Abstract
Background & Aim
Intrinsic synchronous fluctuations of the fMRI signal are indicative of the underlying “functional connectivity” (FC) and serve as a technique to study dynamics of the neuronal networks of the human brain. Earlier studies have characterized the functional connectivity of a distributed network of brain regions involved in swallowing, called brain swallowing network (BSN). The potential modulatory effect of esophageal afferent signals on the BSN, however, has not been systematically studied.
Methods
Fourteen healthy volunteers underwent steady state fMRI across three conditions: 1) transnasal catheter placed in the esophagus without infusion; 2) buffer solution infused at 1ml/min; and 3) acidic solution infused at 1 ml/min. Data were preprocessed according to the standard FC analysis pipeline. We determined the correlation coefficient values of pairs of brain regions involved in swallowing across and calculated average group FC matrices across conditions. Effects of subliminal esophageal acidification and nasopharyngeal intubation were determined.
Results
Subliminal esophageal acid stimulation augmented the overall FC of the right anterior insula and specifically the FC to the left inferior parietal lobule. Conscious stimulation by nasopharyngeal intubation reduced the overall FC of the right posterior insula, particularly the FC to the right prefrontal operculum.
Conclusions
The FC of BSN is amenable to modulation by sensory input. The modulatory effect of sensory pharyngoesophageal stimulation on BSN is mainly mediated through changes in the FC of the insula. The alteration induced by subliminal visceral esophageal acid stimulation is in different insular connections compared to that of conscious somatic pharyngeal stimulation.
Keywords: cortical swallowing network, default mode network, resting connectivity, negative BOLD, buffer
INTRODUCTION
Functional magnetic resonance imaging (fMRI) studies on swallowing have identified consistent positive BOLD activity in the middle cingulate and prefrontal cortex, insula/operculum, lateral peri-central and peri-sylvian cortices that collectively have been identified as the brain swallowing network (BSN) 1-5. Earlier studies have shown that these swallow-associated cortical activity maps are reproducible utilizing paradigm driven event-related fMRI analysis 5. The intrinsic functional connectivity (FC) of the BSN has recently been characterized with use of the resting state functional MRI techniques6.
Functional connectivity (FC) is based on the observation that functionally related brain regions display intrinsic synchronized fluctuations of blood-oxygenation-level-dependent (BOLD) contrast7. Biswal et al. were the first to demonstrate that the BOLD signal time series from the motor cortex were correlated with all the regions within the motor system without performance of any explicit task 8. The FC of various brain networks has been successfully mapped by utilizing these task-independent approaches 9, 10. Seed based FC analysis constitutes the primary strategy that has been applied to the resting state fMRI data 8. In this technique, FC is the correlation between the BOLD signal fluctuation of a region of interest (ROI) and all other voxels in brain, or BOLD signal correlation in between several distributed ROIs 11. ROI(s) are a-priori demarcated as seed regions (anatomically or functionally), and results are depicted as connectivity brain maps and connectivity matrices respectively. The latter approach offers the benefit of reducing the dimensionality of the data from many thousands (the number of brain voxels) by several orders of magnitude to a few brain regions that have been identified as seed regions, thereby reducing the problem of multiple inferences in the analysis 11.
Recent evidence has emerged showing that even cognitive load and subliminal perceptual processing 12-14 may modulate the FC of the brain networks. Gastroesophageal reflux events are common occurrences in healthy individuals and majority of these events are subliminal and therefore not perceived. Swallowing is the main clearing mechanism of acid from the esophagus 15, and previous studies have shown increased swallow frequency during esophageal acid exposure 16. Furthermore, subliminal esophageal acid infusion has been shown to increase cerebral cortical activity 17 and potentiate swallow related cortical BOLD activation in healthy humans 18. Therefore, the interaction of subliminal esophageal acid stimulation (i.e. simulation of unperceived acid reflux events) and cortical governance of swallowing has physiologic importance and may provide the basis for future studies of the pathophysiologic esophageal conditions.
While we have studied the FC of the BSN at rest and during episodic swallows 6, the FC of the BSN during sensory manipulation has not been investigated. This would be an important step in understanding the neurophysiological basis of swallowing in interaction with gastroesophageal reflux. The primary aim of the present study was to determine the effects of subliminal esophageal acid exposure on the FC of the BSN.
METHODS
Study Subjects
Fourteen healthy right-handed volunteers (24 ± 4 years, 7 female) participated in the study. The institutional review board at the Medical College of Wisconsin approved the study. Subjects were recruited by advertisement and all volunteers gave written informed consent before the study. All volunteers were evaluated by a personal interview, detailed health questionnaire (MCW), physical examination and a transnasal upper endoscopy. Exclusion criteria included history of any gastrointestinal disease or functional gastrointestinal disorder. The subjects were asked to fast for 6 hours before the study. We placed a double lumen 2.4 mm external diameter infusion tube transnasally following topical lubricant and anesthetic application. Perfusion ports were placed 5-7cm above the upper border of the lower esophageal sphincter. The location of the lower esophageal sphincter was identified by either transnasal upper GI endoscopy or high resolution manometry. We waited 15 minutes until subjects became accustomed to the transnasal catheter prior to acquiring fMRI data. All subjects tolerated the procedure well and completed the protocol.
