Abstract
Chronic obstructive pulmonary disease is known to be associated with systemic inflammation. We examined the longitudinal association of C-reactive protein (CRP) and lung function in a cohort of 18,110 men and women from the European Prospective Investigation Into Cancer in Norfolk who were 40–79 years of age at baseline (recruited in 1993–1997) and followed-up through 2011. We assessed lung function by measuring forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) at baseline, 4 years, and 13 years. Serum CRP levels were measured using a high-sensitivity assay at baseline and the 13-year follow up. Cross-sectional and longitudinal associations of loge-CRP and lung function were examined using multivariable linear mixed models. In the cross-sectional analysis, 1-standard-deviation increase in baseline loge-CRP (about 3-fold higher CRP on the original milligrams per liter scale) was associated with a −86.3 mL (95% confidence interval: −93.9, −78.6) reduction in FEV1. In longitudinal analysis, a 1-standard-deviation increase in loge-CRP over 13 years was also associated with a −64.0 mL (95% confidence interval: −72.1, −55.8) decline in FEV1 over the same period. The associations were similar for FVC and persisted among lifetime never-smokers. Baseline CRP levels were not predictive of the rate of change in FEV1 or FVC over time. In the present study, we found longitudinal observational evidence that suggested that increases in systemic inflammation are associated with declines in lung function.
Keywords: aging, chronic obstructive pulmonary disease, C-reactive protein, inflammation, longitudinal study, lung function
The interrelationship between lung function and systemic inflammation is not well understood. Patients with chronic obstructive pulmonary disease (COPD, a condition that is characterized by accelerated decline in lung function) have a higher risk of cardiovascular diseases and cardiovascular-related death, weight loss, osteoporosis, etc. (1, 2). It has been speculated that systemic inflammation related to COPD plays a role in the development of extrapulmonary comorbid conditions (3–5). On the other hand, individuals with higher levels of inflammatory markers are at a higher risk of developing COPD (6–8) and a higher risk of hospitalization due to COPD exacerbation (7, 9). Exploring the temporal interrelationship of systemic inflammation and lung function may shed light on and lead to better understanding of the mechanisms involved.
Systemic inflammation has a role in endothelial dysfunction and is associated with the risk of cardiovascular diseases and mortality (10). Therefore, inflammation may also play a role in the decline of lung function. C-reactive protein (CRP), a marker of systemic inflammation, is produced in the liver in response to interleukin-1 and interleukin-6 stimuli. In several studies, investigators have reported an inverse cross-sectional association between CRP and lung function as assessed by spirometry, that is, forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) (11–14). CRP has also been shown to be associated with incident COPD (6–8). A few studies have attempted to assess the likelihood of the association between CRP and lung function and COPD being causal using CRP related genes as unconfounded instruments (6, 7, 11), but their findings have largely been inconclusive because of their limited statistical power for such inferences. CRP genotype polymorphisms are associated with a small difference (about 60%) in serum CRP levels and are of insufficient magnitude to allow for detection of an association with changes in lung function. Therefore, it remains unclear whether systemic inflammation has a causal role in lung function decline and related conditions or whether it is a case of reverse causation, that is, lung function decline causing inflammation.
Investigation of the longitudinal association between CRP and changes in lung function over time in large-scale observational studies should be more informative of the nature of any temporal associations than investigation in cross-sectional studies, but longitudinal studies have been sparse. The few longitudinal studies in which the association of CRP with lung function have been investigated showed greater longitudinal changes in FEV1 or FVC among young adult participants with higher levels of CRP at baseline (15, 16), whereas in other studies, no association was found (8, 12, 14). However, the use of baseline CRP as a predictor variable in these longitudinal analyses may still be subject to confounding by unmeasured factors that vary between participants. An alternative analysis that correlates within-subject changes in lung function and similar changes in CRP levels should be less prone to residual confounding. Two of these previous longitudinal studies (8, 14) were able to quantify such a temporal association between changes in CRP levels during follow up as well as changes in lung function, for which it also found a negative association. On the other hand, data on the longitudinal association of lung function with change in CRP over time is sparse.
The association between CRP and lung function is largely confounded by smoking, but these previous longitudinal studies have generally not had adequate statistical power to quantify differences of associations according to some relevant subgroups, such as in lifetime nonsmokers versus smokers. In the present study, we aimed to examine the longitudinal association of CRP and lung function in greater detail (especially among never-smokers) in a large prospective study of 18,110 participants with repeated measures of CRP, lung function, and other confounders over 13 years of follow up.
MATERIALS AND METHODS
Study participants were recruited in the European Prospective Investigation Into Cancer in Norfolk (EPIC-Norfolk). Details of this study have been previously described (17). Briefly, 25,639 men and women who were 40–79 years of age and residents of Norfolk, England, were recruited into the study using general practice age and sex registers, which were approximately similar to the population age and sex registers in the United Kingdom. Participants attended the baseline health examination between 1993 and 1997, a second health examination approximately 4 years later, and a third health examination approximately 13 years after recruitment. The EPIC-Norfolk Study was approved by the Norfolk Local Research Ethics Committee, and all volunteers gave written informed consent.
At the clinic visits, extensive data on demographic, medical, lifestyle, family history, and dietary characteristics were collected by asking participants to complete a health and lifestyle questionnaire. Participants were categorized by smoking status as current, former, and never smokers, and alcohol consumption was computed as units per week. Medication use (including use of corticosteroids and postmenopausal hormone replacement therapy) was assessed by self-report on the health and lifestyle questionnaire and by examining medications brought by the participants to the clinic visit. Habitual physical activity was assessed using the EPIC-validated short physical activity questionnaire by combining levels of occupational and leisure-time physical activity into 4 categories (18).
