur Flow cytometer within 24 hours. Fluorescence positivity of a particular antibody was determined by comparison to the corresponding isotype control. Cell types were quantified by multiplying the percentage of each cell type, obtained through flow cytometry, with total leukocyte concentration. The concentration for each cell type was reported.
Multiplex Immunoassay
A multiplex cytokine immunoassay containing a mouse cytokine panel (IL-4 and IL-13) was purchased from Lincoplex (Millipore, Billerica, MA). Due to the multiplex technology, the cytokines in the mouse panel were measured simultaneously in each sample. Samples collected from days 7, 14, and 21 exudate withdrawals were run in duplicate. The multiplex immunoassay was run in accordance with manufacturer's instructions for serum or plasma samples. The plate was run on a Luminex® 200 Instrument using Bio-plex manager 4.1 standard software (Bio-Rad Laboratories, Hercules, CA). Raw fluorescence data was analyzed by the software using a 5 parameter logistic method. Minimum detection concentrations were 0.3 pg/ml and 4.7 pg/ml for IL-4 and IL-13, respectively.
Adhesion and Fusion Analyses
On days 7, 14 and 21, adherent cell densities and macrophage fusion were determined on the biomaterial surfaces obtained from explanted cages as described previously21. Surfaces were rinsed twice with warm phosphate buffered saline, subsequently fixed in methanol for 5min, and air dried. May Grunwald reagent was added to surfaces for 5min. Surfaces were rinsed in PBS twice and Giemsa reagent added for 15min immediately. Surfaces were rinsed with distilled water twice and allowed to air dry. Adherent cell densities were determined by counting the number of nuclei from 5 representative 20x fields for each sample and expressed as cells/mm2. Percent fusion was determined by dividing the number of nuclei within foreign body giant cells (containing 3 or more nuclei as identified histologically) by the total number of nuclei in the field. Average nuclei per FBGC was determined by dividing the total number of foreign body nuclei by the number of FBGCs in a field. Cell densities, percent fusion, and average nuclei per FBGC were averaged from the five fields per sample from 5 animals (n=5) in each group.
Statistical Analyses
Data were represented as the mean ± standard error mean (SEM) from 5 animals. Comparisons among material groups were made using ANOVA and statistical significance was determined using a Tukey post hoc test with a 95% confidence interval. Comparisons between BALB/c and nude BALB/c data within the same material group was made using an unpaired student's t-test with statistical significance assigned at the 95% confidence interval (Minitab, Inc., State College, PA).
Results
Exudate Cell Analyses
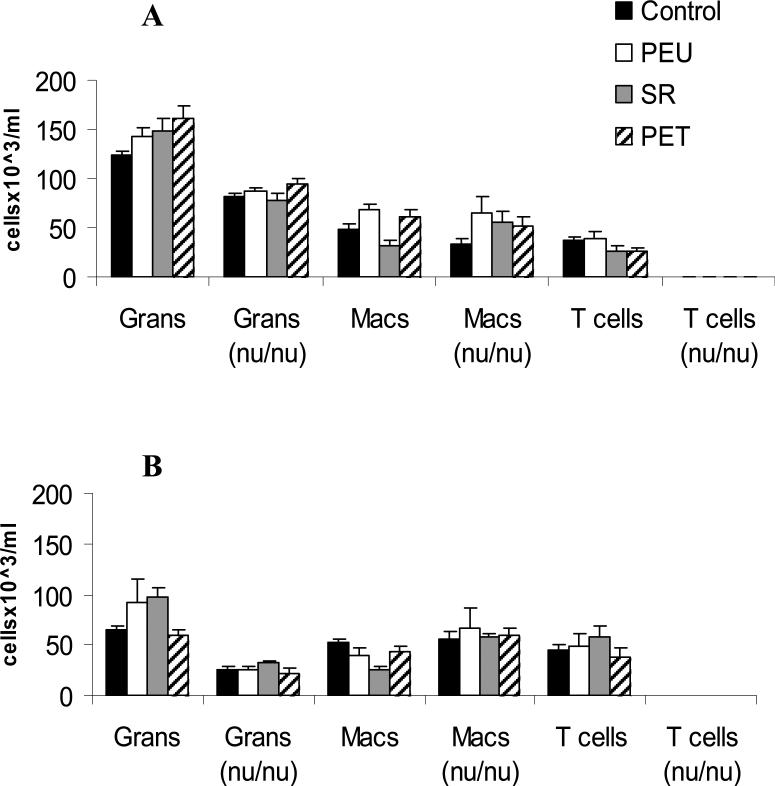
Total leukocyte concentrations (TLCs) decreased with time for all groups. TLCs were significantly lower in nude BALB/c in comparison to BALB/c mice within the same material group. At day 21, TLCs in nude mice were not calculated because most samples were acellular (Table I). The cellular profiles show that BALB/c mice had greater TLCs than their nude counterparts because of the infiltration of T cells and higher levels of granulocytes. Exudate macrophage concentrations were comparable between BALB/c and nude BALB/c mice. No material dependent trends were noted in exudate cell profiles. No B cells were identified in all samples (Figure 1).
