Abstract
Exposure of mice to a brief light stimulus during their nocturnal active phase induces several simultaneous behavioral or physiological responses, including circadian rhythm phase shifts, a drop in core body temperature (Tc), suppression of locomotor activity and sleep. Each response is triggered by light, endures for a relatively fixed interval and does not require additional light for expression. The present studies address the ability of the psychostimulant drugs, methamphetamine (MA), modafinil (MOD) or caffeine (CAF), to modify the light-induced responses. Drug or vehicle (VEH) was injected at CT11 into constant dark-housed mice then exposed to 5 min 100 μW/cm2 light or no light at CT13. Controls (VEH/Light) showed approximately 60 min phase delays. In contrast, response was substantially attenuated by each drug (only 12-15 min delays). Under a LD12:12 photoperiod, VEH/light-treated mice experienced a Tc drop of about 1.3 °C coincident with locomotor suppression and both effects were abolished by drug pretreatment. Each drug elevated activity during the post-injection interval, but there was also evidence for CAF-induced hypoactivity in the dark prior to the photic test stimulus. CAF acutely elevated Tc; MA acutely lowered it, but both drugs reduced Tc during the early dark (ZT12.5-ZT13). The ability of the psychostimulant drugs to block the several effects of light exposure is not the result of drug-induced hyperactivity. The results raise questions concerning the manner in which drugs, activity, sleep and Tc influence behavioral and physiological responses to light.
Keywords: arousal, suprachiasmatic, photosomnolence, thermoregulation, circadian, masking
1.0
The light-type phase response curve describes the relationship between an organism's rhythm phase shift magnitude and the circadian time at which the stimulus occurred (Daan and Pittendrigh, 1976). In general, light exposure early in the subjective night elicits phase delays and phase advances occur if exposure occurs later. Light at night coincidentally suppresses locomotion in nocturnal rodents, a phenomenon labeled ‘negative masking’ (Mrosovsky, 1999). Such light-induced locomotor suppression occurs rapidly and is a prelude to another consequence of light exposure, namely, photosomnolence or light-induced sleep (Morin and Studholme, 2009, Morin et al., 2010).
Several studies suggest that reduced or absent locomotor activity may be necessary for normal, light-induced phase shifts (Ralph and Mrosovsky, 1992, Mistlberger and Antle, 1998, Mistlberger and Holmes, 1999, Edelstein et al., 2003). In addition to its locomotion suppression effects, light also induces a large decline in core body temperature (Tc; (Studholme et al., 2013)). Tc is normally lowest during the daylight hours when most nocturnal species sleep and, regardless of time of day, is lower during sleep than during wake (Obal et al., 1985).
Methamphetamine (MA), modafinil (MOD) and caffeine (CAF) are well known as sleep-prevention pharmaceuticals (see (Edgar and Seidel, 1997) for a discussion). MA maintains wakefulness and induces hyperactivity (Edgar and Seidel, 1997). MOD is used clinically to maintain wakefulness, in part, because it induces less activity than MA (Edgar and Seidel, 1997, Okuro et al., 2010). CAF has more limited effects, but modestly increases locomotor activity (Okuro et al., 2010). In addition, certain doses of each drug are reported to increase Tc (Edgar and Seidel, 1997, Okuro et al., 2010, Phelps et al., 2010).
MA treatment has also been shown to block both light-induced circadian rhythm phase shifts and FOS protein in suprachiasmatic nucleus (SCN) neurons (Moriya et al., 1996, Ono et al., 1996). Similar effects were not found after MOD treatment (Webb et al., 2006). The effect of CAF on either of these responses has not been reported, although the drug attenuates phase shifts induced by wheel running (Antle et al., 2001). Further, the adenosine R1 receptor, a native target for CAF, has been implicated as a modulator of light-induced rhythm shifts, FOS in the SCN, as well as SCN field potential amplitude following optic nerve stimulation (Watanabe et al., 1996, Elliott et al., 2001, Sigworth and Rea, 2003). CAF may also induce phase delays by direct effect on the SCN (Ding et al., 1998).
We have suggested elsewhere that light-induced circadian rhythm phase shifts, locomotor suppression/photosomnolence and reduction of body temperature may be controlled via a common retinal input pathway (Morin, 2013b, Studholme et al., 2013). The present studies were designed to determine whether the three psychostimulant drugs are able to prevent these responses with the expectation that if a drug blocked one of the responses to light, it would block them all. Moreover, because of their similar sleep-prevention attributes, the three drugs were predicted to have similar effects on the light-induced responses despite their differing modes of action and differing effects on general locomotion.
2.0 EXPERIMENTAL PROCEDURES
2.1 Animals and housing conditions
Male C57BLJ/6 mice (Jackson Laboratory, Bar Harbor, ME) were initially housed individually in polycarbonate test cages (45 L × 20 W × 20 H cm). During Experiments 1-3, each cage contained a 16.5 cm diameter stainless steel running wheel and was semi-isolated, along with 4-5 other cages, in an enclosed, light-tight shelf on a rack consisting of 5 such shelves. During Experiments 4-6, the test cages were on stainless steel shelving in a standard animal housing room. Mice were maintained under a 12 h light:12 h dark (LD12:12) photoperiod (light on at 2 AM clock time), except as otherwise described. Ambient temperature was 21±1 °C; food and water were available ad libitum.
Lighting of each shelf in the semi-isolation chamber consisted of a linear array of 48 broad spectrum white light LEDs (LBFA-CW12, Superbrightleds.com) arranged approximately 10 cm from the wheel end of the cage. Irradiance within each chamber was controlled by an 8 bit D-A voltage controller, an LED dimmer (OSRAM OT DIM, Osram Sylvania, Danvers, MA) and custom software (LightControl written by Glenn Hudson, Stony Brook University) that allowed timing of the LED light with one second accuracy. In Experiments 1-3, the LED light turned on (irradiance = 100 μW/cm2) at the pre-programmed time and remained on for 5 min. In Experiments 4-6, a 5 min, 100 μW/cm2 light pulse was also used, but was delivered from a 150W incandescent light source (#120-P38BI-1, Cheaplights.com).
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Stony Brook University (NY) and conducted in accordance with the the United States National Institutes of Health Guidelines regarding the care and use of animals for experimental procedures.
2.2 Data recording and analysis
Each revolution of a running wheel closed a microswitch. Such closures were recorded as revolutions per minute by a data acquisition system (WinCollectRT software written by Glenn Hudson, Stony Brook University). The software also created the raster format representation of the daily locomotor activity of each animal and exported the data to a spreadsheet for further analysis. Independent groups of mice were tested in each experiment.
2.3.1 Experiments 1-3
Mice stably entrained to LD12:12 were exposed to constant dark (DD). After 5 days in DD, a line was eye-fitted through the daily wheel running onsets in DD and extrapolated to day 6. The light pulse was delivered to each shelf at the clock time that would effectively stimulate the largest number of mice on that shelf. Effectively stimulated mice were those that received light exposure during the interval of CT13-CT14 and only these were used in the subsequent analysis. An eye-fitted line through 5 daily, post-stimulus activity onsets was extrapolated back to day 6 and the phase shift (time difference between the extrapolated pre- and post-test activity onsets) was measured on that day with a digital caliper.
For each of these experiments, a modification of a previous procedure (Morin and Studholme, 2009), provided a “wheel running suppression index.” This measure was calculated as the percentage of minutes in a 60 min interval after stimulus onset in which the animal registered zero wheel revolutions.
2.3.2 Experiments 4-6
Mice were housed under LD12:12, then deeply anesthetized with a mixture of ketamine (100 mg/kg, Butler Supply, Dublin, OH) and xylazine (10 mg/kg, Lloyd Laboratories, Shenandoah, IA) and intra-abdominally implanted with a telemetric transmitter (PhysioTel model TA-11-F10; Data Sciences International (DSI), St. Paul, MN) that simultaneously obtained temperature and activity data. ART v4.3 (DSI) software was used for data collection. Animals were allowed to recover from surgery for at least 8 days prior to participation in an experiment.
2.4 Analysis and Statistics
Activity indices obtained from the DSI transmitters and Tc data were graphed for each animal using SigmaPlot 11.0 (Systat Software, San Jose, CA). On the average, 2 units of DSI activity correspond to approximately 1 cm distance moved (Studholme et al., 2013).