At the clinic visits, trained nurses took anthropometric measurements on individuals who were wearing light clothing and no shoes. Height was measured to the nearest millimeter using a free standing stadiometer, and weight was measured to the nearest 100 g using digital scales. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. At each health examination, lung function was assessed as FEV1 and FVC using an electronic turbine spirometer (Micro Medical Ltd, Rochester, United Kingdom), with the higher of 2 consecutive expirations recorded after a practice blow.
Serum high-sensitivity CRP was measured using the Olympus AU640 Chemistry Immuno Analyzer (Olympus Diagnostics, Watford, United Kingdom). Blood samples collected at baseline examination were centrifuged at 2,100g for 15 minutes at 4°C, and serum samples were kept frozen at −80°C until being thawed in 2008 for CRP assaying. CRP was not measured at the second health examination but was measured at the third health examination in fresh blood samples using the Siemens Dimension clinical chemistry analyzer (Siemens Dimension clinical chemistry analyzer, Newark, Delaware).
Statistical analysis
Serum CRP levels and spirometry measures were analyzed as continuous and categorical variables in separate analyses. Serum CRP had a right-skewed distribution, and for continuous analysis it was log-transformed to obtain a normal distribution. For categorical analyses, CRP was divided into 4 clinically relevant categories: ≤1, 1.1–3, 3.1–10, and >10 mg/L.
In cross-sectional analysis using baseline data, multiple linear regression analysis was used to examine the mean differences in FEV1 and FVC across CRP categories. Analyses were adjusted for age, sex, height, BMI, smoking status, physical activity level, alcohol intake, and corticosteroid medication use, as well as menopausal status and hormone replacement therapy use for women only. The associations of baseline CRP with 13-year FEV1 and FVC were also examined using multiple linear regression analyses that were adjusted for the same covariates and length of follow-up time. Missing values for categorical variables were coded as such and were not excluded from the analyses.
In longitudinal analyses, linear mixed models were used to examine the rate of change of spirometry measures across baseline CRP categories. The rates of change of FEV1 and FVC across categories of CRP were modelled as a fixed-effect interactions between follow-up time (in years) and CRP categories, allowing for subject-specific random effects for the intercept and time coefficients. The P values were calculated as a fixed-effects interaction terms between loge-CRP (continuously) and time, with subject-specific random effects for the intercept and time. All covariates were treated as time-varying in longitudinal analysis to account for changes in lifestyle and anthropometric factors over time. The magnitude of longitudinal change in FEV1 (or FVC) associated with a 1-standard-deviation increase in loge-CRP (corresponding to a 3-fold increase in original CRP values) over time was calculated by fitting a linear mixed model that allowed for random subject-specific slopes for loge-CRP and random subject-specific intercepts.
Linear mixed models treat missing data as being missing at random. However, loss to follow up in our study was likely to be due to higher rates of morbidity and mortality, both of which are associated with CRP and spirometry measures. Therefore, to minimize selection bias, we performed a complete case analysis in which we restricted the longitudinal analyses to participants for whom we had adequate data for analysis at both baseline and at least 1 follow-up health examination.
All analyses were repeated in lifetime nonsmokers. The analyses were also repeated stratified by sex. Because the associations were qualitatively similar in sex-stratified analyses, we present all results for both sexes combined. Analyses were also repeated after excluding participants with a CRP level higher than 10 mg/L at either baseline or the 13-year health examination to minimize the potential confounding effect of infections.
We also examined the associations in the reverse direction, that is, baseline FEV1 and FVC as predictors of longitudinal change in CRP levels. All analyses were performed using Stata, version 12.0 (StataCorp LP, College Station, Texas).
RESULTS
We had data on CRP, lung function, and covariates at baseline examination for a total of 18,110 participants in the present analysis. The mean age of participants at baseline was 59 years, and 45% were male (Table 1). Of the 18,110 participants, 10,641 (59%) attended and had adequate data for statistical analysis from the second health examination (4-year follow up), and 5,130 (28%) had adequate data from the third health examination (13-year follow up). The mean duration of follow up was 3.7 (standard deviation (SD), 0.7) years and 13.1 (SD, 1.8) years at the second and third examinations, respectively. Compared with those who attended at least 1 follow-up health examination, participants who died or were subsequently lost to follow up were slightly older (mean age, 59.8 (SD, 9.7) years vs. 58.1 (SD, 8.8) years), had lower FEV1 (mean, 2401 (SD, 757) mL vs. 2553 (SD, 719) mL), had higher CRP levels (median, 1.8 (interquartile range, 0.8–3.9) mg/L vs. 1.4 (interquartile range 0.7–3.0) mg/L), had higher BMIs (mean, 26.6 (SD, 4.07) vs. 26.0 (SD, 3.6)), were more likely to be smokers (15.2% vs. 9.2% current smokers), and were less physically active (35.7% vs. 46.6% physically active).
Table 1.