Data Acquisition
Subjects were supine in a 3.0T General Electric Signa LX scanner (General Electric Medical Systems, Waukesha, WI) equipped with an eight-channel receiver head coil and body quadrature transmit radiofrequency coil. Cardiorespiratory monitoring was performed at a sampling rate of 40 Hz with a pulse oximeter and respiratory bellows equipment provided in the MRI system. Subjects were instructed to relax and keep their eyes closed. Functional MRI data were acquired during 4 distinct 540s scans in three different conditions: 1) awake steady state with eyes closed and infusion tube in place at the beginning and end of the scanning session (tube-i and tube-ii); 2) awake steady state with eyes closed and phosphate buffer solution with pH=7.0-7.5 continuously infusing at 1ml/min (buffer); and 3) awake steady state with eyes closed and subliminal 0.1 normal hydrochloric acid solution with pH =1.0-2.0 continuously infusing at 1ml/min (acid). We started the buffer and acid infusions at least three minutes prior to the onset of their respective scans (during inter-scan resting period) to establish an esophageal equilibrium state with the infusate. To maintain consistency across study conditions we waited three minutes before imaging data acquisition in the tube i and tube ii conditions as well. The acid scan was systematically performed after the buffer infusion in all subjects due to the potential lasting effects of esophageal acid exposure. We obtained a second scan with the infusion tube in place at the end of the study to account for time effects on the functional connectivity of the BSN and to remedy the hypothetical presence of an order effect. An anatomical scan was acquired in the middle of the scanning session after the second functional run using a high-resolution spoiled gradient recalled acquisition (SPGR) technique consisting of 140 sagittal whole brain 1 mm-thick slices over a 240 mm field of view (FOV) and 256 × 224 within slice pixel resolution. The high-resolution anatomical images were used for subsequent superposition of the lower-resolution echo planar blood oxygenation level-dependent (BOLD) contrast image data in each subject. Echo planar images (EPI) were acquired as 34 contiguous 4-mm thick sagittal slices over the whole brain volume in an interleaved fashion without any gap or overlap. EPI images were acquired with a slice-wise pixel resolution of 64 × 64 pixels over a 240 mm field of view yielding a within-slice resolution of 3.75 × 3.75 mm, captured with an echo time (TE) of 23.4 ms and a repetition time (TR) of 2000 ms.
Data Analysis
fMRI signal conditioning and analysis were carried out using Analysis of Functional NeuroImages (AFNI) software 19. Functional EPI images were reconstructed into four-dimensional time dependent datasets wherein each voxel was associated with 540s time series of fluctuating BOLD contrast data. We conditioned the data according to a rigorous series of signal pre-processing steps based on a previously published fMRI connectivity analysis pipeline 20. All participants maintained a peak-to-peak global (EPI volume) head motion of <1 mm. Physiologic (cardiac and respiratory) related signal changes expressed as second order Fourier series expansion were retrospectively corrected 21. In the anatomical data set, the brain was extracted from surrounding tissue 22 and used as the reference for alignment for all EPI datasets 23. The reference anatomical scan was spatially normalized to match the standard Talaraich-Tournoux stereotaxic template 24. We performed slice timing correction and modeled head motion using 12 degrees of freedom indexed by time (motion parameters from a general affine transformation matrix representing shift, rotational and shear motion) 25, which was used to interpolate the time series back to the original acquisition grid. We computed the alignment matrix between the EPI datasets and the anatomical scan. The alignment matrix was then used to register, resample (2 × 2 × 2 mm voxel size), and align all datasets to the anatomical scan grid 23. Voxel-wise extreme signal fluctuations of the signal (spike values) were replaced by a fitted smooth curve to the time series. fMRI BOLD signal trend components were removed over the course of time series voxel by voxel independently using linear least squares. White matter and cerebrospinal fluid containing voxels were identified automatically based on their signal intensity and manually verified within each subject. The average time courses within the white matter and cerebrospinal fluid voxels 26 as well as global noise 27 were extracted for use as nuisance regressors. General linear modeling techniques with orthogonal least squares estimation were used to remove undesirable signal contamination correlated with motion parameters, white matter, cerebrospinal fluid and global noise temporal components as covariates of no interest. Residual time series were then spatially smoothed to full-width -half-maximum (FWHM) of 6 mm and bandpass filtered to a frequency target range of 0.015 – 0.1 Hz using Fourier transformation. Subsequently the first 60 seconds of each run was discarded (scanner equilibration period).
We adopted previously identified positive (increased BOLD signal during swallow task) and negative (decreased BOLD signal during swallow task) activated regions from an earlier study (the same research participants less than 2 weeks apart from current study) and utilized them as seed regions for functionally guided connectivity analysis of the brain swallow network5. The average time series for each seed region was individually extracted (averaged time course across all voxels within a seed region) individually during each condition. We assessed functional connectivity between any two seed regions by calculating the Pearson product moment-correlation coefficient (r) between the regions’ preprocessed 480s time series. We also used the resting state data of these same subjects who participated in our previously published resting state protocol to determine the effects of constant noisome presence of the nasopharyngeal tube on FC of the BSN 6.
Statistical Analysis
Statistical analysis was performed using customized MATLAB® software (MathWorks, Natick, MA). We calculated cross correlation coefficients (CC) between preprocessed 480s time series (240 data points) of the paired ROIs, with values ranging from -1 to +1. Large positive values (i.e. closer to 1) are indicative of high functional connectivity, while large negative values (i.e. closer to -1) are indicative of highly anti-correlated functional connectivity. Small values (i.e. close to zero) are suggestive of low functional connectivity between corresponding pairs. A Pearson product-moment correlation coefficient (r) obtained from 240 data-points (df=238) is considered significant if the absolute r-value is higher than 0.165 (|r| > 0.165, two-tailed p < 0.01). For each subject there were 780 [(40×39)/2] pairwise CC values among 40 ROIs of the deglutition connectome. These CC values were arranged into an individual connectivity matrix for each study condition and a frequency distribution of CC values was determined. The matrices of correlation coefficients, hereafter called FC matrix, were Fisher z-transformed to produce approximately normally-distributed variables. These values were then averaged across subjects to generate a group FC matrix for each condition (tube i, tube ii, buffer and acid). We plotted the CC values as a color matrix to confirm consistency of the swallow network in the presence of the nasopharyngeal tube. We performed a paired t-test between tube-i and tube-ii conditions and plotted cross correlation coefficients of group FC matrices and ran linear regression analyses to confirm reproducibility of the data and identify potential order effect 6. Then we compared CC matrices of buffer and acid conditions to investigate subliminal esophageal infusate effect. Finally, the two CC matrices of tube i and ii conditions were compared to two resting state CC matrices of the same fourteen subjects (resting i and ii) adopted from our previous study 6 to evaluate the effect of nasopharyngeal intubation (tube effect). In all comparisons, false discovery rate (FDR) was used as the statistical method to correct for multiple comparisons 28. FDR controlling procedures exert a less stringent control compared to family-wise error rate procedures (such as the Bonferroni correction). This provides a useful compromise between the loss of power attributable to the Bonferroni correction and the lack of control of Type I errors associated with comparisons unadjusted for multiple comparisons 29. The FDR method has been widely applied in genetic and neuroimaging research, where quantitative analyses took a new dimension and the number of hypotheses tested in an experiment reached thousands. In this statistical method a new quantity called the “q-value”, which is the FDR analogue of the p-value has been utilized to test the null hypothesis 30.