Analysis of the effect of light on Tc was accomplished by evaluating change in Tc calculated by subtracting the lowest Tc measured during a 20 min interval beginning at the time of light stimulus onset from the average Tc during the 20 min prior to stimulus onset time. Light-induced change in activity level was obtained by subtracting each animal's post-stimulus activity level from its average activity level during the 20 min prior to stimulus onset. Because there were large minute-to-minute fluctuations in the activity levels, the actual post-stimulus activity index subtracted was the level represented by the first quartile (25th percentile) of all activity across the 20 min test interval after stimulus onset.
Statistical calculations were performed with SigmaPlot 11.0 (Systat Software, Chicago, Il). Analysis of effects involving four or more treatments were made with parametric one-way analysis of variance (ANOVA) and post-hoc tests (Student-Newman-Keuls (SNK)) for pair-wise comparisons when tests for normality and equal variance were acceptable. Otherwise, non-parametric Kruskal-Wallis analyses of variance on ranks were performed, followed by Dunn's tests for pair-wise comparisons. Statistical comparison of drug vs vehicle effects with respect to activity or Tc during several intervals prior to light exposure was performed with parametric t tests or, if either normality or equal variance tests failed, with non-parametric Mann-Whitney tests.
2.5 Experiment 1. Effect of modafinil on light-induced phase delays
Treatments were administered after 5 days in DD. Each mouse received an intraperitoneal (IP) injection of MOD (product M6940, Sigma-Aldrich, St. Louis, MO) or vehicle (VEH) at CT11 followed by a 5 min light pulse (Light) or no light (NoLight) during the interval of CT13-CT14. Thus, there were four treatment conditions: a) MOD/Light; b) MOD/NoLight; c) VEH/Light; and (d) VEH/NoLight.
MOD was injected IP at a concentration of 150mg/kg. The vehicle used to dissolve the MOD was a mixture of 0.9% saline, 1.5% DMSO, 1.5% Cremophor (C-5135; Sigma-Aldrich, St. Louis, MO (Mitchell et al., 2008)). A stock solution was prepared about 2 hr before injection, heated to 42 °C and kept warm until its application. At that time, the cage of each animal was quickly transferred from the semi-isolation chamber into the dark surrounding room under dim red light. The resident mouse was gently handled, injected, returned to its cage and its cage replaced in the chamber.
2.6 Experiment 2. Effect of methamphetamine on light-induced phase delays
The procedures for this study were the same as in Expt. 1 except that the drug was MA (product M-8750; Sigma-Aldrich, St. Louis, MO) which was injected at a concentration of 2 mg/kg in 0.9% saline vehicle. The solution was prepared about 2 hr before the injection.
2.7 Experiment 3. Effect of caffeine on light-induced phase delays
The procedures for this study were the same as in Expt. 1 except that CAF (product C0750; Sigma-Aldrich, St. Louis, MO) was delivered at a concentration of 40 mg/kg in 0.9% saline vehicle. The solution was prepared about 2 hr before the injection.
2.8 Experiment 4. Effect of modafinil on light-induced locomotor suppression and change in Tc
Mice implanted with DSI transmitters were entrained to LD12:12 for at least 8 days before receiving one of four treatments: MOD/Light; MOD/NoLight; VEH/Light; or VEH/NoLight. IP injections of MOD (150 mg/kg) or vehicle were initiated for each group at approximately ZT11. In this experiment, each animal received a 0.2 ml injection of MOD dissolved in a mixture of 0.9% saline, 10% DMSO and 2% Cremophor which more effectively kept the drug in solution. The 5 min light pulse was turned on at ZT13. DSI-measured activity and Tc responses to light were assessed.
2.9 Experiment 5. Effect of methamphetamine on light-induced locomotor suppression and change in Tc
Mice bearing DSI transmitters were treated as in Experiment 4 except that the drug injected was MA. Drug concentration and vehicle were as described in Experiment 2. After at least 8 days post surgical entrainment, each animal received one of four treatments: MA/Light; MA/NoLight; VEH/Light; or VEH/NoLight, with the light pulse administered at ZT13. DSI-measured activity and Tc responses to light were assessed.
2.10 Experiment 6. Effect of caffeine on light-induced locomotor suppression and change in Tc
Mice bearing DSI transmitters were treated as in Experiment 4 except that the drug injected was 20 or 40 mg/kg CAF and VEH was 0.9% saline. At that time, each animal received one of six treatments: 20 mg/kg CAF/Light; 20 mg/kg CAF/NoLight; 40 mg/kg CAF/Light; 40 mg/kg CAF/NoLight; VEH/Light; or VEH/NoLight. DSI-measured activity and Tc responses to light were assessed.
3.0 RESULTS
3.1 Experiment 1. Effect of modafinil on light-induced phase delays and locomotor suppression
MOD blocked light-induced phase shifts (Figs. 1, 2). ANOVA revealed an overall treatment effect (F=29.88, df=3,60, p<.001). VEH/Light induced a phase delay of −72 ± 7 min which was significantly greater than the −15 ± 7 min shown by mice in response to the MOD/Light treatment. The effect of the VEH/Light treatment was also significantly different from all other treatment effects (Fig. 2). In addition, the MOD/Light and MOD/NoLight groups differed from the VEH/NoLight group.
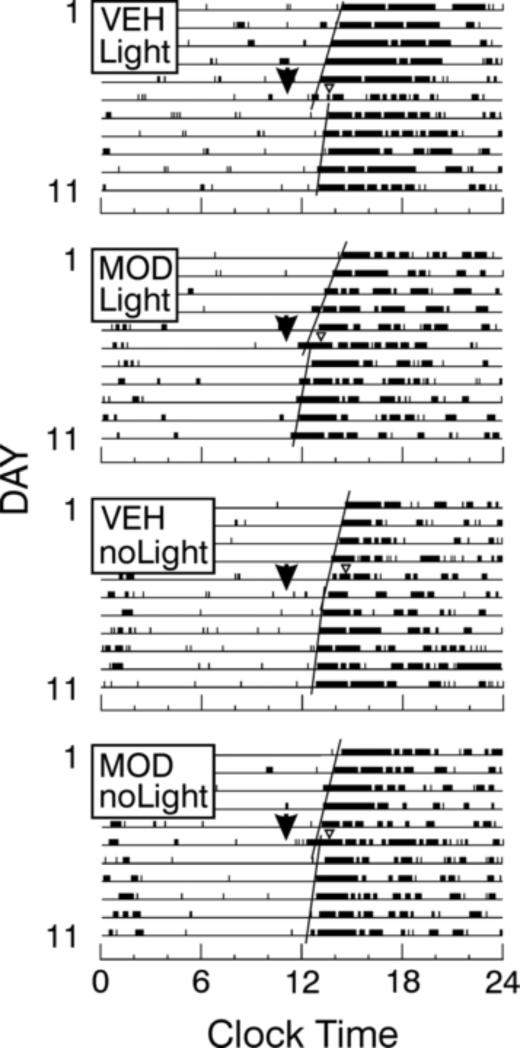
Figure 1.
Raster scan wheel running records of individual mice in DD which are pre-treated (arrowheads) with modafinil (MOD) or vehicle (VEH), then exposed to a 5 min pulse of Light or NoLight at CT13-CT14 (triangle). Only the VEH/Light mice had significant phase shifts (see text).
Figure 2.
Light delivered at CT13-CT14 induces large phase delays (mean ± SEM) which are generally blocked by modafinil (MOD) pre-treatment. Black dots indicate data points of individuals and the number of animals per group is in parentheses. Significant pair-wise differences are indicated by p values (see text).
The treatments had a significant effect on the wheel running suppression index (H=32.90, df=3, p<.001, Kruskall-Wallis ANOVA on ranks; Table 1), with the primary effect being a 90% suppression of wheel running by light. Post hoc analysis showed that MOD blocked the effect of light. The VEH/Light group differed significantly from the VEH/NoLight, the MOD/Light and the MOD/NoLight groups (p<.05 each, Dunn's tests).
Table 1.