Baseline Characteristics of Cohort Participants by Categories of C-Reactive Protein and in All Participants, European Prospective Investigation Into Cancer in Norfolk, 1993–1997
| Characteristic | C-Reactive Protein Level, mg/L |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Participants (n = 18,110) |
≤1 (n = 6,527) |
1.1–3 (n = 6,691) |
3.1–10 (n = 4,052) |
>10 (n = 840) |
|||||||||||
| Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | |
| Age, years | 58.7 (9.2) | 56.1 (8.9) | 59.5 (9.0) | 60.9 (8.9) | 61.5 (8.8) | ||||||||||
| Male sex | 8,126 | 44.9 | 2,958 | 45.3 | 3,096 | 46.3 | 1,702 | 42.0 | 370 | 44.1 | |||||
| FEV1, mL | 2,497 (737) | 2,712 (743) | 2,469 (710) | 2,266 (673) | 2,176 (706) | ||||||||||
| FVC, mL | 3,066 (922) | 3,301 (946) | 3,041 (893) | 2,800 (838) | 2,718 (864) | ||||||||||
| Height, cm | 166.8 (9.1) | 167.7 (9.1) | 166.8 (9.1) | 165.7 (8.9) | 165.6 (9.0) | ||||||||||
| Body mass indexa | 26.2 (3.8) | 24.7 (3.0) | 26.5 (3.5) | 27.9 (4.3) | 27.7 (5.0) | ||||||||||
| Alcohol consumption, units/week | 7.0 (9.2) | 7.2 (8.7) | 7.1 (9.6) | 6.5 (9.5) | 6.6 (9.2) | ||||||||||
| Smoking status | |||||||||||||||
| Current | 2,051 | 11.4 | 581 | 9.0 | 718 | 10.8 | 615 | 15.3 | 137 | 16.5 | |||||
| Former | 7,528 | 41.9 | 2,472 | 38.1 | 2,902 | 43.8 | 1,778 | 44.3 | 376 | 45.1 | |||||
| Never | 8,382 | 46.7 | 3,430 | 52.9 | 3,012 | 45.4 | 1,620 | 40.4 | 320 | 38.4 | |||||
| Physical activity level | |||||||||||||||
| Inactive | 5,422 | 29.9 | 1,506 | 23.1 | 2,042 | 30.5 | 1,530 | 37.8 | 344 | 41.0 | |||||
| Moderately inactive | 5,201 | 28.7 | 1,891 | 29.0 | 1,952 | 29.2 | 1,125 | 27.8 | 233 | 27.7 | |||||
| Moderately active | 4,118 | 22.7 | 1,686 | 25.8 | 1,486 | 22.2 | 794 | 19.6 | 152 | 18.1 | |||||
| Active | 3,369 | 18.6 | 1,444 | 22.1 | 1,210 | 18.1 | 604 | 14.9 | 111 | 13.2 | |||||
| Corticosteroid medication use | 564 | 3.1 | 127 | 2.0 | 200 | 3.0 | 185 | 4.6 | 52 | 6.2 | |||||
| Postmenopause | 7,844 | 78.6 | 2,428 | 68.0 | 2,952 | 82.1 | 2,058 | 87.5 | 406 | 86.4 | |||||
| % Currently using hormone replacement therapy | 2,086 | 20.9 | 504 | 14.1 | 769 | 21.4 | 656 | 27.9 | 157 | 33.4 | |||||
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SD, standard deviation.
a Weight (kg)/height (m)2.
Among participants who attended the baseline and follow-up health examinations, the median CRP level was 1.3 (interquartile range, 0.7–2.6) mg/L at baseline and 2.0 (interquartile range, 1.3–3.5) mg/L at the third health examination. Mean FEV1 at the baseline, second, and third health examinations were 2,699 (SD, 701) mL, 2,610 (SD, 701) mL, and 2,448 (SD, 712) mL, respectively. The corresponding figures for FVC were 3,286 (SD, 911) mL, 3,114 (SD, 878) mL, and 3,000 (SD, 888) mL, respectively. At the baseline assessment, participants with higher serum CRP levels tended to be older, had lower FEV1 and FVC, were less likely to be physically active, and were more likely to smoke, have a higher BMI, have a shorter height, or take corticosteroid medication or hormone replacement therapy (Table 1).
CRP predicting lung function
The baseline standardized loge-CRP had an inverse correlation with baseline standardized FEV1 and FVC (for FEV1, Pearson correlation coefficient (ρ) = −0.26, 95% CI: −0.27, −0.24, and for FVC, ρ = −0.23, 95% CI: −0.24, −0.21; P < 0.001). FEV1 and FVC were highly correlated (ρ = 0.89, 95% CI: 0.89, 0.90; P = 0.001). In cross-sectional multiple linear regression analyses, higher CRP levels were associated with significantly lower FEV1 and FVC (Table 2). A 1-standard-deviation higher baseline loge-CRP (about 3-fold higher CRP on the original milligrams per liter scale) was associated with a −86.3 mL (95% CI: −93.9, −78.6) decrease in FEV1 and a −91.7 mL (95% CI: −101.8, −81.6) decrease in FVC after adjustment for confounders. The inverse associations persisted in analysis stratified by sex (data not shown) and in nonsmokers (Table 2). There was a strong correlation between spirometry measures at baseline and at the 13-year follow up (for FEV1, ρ = 0.83, 95% CI: 0.82, 0.83 and for FVC, ρ = 0.75, 95% CI: 0.73, 0.75; P < 0.001) and between CRP measured at the 2 time points (ρ = 0.41, 95% CI: 0.39, 0.43). Baseline CRP level was also inversely associated with 13-year follow-up FEV1 and FVC adjusted for baseline covariates and follow-up time (Table 3). The association persisted in never smokers.