After studying the individual connection dynamics of the large scale network, we defined the following FC scores for each seed region across different study conditions in order to further investigate the neural components as previously used by investigators for motor networks 6, 31: a) Average CC score of every cortical positive BOLD activated seed to remainder of cortical positive BOLD activated regions; b) Average CC score of every cortical negative BOLD activated seed to the remainder of cortical negative BOLD activated seeds; c) Average CC score of subcortical activated seeds to the remainder of subcortical regions; and d) Average CC score of subcortical activated seeds to the cortical BOLD positive and negative BOLD seeds. We used analysis of variance and post-hoc analysis of FC scores to evaluate the effects of various study conditions.
RESULTS
None of the volunteers reported heartburn, nausea, regurgitation or chest discomfort during or after esophageal acid infusion.
Cross Correlation (CC) Matrix
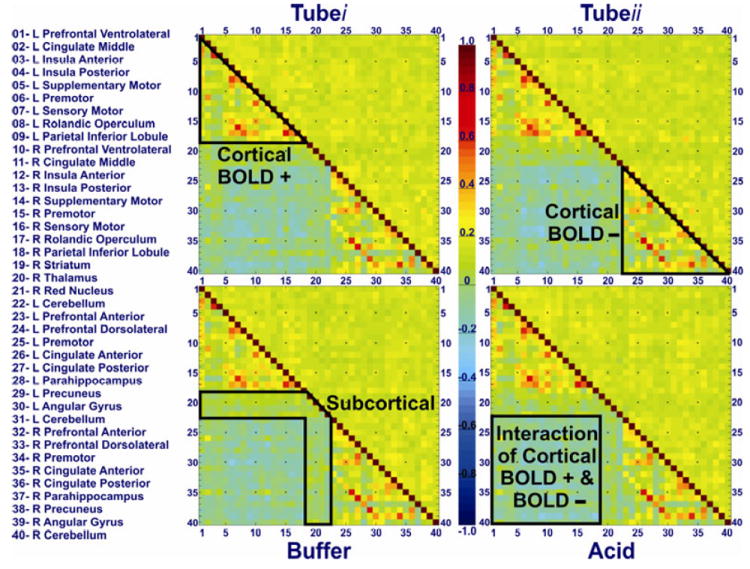
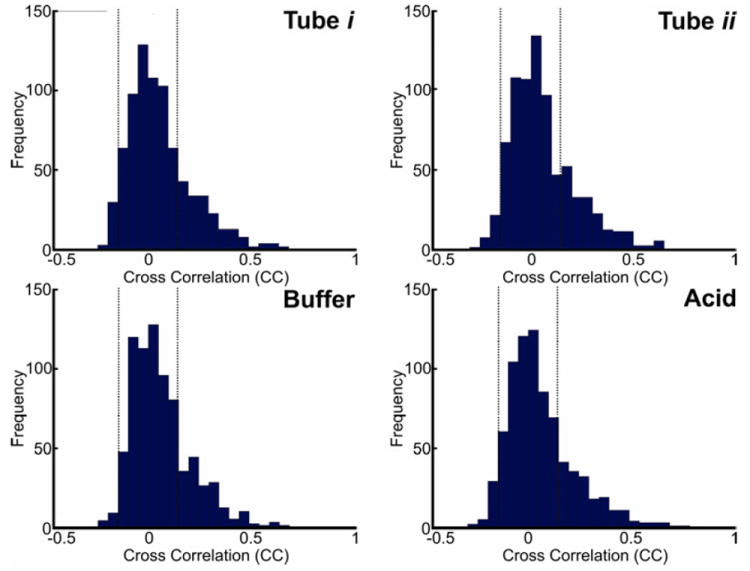
Group CC matrices of tube-i, tube-ii, buffer and acid infusion states are shown in Figure 1. The diagonal of the CC matrix from the upper left to the lower right corners represents connectivity of each seed region to itself, which (by definition) is always 1 (fully correlated). The lower left section of the CC matrix below this diagonal represents CC values and the right upper part of the matrix above the diagonal represents the standard deviation of each corresponding connection. High positive CC values (closer to +1) represent high FC, depicted as warm colors (orange – red). Negative CC values (below 0) represent anti-correlations, demonstrated as cold colors (blue – navy). Smaller CC values (close to 0) represent weak FC as shown in green color (Figure 1). Consistent with our previous report 6, the CC matrices showed a consistent pattern of strong FC amongst cortical BOLD positive activated and negative BOLD activated seeds throughout all study conditions as depicted by the upper left and lower right triangles, respectively. Interaction of BOLD negative activated seeds and BOLD positive activated seeds demonstrated mainly an anti-correlation pattern (or no significant correlation), outlined by a light green-blue square in the lower left section of the CC matrices. Sub-cortical seeds showed persistent low level of FC connectivity across both cortically positive and negative BOLD activated seeds as depicted in the middle yellow band of the FC matrices. Variance of the data was homogeneously distributed as shown by a uniform yellowish green representation of standard deviation above the diagonal of the CC matrices (Figure 1). CC values of the BSN were not normally distributed and were skewed to the right as shown in their frequency histograms of each condition (Figure 2). This pattern was similar across study conditions, and hereafter CC values were z-transformed to assure normalized distribution of the data for further analyses.
Figure 1. Functional connectivity matrix of the brain swallowing network after nasopharyngeal intubation (tube i and tube ii) and during subliminal acid and buffer infusion.
Participants were awake but closed their eyes. Forty regions of interest that showed significant group BOLD activation (either positive or negative) during an independently obtained fMRI scan in the same population were used as seeds for subsequent connectivity analysis as described previously 5, 6. Color scale in the middle shows the spectrum of Pearson product moment-correlation coefficient (CC) values ranging from -1 to 1. The diagonal of the matrix from the upper left to the lower right corners represents connectivity of each seed region with a CC value of 1 (i.e. completely correlated). The lower left section of FC matrix below this diagonal represents CC values and the right upper section of the matrix above the diagonal represents the standard deviation of each corresponding CC. Forty seeds are enumerated on the x- and y- axes, and their corresponding FC range from -0.40 to +1.00. Connections between the first eighteen rows and columns (1-18) of the matrix represent FC of cortical positive BOLD activated seeds (top left demarcated triangle of FC matrix) and the last eighteen rows and columns (23-40) denote FC of negative BOLD activated seeds (bottom right demarcated triangle). Middle four (19-22) ROIs represent FC of subcortical positive BOLD activated seeds (demarcated middle band). Cells corresponding to the interface of the first eighteen and last eighteen rows represent the interaction of FC between positively and negatively BOLD activated seed regions (bottom left demarcated square of FC matrix).