Effect of stimulatory drugs on the wheelrunning suppression index (median percent suppression, inter-quartile range) in mice tested in constant darkness.
| Treatment | Modafinil | Methamphetamine | Caffeine |
|---|---|---|---|
| VEH/Light | 90.0 (87.9/92.1) (n=17)a,b,c | 90.0 (86.7/90) (n=11)a,b | 80.0 (73.3/95.0) (n=7)a |
| VEH/NoLight | 36.7 (23.3/70.0) (n=14)a,d | 11.7 (8.3/16.7) (n=16)a,d | 24.6 (13.1/29.5) (n=9)a,b,c |
| Drug/Light | 1.6 ± (0.0/12.5) (n=16)b,d | 8.3 (1.7/13.3) (n=13)b,c | 65.6 (24.6/82.0) (n=7)b |
| Drug/NoLight | 17.5 (4.6/26.7) (n=16)c | 56.7 (36.7/77.5) (n=19)c,d | 54.1 (39.3/77.1) (n=12)c |
A common superscript denotes a statistically significant between-group difference (see text).
3.2 Experiment 2. Effect of methamphetamine on light-induced phase delays and locomotor suppression
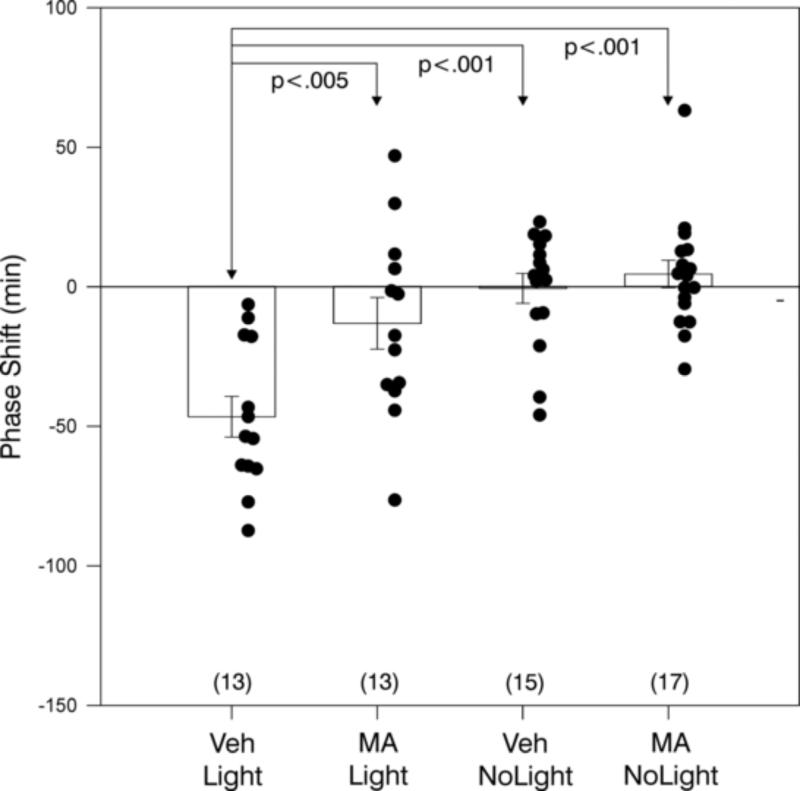
MA blocked light-induced phase shifts (Fig. 3). The raster plot running records (not shown) were very similar to those in Experiment 1. There was an overall treatment effect (F=11.61, df=3,54, p<.001, ANOVA). VEH/Light treatment induced a phase delay of −45 ± 7 min which was significantly greater than the phase shifts of all other groups (Fig. 3). There were no other significant pair-wise comparisons.
Figure 3.
Light delivered at CT13-CT14 induces large phase delays (mean ± SEM) which are generally blocked by methamphetamine (MA) pre-treatment. Black dots indicate data points of individuals and the number of animals per group is in parentheses. Significant pair-wise comparisons are indicated by p values (see text).
The treatments had a significant effect on the wheel running suppression index (H=50.44, df=3, p<.001, Kruskall-Wallis ANOVA on ranks; Table 1). Post hoc analysis showed that MA blocked the effect of light. In this study, MA appeared to interact with the photic treatment, ameliorating the suppressive effect of light. The VEH/Light group differed significantly from the VEH/NoLight and the MA/Light groups (p<.05 each, Dunn's tests). The MA/NoLight group also differed significantly from the MA/Light and the VEH/NoLight groups (p<.05 each, Dunn's tests).
3.3 Experiment 3. Effect of caffeine on light-induced phase delays and locomotor suppression
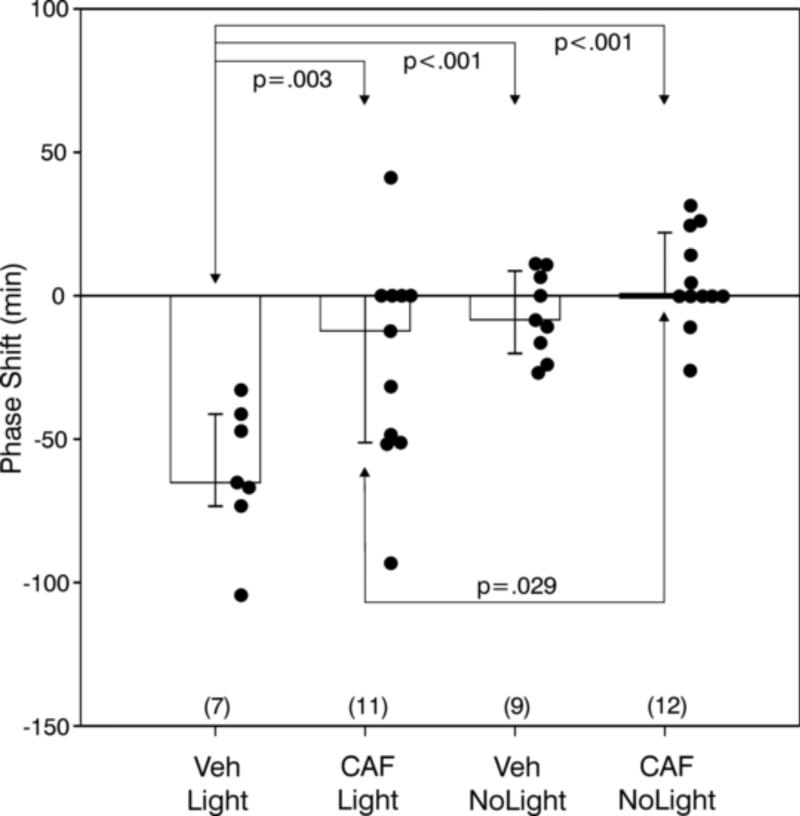
The 40 mg CAF treatment blocked normal light-induced phase shifts (Fig. 4). The raster plot running records (not shown) were very similar to those in Experiments 1 and 2. ANOVA revealed an overall treatment effect (F=11.44, df=3,35, p<.001). VEH/Light treatment induced a phase delay of −61.6 ± 9.1 min which was significantly greater than the phase shifts of all other groups (Fig. 4). In addition, the CAF/Light treatment differed from the CAF/NoLight treatment.
Figure 4.
Light delivered at CT13-CT14 induces large phase delays (mean ± SEM) which are generally blocked by 40 mg caffeine (CAF) pre-treatment. Black dots indicate data points of individuals and the number of animals per group is in parentheses. Significant pair-wise comparisons are indicated by p values (see text)..
There was a significant treatment effect on the wheel running suppression index (F=8.49, df=3,31, p<.001, ANOVA). Post hoc analysis (SNK tests) showed (Table 1) that the VEH/NoLight group differed significantly from the VEH/Light (p<.001), the CAF/NoLight (p=.004) and the CAF/Light (p=.013) groups. The data tended toward differences between VEH/Light and CAF/Light or CAF/NoLight (p=.083 and .07, respectively).
3.4 Drug effects across Experiments 1-3
A few animals in each drug treatment group appeared to show robust phase shifts to light. Although there were relatively few of these cases, it is possible that the drugs did not completely block the effect of light. To test this further, data from the three drug studies were combined in order to compare a larger drug/Light data set to a larger drug/NoLight data set. A Fisher's exact test showed that the two treatments differed with respect to the proportion of animals showing measured phase delays, no matter how small (p=.016; 29/40 vs 21/46 for drug/Light and drug/NoLight, respectively). This supports the idea that light is indeed exerting a modest effect on rhythm phase despite the drug treatment.