Table 2.
Cross-sectional and Longitudinal Association of Lung Function With Baseline Levels of C-Reactive Protein, European Prospective Investigation Into Cancer in Norfolk, 1993–2011
| CRP Level by Outcome, mg/L | Cross-sectional Association (n = 18,110) |
Longitudinal Association (n = 11,945) |
||||||
|---|---|---|---|---|---|---|---|---|
| Age- and Sex-adjusted |
Multivariable-adjusted |
Age- and Sex-adjustedc |
Multivariable-adjustedd |
|||||
| Mean Differencea | 95% CI | Mean Differencea | 95% CI | Annual Changeb | 95% CI | Annual Changeb | 95% CI | |
| All Participants | ||||||||
| FEV1 | ||||||||
| ≤1 | 0 | Referent | 0 | Referent | −19.39 | −20.42, −18.36 | −20.86 | −22.75, −18.96 |
| 1.1–3 | −127.88 | −145.53, −110.23 | −105.62 | −122.25, −88.99 | −20.45 | −21.56, −19.34 | −21.33 | −23.25, −19.42 |
| 3.1–10 | −249.22 | −269.65, −228.80 | −196.21 | −216.28, −176.15 | −19.50 | −21.11, −17.90 | −20.19 | −22.42, −17.96 |
| >10 | −334.55 | −371.53, −297.58 | −262.34 | −297.00, −227.67 | −19.24 | −23.22, −15.26 | −19.82 | −24.12, −15.51 |
| P value | <0.001 | <0.001 | 0.28 | 0.87 | ||||
| FVC | ||||||||
| ≤1 | 0 | Referent | 0 | Referent | −21.69 | −23.34, −20.05 | −34.33 | −37.30, −31.35 |
| 1.1–3 | −147.20 | −170.56, −123.84 | −106.76 | −128.78, −84.74 | −21.44 | −23.21, −19.67 | −32.59 | −35.60, −29.58 |
| 3.1–10 | −295.96 | −322.99, −268.92 | −209.76 | −236.33, −183.19 | −22.58 | −25.13, −20.03 | −33.53 | −37.04, −30.02 |
| >10 | −378.31 | −427.25, −329.37 | −272.44 | −318.34, −226.55 | −22.51 | −28.81, −16.22 | −32.01 | −38.76, −25.25 |
| P value | <0.001 | <0.001 | 0.21 | 0.75 | ||||
| Never Smokerse | ||||||||
| FEV1 | ||||||||
| ≤1 | 0 | Referent | 0 | Referent | −17.63 | −19.09, −16.16 | −17.16 | −19.90, −14.41 |
| 1.1–3 | −104.28 | −127.92, −80.63 | −81.64 | −104.06, −59.21 | −19.22 | −20.91, −17.54 | −18.53 | −21.36, −15.69 |
| 3.1–10 | −189.76 | −218.58, −160.94 | −151.00 | −179.54, −122.46 | −17.93 | −20.43, −15.43 | −17.15 | −20.52, −13.78 |
| >10 | −263.02 | −317.90, −208.14 | −201.87 | −253.25, −150.49 | −17.56 | −23.66, −11.45 | −15.64 | −22.10, −9.18 |
| P value | <0.001 | <0.001 | 0.36 | 0.65 | ||||
| FVC | ||||||||
| ≤1 | 0 | Referent | 0 | Referent | −20.65 | −22.89, −18.42 | −31.57 | −35.69, −27.45 |
| 1.1–3 | −118.56 | −150.30, −86.83 | −77.76 | −107.61, −47.91 | −21.28 | −23.85, −18.71 | −30.57 | −34.83, −26.30 |
| 3.1–10 | −244.73 | −283.40, −206.05 | −173.89 | −211.88, −135.91 | −21.25 | −25.06, −17.44 | −30.87 | −35.94, −25.81 |
| >10 | −273.65 | −347.30, −200.00 | −175.15 | −243.54, −106.77 | −28.47 | −37.74, −19.19 | −35.99 | −45.70, −26.28 |
| P value | <0.001 | <0.001 | 0.05 | 0.38 | ||||
Abbreviations: CI, confidence interval; CRP, C-reactive protein; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
a Mean difference in spirometry measures in each category of CRP and the reference category (CRP ≤1 mg/L). Multivariable cross-sectional analyses were adjusted for baseline covariates: age, sex, height, body mass index (weight in kilograms divided by height in meters squared), smoking status, physical activity level, alcohol intake, corticosteroid medication use, menopausal status, and hormone replacement therapy use.
b Annual change calculated as an interaction of baseline CRP categories with time in participants who had at least 1 follow-up health examination.
c Adjusted for baseline age and sex.
d Multivariable analyses were additionally adjusted for height, body mass index, smoking status, physical activity level, alcohol intake, corticosteroid medication use, menopausal status, and hormone replacement therapy use, all of which were treated as time-varying variables.
e The number of never smokers available for analysis was 8,382 participants for the cross-sectional analysis and 5,419 participants for the longitudinal analysis.
Table 3.