Figure 2. Frequency histogram of cross correlation (CC) values of the brain swallowing network (BSN) across study conditions (tube i, tube ii, buffer and acid).
CC values of the BSN were not normally distributed and the histogram was similarly skewed to the right across all study conditions. CC values were Fisher z-transformed to assure normalized distribution of the data for further analyses. The absolute CC values higher than 0.165 (|r| > 0.165, two-tailed p < 0.01) are considered statistically significant. The dotted vertical line demonstrates that the majority of CC values were located in interaction section of BOLD positive and negative regions (76%) and were not statistically significant.
In the cortical BOLD positive component of the FC matrix (top left triangle of FC matrix) consisting of 153 possible connections, 79, 89, 76 and 82 of them displayed significant FC (r>0.165) during tube-i, tube-ii, buffer and acid conditions, respectively (50-58% significantly correlated and 0% significantly anti-correlated). In the BOLD negative component of the FC matrix (bottom right triangle of all matrices) consisting of 153 possible connections, 64, 71, 66 and 59 of the connections displayed significant FC (r>0.165) during tube-i, tube-ii, buffer and acid conditions, respectively (39-46% significantly correlated and 0% significantly anti-correlated).
Within the interaction of BOLD positive and negative component of the FC matrix (bottom left square of all matrices), however, only 1 out of possible 324 connections (left middle cingulate and left anterior cingulate) displayed significant correlation (r>0.165) across all study conditions. 42, 42, 40 and 40 of the connections displayed significant anti-correlation (r<-0.165) during tube-i, tube-ii, buffer and acid conditions respectively (0% significantly correlated and 12-13% significantly anti-correlated). In the subcortical BOLD positive component of the FC matrix (middle band the BSN matrix), no link amongst all 150 connections displayed significant FC (0% significantly correlated or anti-correlated).
Effects of subliminal acid infusion
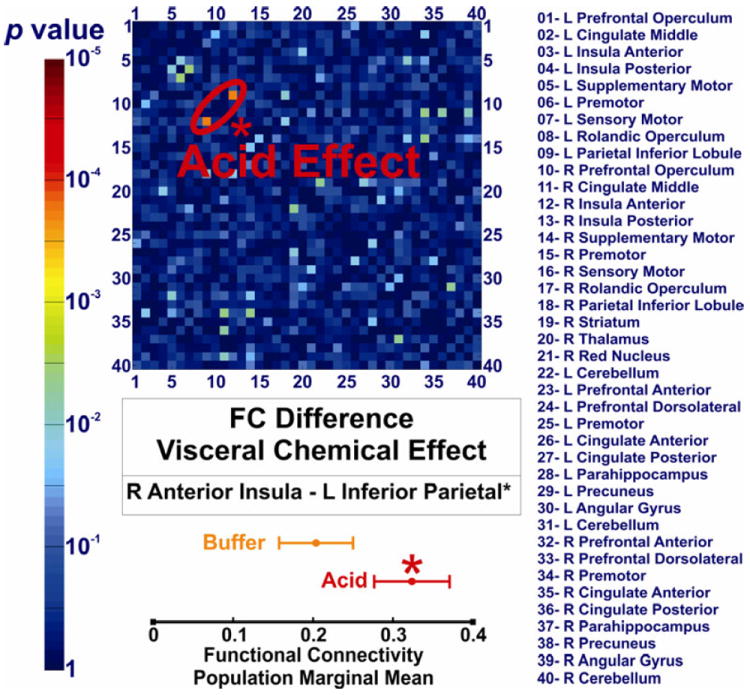
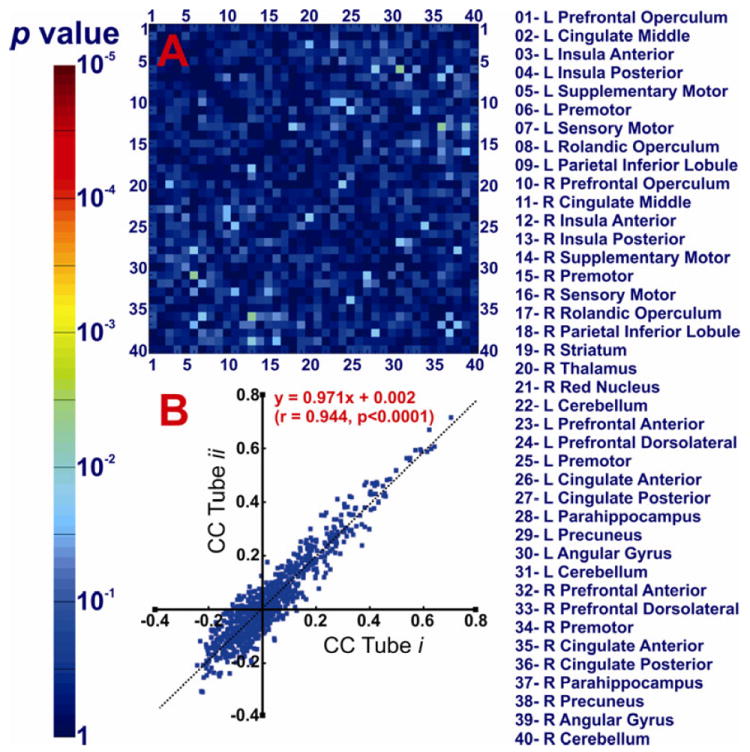
We compared the FC of all 780 connections of the swallow network across the acid and buffer conditions using paired t-tests. Significant difference was observed in cortical positive BOLD activated regions. Comparison of the acid and buffer infusion states showed that the FC of right anterior insula and contralateral inferior parietal lobule (Figure 3) is significantly increased during subliminal esophageal acid stimulation (q<0.05). We compared tube-i and tube-ii (pre-infusion and post-infusion) to study the possible order effect on the observed differences between buffer and acid conditions. If the time/sequence of scan during fMRI session was an effective factor resulting in some differences, the post-infusion scan should also show similar difference compared to the pre-infusion scan. This comparison did not show any significant FC difference (q>0.05, Figure 4A). Furthermore, as shown in Figure 4B, the linear regression analysis of group FC matrix during tube-i and tube-ii conditions demonstrated highly reproducible values (r=0.944, p <10-5). Thus the observed difference between the acid and buffer conditions could not have been simply related to the timing of scan or order effect. We then performed a FC score analysis of all the regions comprising the BSN. Acid infusion significantly increased the FC score of the right anterior insula region (p<0.05), further confirming the role of right anterior insula in cortical processing of esophageal acid exposure (Figure 5). No other cortical BOLD positive, cortical BOLD negative or subcortical region showed a significant difference across study conditions.