Table I.
Total Exudate Leukocyte Concentrationsa
| Total Leukocyte Concentrations (cells×103/ml) | ||
|---|---|---|
| Day 7 | BALB/c | nu/nu |
| Control | 219±7 | 121±2* |
| PEU | 258±2 | 160±8* |
| SR | 220±9 | 134±17* |
| PET | 252±10 | 154±5* |
| Day 14 | ||
| Control | 181±11 | 82±8* |
| PEU | 177±13 | 92±24 |
| SR | 188±14 | 86±5* |
| PET | 159±14 | 98±8* |
| Day 21 | ||
| Control | 43±12 | ---- |
| PEU | 27±4 | ---- |
| SR | 34±13 | ---- |
| PET | 44±6 | ---- |
Data represent the mean ± SEM from five mice.
indicates statistical significance (p<0.05) in comparison to the BALB/c group.
Figure 1.
Quantification of Exudate T cells, granulocytes, and macrophages by flow cytometry. Exudates were collected from BALB/c mice and nude BALB/c mice at days 7 (A) and days 14 (B). The data represent the mean ±SEM from five animals.
IL-4 and IL-13 Levels at the Implant Site
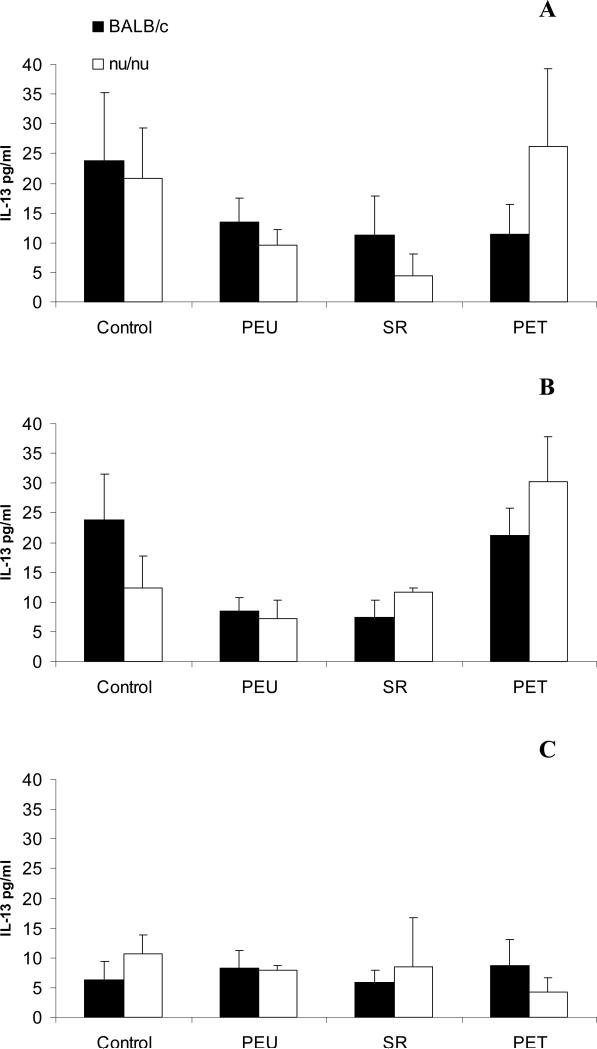
IL-4 was not detected in any exudate samples from all animals. IL-13 was detected at concentrations below 40pg/ml. IL-13 levels were comparable between BALB/c and nude BALB/c mice within the same group, and between control and polymer implanted groups at all time points. By day 21, IL-13 concentrations were below 15pg/ml (Figure 2).