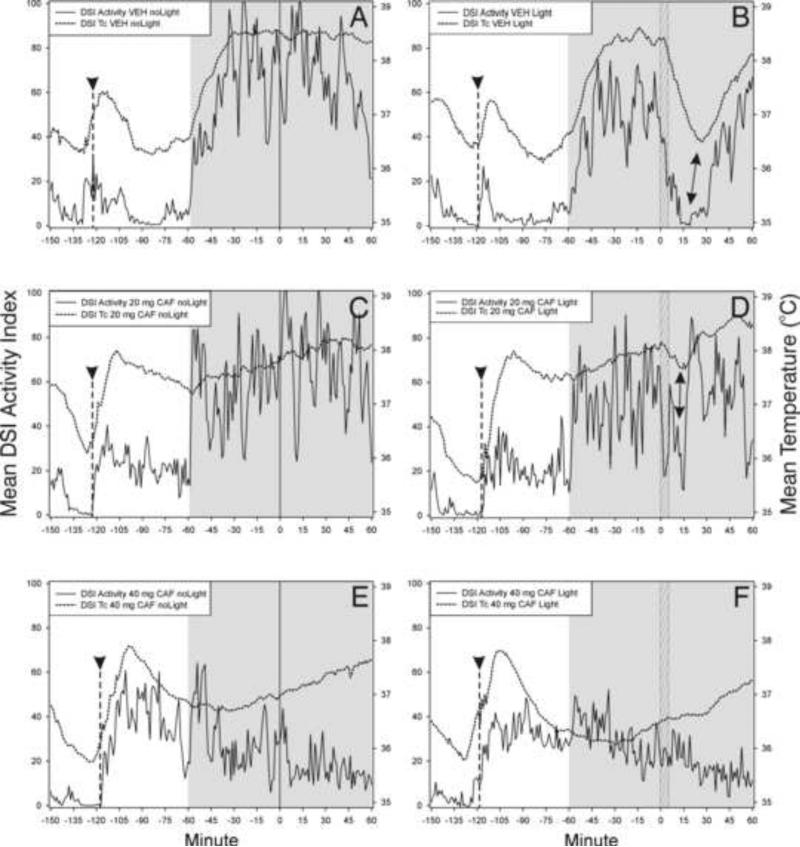
3.5 Experiment 4. Effect of MOD on light-induced change in Tc and activity
The normal light-induced drop in Tc was blocked by MOD treatment (Fig. 5). Exposure of VEH-injected mice to a light pulse at ZT13 resulted in a median Tc drop of 1.24 °C (1.16/1.62 inter-quartile range) that reached its nadir approximately about 20 min after light onset. The amount that Tc dropped was dependent upon treatment (H=22.98, df=3, p<.001, Kruskal-Wallis test). Pair-wise comparisons showed that the drop was greater for VEH/Light-treated mice than for Veh/NoLight (0.15 °C (0.01/0.33)), MOD/Light (0.25°C (0.11/0.43)) and MOD/NoLight-treated (0.06 (−0.03/0.14)) mice (p<.05, Dunn's tests).
Figure 5.
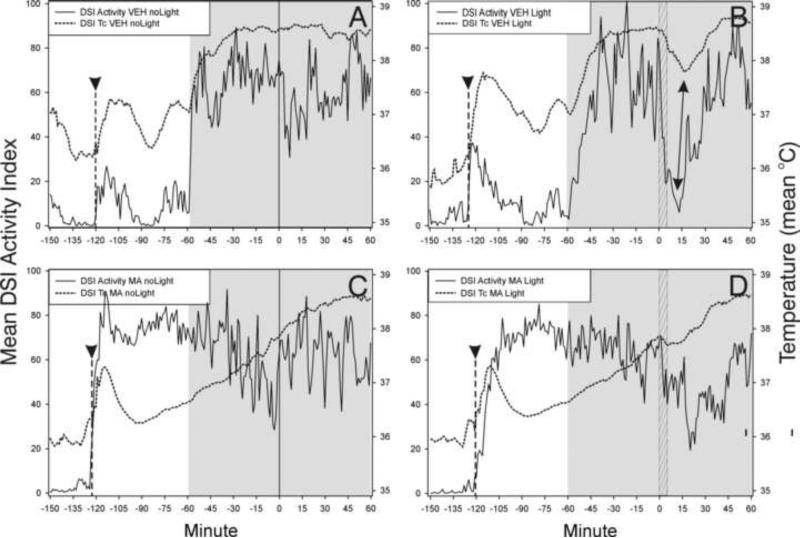
Modafinil (MOD; Panel D) blocks both light-induced locomotor suppression and the associated Tc drop in vehicle-treated mice (Panel B; double-headed arrow). Arrowhead and dashed vertical line - time of VEH (panels A, B) or MOD injection (panels C, D); shaded area - early dark phase (ZT12-ZT14). The light pulse was administered for 5 min beginning at minute 0 (ZT13; hatched vertical bar, panels B,D). Dotted data line - Tc obtained with DSI transmitters; solid data line - DSI activity index. N=7/group.
MOD also blocked the normal light-induced locomotor suppression (Fig. 5), as indicated by the first quartile responses which revealed a significant treatment effect (H=17.52, df=3, p<.001, Kruskal-Wallis test). The greatest treatment effect was in VEH/Light-treated mice which had a decrease in DSI-detected activity (median (25/75%iles)) of 70.3 (32.8/86.8). Pair-wise comparisons indicating that activity by this group was significantly greater than that for all other groups ([<.05, Dunn's tests). There were no other significant differences. VEH/NoLight mice had a median activity change of 11.0 (1.9/29.6); for MOD/Light mice it was 18.9 (−0.3/43.5); and for MOD/NoLight mice the change was 8.0 (2.3/19.6).
3.6 Experiment 5. Effect of MA on light-induced change in Tc and activity
The normal light-induced drop in Tc was blocked by MA treatment (Fig. 6). Exposure of VEH-injected mice to a light pulse at ZT13 resulted in a median Tc drop of 1.03 °C (0.45/1.45 inter-quartile range). The amount that Tc dropped was dependent upon treatment (H=20.08, df=3, p<.001, Kruskal-Wallis test). Pair-wise comparisons showed that the drop was greater for VEH/Light-treated mice than for the Veh/NoLight (0.13 °C (0.09/0.19)), MA/Light (−0.14°C (−0.28/0.00)) and MA/NoLight (−0.30 (−0.34/−0.11) groups (p<.05, SNK tests). The VEH/NoLight group had a Tc drop that was significantly greater than that for the MA/NoLight and the MA/Light groups (p<.05 each).
Figure 6.
Methamphetamine (MA; Panel D) blocks both light-induced locomotor suppression and the associated Tc drop in vehicle-treated mice (Panel B; double-headed arrow). Panel A - effect of VEH/noLight; Panel C - effect of MA/noLight. Arrowhead and dashed vertical line - approximate time of VEH (panels A,B) or MA injection (panels C,D); shaded area - early dark phase (ZT12-ZT14). The light pulse was administered for 5 min beginning at minute 0 (ZT13; hatched vertical bar, panels B,D). Dotted data line - Tc obtained with DSI transmitters ; solid data line - DSI activity index. N = 7/group.
MA blocked the normal light-induced locomotor suppression (Fig. 6), as indicated by the first quartile responses which revealed a significant treatment effect (H=11.92, df=3, p=.008, Kruskal-Wallis test). The greatest treatment effect was in VEH/Light-treated mice which had a decrease in DSI-detected activity (median (25/75%iles)) of 55.7 (43.5/84.4). Pair-wise comparisons showed that activity change by this group was significantly greater than that of all other groups (p<0.05, SNK tests). There were no other between-group differences. VEH/NoLight mice had a median activity change of 21.1 (9.2/33.5); for MA/Light mice it was 10.1 (5.3/41.8); and for MA/NoLight mice the change was 13.9 (−9.8/18.2)).
3.7 Experiment 6. Effect of CAF on light-induced change in Tc and activity
The normal light-induced drop in Tc was blocked by 40 mg/kg CAF; 20 mg/kg CAF appeared to have a partial effect (Fig. 7). Exposure of VEH-injected mice to a light pulse at ZT13 resulted in a median Tc drop of 1.71 °C (1.36 /1.88 inter-quartile range). The amount that Tc dropped was dependent upon treatment (H=22.80, df=5, p<.001, Kruskal-Wallis test). Pair-wise comparisons indicated that the change induced by the VEH/Light treatment was significantly greater than that induced by all other treatments (p<.05 in each case, SNK tests). There were no other significant differences. VEH/NoLight mice had a median Tc change of −0.24 (−0.09/−0.46); 20 mg CAF/Light = −0.54 (0.08/−0.76); 20 mg CAF/NoLight = −0.04 (0.04/−0.19); 40 mg CAF/Light = 0.01 (0.08/−0.13) and 40 mg CAF NoLight = 0.01 (0.07/−0.12).
