Abstract
Lymphocytes have been shown to be involved in modulating monocyte and macrophage behavior in the foreign body reaction. Lymphocyte effects on biomaterial-adherent macrophage and foreign body giant cell (FBGC) behavior were further investigated by culturing monocytes alone or together with lymphocytes, either in direct co-cultures or indirectly in transwells, on a series of polyethylene terephthalate (PET)-based photograft co-polymerized material surfaces displaying distinct hydrophobic, hydrophilic/neutral, hydrophilic/anionic, and hydrophilic/cationic chemistries. After periods of 3, 7, and 10 days, cytokine production was quantified by ELISA and normalized to adherent macrophage/FBGC density to yield a measure of adherent macrophage/FBGC activation. Interactions with lymphocytes enhanced adherent macrophage and FBGC production of pro-inflammatory IL-1β, TNF-α, IL-6, IL-8, and MIP-1β on the hydrophobic and hydrophilic/cationic surfaces but had no effect on anti-inflammatory IL-10 production indicating lymphocytes promote a pro-inflammatory response to biomaterials. Lymphocytes also did not significantly influence MMP-9, TIMP-1, and TIMP-2 production. Interactions through indirect (paracrine) signaling showed a significant effect in enhancing adherent macrophage/FBGC activation at early time points while interactions via direct (juxtacrine) mechanisms dominated at later time points. Biomaterial surface chemistries differentially affected the observed responses as hydrophilic/neutral and hydrophilic/anionic surfaces evoked the highest levels of activation relative to the other surfaces but did not facilitate lymphocyte enhancement of adherent macrophage/FBGC activation.
Keywords: biomaterials, foreign body reaction, lymphocytes, macrophages, activation
Introduction
Biomaterials introduced into the body elicit a biological response involving degradative and phagocytic activities leading to potential implant damage and failure. Macrophages lie at the center of this foreign body reaction as the precursor cells that fuse to form foreign body giant cells (FBGC). Once activated, macrophages guide the tissue response through the production of inflammatory mediators including cytokines, chemokines, and extracellular matrix (ECM)-modifying proteins. Lymphocytes appear transiently during the chronic inflammatory response and have the opportunity to interact with the macrophages at the tissue/material interface.1 We have shown previously that lymphocytes are capable of adhering to biomaterial surfaces primarily through contact with macrophages/FBGCs in co-cultures; however, lymphocytes enhanced monocyte adhesion and macrophage fusion to form FBGCs through indirect or paracrine signaling.2 Therefore, both direct (juxtacrine) interactions via cell-cell adhesion mechanisms and indirect (paracrine) interactions, mediated by cytokine and chemokine mechanisms, participate in lymphocyte and macrophage interactions at the tissue/material interface. However, we have yet to gain a complete understanding of how lymphocytes and macrophages interact at the implant site and affect macrophage behavior.
The lymphocyte population, which includes T lymphocytes (T cells), natural killer (NK) cells, and B lymphocytes (B cells), participate in the host defense against foreign pathogens. T cells are divided into primarily CD8+ cytotoxic T cells and CD4+ type 1 and type 2 helper T cells (Th1 and Th2). Upon activation, these lymphocytes produce distinct cytokines and perform specific roles. CD8+ T cells and NK cells mediate cellular killing. Th1 and NK cells produce IFN-γ while Th2 cells secrete IL-4, IL-5, IL-10, IL-13.3 B cells respond when activated by producing antigen-specific antibodies. Therefore, lymphocytes utilize direct and indirect mechanisms in responding to stimuli and communication with each other as well as with other cell types.
Lymphocytes are capable of activating or enhancing the activation of macrophages through antigen-dependent and antigen-independent direct cell-cell contact and indirect interactions. T-cell receptor (TCR)-activated T lymphocytes and cytokine-activated T lymphocytes are capable of activating macrophages albeit resulting in differential production of inflammatory mediators.4 Membrane TNF-α, CD69, and the CD40-CD40L signaling pathways appear to be involved in the contact-mediated activation.4 Lymphocyte stimulation of macrophages can also occur by non-contact mechanisms through secretion of soluble factors. T cell-derived IL-4, IL-13, and IFN-γ or natural killer cell-derived IFN-γ can induce distinct forms of macrophage activation.3,5,6 These different stimuli, contact or cytokine-mediated, induce activated macrophages to secrete a multitude of cytokines and chemokines such as IL-1β, TNF-α, IL-6, IL-8, IL-10 with the profiles dependent on stimuli.4,5,7
Monocyte/macrophage cytokine responses to biomaterials have been examined both in vitro and in vivo. Many studies have shown differential monocyte/macrophage responses to various biomaterials using inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8 as indicators of cellular activation.8–12 Recent proteomic investigations in our laboratory have shown material-dependent production of cytokines such as IL-1β, IL-6, IL-10; chemokines such as IL-8, MIP-1β, MCP-1; and ECM-remodeling proteins such as MMP-9, TIMP-1, and TIMP-2 from adherent macrophages/FBGCs populations and co-cultures with lymphocytes.13,14
To further investigate lymphocyte effects on adherent macrophage behavior, monocytes were cultured alone or together with lymphocytes on polyethylene terephthalate (PET)-based photograft co-polymerized material surfaces displaying distinct hydrophobic, hydrophilic/neutral, hydrophilic/anionic, and hydrophilic/cationic chemistries. We hypothesized that lymphocytes differentially modulate the production of macrophage-derived inflammatory mediators in response to biomaterial surfaces. This study examined the role of paracrine (indirect) and juxtacrine (direct) lymphocyte interactions on macrophage activation as measured by macrophage-derived cytokines, chemokines, and extracellular matrix-modifying proteins. In addition, varying biomaterial surface chemistries were utilized to explore how these interactions were influenced by surface properties. The results from this investigation provide additional insight into the role lymphocytes play in the foreign body reaction and the interfacial interaction mechanisms that exist between lymphocytes and macrophages at the biomaterial implant site.