Figure 2.
IL-13 levels in exudate supernatant at days 7 (A), 14 (B), and 21 (C) post-implantation in BALB/c and nude BALB/c mice. Data represent mean±SEM, n=5.
Adherent Cell Density
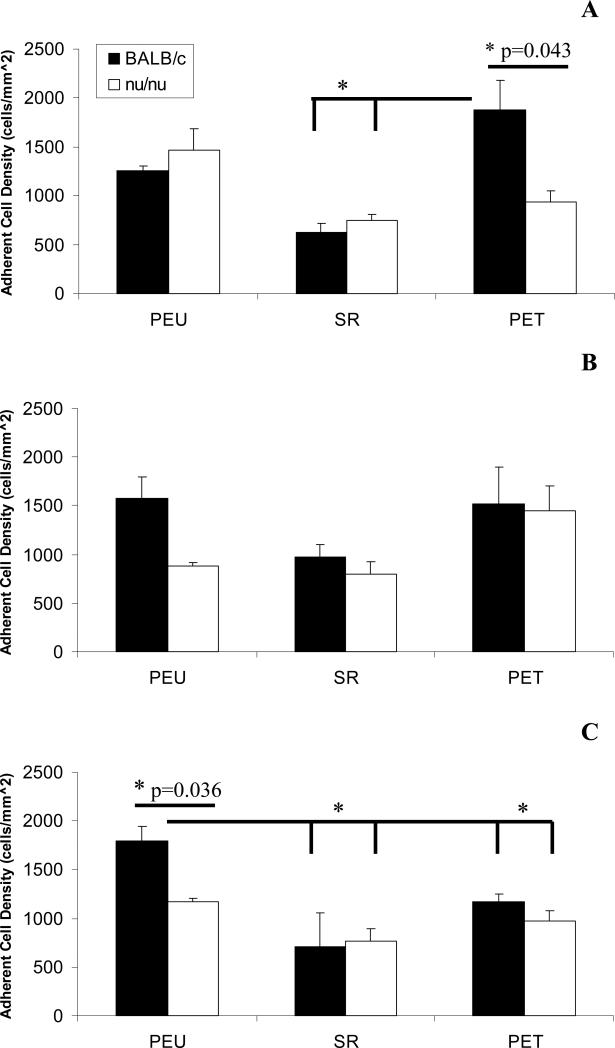
At day 7, adherent cell density on PET surfaces was significantly higher in BALB/c mice in comparison to nude mice implanted with PET and both BALB/c and nude mice implanted with SR. At day 14 all groups had comparable levels of adherent cell density. At day 21, adherent cell density on PEU surfaces implanted in BALB/c mice was significantly greater in comparison to nude mice implanted with PEU and animals implanted with SR and PET. There were no other statistically significant differences at this time point among other material groups. No apparent adherent cell density trend was noted at any time points (Figure 3).
Figure 3.
Macrophage adherent cell density at days 7 (A), 14 (B), and 21 (C) post-implantation in BALB/c and nude BALB/c mice. Densities were obtained from 5 representative 20X fields objective fields per sample. The data represent the mean ± SEM for five animals. * indicates statistical significance p<0.05.