Figure 3. Effect of subliminal visceral chemical stimulation (esophageal acid exposure) on the functional connectivity of the brain swallowing network.
The corresponding p values of comparing 780 functional connectivity values among forty seed regions in acid and buffer conditions are demonstrated as a color scale. Forty seed regions are listed and shown on x- and y- axes numerically. The only connection that is significantly different is between right anterior insula and left inferior parietal lobule. This difference survived the false discovery rate (FDR) correction for multiple comparisons (q < 0.05).
Figure 4. Comparison of the tube i and tube ii conditions to investigate the potential order effect on the functional connectivity of the brain swallowing network.
A) The corresponding p values of comparing 780 functional connectivity values among forty seed regions in tube i and ii conditions are demonstrated as a color scale. Forty seed regions are listed and shown on x- and y- axes numerically. No connection demonstrated a significant difference between tube i at the beginning of scanning session, and tube ii at the end of scanning session. B) Linear regression analysis of the cross correlation (CC) values shows highly reproducible values between tube i and ii conditions. This result is suggesting that timing of the scan in the session had no systematic effect on CC values. These findings are indicative of no measurable order effect on the functional connectivity of the brain swallowing network.
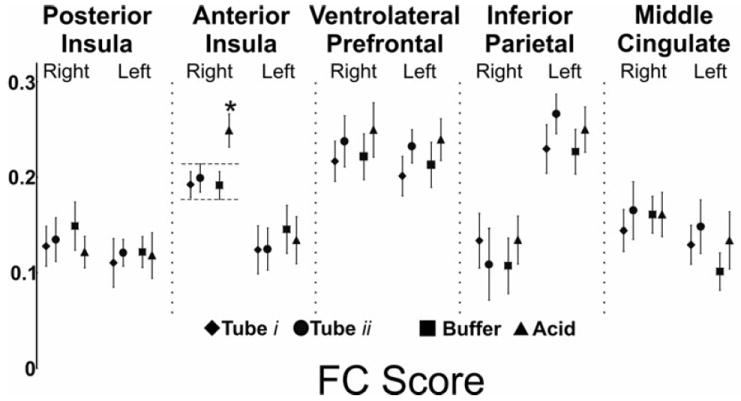
Figure 5. Effect of subliminal visceral chemical stimulation (esophageal acid) on the functional connectivity score of brain swallowing network.
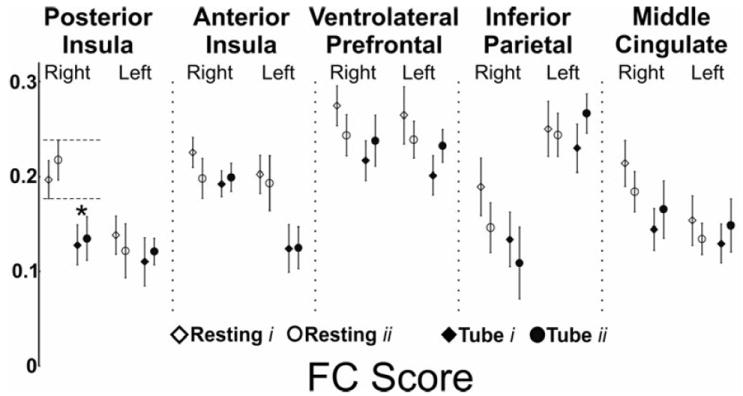
We measured the average functional connectivity score of every seed region across all study conditions. Analysis of variance and post-hoc analysis showed that FC score of the right anterior insula during acid infusion is significantly higher during acid infusion compared to all other study conditions including buffer infusion (p < 0.05).
Effects of nasopharyngeal intubation
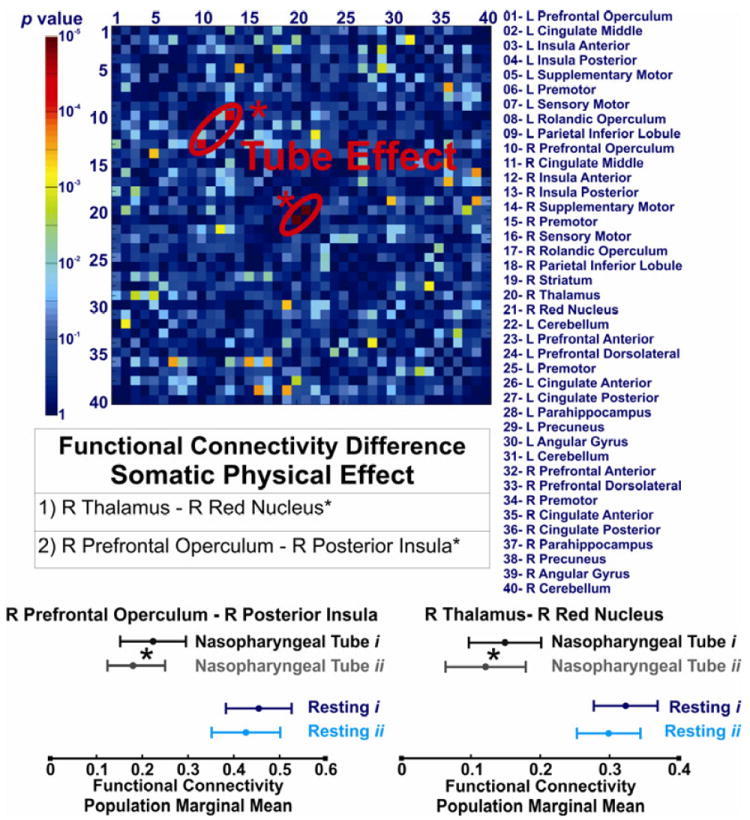
Significant differences were observed in cortical and subcortical positive BOLD activated regions. Intrinsic FC between the right posterior insula and the ipsilateral ventrolateral prefrontal cortex was significantly diminished with nasopharyngeal intubation (q<0.05, Figure 6). Amongst the subcortical regions, the FC between the right thalamus and the red nucleus was significantly diminished after nasopharyngeal intubation (q<0.05, Figure 6). We then performed an analysis of FC scores of all the BSN regions. This analysis showed that the FC score of the right posterior insula significantly changed during nasopharyngeal intubation (Figure 7, p<0.05). No other cortical BOLD positive, cortical BOLD negative or subcortical region showed any significant difference between resting and intubated state or across various infusion states.