Materials and Methods
Biomaterial Surfaces
Synthesis of the PET-based photograft copolymerized surfaces is described elsewhere. 15 Briefly, sheets of Mylar® PET were initially coated with poly(benzyl N,N-diethyldithiocarbamate-co-styrene) (BDEDTC) and then photograft copolymerized with acrylamide (PAAm), sodium salt of acrylic acid (PAANa), and N-methiodide of dimethylamino propylacrylamide (DMAPAAmMeI) to provides surfaces with distinct hydrophobic, hydrophilic/neutral, hydrophilic/anionic, and hydrophilic/cationic surface characteristics, respectively. Biomaterial surfaces were punched into 1.5 cm diameter disks and sterilized via a 100% ethanol wash prior to use. The material samples were placed into 24-well tissue culture polystyrene (TCPS) plates (Fisher Scientific, Pittsburgh, PA) and secured in place at the bottom of the well under sterile conditions by silicone rings that were subjected to 5 minutes of sonication and autoclave-sterilization. Prior to cell seeding, surfaces in each well were washed with phosphate-buffered saline containing magnesium chloride and calcium chloride (Invitrogen, Grand Island, NY) (PBS++) to remove any residual ethanol from sterilization.
Monocyte and Lymphocyte Isolation and Culture
Monocytes and lymphocytes were isolated from non-medicated peripheral blood of healthy adult volunteers utilizing a non-adherent centrifugation method as previously described.16 The monocyte and lymphocyte populations were separately suspended in serum free medium (SFM) (Invitrogen, Grand Island, NY) supplemented with L-glutamine, antibiotics, and antimycotics along with 20% autologous serum (AS) for plating. 5×105 monocytes were plated on each of the biomaterial surfaces and incubated at 37°C and 5% CO2. After 2 hours, non-adherent cells were removed, surfaces were washed once with warm PBS++, and cultured in 1 mL of supplemented SFM containing 20% AS with and without 1.5×106 lymphocytes. The lymphocyte population was added both directly to the adherent monocytes (direct culture) and in 0.02 µm Anapore membrane transwell inserts (Nunc, Naperville, IL) separated from the adherent monocytes (indirect culture). Supernatants, collected after 3, 7, and 10 days, were centrifuged at 3000 rpm and cell-free supernatants were stored at −20°C for analysis.
Determination of Adherent Cell Densities
Adherent cells were washed twice with PBS++ to remove non-adherent cells and residual media, fixed with 100% methanol for 5 minutes, air-dried, and stained with May-Grünwald/Giemsa. The staining process involved the addition of May-Grünwald reagent for 5 minutes followed by a PBS++ wash and then addition of the Giemsa reagent. After incubation for 15 minutes at room temperature, the surfaces were finally washed twice with dH20 and allowed to air-dry overnight. The adherent monocyte, macrophage, and foreign body giant cell density (cells/mm2) was determined by counting nuclei from 5 fields under 20X objective using optical microscopy. Cells containing greater than 3 nuclei were considered foreign body giant cells.
Determination of Macrophage/FBGC Activation
Cytokine production was quantified by utilizing ELISA (R&D systems, Minneapolis, MN) for IL-1β, TNF-α, IL-6, IL-8, IL-10, MMP-9, TIMP-1, and TIMP-2, performed according to manufacturer’s protocol, along with an EL808 ultra microplate reader and KC Junior software (Bio-Tek Instruments, Inc., Winooski, Vermont). To represent cellular activation, the concentration of cytokines produced was divided by the adherent macrophage/FBGC density which yields the amount of cytokines produced per adherent cell.
Statistical Analysis
All results were obtained by repeating experiments at least 4 times utilizing different donors (n ≥ 4) and presented as an average ± the standard error of the mean (SEM). All data were analyzed by Minitab statistical software (Minitab Inc., State College, PA) and comparisons made utilizing ANOVA and the Tukey post hoc test to determine significant differences.