Foreign Body Giant Cell Formation
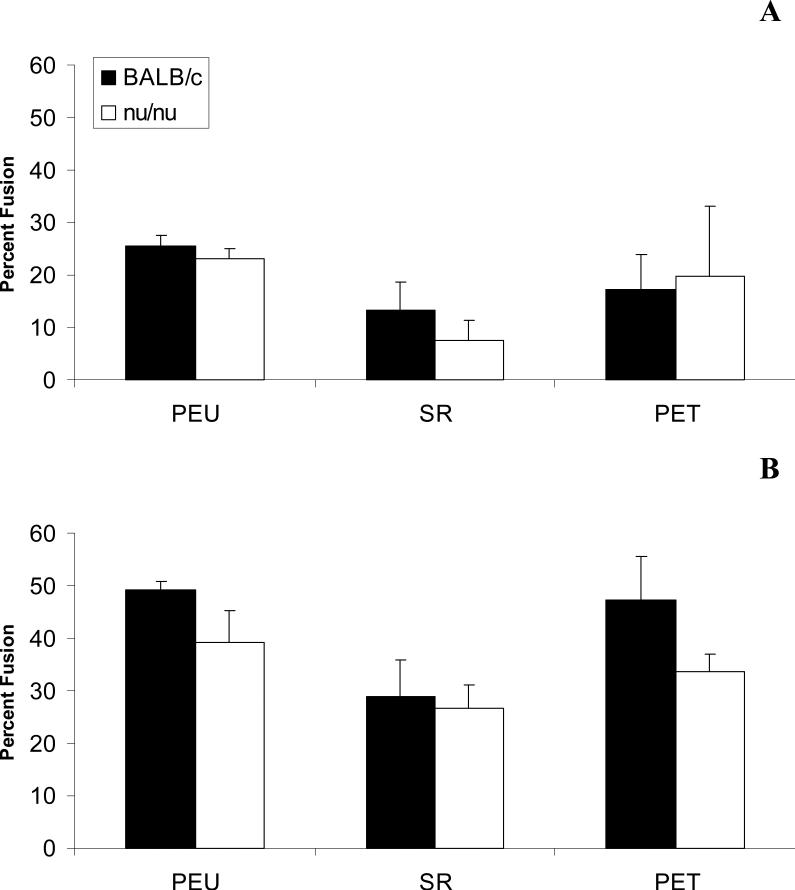
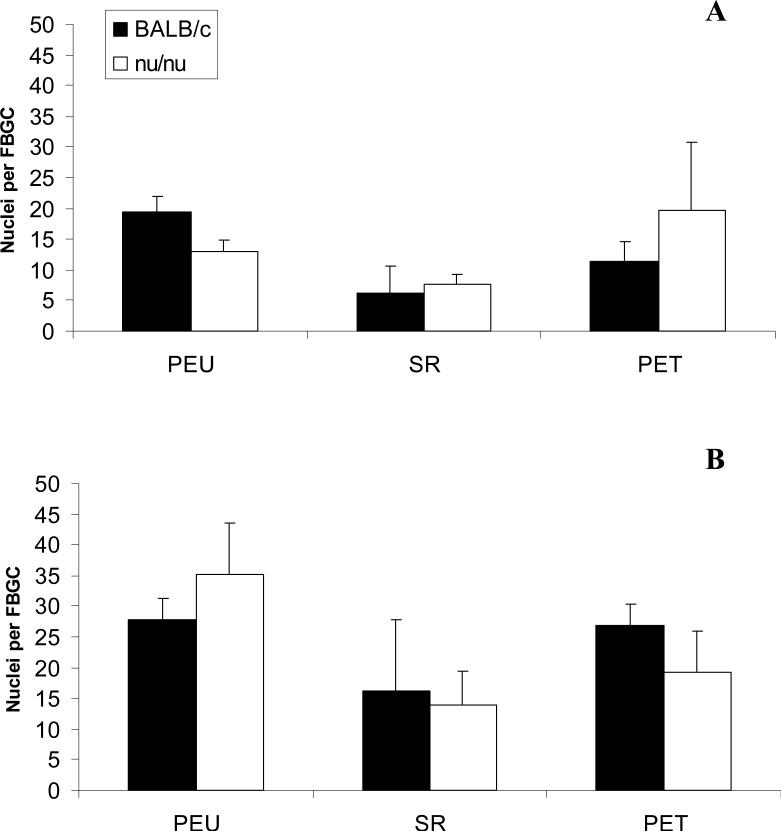
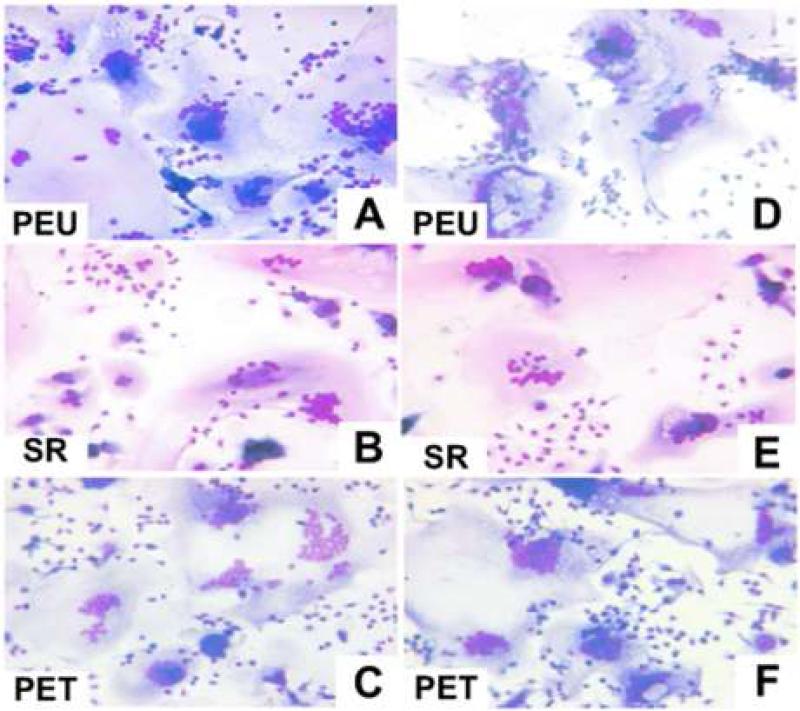
Foreign body giant cells were not seen on nearly all surfaces at day 7. Out of all the fields counted, only 4 fields had several small foreign body giant cells (3 nuclei per cell). Percent fusion was comparable between all material groups at days 14 and 21. Fusion percentages increased with time but did not exceed an average of 50% for any material. Fusion was comparable between BALB/c and nude mice (Figure 4). Average nuclei per FBGC also increased from day 14 to day 21, but no significant differences were noted between material groups or BALB/c and nude BALB/c mice (Figure 5). Furthermore, foreign body giant cells appeared morphologically similar by optical microscopy between BALB/c and nude mice (Figure 6).
Figure 4.
Percent Fusion at days 14 (A) and 21 (B) post-implantation in BALB/c and nude BALB/c mice. Percent Fusion was obtained from 5 representative 20X fields objective fields per sample. The data represent the mean ± SEM for five animals.
Figure 5.
Average nuclei per foreign body giant cell (FBGC) at days 14 (A) and 21 (B) post-implantation in BALB/c and nude BALB/c mice. Average nuclei per FBGC was obtained from 5 representative 20X fields objective fields per sample. The data represent the mean ± SEM for five animals.
Figure 6.
Optical Micrographs of adherent macrophages following 21 days of implantation in BALB/c (A, B, C) and nude BALB/c (D, E, F) mice. All micrographs were taken at a 20X magnification.
Discussion
Our goal was to determine if T cells influence foreign body giant cell formation on synthetic biomaterials in vivo. Previous work in our laboratory has shown that the addition of a lymphocyte cell suspension containing 80% T lymphocytes to monocytes leads to enhanced macrophage adhesion and fusion on biomaterial surfaces2, 3. Furthermore it has long been hypothesized in the biomaterials field that the IL-4 and/or IL-13 produced in vivo to induce macrophage fusion is secreted from T cells. This study shows that foreign body giant cell formation can progress normally in a T cell deficient setting.