Association of Contemporaneous and Follow-up Values of Forced Expiratory Volume in 1 Second With Baseline C-Reactive Protein in Participants Who Attended the Baseline and 13-Year Follow-up Health Examinations, European Prospective Investigation Into Cancer in Norfolk, 1993–2011
| Exposure | Baseline Valuesa |
13-Year Follow-upb Values |
||
|---|---|---|---|---|
| Mean Differencec | 95% CI | Mean Differencec | 95% CI | |
| Baseline CRP for all participants, mg/L (n = 5,130) | ||||
| ≤1 | 0 | Referent | 0 | Referent |
| 1.1–3 | −95.4 | −122.1, −68.6 | −93.3 | −121.1, −65.5 |
| 3.1–10 | −169.4 | −204.7, −134.1 | −145.3 | −182.0, −108.6 |
| >10 | −196.4 | −269.8, −123.0 | −188.8 | −265.0, −112.7 |
| Loge-CRP per 1-SD | −70.7 | −84.0, −57.5 | −66.1 | −79.9, −52.4 |
| Baseline CRP for never smokers, mg/L (n = 2,852) | ||||
| ≤1 | 0 | Referent | 0 | Referent |
| 1.1–3 | −72.6 | −108.0, −37.1 | −77.9 | −114.3, −41.5 |
| 3.1–10 | −164.6 | −213.7, −115.5 | −134.8 | −185.2, −84.4 |
| >10 | −158.5 | −259.6, −57.4 | −202.9 | −306.7, −99.2 |
| Loge-CRP per 1-SD | −66.1 | −83.6, −48.5 | −64.7 | −82.7, −46.7 |
Abbreviations: CI, confidence interval; CRP, C-reactive protein; SD, standard deviation.
a All analyses were adjusted for baseline covariates: age, sex, height, body mass index (weight in kilograms divided by height in meters squared), smoking status, physical activity level, alcohol intake, corticosteroid medication use, menopausal status, and hormone replacement therapy use.
b Adjusted for all covariates mentioned above and additionally adjusted for follow-up time.
c Mean difference in forced expiratory volume in 1 second in each category of CRP and the reference category (CRP ≤1 mg/L).
In longitudinal analyses, although there was evidence of significant declines in FEV1 (mean, −18 (SD, 33) mL/year,) and FVC (mean, −21 (SD, 51) mL/year,) over time, the annualized rates of change were not significantly associated with baseline CRP levels (P > 0.2 for continuous baseline loge-CRP*time interaction; Table 2). However, longitudinal change in CRP was inversely associated with longitudinal change in FEV1 (Figure 1) and FVC. A 1-standard-deviation increment in loge-CRP (corresponding to 3-fold higher CRP on the original milligrams per liter scale) over 13 years of follow up was associated with a decline in FEV1 of −69.5 mL (95% CI: −77.5, −61.5) and decline in FVC of −101.2 mL (95% CI: −11.2, −89.9) after adjustment for age, sex, and follow-up time. After adjustment for changes in other covariates in multivariable models, the associations were attenuated but remained statistically significant. The corresponding figures were a −64.0 mL (95% CI: −72.1, −55.8) decline in FEV1 and a −84.0 mL (95% CI: −95.3, −72.8) decline in FVC, respectively. After excluding participants with a CRP level greater than 10 mg/L, results were attenuated but persisted. The corresponding figures were a −53.6 (95% CI: −65.8, −41.5) decline in FEV1 and a −83.3 (−101.0, −65.6) decline in FVC. Restricting the analysis to lifetime nonsmokers did not considerably change the results (not shown).
Figure 1.
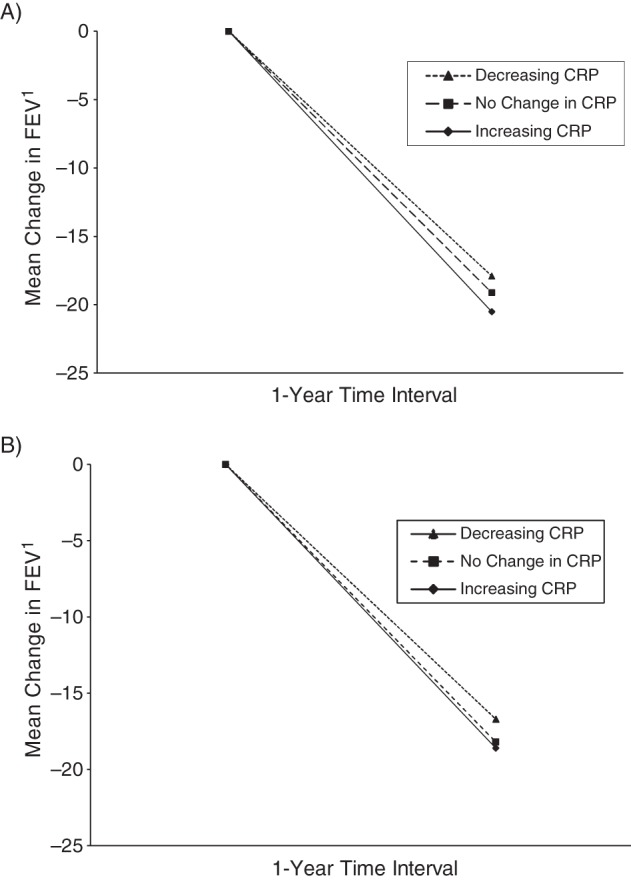
Mean annual change in forced expiratory volume in 1 second (FEV1) by change in C-reactive protein (CRP) level over 13 years among all participants (A) and never smokers (B) in the European Prospective Investigation into Cancer in Norfolk cohort study, 1993–2011. No change in CRP is defined was a difference of less than 1 mg/L in CRP measured at baseline and the 13-year follow up.