Figure 6. Effect of perceived somatic physical stimulation (nasopharyngeal tube presence) on the functional connectivity of the brain swallowing network.
The corresponding p values of comparing 780 functional connectivity values among forty seed regions in resting and tube conditions are demonstrated as a color scale. Forty seed regions are listed and shown on x- and y- axes numerically. The following connections showed significant difference during presence of nasopharyngeal tube: between the right thalamus and the right red nucleus in subcortical regions and between the right posterior insula and the right prefrontal operculum in cortical BOLD positive seeds. These differences survived the false discovery rate (FDR) correction for multiple comparisons (q < 0.05).
Figure 7. Effect of perceived somatic physical stimulation (nasopharyngeal tube presence) on the functional connectivity score (FC) of the brain swallowing network.
We measured the average functional connectivity score of every seed region both at rest and after nasopharyngeal intubation. Analysis of variance and post-hoc analysis showed that FC score of the right posterior insula during presence of nasopharyngeal tube is significantly diminished compared to the pure resting state (p < 0.05).
DISCUSSION
In this study, we determined the alterations in the functional connectivity (FC) of brain regions involved in swallowing during sensory stimulation. We found that although the overall architecture of the brain swallowing network (BSN) is stable, the FC of a subset of the BSN regions is modulated by pharyngoesophageal sensory input. Subliminal esophageal acid stimulation augments the FC of right anterior insula to the BOLD positive component of the BSN and specifically between the right anterior insula and the left inferior parietal lobule. The conscious somatic stimulation induced by nasopharyngeal intubation significantly reduces the FC of right posterior insula to the BOLD positive component of the BSN and particularly the FC between the right posterior insula and the right prefrontal operculum, and diminishes the FC between the thalamus and the red nucleus. The functional connectivity of the entire negative BOLD component was not affected by the conscious somatic or subliminal visceral pharyngoesophageal sensory stimulation.
Determining the effects of sensory stimulation on FC of cortical regions associated with swallowing activity is important from a physiological and even pathophysiological viewpoint. Our earlier studies have documented the accentuating effect of esophageal acid stimulation on cortical bold activity associated with swallowing 18. Given the fact that esophageal reflux occurs in various degrees in healthy individuals and patients with gastroesophageal reflux disease, and that swallowing plays an essential mechanistic role in the clearance of the potentially hazardous chemicals from the esophagus 15, modulatory effects of esophageal acid stimulation has both physiologic and potentially clinical relevance. The present study further supports the finding of a previous study that subliminal esophageal acid stimulation modulates the underlying fMRI activity of cortical regions governing the swallow motor function18. Furthermore, it shows that this modulation extends beyond a temporary increase in swallow related cortical activity and includes how these regions functionally connect to each other at rest without an explicit task. These findings provide a window into the depth of involvement of the cerebral cortex not only in conscious but also unperceived physiologic stimulations occurring in the upper gastrointestinal tract. In addition, they clearly establish the influence of an unperceived signal beyond a sensory network and onto a motor related network such as swallowing.
Another important finding of the present study is the observation that mere transnasal esophageal intubation of healthy volunteers affects the functional connectivity of a distinct subset of the swallow-related cortical regions. Considering the invasive nature of the transnasal placement of infusion catheter this effect is not unexpected. It is reassuring that alterations in these FC findings, at the last phase of study was not different from that of the beginning of the study alleviating our concern about the order effect as a cause of observed subliminal acid induced changes. Limitations of current technology have not allowed for systematic and simultaneous investigation of the effects of these modulations on swallowing frequency, efficacy and biomechanical characteristics. However, it is important to remember that the acid effect compared to buffer, manifested itself above and beyond the alteration induced by the presence of the infusion tube and was observed at a stimulation level that was not perceived by subjects.
Although functional brain imaging has greatly advanced our understanding of the neural basis of swallowing and has validated the crucial role of cerebral cortex during deglutition in humans 1, 3, 32, 33, the exact neurophysiology of cortical control of deglutition and functional role of swallow-related cortical regions remain elusive 34. The lateral sensorimotor cortex, both primary motor and sensory (MI and SI), plays a fundamental role not only in the execution of the semiautomatic deglutitive motor sequence35, 36 but also monitoring of sensory signals from oropharyngeal structures37, 38. Earlier studies have shown that when the network was engaged in the performance of episodic swallow task the FC of bilateral sensorimotor regions was augmented signifying the crucial role of this region in execution of the swallow task 6. Supplementary motor area (SMA) and mid-cingulate cortex activations overlap with brain regions identified in speech production and word reading 39 that employ similar oropharyngeal neuromuscular apparatus. These two areas are also activated in a range of other volitional motor activities 40 and probably play a role in attention and motor planning 41. It is not surprising that during current study which sensory stimulation was not associated with a constant volitional response, these areas did not show any alteration in the functional connectivity.
The insula is one of the primitive regions of the brain and receives direct afferents from the thalamus. It is believed to serve as the central cortical processing region of the visceral afferents 42-44 and is organized in a hierarchical fashion 45, 46. In addition to swallowing 17, 41,47,56, 59-61, it is involved in cognitive, socio-emotional, gustatory and sensorimotor functions 48. The insula has shown changes in fMRI activity during esophageal acid exposure 17, esophageal distention49 and esophageal distention 50, and anterior insula is the proposed final site in intra-insular processing of visceral afferent signals before these signals are projected to other cortical regions such as prefrontal cortex 51. A recent study has revealed that right anterior insula is the most active among various insular regions associated with swallowing during gustatory or electrical sensory stimulation 52. Our finding of altered functional connectivity of the right anterior insula with other cortical regions involved in swallowing during esophageal acid exposure concurs with the pivotal role of insula in sensory aspects of swallowing. The inferior parietal lobule has shown activity associated with deglutition and is considered a component of the brain swallowing network 34, 47. The lateral part of the inferior parietal lobule, parietal operculum (BA 40,43), probably represents the neuroanatomical correlate of secondary somatosensory cortex (SII)53 and has been viewed as a projection area of visceral stimulation 44. In that, the current study also shows amplified FC of right anterior insula to this region during subliminal esophageal acid infusion pointing to the putative visceral sensory role of this region.