Despite significantly decreased exudate leukocyte concentrations in nude mice, adherent cell densities were comparable between BALB/c and nude BALB/c mice except for the PEU group (Figure 3). Nude mice implanted with PEU had significantly lower adherent cell density than BALB/c mice implanted with the same surface at day 21. The cellular exudate profiles show that BALB/c and nude mice in all material groups had comparable concentrations of macrophages at days 7 and 14 (Figure 1). At day 21, most samples from nude mice were acellular. Despite the disparity in leukocyte infiltration between BALB/c and nude BALB/c mice it is apparent that adherent cell density was not affected significantly.
Foreign body giant cell formation in all material groups, inclusive of PEU, was not impacted by the absence of T cells. Nude mice had comparable percent fusion to their immunocompetent counterparts for all material groups (Figure 4). The number of nuclei in FBGCs from nude mice was comparable to the number of nuclei in FBGCs from BALB/c mice and these FBGCs were morphologically indistinguishable as analyzed by optical microscopy (Figures 5&6). These data contrast with what was expected because in vitro lymphocytes enhance macrophage fusion. However in the in vitro setting the added lymphocyte population is not exclusively T cells (~20% non-T cells)2, indicating the possibility that lymphocyte subsets other than T cells contributed to the effects on macrophage surface behavior. Our study shows that in the absence of T cells, foreign body giant cell formation is not affected.
We also investigated whether IL-4 and IL-13 concentrations were impacted by T cell deficiency because T cell may be a possible source of these cytokines. Al-Saffar et al.'s study shows that IL-4 is produced by mast cells at the bone-material interface10. However, orthopedic joint revisions use materials that contain metal and this study does not eliminate the possibility of a classic hypersensitivity reaction to a metal component of the biomaterial, such as nickel or chromium. A metal hypersensitivity reaction in humans would require T cell directed mechanisms22. In our cage implant system, we use metal mesh but in order to induce metal allergies in murine models the animals have to be sensitized appropriately23.
In vitro FBGC formation can be induced by either IL-4 or IL-135, 7. Neutralization of IL-4 in vivo significantly reduced foreign body giant cell formation on a poly(ether urethane) urea surface following 7 days of implantation in BALB/c mice6. In this study, IL-4 was not detected at days 7, 14, and 21, and fusion was not present on material surfaces at day 7. Macrophage fusion is material dependent and the poly(ether urethane) urea surface may promote earlier FBGC formation in mice than the surfaces used in this study24. The study indicates that IL-4 may be present before day 7, but we did not detect IL-4 at day 7 or subsequent time points. Brodbeck et al. has measured IL-4 mRNA expression in exudate leukocytes surrounding material implants at days 7, 14, and 21 in BALB/c mice25. However measurement of IL-4 mRNA does not indicate that IL-4 was produced by exudate leukocytes especially since mast cells, basophils, and eosinophils have constitutive IL-4 and IL-13 transcripts9. Brodbeck et al. did not see material dependency in IL-4 mRNA expression, but did see increased IL-13 mRNA expression in exudate leukocytes surrounding surfaces that promoted fusion25. We were able to measure IL-13 at all time points and found IL-13 concentrations to be comparable between material implanted mice and empty cage control mice. We also did not have a correlation between IL-13 concentrations and fusion (Figure 2).
Our data indicate that T cells are not necessary for the production of IL-13 at the implant site. However whether IL-13 is necessary to induce fusion in vivo has not been investigated. It was hypothesized that neutralization of IL-4 at the implant site did not abrogate macrophage fusion because of the actions of IL-13 which was not blocked. In vitro studies with murine monocytes have shown that macrophage fusion is STAT6 dependent26. Both IL-4 and IL-13 signal through STAT6 indicating that signaling pathways induced by either cytokine are necessary for FBGC fusion, indicating that IL-13 is a likely fusion inducer in vivo27. In vitro, IL-4 or IL-13 is added at day 3 or day 1 in order to induce fusion in human and murine monocyte cultures, respectively5, 26. In vitro, the fusion inducing stimulus is required early on in adherent macrophage development. In vivo, foreign body giant cell formation progresses at a much slower rate, but it is not known when adherent macrophages need to receive IL-4 and/or IL-13 stimuli to undergo fusion.