Figure 7.
Caffeine (CAF; 40 mg) treatment blocks (panel F) both light-induced locomotor suppression and the associated drop in Tc; 20 mg has a partial effect (panel D; double-ended arrows) compared to the effect of vehicle (VEH; panel B). Arrowhead and dashed vertical line - approximate time of VEH (panels A,B) or CAF (panels C-F) injection; shaded area - early dark phase (ZT12-ZT14). The light pulse was administered for 5 min beginning at minute 0 (ZT13; hatched vertical bar, panels B,D,F). Dotted data line - Tc obtained with DSI transmitters; solid data line - DSI activity index. N=9-11/group.
The 40 mg CAF also blocked the normal light-induced locomotor suppression; 20 mg CAF appeared to have a partial effect (Fig. 7). The first quartile responses revealed a significant treatment effect (H=11.82, df=5, p=.037, Kruskal-Wallis test). The greatest treatment effect was in VEH/Light-treated mice which had decrease in DSI-detected activity (median (25/75%iles)) of 48.3 (29.3/83.6). Pair-wise comparisons indicating that the activity suppressing effect of VEH/Light treatment was significantly greater than that induced by all other treatments (p<.05 each, SNK tests). There were no other significant differences. Activity change of each other group was as follows: VEH/NoLight 12.7 (10.1/27.1); 20 mg CAF/Light 29.7 (15.0/38.2); 20 mg CAF/NoLight 24.7 (6/36.5); 40 mg CAF/Light 22.0 (10.3/35.7) and 40 mg CAF NoLight 17.8 (8.7/26.9).
3.8 Pharmacological Effects of Psychostimulant Drugs on Activity and Core Temperature
The effects of MOD, MA and CAF on activity and Tc were assessed in Experiments 4-6 during three intervals prior to the above described tests of the effect of light. The post-injection interval began at the time of drug injection (ZT11) and ended at ZT11.5. The pre-darkness interval began 30 min prior to the onset of the daily dark period (ZT 11.5) and ended at the onset of darkness (ZT12). The pre-light test interval began during the dark at ZT12.66 and ended at the time of light stimulus onset (ZT13) 20 min later.
3.8.1 During the Post-injection Interval
In general, injection and the related handling resulted in an immediate, but transient (30 min), increase in both activity and Tc, regardless of whether animals received vehicle or drug (Figs. 5-7). MOD had no acute effect on Tc (Table 2), but induced elevated activity at this time. MA injection reduced Tc relative to the effect of VEH treatment, while elevating activity (Table 2). CAF treatment increased activity during the 30 min post-injection interval by a combination of higher and more prolonged activity. CAF elevated both activity and Tc in a dose dependent manner.
Table 2.
Pharmacological effects on Tc and DSI activity (medians, inter-quartile range) during the 30 min post-injection interval (ZT11-ZT11.5).
| Tc | Activity | |
|---|---|---|
| VEH (20) | 37.05 (36.83/37.31)NS | 11.4 (8.2/15.8)a |
| MOD (19) | 37.07 (36.69/37.34)NS | 27.7 (15.9/36.6)a |
| VEH (14) | 37.23 (36.99/37.35)b | 14.9 (9.3/18.8)b |
| MA (14) | 36.68 (36.62/36.85)b | 72.3 (38.4/82.4)b |
| VEH (14) | 37.05 (36.74/37.30)c | 6.2 (4.2/12.1)d,e |
| CAF 20mg (14) | 37.32 (37.03/37.75) | 22.5 (17.6/28.3)d |
| CAF 40mg (14) | 37.54 (37.11/37.79)c | 36.4 (24.9/40.3)e |
NSNot significant, Mann-Whitney test; Differ
<.001, Mann-Whitney tests
<.001, Mann-Whitney tests
p<.05, SNK tests after significant Kruskal-Wallis ANOVA.
p<.05, SNK tests after significant Kruskal-Wallis ANOVA.
p<.05, SNK tests after significant Kruskal-Wallis ANOVA.
3.8.2 During the Pre-darkness Interval
During the pre-darkness interval, both Tc and activity were significantly elevated by MOD relative to VEH treatment (Table 3). In contrast, MA treatment resulted in significantly lower Tc despite the fact that activity was greatly elevated. CAF increased activity in a dose-dependent fashion. Tc was significantly increased by 40, but not 20, mg CAF.
Table 3.
Pharmacological effects on Tc (°C) and DSI activity (median, inter-quartile range) during the 30 min interval prior to daily dark onset (ZT11.5-ZT12).
| Tc | Activity | |
|---|---|---|
| VEH | 36.81 (36.56/37.11)a | 5.5 (2.7/8.3)b |
| MOD | 37.57 (37.19/37.78)a | 45.5 (30.5/57.2)b |
| VEH | 36.86 (36.65/37.09)c | 6.4 (3.9/9.5)d |
| MA | 36.61 (36.24/36.86)c | 75.4 (57.4/88.3)d |
| VEH* | 36.8 (36.4/37.0)e | 4.2 (1.5/5.8)f |
| CAF 20mg | 37.6 (37.5/37.7)e | 19.2 (16.2/23.1)f |
| CAF 40mg | 37.1 (36.7/37.3)e | 32.9 (25.2/41.7)f |
Differ
p=.007
p<.001, Mann-Whitney tests
p=.018
p<.001, Mann-Whitney tests
Differ p<.05, SNK tests after significant Kruskal-Wallis ANOVA.
Differ p<.05, SNK tests after significant Kruskal-Wallis ANOVA.
3.8.3 During the Pre-light Test Interval
MOD caused significantly reduced Tc during the 20 min pre-light test interval, but had no effect on activity (Table 4). MA treatment also decreased Tc during this interval, but activity was unaffected. CAF treatment significantly reduced both Tc and activity in a dose-dependent manner.
Table 4.
Pharmacological effects on Tc and activity (median, inter-quartile range) during the 20 min interval of darkness (ZT1240-ZT1300) immediately prior to the light pulse test.
| Tc | Activity | |
|---|---|---|
| VEH | 38.50 (38.19/38.65)a | 61.5 (38.3/88.3)ns |
| MOD | 38.00 (37.63/38.45)a | 59.5 (41.7/70.5)ns |
| VEH | 38.57 (38.43/38.73)b | 56.4 (43.1/88.3)ns |
| MA | 37.66 (337.42/37.76)b | 45.8 (33.4/69.5)ns |
| VEH | 38.61 (38.48/38.83)c | 62.6 (47.7/82.7)c |
| CAF 20mg | 37.96 (37.36/38.26)d | 51.9 (38.3/78.6)d |
| CAF 40mg | 36.74 (36.45/37.08)c,d | 25.2 (20.6/28.1)c,d |
NSNot significant, Mann-Whitney test
Differ p=.018
Differ p<.001, Mann-Whitney tests
Differ p<.05, SNK tests after significant Kruskal-Wallis ANOVA.
Differ p<.05, SNK tests after significant Kruskal-Wallis ANOVA.
4.0 DISCUSSION
Brief light exposure early in the subjective night induces multiple, simultaneous changes in physiology and behavior. The best known of these is alteration of circadian rhythm phase. The same light stimulus that induces rhythm phase shifts also triggers mechanisms that suppress locomotor activity, induce sleep (photosomnolence) and reduce Tc (Morin and Studholme, 2009, Morin and Studholme, 2011, Studholme et al., 2013). The present studies demonstrate that each of these responses to light is blocked or attenuated by pre-treatment with the psychostimulant drugs, MA, MOD or CAF. The results are consistent with the notion that the several light-induced responses are regulated by a shared input pathway (Morin, 2013b, Studholme et al., 2013).
4.1 Psychostimulant Drugs Block Multiple Effects of Nocturnal Light
Light-induced sleep occurs when there is simultaneous suppression of locomotion and Tc (Morin and Studholme, 2009, Morin et al., 2010, Morin and Studholme, 2011, Studholme et al., 2013). Sleep is generally incompatible with locomotion. There are several possible explanations for the ability of psychostimulant drugs to block photosomnolence, including but not limited to, the following: (1) A direct effect on the motor system to promote locomotion with the ensuing activity indirectly preventing sleep. (2) Direct activation of a separate “arousal” system, with consequent prevention of sleep. (3) Deactivation of the sleep induction system independent of any effect on the motor or arousal systems. (4) A direct effect on some other system (e.g., the thermoregulatory system) with a consequent indirect action on the sleep or locomotor systems. (5) Blockade of transmission of photic information to the non-image forming visual system thereby preventing any effects of light irrespective of the level of locomotion or Tc.