Results
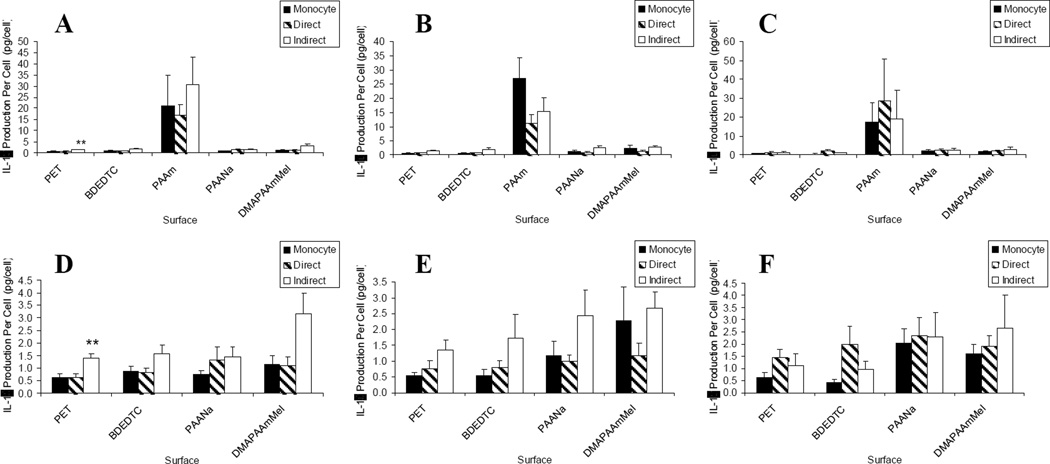
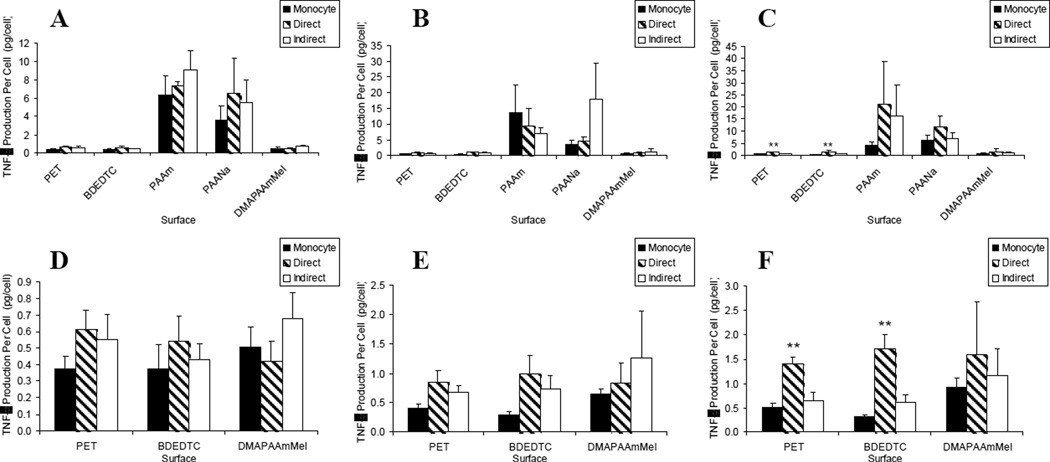
IL-1β, TNF-α, IL-6, IL-8, MIP-1β, IL-10, MMP-9, TIMP-1 and TIMP-2, were quantified in terms of production of these inflammatory mediators per adherent cell. Adherent macrophages and foreign body giant cells on various biomaterial surfaces were introduced to lymphocytes both directly and indirectly in order to examine the effects of the two mechanisms of interaction on adherent cell activation as measured by inflammatory mediator production. We previously demonstrated material-dependent production of these cytokines, chemokines, and ECM-modifying proteins by adherent macrophages and foreign body giant cells.13 In all cultures, regardless of the presence of lymphocytes or period of culture time, the hydrophilic/neutral surface, PAAm, induced the highest level of activation which is consistent with our previous work.13 This is demonstrated in Figures 1–7. The hydrophilic/anionic surface, PAANa, also showed increased levels of activation in terms of TNF-α and MIP-1β production (Figures 1 and 5).
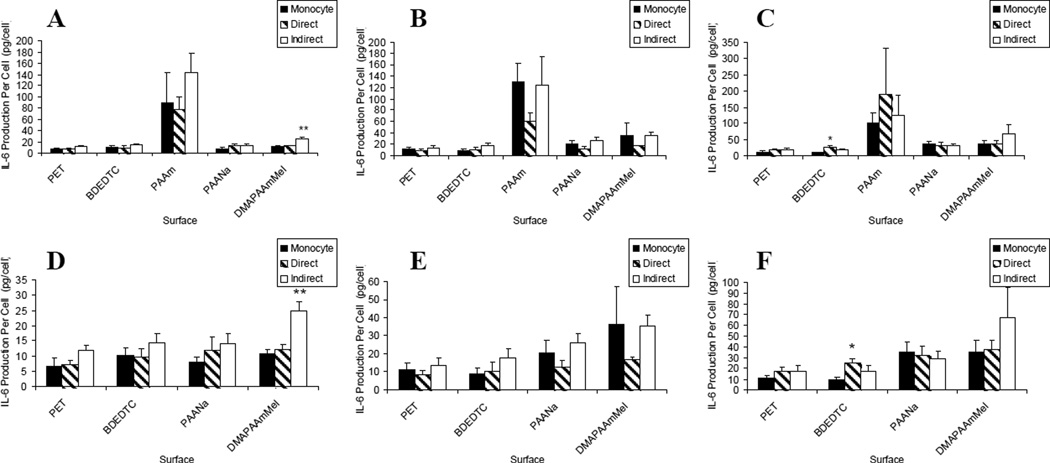
Figure 1.