In vivo there are innate immune pathways, most likely mediated by mast cells, that can lead to alternative activation of macrophages during the acute inflammatory response to surgical injury alone13. Our data show that IL-13 is present at the empty cage control implant site indicating that IL-13 production may be produced solely to injury stimuli. However, all of our time points in this study are past the acute inflammatory stage indicating that there is IL-13 production during chronic inflammatory and wound healing phases at the biomaterial implant site. Mast cells have been implicated in several chronic inflammatory responses that do not have adaptive immune mechanisms, such as silicosis28. In Th2 type granuloma formation, which is another host response that features IL-4/IL-13 induced multinucleated giant cells, there are multiple and redundant sources of IL-4 namely T cell, NK cells, and mast cells. 29. Therefore our study does not exclude the possibility that T cells are playing roles in FBGC formation. T cells can be a redundant source of IL-4 and/or IL-13 in vivo or may participate in other FBGC formation mechanisms not yet identified. However this study does show that T cells are not necessary for a normal foreign body response to synthetic biomaterials.
Acknowledgments
Contract Grant Sponsor: National Institutes of Health (NIH)
Contract Grant Number: EB-000282
References
- 1.Anderson JM. Inflammatory response to implants. ASAIO Trans. 1988;34(2):101–7. doi: 10.1097/00002480-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Brodbeck WG, Macewan M, Colton E, Meyerson H, Anderson JM. Lymphocytes and the foreign body response: lymphocyte enhancement of macrophage adhesion and fusion. J Biomed Mater Res A. 2005;74(2):222–9. doi: 10.1002/jbm.a.30313. [DOI] [PubMed] [Google Scholar]
- 3.MacEwan MR, Brodbeck WG, Matsuda T, Anderson JM. Student Research Award in the Undergraduate Degree Candidate category, 30th Annual Meeting of the Society for Biomaterials, Memphis, Tennessee, April 27-30, 2005. Monocyte/lymphocyte interactions and the foreign body response: in vitro effects of biomaterial surface chemistry. J Biomed Mater Res A. 2005;74(3):285–93. doi: 10.1002/jbm.a.30316. [DOI] [PubMed] [Google Scholar]
- 4.Chang DT, Jones JA, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM. Lymphocyte/Macrophage interactions: biomaterial surface dependent cytokine, chemokine, and matrix protein production. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31630. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNally AK, Anderson JM. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am J Pathol. 1995;147(5):1487–99. [PMC free article] [PubMed] [Google Scholar]
- 6.Kao WJ, McNally AK, Hiltner A, Anderson JM. Role for interleukin-4 in foreign-body giant cell formation on a poly(etherurethane urea) in vivo. J Biomed Mater Res. 1995;29(10):1267–75. doi: 10.1002/jbm.820291014. [DOI] [PubMed] [Google Scholar]
- 7.DeFife KM, Jenney CR, McNally AK, Colton E, Anderson JM. Interleukin-13 induces human monocyte/macrophage fusion and macrophage mannose receptor expression. J Immunol. 1997;158(7):3385–90. [PubMed] [Google Scholar]
- 8.Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77(9):1859–70. [PubMed] [Google Scholar]
- 9.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174(2):1063–72. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 10.Al-Saffar N, Iwaki H, Revell PA. Direct activation of mast cells by prosthetic biomaterial particles. J Mater Sci Mater Med. 1998;9(12):849–53. doi: 10.1023/a:1008952329788. [DOI] [PubMed] [Google Scholar]
- 11.Tang L, Jennings TA, Eaton JW. Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc Natl Acad Sci U S A. 1998;95(15):8841–6. doi: 10.1073/pnas.95.15.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shelburne CP, Ryan JJ. The role of Th2 cytokines in mast cell homeostasis. Immunol Rev. 2001;179:82–93. doi: 10.1034/j.1600-065x.2001.790109.x. [DOI] [PubMed] [Google Scholar]