Limited previous testing has shown that MA can block light-induced phase shifts and FOS protein expression in the retinorecipient rat or hamster SCN (Moriya et al., 1996, Ono et al., 1996, Watanabe et al., 1996). The present data also show that MA pre-treatment blocks such phase shifts. In contrast, the present MOD data differ from those in a previous hamster study in which the drug failed to modify light-induced phase advances or delays (Webb et al., 2006). The different results may be explained by the species difference, light stimulus parameters or drug doses. Importantly, there is no evidence that drug-induced hyperactivity was responsible for blockade of the light effects. Notably, none of the drugs augmented locomotion during the early dark hours immediately prior to photic stimulus exposure when activity was already high. In fact, activity was actually reduced by 60% in mice receiving 40 mg CAF. The effect of each drug may have been more specific to blocking stimulus input than to enhancing motor output.
Acute CAF treatment of a rat SCN slice preparation induces phase delays (Ding et al., 1998). Chronic exposure to the drug lengthens the circadian period of clock gene expression recorded in mouse or human cell lines and in cultured liver explants (Oike et al., 2011). Ingested coffee or CAF also lengthens the period of the mouse circadian locomotor rhythm. A single experiment involving CAF-treated humans represents the extent of research on the interaction between light and drug effects (Wright et al., 2000). Those data show that bright nocturnal light plus CAF elevates human Tc beyond that found in response to either stimulus alone. Such a response, for a diurnal species, is consistent with the present results from nocturnal mice in which night-time light decreases Tc (present data and (Studholme et al., 2013)). Indirect evidence of an interaction between light and CAF comes from studies showing that an adenosine R1 receptor agonist, N6-cyclohexyladenosine, inhibits both light-induced phase delays and FOS protein expression in hamster SCN (Watanabe et al., 1996, Antle et al., 2001, Elliott et al., 2001). Enhanced locomotion in response to intra-SCN application of the agonist suggests that it may exert its effects through a direct action on the circadian clock (Elliott et al., 2001).
4.2 Activity Interferes with Light-induced Phase Shifts
Several studies have convincingly demonstrated that locomotion is not compatible with normal photically-induced phase shifts by hamsters or mice (Ralph and Mrosovsky, 1992, Mistlberger and Antle, 1998, Mistlberger and Holmes, 1999, Edelstein et al., 2003). Such observations are consistent with the view that various elements of a broad pattern of response to nocturnal light are causally linked. According to this view, light-induced locomotor suppression and/or sleep is necessary to enable the occurrence of normal phase shifts. This perspective is complicated by the associated light-induced drop in Tc. All treatments that effectively prevented light-induced locomotor suppression also blocked the expected drop in Tc. Therefore, it is not presently possible to state whether the simple, light-induced absence of activity enables phase shifts, or whether the shifts are facilitated by the simultaneous photosomnolence, the light-induced drop in Tc, or a combination of these events.
A different, but likely related, aspect of the foregoing issue has been discussed at length by Webb et al. (2006). These investigators noted that hamster phase advances are induced during the subjective day by a non-photic, locomotion-related stimulus. The phase shifts do not occur simply because the animals are sleep deprived, awake or active, as evidenced by the fact that wakefulness induced by confinement to a pedestal over water or arousal induced by physical restraint or by CAF- or MOD-treatment (and the associated induced locomotion) do not yield phase shifts (Antle et al., 2001, Mistlberger et al., 2003, Webb et al., 2006) (and present data). The effective stimulus or sensory pathway for behaviorally-induced phase shifts is not yet known, although some level of forward locomotion appears necessary (see (Mistlberger et al., 2003) for a thorough discussion).
4.4 Core Temperature, Sleep and Circadian Rhythm Regulation
Reduced Tc typically occurs in nocturnal animals during the daytime sleep interval when there is little or no locomotion (Refinetti and Menaker, 1992). Such reduced Tc could theoretically occur because of the absence of locomotor activity. Data from several mammalian species support the view that, at best, there is only a weak link between locomotor activity level and measured Tc (Brown and Refinetti, 1996, Decoursey et al., 1998, Van den Heuvel et al., 1998, Gebczynski and Taylor, 2004), although the strength of the link may vary with strain or species (Weinert and Waterhouse, 1998).
In the C57BL6/J mouse, five situations have been documented during which Tc appears causally unrelated to the level of activity (Studholme et al., 2013). The present data (Tables 2-4) provide additional examples in which Tc and activity do not show parallel changes. The most obvious is the MA-induced reduction in Tc that occurs between ZT11 and ZT12 when there is a simultaneous activity increase.
One potentially important aspect of reduced Tc concerns its effects on other measures. For example, although the light-induced drop in Tc is unlikely to directly cause circadian rhythm phase shifts, it could very well function to permit or facilitate such shifts in response to other stimuli. In the present studies, the failure to obtain a light-induced drop in Tc after drug treatment may have been either a direct or indirect effect of the drugs. In either case, drug-induced prevention of the Tc drop may have precluded phase shifts, locomotor suppression and photosomnolence. Such a permissive role for Tc in circadian rhythm regulation has not been widely explored, although it has been previously acknowledged (Aschoff, 1982).
4.5 Possible Neural Circuits Mediating the Effects of Nocturnal Light
The similar effects of the three drugs employed in these studies may result from the fact that they all act on the dopamine (DA) system to some degree. MA-induced hyperactivity in rats arises primarily from DA release, but also involves serotonin-mediated effects and actions of other transmitter systems (Fleckenstein et al., 2007, Steed et al., 2011). MOD appears to influence general activity primarily via the DA R1 receptor, but also modifies locomotor activity through multiple systems in mice and rats (Minzenberg and Carter, 2008, Young et al., 2011). CAF directly antagonizes receptors of the rat adenosine system with its locomotion facilitating actions likely occurring via consequent DA release (Josselyn and Beninger, 1991).
A proposed model (Morin, 2013b) suggests that light acts via the SCN to induce phase shifts, locomotor suppression and the Tc drop. The model also provides for feedback of light effects onto the SCN through the geniculohypothalamic tract. Antle et al. (2001) suggest that sleep-modulatory adenosine R1 agonists could alter the circadian clock by via serotonin release from the IGL-afferent dorsal raphe projection (Meyer-Bernstein and Morin, 1996). It is also possible that orexin/hypocretin neurons contributing to sleep/arousal regulation modify circadian rhythm function via input to the IGL (Vidal et al., 2005).
A circuit by which nocturnal light might induce sleep consists of retinal projections to the SCN, rhythmic output from the SCN to the paraventricular thalamus, and passage of that output from there, rostrally to the nucleus accumbens (Krettek and Price, 1977, Moga et al., 1995, Yamazaki et al., 1998, Pinto et al., 2003, Sleipness et al., 2007, Qiu et al., 2010, Qiu et al., 2012, Morin, 2013a). The thalamic projections to the rat accumbens converge upon cellular targets also receiving synaptic DA input (Pinto et al., 2003). CAF promotes arousal via adenosine 2a receptors in the accumbens shell (Huang et al., 2005, Lazarus et al., 2011). Adenosine 2a receptors are also co-expressed with DA D2 receptors in the accumbens and both receptor types are essential for the arousal effects of MOD in mice (Qu et al., 2008). Also in the mouse, DA D2 receptors are necessary for light-induced locomotor suppression (Doi et al., 2006). Similarly, DA activity in the rat accumbens is necessary for amphetamine-induced locomotion (Kelly and Iversen, 1976, Pijnenburg et al., 1976)
An alternative circuit providing rhythmic input to the accumbens may include rhythmically modulated ventral tegmental area DA cells which receive multisynaptic input from the SCN, with the medial preoptic nucleus as an intermediate relay site (Dahan et al., 2007, Luo et al., 2008, Luo and Aston-Jones, 2009). The medial preoptic contains neurons activated by adenosine 2A receptors (Gallopin et al., 2005), is critical to both normal sleep and thermoregulation (McGinty et al., 2001) and is an area directly innervated by both the retina and SCN (Morin, 2013a). Descending projections to the VTA likely constitute a major pathway through which the nucleus accumbens modulates sleep, the VTA having connections with brainstem nuclei involved in sleep state switching (Saper et al., 2010, Holmstrand and Sesack, 2011).