IL-1β production: lymphocyte effects on macrophages/FBGCs over (A) 3, (B) 7, and (C) 10 days of culture. Results on the PAAm surface were removed for demonstrating lymphocyte effects on the other surfaces over (D) 3, (E) 7, and (F) 10 days of culture. Data represents the mean ± standard error of the mean (SEM), n = 4–5. **Significance compared to direct and monocyte-only cultures (p < 0.05).
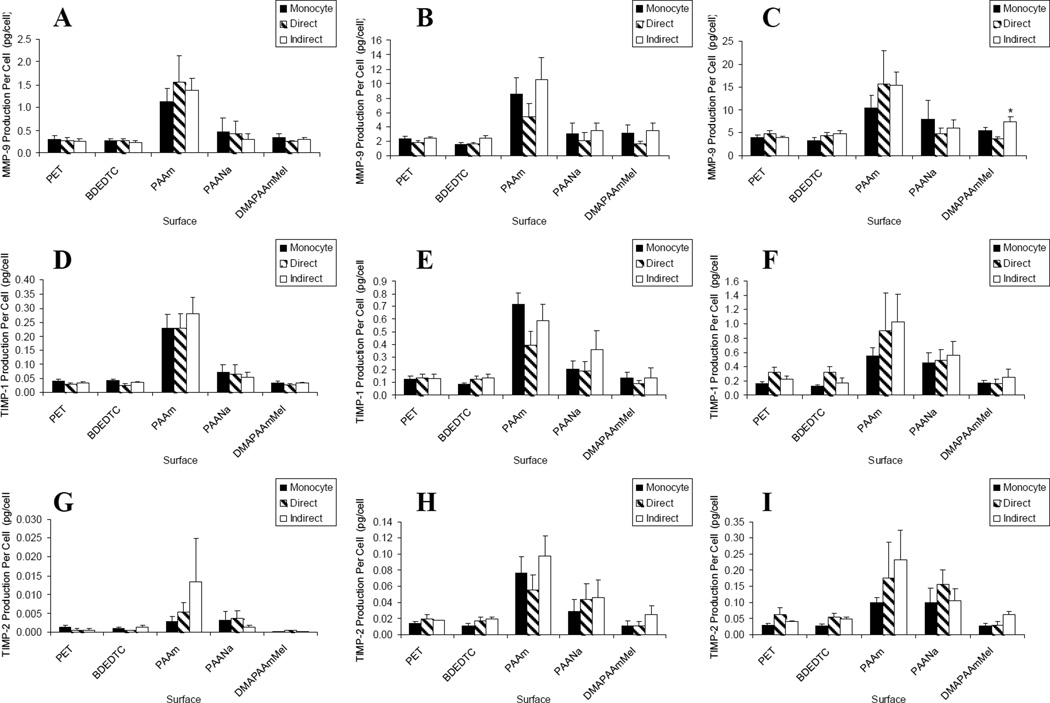
Figure 7.
Lymphocyte effects on macrophage/FBGC production of MMP-9 over (A) 3, (B) 7, and (C) 10 days of culture; TIMP-1 over (A) 3, (B) 7, and (C) 10 days of culture; and TIMP-2 over (A) 3, (B) 7, and (C) 10 days of culture. Data represents the mean ± standard error of the mean (SEM), n = 4–5. *Significance compared to direct culture (p < 0.05).
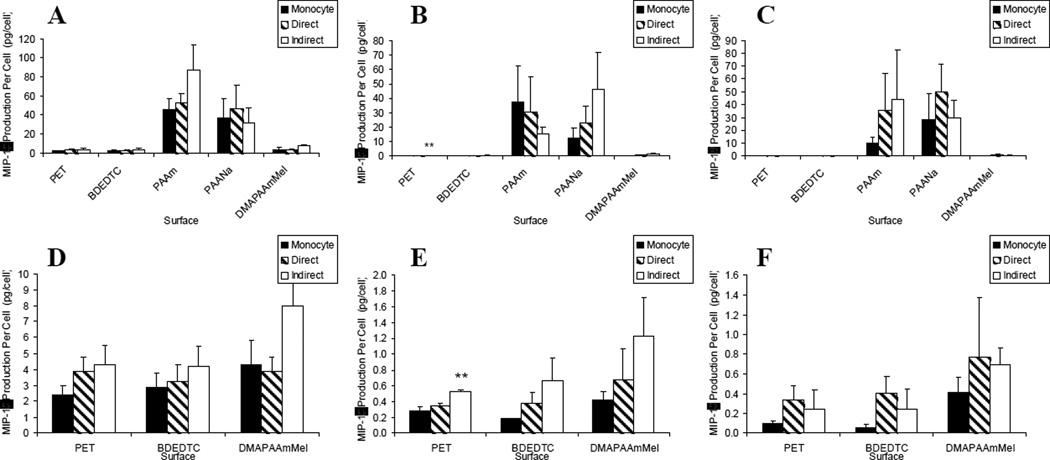
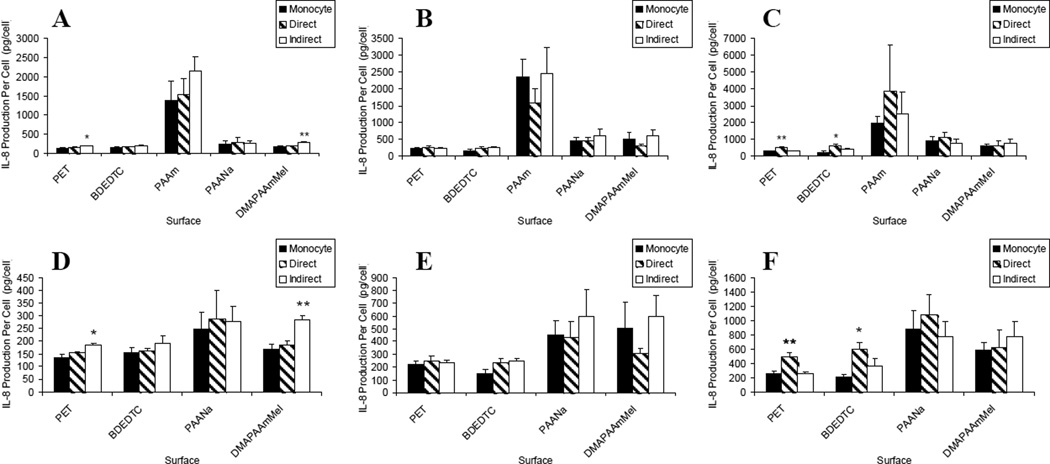
Figure 5.
MIP-1β production: lymphocyte effects on macrophages/FBGCs over (A) 3, (B) 7, and (C) 10 days of culture. Results on the PAAm and PAANa surfaces were removed for demonstrating lymphocyte effects on the other surfaces over (D) 3, (E) 7, and (F) 10 days of culture. Data represents the mean ± standard error of the mean (SEM), n = 4–5. **Significance compared to direct and monocyte-only cultures (p < 0.05).
Pro-inflammatory Mediators
IL-1β production per cell was highest on the hydrophilic/neutral surface, PAAm, over all time points and cultures as shown in Figure 1(a–c). The presence of lymphocytes, interacting via soluble factors, showed a general trend towards increased activation at day 3 and day 7 of culture. This increase over adherent macrophage/FBGC cultures and direct co-culture with lymphocytes was significant only on the hydrophobic PET surface [Figure 1(d–e)]. The hydrophobic surface, BDEDTC, and the hydrophilic/cationic surface, DMAPAAmMeI, showed trends of increased activation only during the early time period of the cultures.