- 13.Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, Allison JP, Allen JE. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179(6):3926–36. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 14.McNally AK, DeFife KM, Anderson JM. Interleukin-4-induced macrophage fusion is prevented by inhibitors of mannose receptor activity. Am J Pathol. 1996;149(3):975–85. [PMC free article] [PubMed] [Google Scholar]
- 15.Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202(3):345–51. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Luyn MJ, Khouw IM, van Wachem PB, Blaauw EH, Werkmeister JA. Modulation of the tissue reaction to biomaterials. II. The function of T cells in the inflammatory reaction to crosslinked collagen implanted in T-cell-deficient rats. J Biomed Mater Res. 1998;39(3):398–406. doi: 10.1002/(sici)1097-4636(19980305)39:3<398::aid-jbm8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez A, Voskerician G, Meyerson H, Macewan SR, Anderson JM. T cell subset distributions following primary and secondary implantation at subcutaneous biomaterial implant sites. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31562. [DOI] [PubMed] [Google Scholar]
- 18.Miosge L, Zamoyska R. Signalling in T-cell development: is it all location, location, location? Curr Opin Immunol. 2007;19(2):194–9. doi: 10.1016/j.coi.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Dadsetan M, Christenson E, Unger F, Ausborn M, Kissel T, Hiltner A, Anderson JM. In vivo biomcompatibility and biodegradation of poly(ethylene carbonate). J Control Release. 2003;93(3):259–270. doi: 10.1016/j.jconrel.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Suggs LJ, Shive MS, Garcia CA, Anderson JM, Mikos AG. In vitro cytotoxicity and in vivo biocompatibility of poly(propylene fumarate-co-ethylene glycol) hydrogels. J Biomed Mater Res. 1999;46:22–32. doi: 10.1002/(sici)1097-4636(199907)46:1<22::aid-jbm3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 21.Brodbeck WG, Patel J, Voskerician G, Christenson E, Shive MS, Nakayama Y, Matsuda T, Ziats NP, Anderson JM. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proceedings of the National Academy of Sciences. 2002;99(16):10287–10292. doi: 10.1073/pnas.162124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasser S. Orthopedic metal immune hypersensitivity. Orthopedics. 2007;30(8 Suppl):89–91. [PubMed] [Google Scholar]
- 23.Sato N, Kinbara M, Kuroishi T, Kimura K, Iwakura Y, Ohtsu H, Sugawara S, Endo Y. Lipopolysaccharide promotes and augments metal allergies in mice, dependent on innate immunity and histidine decarboxylase. Clin Exp Allergy. 2007;37(5):743–51. doi: 10.1111/j.1365-2222.2007.02705.x. [DOI] [PubMed] [Google Scholar]
- 24.Jones JA, Dadsetan M, Collier TO, Ebert M, Stokes KS, Ward RS, Hiltner PA, Anderson JM. Macrophage behavior on surface-modified polyurethanes. J Biomater Sci Polym Ed. 2004;15(5):567–84. doi: 10.1163/156856204323046843. [DOI] [PubMed] [Google Scholar]
- 25.Brodbeck WG, Voskerician G, Ziats NP, Nakayama Y, Matsuda T, Anderson JM. In vivo leukocyte cytokine mRNA responses to biomaterials are dependent on surface chemistry. J Biomed Mater Res A. 2003;64(2):320–9. doi: 10.1002/jbm.a.10425. [DOI] [PubMed] [Google Scholar]
- 26.Moreno JL, Mikhailenko I, Tondravi MM, Keegan AD. IL-4 promotes the formation of multinucleated giant cells from macrophage precursors by a STAT6-dependent, homotypic mechanism: contribution of E-cadherin. J Leukoc Biol. 2007 doi: 10.1189/jlb.0107058. [DOI] [PubMed] [Google Scholar]
- 27.Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol. 2000;105(6 Pt 1):1063–70. doi: 10.1067/mai.2000.107604. [DOI] [PubMed] [Google Scholar]
- 28.Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2007;217:304–28. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 29.Metwali A, de Andres B, Blum A, Elliott D, Li J, Qadir K, Sandor M, Weinstock J. Th2-type granuloma development in acute murine schistosomiasis is only partly dependent on CD4+ T cells as the source of IL-4. Eur J Immunol. 2002;32(5):1242–52. doi: 10.1002/1521-4141(200205)32:5<1242::AID-IMMU1242>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]