Lung function predicting CRP
In cross-sectional multiple regression analysis, participants with higher FEV1 or FVC had lower levels of CRP that persisted in lifetime nonsmokers. However, baseline FEV1 and FVC were not predictive of the rate of change of CRP over time (Appendix Table 1). Nevertheless, longitudinal changes in FEV1 and FVC were inversely associated with longitudinal changes in CRP (data not shown).
DISCUSSION
In the present study, we found a cross-sectional negative association between serum CRP and spirometry measures (FEV1 and FVC). A 1-standard-deviation higher loge-CRP was cross-sectionally associated with an approximately 90 mL lower FEV1 and FVC. Changes in levels of serum CRP over time were also negatively associated with changes in spirometry measures but to a lesser extent to than found in cross-sectional analysis, such that 1-standard-deviation increase in loge-CRP levels over time was associated with an approximately 64-mL decline in FEV1 and an approximately 84-mL decline in FVC. However, baseline CRP levels were not predictive of the rate of decline in spirometry measures over time. These associations were largely independent of age, smoking status, BMI, height, physical activity level, corticosteroid medication use, and hormone replacement therapy use.
The cross-sectional negative associations between CRP and spirometry measures have been previously demonstrated in several studies (6, 7). Similar to our findings, Fogarty et al. (8) found that in a younger community cohort, a 1-mg/L increase in CRP was cross-sectionally associated with a 9-mL decrease in FEV1 and an 11-mL decrease in FVC, and Jiang et al. (12) found that in an older cohort, every 2.8-mg/L increase in CRP was associated with an approximately 14-mL decrease in FEV1.
To the best of our knowledge, only a few studies have assessed the longitudinal association of CRP and lung function. Similar to Jiang et al. (12), who studied 5,800 elderly participants older than 65 years of age, we also observed a somewhat lower rate of decline of FEV1 and FVC in participants with higher baseline CRP levels; however, the differences did not reach statistical significance. We restricted the analyses to persons who attended and had adequate data at both the baseline and follow-up health examinations to avoid a bias caused by lower survival in those with worse lung function and higher CRP. Fogarty et al. (8) and Hancox et al. (13) found no significant association between baseline CRP levels and rate of decline in lung function in younger adult populations. However, contrary to our findings, Rasmussen et al. (15) reported a greater decline in FEV1 in persons with higher baseline CRP among young adults aged 20 years, and Kalhan et al. (16) found a greater decline in FVC, but not FEV1, in young adults with higher baseline CRP in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Similar to our findings, Shaaban et al. (14) found no association between baseline FEV1 and the subsequent rate of change in CRP over time in 531 adults, but they did find an inverse association between change in CRP and change in FEV1 over time.
A limitation of the present study was that CRP at baseline was measured in serum samples that were kept frozen for approximately 15 years. However, previous studies have shown that CRP is relatively stable in frozen plasma (19). CRP at the third health examination was measured in fresh serum samples using a different chemistry analyzer. Differences in methods of measurement of CRP between the 2 time points may have increased the measurement error. However, the correlation coefficient between the 2 measurements of CRP was similar to those of systolic and diastolic blood pressure, total cholesterol, and triglycerides measured at the same time points (20).
Knowledge of biological mechanisms justifies the plausibility of associations in both directions. CRP is a marker of an age-related proinflammatory process described as “inflamm-aging” (21–23). According to this theory, long-term exposure to a variety of antigens (infection, food, etc.) results in progressive filling of the immune system by activated immune cells and proinflammatory cytokines. This process may lead to a reduced capacity to respond to infectious and stress factors later in life and may cause long-term tissue damage. More specifically, chronic inflammation enhances telomere shortening, which in turn has been shown to lead to senescence of lung alveolar and endothelial cells (24, 25). Furthermore, endothelial dysfunction induced by systemic inflammation (26) may lead to pulmonary vascular filtration and lung tissue damage. Further research is required to understand whether a reduced host-defence capacity associated with inflamm-aging, cellular senescence induced by telomere shortening, and also potential lung tissue damage associated with higher inflammatory status and endothelial dysfunction account for a decline in respiratory measures observed in association with increase in CRP.
On the other hand, several studies have found higher levels of inflammatory markers (CRP, interleukin-6, tumor necrosis factor-α) in patients with COPD than in controls (4). The mechanisms by which COPD patients develop systemic inflammation are not well known. Pulmonary epithelial cells have been shown to express CRP and interleukin-6 (25, 27, 28). It has been postulated that the local inflammatory process in the airways associated with COPD might spill into the systemic circulation or otherwise lead to general systemic inflammation (1, 4, 29, 30). Other potential mechanisms include hypoxia and tissue damage caused by oxidative stress in persons with lower lung function and COPD (1, 31) and a reduced ability for physical activity that may in turn lead to increased systemic inflammation (32). Conversely, there might be a common genetic (29) or environmental risk factor (such as smoking, air pollutants, lower intake of antioxidants or vitamins, etc.) (1, 4, 33) that predisposes persons to both decline in lung function (and COPD) and systemic inflammation. In the present study, we were unable to control for potential confounding effect of air pollution and subclinical infections. However, because study participants all lived in the same region with no large industrial cities, the variability in the levels of air pollution between participants was not likely to be large, so the potential confounding effect by air pollutants should not be substantial in the present analysis. We also attempted to minimize the confounding effect of infections by excluding participants with a CRP level greater than 10 mg/L in subsidiary analysis, which did not produce different results. The association observed in the present study between decline in lung function and increase in CRP levels over time, which persisted among lifetime nonsmokers, supports the hypothesis that systemic inflammation may be the link between COPD and extrapulmonary comorbid conditions, such as cardiovascular diseases.