Although TNF-α was most highly produced from adherent macrophages and FBGCs on the hydrophilic/neutral and hydrophilic/anionic surfaces, lymphocytes did not alter activation on these two surfaces [Figure 2(a–c)]. Lymphocytes did not significantly influence activation until day 10 on the two hydrophobic surfaces, PET and BDEDTC, as illustrated in Figure 2(d–f). At that time, TNF-α production per cell was increased in direct lymphocyte co-cultures compared to both the monocyte-only culture and indirect co-culture (p < 0.01).
Figure 2.
TNF-α production: lymphocyte effects on macrophages/FBGCs over (A) 3, (B) 7, and (C) 10 days of culture. Results on the PAAm and PAANa surfaces were removed for demonstrating lymphocyte effects on the other surfaces over (D) 3, (E) 7, and (F) 10 days of culture. Data represents the mean ± standard error of the mean (SEM), n = 4-5. **Significance compared to indirect and monocyte-only cultures (p < 0.01).
IL-6 and IL-8 material-dependent activation were similar to IL-1β in that the hydrophilic/neutral surface, PAAm, induced the greatest activation [Figure 3 and 4 (a–c)]. These were similar culture trends early (day 3) with indirect co-cultures increasing IL-6 and IL-8 production per cell significantly on DMAPAAmMeI over the other two culture types and also significantly increasing IL-8 production per cell relative to adherent macrophage/FBGC cultures on PET. By day 10, direct lymphocyte/macrophage co-cultures on BDEDTC showed increased IL-6 and IL-8 over the monocyte culture without lymphocytes (p < 0.05). Additionally at day 10, direct co-cultures on PET induced a higher level of IL-8 production per cell compared to the other two culture types (p < 0.01).
Figure 3.
IL-6 production: lymphocyte effects on macrophages/FBGCs over (A) 3, (B) 7, and (C) 10 days of culture. Results on the PAAm surface were removed for demonstrating lymphocyte effects on the other surfaces over (D) 3, (E) 7, and (F) 10 days of culture. Data represents the mean ± standard error of the mean (SEM), n = 4–5. **Significance compared to direct and monocyte-only cultures (p < 0.01). *Significance compared to monocyte-only culture (p < 0.05).
Figure 4.