The thalamus, putamen (dorsal striatum) and cerebellum 47, 54-56 have previously shown activation associated with volitional swallowing. Striatum is usually considered the gateway of primary motor cortex to thalamus, which in turn has projections to SMA, and may play a role in sensorimotor integration 57, 58. Cerebellar cortex may play an important role in the adaptive coordination 59, and timing of the stereotypical contractions of complex oropharyngeal musculature during deglutition 47. Swallow related BOLD activity of the red nucleus has not been reported in previous studies, but another study focusing on the subcortical regions reported fMRI activity in adjacent substantia nigra 54. Red nucleus receives projections from motor cortex (BA 4, 6 and 8) and deep cerebellar nuclei through medial cerebellar systems and plays an important role in motor control 60. Since very little is known about the function of the red nucleus in humans and no direct anatomic rubrothalamic connections are described in the literature, we hesitate to speculate regarding the importance of diminished FC of thalamus-red nucleus to statistically insignificant level (r<0.165) during nasopharyngeal intubation. Furthermore, the pathophysiologic impact of the observed alterations at a functional level is not currently known and whether any of these functional connectivity modulations can affect behavioral changes merits further investigation with availability of newer technology and equipment.
In summary, following our previous report of modulation of FC of bilateral sensorimotor region within the swallow network during engagement in repetitive swallowing 6, the present study shows modulation of right posterior insular FC during conscious nasopharyngeal tube presence, and alteration of right anterior insular FC during subliminal esophageal acid exposure in healthy humans. Taken together, these findings indicate that the brain swallowing network is distinctly responsive to both perceived somatic physical and unperceived visceral chemical afferent input from the deglutitive axis.
Acknowledgments
Supported in part by NIH Grant 5R01DK025731-29 and 2T32DK061923-06
Abbreviations
- fMRI
functional magnetic resonance imaging
- BOLD
Blood oxygenation level dependent
- FC
functional connectivity
- CC
cross-correlation coefficient
- BSN
brain swallowing network
Footnotes
Dr. Babaei had a major role in all aspects of this manuscript including study concept and design, data acquisition, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. Dr. Siwiec contributed to data analysis and interpretation and critical revision of the manuscript. Mark Kern and B. Douglas Ward contributed to data analysis and interpretation of data along with statistical analysis. Dr. Li and Dr. Shaker had a major role in study concept and design, drafting of the manuscript, supervision and critical revision of the manuscript.
The authors have no conflict of interest to disclose.
References
- 1.Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol. 1999;277:G219–25. doi: 10.1152/ajpgi.1999.277.1.G219. [DOI] [PubMed] [Google Scholar]
- 2.Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol. 2001;280:G531–8. doi: 10.1152/ajpgi.2001.280.4.G531. [DOI] [PubMed] [Google Scholar]
- 3.Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85:938–50. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- 4.Mosier KM, Liu WC, Maldjian JA, Shah R, Modi B. Lateralization of cortical function in swallowing: a functional MR imaging study. AJNR Am J Neuroradiol. 1999;20:1520–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Babaei A, Ward BD, Ahmad S, Patel A, Nencka A, Li SJ, Hyde J, Shaker R. Reproducibility of swallow-induced cortical BOLD positive and negative fMRI activity. American journal of physiology Gastrointestinal and liver physiology. 2012;303:G600–9. doi: 10.1152/ajpgi.00167.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babaei A, Ward BD, Siwiec R, Ahmad S, Kern M, Nencka A, Li SJ, Shaker R. Functional Connectivity of the Cortical Swallowing Network in Humans. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 8.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 9.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106:1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margulies DS, Bottger J, Long X, Lv Y, Kelly C, Schafer A, Goldhahn D, Abbushi A, Milham MP, Lohmann G, Villringer A. Resting developments: a review of fMRI post-processing methodologies for spontaneous brain activity. Magma. 2010;23:289–307. doi: 10.1007/s10334-010-0228-5. [DOI] [PubMed] [Google Scholar]
- 12.Barnes A, Bullmore ET, Suckling J. Endogenous human brain dynamics recover slowly following cognitive effort. PLoS One. 2009;4:e6626. doi: 10.1371/journal.pone.0006626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton AT, Morgan VL, Rogers BP, Gore JC. Modulation of steady state functional connectivity in the default mode and working memory networks by cognitive load. Human brain mapping. 2011;32:1649–59. doi: 10.1002/hbm.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logothetis NK, Murayama Y, Augath M, Steffen T, Werner J, Oeltermann A. How not to study spontaneous activity. Neuroimage. 2009;45:1080–9. doi: 10.1016/j.neuroimage.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Helm JF, Dodds WJ, Riedel DR, Teeter BC, Hogan WJ, Arndorfer RC. Determinants of esophageal acid clearance in normal subjects. Gastroenterology. 1983;85:607–12. [PubMed] [Google Scholar]
- 16.Helm JF, Dodds WJ, Hogan WJ. Salivary response to esophageal acid in normal subjects and patients with reflux esophagitis. Gastroenterology. 1987;93:1393–7. doi: 10.1016/0016-5085(87)90270-8. [DOI] [PubMed] [Google Scholar]
- 17.Kern MK, Birn RM, Jaradeh S, Jesmanowicz A, Cox RW, Hyde JS, Shaker R. Identification and characterization of cerebral cortical response to esophageal mucosal acid exposure and distention. Gastroenterology. 1998;115:1353–62. doi: 10.1016/s0016-5085(98)70013-7. [DOI] [PubMed] [Google Scholar]
- 18.Kern M, Chai K, Lawal A, Shaker R. Effect of esophageal acid exposure on the cortical swallowing network in healthy human subjects. Am J Physiol Gastrointest Liver Physiol. 2009;297:G152–8. doi: 10.1152/ajpgi.00062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 20.Babaei A, Ward BD, Li SJ, Shaker R. Reproducibility of the Resting and Active State Connectivity of the “Deglutition Connectome”. Gastroenterology. 2011;140:S-368. [Google Scholar]