A strength of the present study is the large sample size and the ability to investigate the associations among never smokers. Smoking is a major confounder of the association between CRP and lung function measurements, and adjustment for smoking status or pack-years of cigarette smoked cannot fully eliminate the possibility of residual confounding. In the present study, the associations persisted and were only slightly attenuated among lifetime nonsmokers. Another strength of the present study is the availability of data on multiple confounders and also repeated measures of all variables, which enabled us to better account for the confounding effect of changes in lifestyle factors over time.
Although we observed in longitudinal analysis that changes in CRP were associated with changes in lung function and vice versa, it is not possible to discern the most plausible direction of associations or to infer causality from an observational study. Interventional studies are required to assess the effect of reducing inflammation on changes in lung function and vice versa.
In the present study, we found that lung function and systemic inflammation are inversely interrelated. As lung function improves, systemic inflammation declines, and as persons develop more inflammation, lung function deteriorates.
ACKNOWLEDGMENTS
Author affiliations: Department of Public Health and Primary Care, University of Cambridge, Cambridge, United Kingdom (Sara Ahmadi-Abhari, Stephen Kaptoge, Robert N. Luben, Kay-Tee Khaw); and Medical Research Council Epidemiology Unit, Institute of Metabolic Sciences, Cambridge, United Kingdom (Nicholas J. Wareham).
We thank the general practitioners and the European Prospective Investigation Into Cancer in Norfolk (EPIC-Norfolk) study team for their contribution.
The EPIC-Norfolk study is supported by funding from the Medical Research Council and Cancer Research UK. Sara Ahmadi-Abhari is supported by the Gates Cambridge scholarship.
The funding sources did not have a role in the design and conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript.
Conflict of interest: none declared.
Appendix Table 1.
Cross-sectional and Longitudinal Associations of C-Reactive Protein With Baseline Quartiles of Spirometry Measures in the European Prospective Investigation Into Cancer in Norfolk, 1993–2011
| Quartile of Spirometry Measure | Cross-sectional Association (n = 18,110) |
Longitudinal Association (n = 5,130) |
||||||
|---|---|---|---|---|---|---|---|---|
| Age- and Sex-adjusted |
Multivariable-adjusted |
Age- and Sex-adjusted |
Multivariable-adjusted |
|||||
| Mean Differencea | 95% CI | Mean Differencea | 95% CI | Annual Changeb | 95% CI | Annual Changeb | 95% CI | |
| All Participants | ||||||||
| FEV1c | ||||||||
| 1 | 0 | Referent | 0 | Referent | 0.02 | 0.02, 0.03 | 0.02 | 0.01, 0.03 |
| 2 | −0.21 | −0.25, −0.16 | −0.17 | −0.21, −0.13 | 0.03 | 0.03, 0.04 | 0.03 | 0.02, 0.04 |
| 3 | −0.43 | −0.48, −0.38 | −0.32 | −0.36, −0.27 | 0.04 | 0.04, 0.04 | 0.03 | 0.02, 0.04 |
| 4 | −0.73 | −0.78, −0.67 | −0.54 | −0.59, −0.48 | 0.04 | 0.04, 0.05 | 0.03 | 0.02, 0.04 |
| P value | <0.001 | <0.001 | <0.001 | 0.1 | ||||
| FVCd | ||||||||
| 1 | 0 | Referent | 0 | Referent | 0.02 | 0.02, 0.03 | 0.02 | 0.01, 0.03 |
| 2 | −0.20 | −0.24, −0.15 | −0.14 | −0.19, −0.10 | 0.04 | 0.03, 0.04 | 0.03 | 0.02, 0.04 |
| 3 | −0.36 | −0.40, −0.31 | −0.24 | −0.29, −0.20 | 0.04 | 0.04, 0.04 | 0.03 | 0.02, 0.04 |
| 4 | −0.61 | −0.67, −0.56 | −0.43 | −0.48, −0.37 | 0.04 | 0.04, 0.05 | 0.03 | 0.02, 0.04 |
| P value | <0.001 | <0.001 | <0.001 | 0.07 | ||||
| Never Smokerse | ||||||||
| FEV1c | ||||||||
| 1 | 0 | Referent | 0 | Referent | 0.03 | 0.03, 0.04 | 0.02 | 0.00, 0.03 |
| 2 | −0.19 | −0.26, −0.13 | −0.17 | −0.23, −0.11 | 0.04 | 0.03, 0.04 | 0.02 | 0.01, 0.04 |
| 3 | −0.36 | −0.44, −0.29 | −0.28 | −0.34, −0.21 | 0.05 | 0.04, 0.05 | 0.03 | 0.02, 0.04 |
| 4 | −0.62 | −0.71, −0.52 | −0.44 | −0.53, −0.35 | 0.05 | 0.05, 0.06 | 0.03 | 0.02, 0.05 |
| P value | <0.001 | <0.001 | <0.001 | 0.004 | ||||
| FVCd | ||||||||
| 1 | 0 | Referent | 0 | Referent | 0.03 | 0.02, 0.04 | 0.01 | 0.00, 0.03 |
| 2 | −0.17 | −0.24, −0.11 | −0.14 | −0.20, −0.08 | 0.04 | 0.04, 0.05 | 0.03 | 0.01, 0.04 |
| 3 | −0.32 | −0.39, −0.25 | −0.21 | −0.27, −0.14 | 0.05 | 0.04, 0.05 | 0.03 | 0.01, 0.04 |
| 4 | −0.50 | −0.59, −0.42 | −0.33 | −0.41, −0.24 | 0.05 | 0.05, 0.06 | 0.03 | 0.01, 0.04 |
| P value | <0.001 | <0.001 | <0.001 | 0.004 | ||||
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
a Mean difference in loge-CRP levels between quartiles 2, 3, and 4 of spirometry measures and the lowest (reference) quartile. All analyses were adjusted for baseline covariates: age, sex, height, body mass index (weight in kilograms divided by height in meters squared), smoking status, physical activity level, alcohol intake, corticosteroid medication use, menopausal status, and hormone replacement therapy use.