IL-8 production: lymphocyte effects on macrophages/FBGCs over (A) 3, (B) 7, and (C) 10 days of culture. Results on the PAAm surface were removed for demonstrating lymphocyte effects on the other surfaces over (D) 3, (E) 7, and (F) 10 days of culture. Data represents the mean ± standard error of the mean (SEM), n = 4–5. **Significance compared to other two culture types (p < 0.01). *Significance compared to monocyte-only culture (p < 0.05).
In terms of MIP-1β production, the material-dependent activation was the same as for TNF-α where PAAm and PAANa induced a higher level of MIP-1β production over all other surfaces as shown in Figure 5(a–c). In Figure 5(d–f), we see similar culture and time-dependent trends as the other pro-inflammatory cytokines and chemokines with indirect co-cultures significantly increasing IL-8 production per cell on the PET surface on day 7.
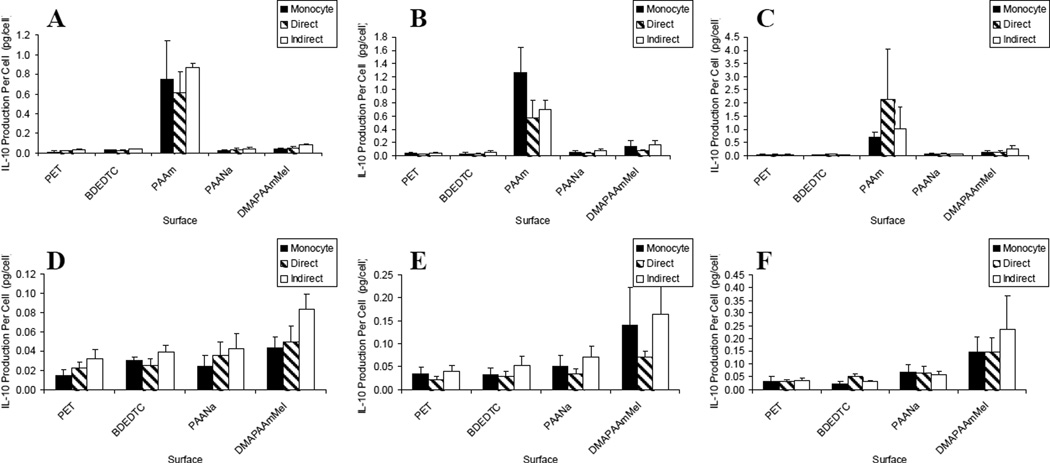
Anti-inflammatory Cytokine
IL-10 was the only anti-inflammatory mediator quantified. The data in Figure 6(a–c) shows that once again, PAAm was highly activating. However, unlike the pro-inflammatory cytokines and chemokines, IL-10 production per cell was not significantly affected by the presence of lymphocytes [Figure 6(d–f)].
Figure 6.
IL-10 production: lymphocyte effects on macrophages/FBGCs over (A) 3, (B) 7, and (C) 10 days of culture. Results on the PAAm surface were removed for demonstrating lymphocyte effects on the other surfaces over (D) 3, (E) 7, and (F) 10 days of culture. Data represents the mean ± standard error of the mean (SEM), n = 4–5.
Matrix metalloproteinases and Tissue Inhibitors
As shown in Figure 7, other than higher levels of MMP-9, TIMP-1, and TIMP-2 production on hydrophilic/neutral surfaces, lymphocyte containing co-cultures did not significantly influence production of these extracellular matrix-modifying proteins from adherent macrophages and FBGCs.
Discussion
Lymphocyte involvement in the biological response to biomaterial implants has received little attention despite their presence at the implant site which allows opportunity to interact with macrophages as well as other cell types. Lymphocyte effects on macrophage behavior in the foreign body reaction are now becoming identified. Brodbeck et al., previously demonstrated that lymphocytes can increase the level of monocyte adhesion on the biomaterial surface as well as macrophage fusion to form FBGCs.2 In light of these observations, we hypothesized that lymphocytes increased adherent macrophage activation. The objectives of the current investigation were to determine the effect lymphocytes have on macrophage activation as measured by the production of inflammatory mediators and how the response could be influenced by biomaterial surface chemistry.
Lymphocytes were cultured directly and indirectly with a population of adherent monocytes, macrophages, and FBGCs. Initially at day 3, increases in macrophage/FBGC activation were mediated by lymphocyte-derived soluble factors. The enhancement via indirect (paracrine) mechanisms was observed in IL-1β, IL-6, and IL-8 production. Direct co-cultures allowing both engagement of direct cell-cell contact and indirect interactions between lymphocytes and adherent macrophages/FBGCs failed to result in increased activation compared to cultures without lymphocytes. These results suggest that initial cell-cell interactions may inhibit the action of the soluble signal. As time progressed, indirect soluble factors no longer significantly increased or altered adherent cellular activation. Lymphocyte-derived IFN-γ and TNF are capable of activating and enhancing macrophage production of cytokines such as IL-1, TNF, and IL-6.5 CD8+ T cells, CD4+ Th1 cells, and NK cells are capable of producing IFN-γ and TNF when given appropriate stimulation.3,6 Previous investigations of inflammatory mediators from lymphocyte/macrophage co-cultures by protein cytokine array failed to detect IFN-γ, but did detect TNF-α.14 Preliminary results utilizing ELISA with higher sensitivity (minimum detection of 8 pg/mL) show that IFN-γ is produced in lymphocyte/macrophage co-cultures (unpublished data) suggesting that the failure to detect IFN-γ by protein array (minimum detection of 100 pg/mL) could be due to limitations in the sensitivity of the array. However, the specific cellular source of IFN-γ in the lymphocyte population is currently unknown.