- 21.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2000;44:162–7. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–48. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talaraich J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. 1988 [Google Scholar]
- 25.Ernst T, Speck O, Itti L, Chang L. Simultaneous correction for interscan patient motion and geometric distortions in echoplanar imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1999;42:201–5. doi: 10.1002/(sici)1522-2594(199907)42:1<201::aid-mrm27>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 26.Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of neurophysiology. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–48. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- 29.Gui J, Tosteson TD, Borsuk M. Weighted multiple testing procedures for genomic studies. BioData mining. 2012;5:4. doi: 10.1186/1756-0381-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Storey J. The positive false discovery rate: a Bayesian interpretation and the q-value. Annals of Statistics. 2003;31 [Google Scholar]
- 31.He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–18. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Hamdy S, Rothwell JC, Brooks DJ, Bailey D, Aziz Q, Thompson DG. Identification of the cerebral loci processing human swallowing with H2(15)O PET activation. J Neurophysiol. 1999;81:1917–26. doi: 10.1152/jn.1999.81.4.1917. [DOI] [PubMed] [Google Scholar]
- 33.Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol. 2001;280:G354–60. doi: 10.1152/ajpgi.2001.280.3.G354. [DOI] [PubMed] [Google Scholar]
- 34.Soros P, Inamoto Y, Martin RE. Functional brain imaging of swallowing: an activation likelihood estimation meta-analysis. Hum Brain Mapp. 2009;30:2426–39. doi: 10.1002/hbm.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin RE, Kemppainen P, Masuda Y, Yao D, Murray GM, Sessle BJ. Features of cortically evoked swallowing in the awake primate (Macaca fascicularis) J Neurophysiol. 1999;82:1529–41. doi: 10.1152/jn.1999.82.3.1529. [DOI] [PubMed] [Google Scholar]
- 36.Narita N, Yamamura K, Yao D, Martin RE, Sessle BJ. Effects of functional disruption of lateral pericentral cerebral cortex on primate swallowing. Brain Res. 1999;824:140–5. doi: 10.1016/s0006-8993(99)01151-8. [DOI] [PubMed] [Google Scholar]
- 37.Teismann IK, Steinstraeter O, Stoeckigt K, Suntrup S, Wollbrink A, Pantev C, Dziewas R. Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci. 2007;8:62. doi: 10.1186/1471-2202-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zald DH, Pardo JV. Cortical activation induced by intraoral stimulation with water in humans. Chemical senses. 2000;25:267–75. doi: 10.1093/chemse/25.3.267. [DOI] [PubMed] [Google Scholar]
- 39.Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT. Stuttered and fluent speech production: an ALE meta-analysis of functional neuroimaging studies. Human brain mapping. 2005;25:105–17. doi: 10.1002/hbm.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paus T, Petrides M, Evans AC, Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol. 1993;70:453–69. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- 41.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu CL, Wu YT, Yeh TC, Chen LF, Chang FY, Lee SD, Ho LT, Hsieh JC. Neuronal correlates of gastric pain induced by fundus distension: a 3T-fMRI study. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16:575–87. doi: 10.1111/j.1365-2982.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- 43.Ladabaum U, Minoshima S, Hasler WL, Cross D, Chey WD, Owyang C. Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology. 2001;120:369–76. doi: 10.1053/gast.2001.21201. [DOI] [PubMed] [Google Scholar]
- 44.Derbyshire SW. A systematic review of neuroimaging data during visceral stimulation. The American journal of gastroenterology. 2003;98:12–20. doi: 10.1111/j.1572-0241.2003.07168.x. [DOI] [PubMed] [Google Scholar]
- 45.Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- 46.Craig AD. How do you feel--now? The anterior insula and human awareness. Nature reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 47.Zald DH, Pardo JV. The functional neuroanatomy of voluntary swallowing. Ann Neurol. 1999;46:281–6. [PubMed] [Google Scholar]
- 48.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain structure & function. 2010;214:519–34. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Binkofski F, Schnitzler A, Enck P, Frieling T, Posse S, Seitz RJ, Freund HJ. Somatic and limbic cortex activation in esophageal distention: a functional magnetic resonance imaging study. Ann Neurol. 1998;44:811–5. doi: 10.1002/ana.410440516. [DOI] [PubMed] [Google Scholar]
- 50.Aziz Q, Thompson DG, Ng VW, Hamdy S, Sarkar S, Brammer MJ, Bullmore ET, Hobson A, Tracey I, Gregory L, Simmons A, Williams SC. Cortical processing of human somatic and visceral sensation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:2657–63. doi: 10.1523/JNEUROSCI.20-07-02657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature reviews Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 52.Humbert IA, Joel S. Tactile, gustatory, and visual biofeedback stimuli modulate neural substrates of deglutition. Neuroimage. 2012;59:1485–90. doi: 10.1016/j.neuroimage.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex. 2006;16:268–79. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia. 2003;18:71–7. doi: 10.1007/s00455-002-0088-x. [DOI] [PubMed] [Google Scholar]
- 55.Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum Brain Mapp. 2009;30:3209–26. doi: 10.1002/hbm.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lowell SY, Poletto CJ, Knorr-Chung BR, Reynolds RC, Simonyan K, Ludlow CL. Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage. 2008;42:285–95. doi: 10.1016/j.neuroimage.2008.04.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaji R. Basal ganglia as a sensory gating devise for motor control. The journal of medical investigation : JMI. 2001;48:142–6. [PubMed] [Google Scholar]
- 58.Mosier K, Patel R, Liu WC, Kalnin A, Maldjian J, Baredes S. Cortical representation of swallowing in normal adults: functional implications. Laryngoscope. 1999;109:1417–23. doi: 10.1097/00005537-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 59.Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annual review of neuroscience. 1992;15:403–42. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- 60.Hicks TP, Onodera S. The mammalian red nucleus and its role in motor systems, including the emergence of bipedalism and language. Progress in neurobiology. 2012;96:165–75. doi: 10.1016/j.pneurobio.2011.12.002. [DOI] [PubMed] [Google Scholar]