It is also possible that psychostimulant drugs act in the retina to directly block non-image forming visual responses, including phase shifts, photosomnolence and locomotor suppression (Hattar et al., 2003, Panda et al., 2003, Morin and Studholme, 2011). Histological data provides convincing evidence that photic information is not being properly transmitted to the SCN in animals treated with MA (Moriya et al., 1996, Ono et al., 1996, Watanabe et al., 1996). The combined behavioral and histological results support the more general view that RHT function is inhibited by psychostimulant drugs which could occur through inhibition of light-induced retinal DA release (Witkovsky et al., 2000, Van Hook et al., 2012).
An inconsistency with a retino-centric drug action is the fact that MA blocks field potentials in the SCN that are induced by hamster or rat optic nerve stimulation (Moriya et al., 1996, Ono et al., 1996). The MA effects may occur via inhibition of serotonin release in the SCN, thereby by-passing a retinal action. It is possible that MA and the other drugs tested here inhibit responses to nocturnal light by acting at multiple sites in the central nervous system.
5.0 Conclusions
The present results support previous observations that that nocturnal light simultaneously induces phase shifts, locomotor suppression, photosomnolence and a decline in Tc. The results also show that each response is substantially blocked by MOD, MA and CAF, drugs known to enhance wakefulness, activity and to elevate Tc, but not simply because of induced hyperactivity. The data support the suggestion that an input pathway to the SCN governs the several responses to light (Morin, 2013b), although the site of drug action is not clear. The locomotor suppression response employed here and in similar investigations is very easily measured (Morin and Studholme, 2009, Morin et al., 2010, Morin and Studholme, 2011, Studholme et al., 2013) and may be used as a rapid and highly productive proxy for studying the effects of light on the circadian visual and sleep systems.
Three stimulant drugs are tested for their effects on responses to light
Methamphetamine, modafinil and caffeine pretreatment block responses to light
Phase shifts, activity suppression and the drop in core temperature are blocked
Response blockade can occur without drug-induced activity increases
Light may modulate all three responses via a common input pathway.
ACKNOWLEDGEMENTS
Supported by NIH grant NS061804 to LPM. We are grateful to Max Grachev and Steven Mirabella for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Antle MC, Steen NM, Mistlberger RE. Adenosine and caffeine modulate circadian rhythms in the Syrian hamster. Neuroreport. 2001;12:2901–2905. doi: 10.1097/00001756-200109170-00029. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Daan S, Honma K-I. Zeitgebers, entrainment, and masking: some unsettled questions. In: Aschoff J, Daan S, Groos GA, editors. Vertebrate Circadian Rhythms. Springer-Verlag; New York: 1982. pp. 13–21. [Google Scholar]
- Brown CM, Refinetti R. Daily rhythms of metabolic heat production, body temperature, and locomotor activity in golden hamsters. J Therm Biol. 1996;21:227–230. [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol A. 1976;106:253–266. [Google Scholar]
- Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- Decoursey PJ, Pius S, Sandlin C, Wethey D, Schull J. Relationship of circadian temperature and activity rhythms in two rodent species. Physiol Behav. 1998;65:457–463. doi: 10.1016/s0031-9384(98)00187-5. [DOI] [PubMed] [Google Scholar]
- Ding JM, Buchanan GF, Tischkau SA, Chen D, Kuriashkina L, Faiman LE, Alster JM, McPherson PS, Campbell KP, Gillette MU. A neuronal ryanodine receptor mediates light-induced phase delays of the circadian clock. Nature. 1998;394:381–384. doi: 10.1038/28639. [DOI] [PubMed] [Google Scholar]
- Doi M, Yujnovsky I, Hirayama J, Malerba M, Tirotta E, Sassone-Corsi P, Borrelli E. Impaired light masking in dopamine D2 receptor-null mice. NatNeurosci. 2006;9:732–734. doi: 10.1038/nn1711. [DOI] [PubMed] [Google Scholar]
- Edelstein K, De la Iglesia HO, Schwartz WJ, Mrosovsky N. Behavioral arousal blocks light-induced phase advances in locomotor rhythmicity but not light-induced Per1 and fos expression in the hamster suprachiasmatic nucleus. Neuroscience. 2003;118:253–261. doi: 10.1016/s0306-4522(02)00908-9. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Seidel WF. Modafinil induces wakefulness without intensifying motor activity or subsequent rebound hypersomnolence in the rat. J PharmacolExpTher. 1997;283:757–769. [PubMed] [Google Scholar]
- Elliott KJ, Weber ET, Rea MA. Adenosine A(1) receptors regulate the response of the hamster circadian clock to light. Eur J Pharmacol. 2001;414:45–53. doi: 10.1016/s0014-2999(01)00786-5. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Ann Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Luppi PH, Cauli B, Urade Y, Rossier J, Hayaishi O, Lambolez B, Fort P. The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A(2A) receptors in the ventrolateral preoptic nucleus. Neuroscience. 2005;134:1377–1390. doi: 10.1016/j.neuroscience.2005.05.045. [DOI] [PubMed] [Google Scholar]
- Gebczynski AK, Taylor JRE. Daily variation of body temperature, locomotor activity and maximum nonshivering thermogenesis in two species of small rodents. J Therm Biol. 2004;29:123–131. [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrand EC, Sesack SR. Projections from the rat pedunculopontine and laterodorsal tegmental nuclei to the anterior thalamus and ventral tegmental area arise from largely separate populations of neurons. Brain Struct Funct. 2011;216:331–345. doi: 10.1007/s00429-011-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2(A), but not A(1), receptors mediate the arousal effect of caffeine. Nature Neuroscience. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Beninger RJ. Behavioral-effects of intrastriatal caffeine mediated by adenosinergic modulation of dopamine. Pharmacol Biochem Behav. 1991;39:97–103. doi: 10.1016/0091-3057(91)90403-o. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons - abolition of psychostimulant-induced locomotor-activity in rats. Eur J Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Cortical Projections of Mediodorsal Nucleus and Adjacent Thalamic Nuclei in Rat. J Comp Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Shen HY, Cherasse Y, Qu WM, Huang ZL, Bass CE, Winsky-Sommerer R, Semba K, Fredholm BB, Boison D, Hayaishi O, Urade Y, Chen JF. Arousal Effect of Caffeine Depends on Adenosine A(2A) Receptors in the Shell of the Nucleus Accumbens. J Neurosci. 2011;31:10067–10075. doi: 10.1523/JNEUROSCI.6730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Aston-Jones G. Circuit projection from suprachiasmatic nucleus to ventral tegmental area: a novel circadian output pathway. EurJ Neurosci. 2009;29:748–760. doi: 10.1111/j.1460-9568.2008.06606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Georges FE, Aston-Jones GS. Novel neurons in ventral tegmental area fire selectively during the active phase of the diurnal cycle. Eur J Neurosci. 2008;27:408–422. doi: 10.1111/j.1460-9568.2007.05985.x. [DOI] [PubMed] [Google Scholar]
- McGinty D, Alam MN, Szymusiak R, Nakao M, Yamamoto M. Hypothalamic sleep-promoting mechanisms: coupling to thermoregulation. Arch Ital Biol. 2001;139:63–75. [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Morin LP. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J Neurosci. 1996;16:2097–2111. doi: 10.1523/JNEUROSCI.16-06-02097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: A review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Antle MC. Behavioral inhibition of light-induced circadian phase resetting is phase and serotonin dependent. Brain Res. 1998;786:31–38. doi: 10.1016/s0006-8993(97)01269-9. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Antle MC, Webb IC, Jones M, Weinberg J, Pollock MS. Circadian clock resetting by arousal in Syrian hamsters: the role of stress and activity. AmJPhysiol. 2003;285:R917–R925. doi: 10.1152/ajpregu.00222.2003. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Holmes MM. Morphine-induced activity attenuates phase shifts to light in C57BL/6J mice. Brain Res. 1999;829:113–119. doi: 10.1016/s0006-8993(99)01370-0. [DOI] [PubMed] [Google Scholar]