Direct cell-cell contact between the lymphocytes and macrophages over the 10 days of culture resulted in enhancement of pro-inflammatory cytokines TNF-α, IL-6, and IL-8 although initially there was no such increase despite significant involvement of soluble factors in the indirect culture system. This time-varying response in direct co-cultures could possibly be due to macrophage or FBGC phenotypic changes over the 10 days of culture. This is the first evidence of lymphocyte contact-mediated modulation of biomaterial surface-adherent macrophage and FBGC behavior. Not only are activated T cells capable of influencing monocyte/macrophage responses through soluble factors, but they are also able to induce monocyte/macrophage production of cytokines and chemokines via membrane molecules such as membrane TNF-α, CD69, CD40, and LFA-1.4,17,18 T cells can be activated by TCR-dependent and TCR-independent, or cytokine-stimulated, mechanisms. The route of T cell activation dictates the contact-mediated monocyte/macrophage cytokine profile. For instance, lymphocytes activated via the TCR induced both TNF-α and IL-10 while lymphocytes activated via cytokines such as IL-6, TNF-α, IL-2 or IL-15 induced TNF-α but not IL-10.4,17,19 Due to lymphocyte contact-mediated induction of greater levels of only TNF-α and not IL-10 in the lymphocyte/macrophage co-cultures on biomaterial surfaces in this study, we suggest that the observed responses are most probably due to cytokine-activated T lymphocytes.
Lymphocytes appear to promote and perpetuate significant pro-inflammatory responses as the results show lymphocyte enhancement of pro-inflammatory cytokines and chemokines with no significant effect on IL-10, an anti-inflammatory cytokine known to suppress immune and inflammatory responses. T cells could be the effector population responsible for the observed responses through secretion of soluble factors such as IFN-γ as well as providing contact-mediated signals.3 The lack of detection of other lymphokines in similar lymphocyte/macrophage co-cultures suggest that the T lymphocytes could be in a state of hyporesponsiveness.14 If that is the case, NK cells could be an alternative source of the soluble mediator(s).20 Despite being hyporesponsive, T cell are still capable of increasing monocyte/macrophage activation via contact mechanisms.4 The lymphocytes in the lymphocyte/macrophage co-cultures on these PET-based surfaces, showed no influence on MMP-9, TIMP-1, or TIMP-2 production. MMP-9, TIMP-1, and TIMP-2 analyses were selected for this investigation since these are matrix-modifying proteins detected previously with biomaterial-adherent monocytes/macrophages/FBGCs.13
Material surface chemistries are known to elicit variable cellular responses. In this study, material surface chemistries induced varying levels of macrophage activation. Hydrophilic/neutral surfaces were the most highly activating surfaces while hydrophilic/anionic surfaces showed instances of high activation for particular cytokines. These results were consistent with our previous findings exploring material surface effects on cytokine, chemokine, and ECM-modulating protein production from adherent macrophages and FBGCs.13 The presented results further emphasize the fact that macrophage/FBGC activation does not correlate with adherent cellular density.
The current findings demonstrate that activation for pro-inflammatory cytokines and chemokines is enhanced by lymphocytes in both direct and indirect co-cultures but at different time points. Hydrophilic/neutral and hydrophilic/anionic surfaces induced the highest levels of activation per cell but the presence of lymphocytes on these surfaces did not significantly influence inflammatory mediator production when compared to cultures without lymphocytes. It is possible that the cells were maximally activated to produce the pro-inflammatory cytokines; if so, the presence of lymphocytes would have no further effect.
Overall, interactions through indirect soluble factor mechanisms show significant effects on adherent macrophage and FBGC activation at early time points while interactions through direct cell-cell mechanisms dominate at later time points. These direct and indirect lymphocyte interactions are pro-inflammatory as they enhance pro-inflammatory cytokines but do not influence IL-10, an anti-inflammatory cytokine, or particular ECM-modulating proteins. The results from this investigation show lymphocyte enhancement of adherent macrophage/FBGC activation via both direct and indirect mechanisms of interaction and further demonstrate lymphocyte involvement in the inflammatory and foreign body responses. Moreover, our findings support our hypothesis that biomaterial material chemistries differentially affect lymphocyte modulation of adherent macrophage and FBGC-derived cytokines and chemokines. Further investigations are ongoing for the specific lymphocyte-derived molecules, soluble and membrane-bound, which mediate the observed macrophage and FBGC responses on biomaterial surfaces. Our findings contribute to gaining a better understanding of the biomaterial-dependent and independent cellular and molecular interactions occurring in the foreign body reaction at the surface of implanted biomaterials in order to provide insight for the design of biomaterials with improved biocompatibility and optimal functional application.