- Mitchell HA, Bogenpohl JW, Liles LC, Epstein MR, Bozyczko-Coyne D, Williams M, Weinshenker D. Behavioral responses of dopamine beta-hydroxylase knockout mice to modafinil suggest a dual noradrenergic-dopaminergic mechanism of action. Pharmacology Biochemistry and Behavior. 2008;91:217–222. doi: 10.1016/j.pbb.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol. 1995;359:221–238. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- Morin LP. Neuroanatomy of the extended circadian rhythm system. Exp Neurol. 2013a;243:4–20. doi: 10.1016/j.expneurol.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP. Nocturnal Light and Nocturnal Rodents: Similar regulation of disparate functions? J Biol Rhythms. 2013b;28:95–106. doi: 10.1177/0748730413481921. [DOI] [PubMed] [Google Scholar]
- Morin LP, Lituma PJ, Studholme KM. Two components of nocturnal locomotor suppression by light. J Biol Rhythms. 2010;25:197–207. doi: 10.1177/0748730410369890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Studholme KM. Millisecond light pulses make mice stop running, then display prolonged sleep-like behavior in the absence of light. J Biol Rhythms. 2009;24:497–508. doi: 10.1177/0748730409349059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Studholme KM. Separation of function for classical and ganglion cell photoreceptors with respect to circadian rhythm entrainment and induction of photosomnolence. Neuroscience. 2011;199:213–224. doi: 10.1016/j.neuroscience.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya T, Yamanouchi S, Fukushima T, Shimazoe T, Shibata S, Watanabe S. Involvement of 5-HT1A receptor mechanisms in the inhibitory effects of methamphetamine on photic responses in the rodent suprachiasmatic nucleus. Brain Res. 1996;740:261–267. doi: 10.1016/s0006-8993(96)00860-8. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Masking: history, definitions, and measurement. ChronobiolInt. 1999;16:415–429. doi: 10.3109/07420529908998717. [DOI] [PubMed] [Google Scholar]
- Obal F, Jr., Rubicsek G, Alfoldi P, Sary G, Obal F. Changes in the brain and core temperatures in relation to the various arousal states in rats in the light and dark periods of the day. Pflugers Arch. 1985;404:73–79. doi: 10.1007/BF00581494. [DOI] [PubMed] [Google Scholar]
- Oike H, Kobori M, Suzuki T, Ishida N. Caffeine lengthens circadian rhythms in mice. Biochem Biophys Res Commun. 2011;410:654–658. doi: 10.1016/j.bbrc.2011.06.049. [DOI] [PubMed] [Google Scholar]
- Okuro M, Fujiki N, Kotorii N, Ishimaru Y, Sokoloff P, Nishino S. Effects of paraxanthine and caffeine on sleep, locomotor activity, and body temperature in orexin/ataxin-3 transgenic narcoleptic mice. Sleep. 2010;33:930–942. doi: 10.1093/sleep/33.7.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Watanabe A, Matsumoto Y, Fukushima T, Nishikawa Y, Moriya T, Shibata S, Watanabe S. Methamphetamine modifies the photic entraining responses in the rodent suprachiasmatic nucleus via serotonin release. Neuroscience. 1996;72:213–224. doi: 10.1016/0306-4522(95)00500-5. [DOI] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Phelps G, Speaker HA, Sabol KE. Relationship between methamphetamine-induced behavioral activation and hyperthermia. Brain Res. 2010;1357:41–52. doi: 10.1016/j.brainres.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Pijnenburg AJJ, Honig WMM, Vanderheyden JAM, Vanrossum JM. Effects of chemical stimulation of mesolimbic dopamine system upon locomotor activity. Eur J Pharmacol. 1976;35:45–58. doi: 10.1016/0014-2999(76)90299-5. [DOI] [PubMed] [Google Scholar]
- Pinto A, Jankowski M, Sesack SR. Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens shell: Ultrastructural characteristics and spatial relationships with dopamine afferents. J Comp Neurol. 2003;459:142–155. doi: 10.1002/cne.10596. [DOI] [PubMed] [Google Scholar]
- Qiu MH, Liu W, Qu WM, Urade Y, Lu J, Huang ZL. The role of nucleus accumbens core/shell in sleep-wake regulation and their involvement in modafinil-induced arousal. PLoS ONE. 2012;7:e45471. doi: 10.1371/journal.pone.0045471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu MH, Vetrivelan R, Fuller PM, Lu J. Basal ganglia control of sleep-wake behavior and cortical activation. Eur J Neurosci. 2010;31:499–507. doi: 10.1111/j.1460-9568.2009.07062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y. Dopaminergic D 1 and D 2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28:8462–8469. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Mrosovsky N. Behavioral inhibition of circadian responses to light. J Biol Rhythms. 1992;7:353–359. doi: 10.1177/074873049200700408. [DOI] [PubMed] [Google Scholar]
- Refinetti R, Menaker M. The circadian rhythm of body temperature. Physiol Behav. 1992;51:613–637. doi: 10.1016/0031-9384(92)90188-8. [DOI] [PubMed] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep State Switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth LA, Rea MA. Adenosine A1 receptors regulate the response of the mouse circadian clock to light. Brain Res. 2003;960:246–251. doi: 10.1016/s0006-8993(02)03896-9. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: Dependence on the suprachiasmatic nucleus. Brain Res. 2007;1129:34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- Steed E, Jones CA, McCreary AC. Serotonergic involvement in methamphetamine-induced locomotor activity: A detailed pharmacological study. Behav Brain Res. 2011;220:9–19. doi: 10.1016/j.bbr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Studholme KM, Gompf HS, Morin LP. Brief light stimulation during the mouse nocturnal activity phase simultaneously induces a decline in core temperature and locomotor activity followed by EEG-determined sleep. AmJPhysiol RegulIntegrComp Physiol. 2013;304:R459–R471. doi: 10.1152/ajpregu.00460.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel CJ, Noone JT, Lushington K, Dawson D. Changes in sleepiness and body temperature precede nocturnal sleep onset: Evidence from a polysomnographic study in young men. J Sleep Res. 1998;7:159–166. doi: 10.1046/j.1365-2869.1998.00112.x. [DOI] [PubMed] [Google Scholar]
- Van Hook MJ, Wong KY, Berson DM. Dopaminergic modulation of ganglion-cell photoreceptors in rat. Eur J Neurosci. 2012;35:507–518. doi: 10.1111/j.1460-9568.2011.07975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal L, Blanchard J, Morin LP. Hypothalamic and zona incerta neurons expressing hypocretin, but not melanin concentrating hormone, project to the hamster intergeniculate leaflet. Neuroscience. 2005;134:1081–1090. doi: 10.1016/j.neuroscience.2005.03.062. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Moriya T, Nisikawa Y, Araki T, Hamada T, Shibata S, Watanabe S. Adenosine A1-receptor agonist attenuates the light-induced phase shifts and fos expression in vivo and optic nerve stimulation-evoked field potentials in the suprachiasmatic nucleus in vitro. Brain Res. 1996;740:329–336. doi: 10.1016/s0006-8993(96)00881-5. [DOI] [PubMed] [Google Scholar]
- Webb IC, Pollock MS, Mistlberger RE. Modafinil [2-[(diphenylmethyl)sulfinyl]acetamide] and circadian rhythms in syrian hamsters: assessment of the chronobiotic potential of a novel alerting compound. J PharmacolExpTher. 2006;317:882–889. doi: 10.1124/jpet.105.099010. [DOI] [PubMed] [Google Scholar]
- Weinert D, Waterhouse J. Diurnally changing effects of locomotor activity on body temperature in laboratory mice. Physiol Behav. 1998;63:837–843. doi: 10.1016/s0031-9384(97)00546-5. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Gabriel R, Haycock JW, Meller E. Influence of light and neural circuitry on tyrosine hydroxylase phosphorylation in the rat retina. J Chem Neuroanat. 2000;19:105–116. doi: 10.1016/s0891-0618(00)00055-7. [DOI] [PubMed] [Google Scholar]
- Wright KP, Myers BL, Plenzler SC, Drake CL, Badia P. Acute effects of bright light and caffeine on nighttime melatonin and temperature levels in women taking and not taking oral contraceptives. Brain Res. 2000;873:310–317. doi: 10.1016/s0006-8993(00)02557-9. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Kerbeshian MC, Hocker CG, Block GD, Menaker M. Rhythmic properties of the hamster suprachiasmatic nucleus in vivo. J Neurosci. 1998;18:10709–10723. doi: 10.1523/JNEUROSCI.18-24-10709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kooistra K, Geyer MA. Dopamine Receptor Mediation of the Exploratory/Hyperactivity Effects of Modafinil. Neuropsychopharmacology. 2011;36:1385–1396. doi: 10.1038/npp.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]