Acknowledgments
Contract Grant Sponsors: National Institute of Health (NIH)
National Institute of Biomedical Imaging and Bioengineering (NIBIB)
Contract Grant Numbers: EB-000275
EB-000282
T32 GM07250
References
- 1.Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 2.Brodbeck WG, Macewan M, Colton E, Meyerson H, Anderson JM. Lymphocytes and the foreign body response: lymphocyte enhancement of macrophage adhesion and fusion. J Biomed Mater Res A. 2005;74(2):222–229. doi: 10.1002/jbm.a.30313. [DOI] [PubMed] [Google Scholar]
- 3.Santana MA, Rosenstein Y. What it takes to become an effector T cell: the process, the cells involved, and the mechanisms. J Cell Physiol. 2003;195(3):392–401. doi: 10.1002/jcp.10258. [DOI] [PubMed] [Google Scholar]
- 4.Monaco C, Andreakos E, Kiriakidis S, Feldmann M, Paleolog E. T-cell-mediated signalling in immune, inflammatory and angiogenic processes: the cascade of events leading to inflammatory diseases. Curr Drug Targets Inflamm Allergy. 2004;3(1):35–42. doi: 10.2174/1568010043483881. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 6.O'Connor GM, Hart OM, Gardiner CM. Putting the natural killer cell in its place. Immunology. 2006;117(1):1–10. doi: 10.1111/j.1365-2567.2005.02256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger D, Dayer JM. Cytokines, acute-phase proteins, and hormones: IL-1 and TNF-alpha production in contact-mediated activation of monocytes by T lymphocytes. Ann N Y Acad Sci. 2002;966:464–473. doi: 10.1111/j.1749-6632.2002.tb04248.x. [DOI] [PubMed] [Google Scholar]
- 8.Hwang JJ, Jelacic S, Samuel NT, Maier RV, Campbell CT, Castner DG, Hoffman AS, Stayton PS. Monocyte activation on polyelectrolyte multilayers. J Biomater Sci Polym Ed. 2005;16(2):237–251. doi: 10.1163/1568562053115480. [DOI] [PubMed] [Google Scholar]
- 9.DeFife KM, Yun JK, Azeez A, Stack S, Ishihara K, Nakabayashi N, Colton E, Anderson JM. Adhesion and cytokine production by monocytes on poly(2-methacryloyloxyethyl phosphorylcholine-co-alkyl methacrylate)-coated polymers. J Biomed Mater Res. 1995;29(4):431–439. doi: 10.1002/jbm.820290403. [DOI] [PubMed] [Google Scholar]
- 10.Ma N, Petit A, Yahia L, Huk OL, Tabrizian M. Cytotoxic reaction and TNF-alpha response of macrophages to polyurethane particles. J Biomater Sci Polym Ed. 2002;13(3):257–272. doi: 10.1163/156856202320176510. [DOI] [PubMed] [Google Scholar]
- 11.Sethi RK, Neavyn MJ, Rubash HE, Shanbhag AS. Macrophage response to cross-linked and conventional UHMWPE. Biomaterials. 2003;24(15):2561–2573. doi: 10.1016/s0142-9612(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 12.Marques AP, Reis RL, Hunt JA. Cytokine secretion from mononuclear cells cultured in vitro with starch-based polymers and poly-L-lactide. J Biomed Mater Res A. 2004;71(3):419–429. doi: 10.1002/jbm.a.30155. [DOI] [PubMed] [Google Scholar]
- 13.Jones JA, Chang DT, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31221. [DOI] [PubMed] [Google Scholar]
- 14.Chang DT, Jones JA, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM. Lymphocyte/macrophage interactions: biomaterial surface dependent cytokine, chemokine, and matrix protein production. J Biomed Mater Res. doi: 10.1002/jbm.a.31630. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakayama Y, Anderson JM, Matsuda T. Laboratory-scale mass production of a multi-micropatterned grafted surface with different polymer regions. J Biomed Mater Res. 2000;53(5):584–591. doi: 10.1002/1097-4636(200009)53:5<584::aid-jbm19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.McNally AK, Anderson JM. Complement C3 participation in monocyte adhesion to different surfaces. Proc Natl Acad Sci U S A. 1994;91(21):10119–10123. doi: 10.1073/pnas.91.21.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennan FM, Foey AD, Feldmann M. The importance of T cell interactions with macrophages in rheumatoid cytokine production. Curr Top Microbiol Immunol. 2006;305:177–194. doi: 10.1007/3-540-29714-6_9. [DOI] [PubMed] [Google Scholar]
- 18.Parry SL, Sebbag M, Feldmann M, Brennan FM. Contact with T cells modulates monocyte IL-10 production: role of T cell membrane TNF-alpha. J Immunol. 1997;158(8):3673–3681. [PubMed] [Google Scholar]
- 19.Sebbag M, Parry SL, Brennan FM, Feldmann M. Cytokine stimulation of T lymphocytes regulates their capacity to induce monocyte production of tumor necrosis factor-alpha, but not interleukin-10: possible relevance to pathophysiology of rheumatoid arthritis. Eur J Immunol. 1997;27(3):624–632. doi: 10.1002/eji.1830270308. [DOI] [PubMed] [Google Scholar]
- 20.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18(4):391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]