b Annual change was calculated as an interaction of baseline FEV1 or FVC quartiles with time in participants who had at least 1 follow-up health examination. All regression models were adjusted for baseline age and sex. Multivariable models were additionally adjusted for height, body mass index, smoking status, physical activity level, alcohol intake, corticosteroid medication use, menopausal status, and hormone replacement therapy use, all of which were treated as time-varying variables.
c The ranges of values for each quartile of FEV1 were as follows: quartile 1: 11–198 mL; quartile 2: 199–243 mL; quartile 3: 244–295 mL; and quartile 4: 296–592 mL.
d The ranges of values for each quartile of FVC were as follows: quartile 1: 15–239 mL; quartile 2: 240–295 mL; quartile 3: 296–362 mL; and quartile 4: 363–738 mL.
e The number of never smokers available for analysis was 8,382 participants for the cross-sectional analysis and 2,852 participants for the longitudinal analysis.
REFERENCES
- 1.Agusti A, Soriano JB. COPD as a systemic disease. COPD. 2008;5(2):133–138. doi: 10.1080/15412550801941349. [DOI] [PubMed] [Google Scholar]
- 2.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 3.Eid AA, Ionescu AA, Nixon LS, et al. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(8):1414–1418. doi: 10.1164/ajrccm.164.8.2008109. [DOI] [PubMed] [Google Scholar]
- 4.Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Eeden S, Leipsic J, Paul Man SF, et al. The relationship between lung inflammation and cardiovascular disease. Am J Respir Crit Care Med. 2012;186(1):11–16. doi: 10.1164/rccm.201203-0455PP. [DOI] [PubMed] [Google Scholar]
- 6.van Durme YM, Verhamme KM, Aarnoudse AJ, et al. C-reactive protein levels, haplotypes, and the risk of incident chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(5):375–382. doi: 10.1164/rccm.200810-1540OC. [DOI] [PubMed] [Google Scholar]
- 7.Dahl M, Vestbo J, Zacho J, et al. C reactive protein and chronic obstructive pulmonary disease: a Mendelian randomisation approach. Thorax. 2011;66(3):197–204. doi: 10.1136/thx.2009.131193. [DOI] [PubMed] [Google Scholar]
- 8.Fogarty AW, Jones S, Britton JR, et al. Systemic inflammation and decline in lung function in a general population: a prospective study. Thorax. 2007;62(6):515–520. doi: 10.1136/thx.2006.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl M, Vestbo J, Lange P, et al. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(3):250–255. doi: 10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]
- 10.Kaptoge S, Di AE, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolton CE, Schumacher W, Cockcroft JR, et al. The CRP genotype, serum levels and lung function in men: the Caerphilly Prospective Study. Clin Sci (Lond) 2011;120(8):347–355. doi: 10.1042/CS20100504. [DOI] [PubMed] [Google Scholar]
- 12.Jiang R, Burke GL, Enright PL, et al. Inflammatory markers and longitudinal lung function decline in the elderly. Am J Epidemiol. 2008;168(6):602–610. doi: 10.1093/aje/kwn174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancox RJ, Poulton R, Greene JM, et al. Systemic inflammation and lung function in young adults. Thorax. 2007;62(12):1064–1068. doi: 10.1136/thx.2006.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaaban R, Kony S, Driss F, et al. Change in C-reactive protein levels and FEV1 decline: a longitudinal population-based study. Respir Med. 2006;100(12):2112–2120. doi: 10.1016/j.rmed.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen F, Mikkelsen D, Hancox RJ, et al. High-sensitive C-reactive protein is associated with reduced lung function in young adults. Eur Respir J. 2009;33(2):382–388. doi: 10.1183/09031936.00040708. [DOI] [PubMed] [Google Scholar]
- 16.Kalhan R, Tran BT, Colangelo LA, et al. Systemic inflammation in young adults is associated with abnormal lung function in middle age. PLoS One. 2010;5(7):e11431. doi: 10.1371/journal.pone.0011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day N, Oakes S, Luben R, et al. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer. 1999;80(suppl 1):95–103. [PubMed] [Google Scholar]
- 18.Wareham NJ, Jakes RW, Rennie KL, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation Into Cancer and Nutrition (EPIC) Study. Public Health Nutr. 2003;6(4):407–413. doi: 10.1079/PHN2002439. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson TK, Boman K, Jansson JH, et al. Comparison of soluble thrombomodulin, von Willebrand factor, tPA/PAI-1 complex, and high-sensitivity CRP concentrations in serum, EDTA plasma, citrated plasma, and acidified citrated plasma (Stabilyte) stored at -70 degrees C for 8–11 years. Thromb Res. 2005;116(3):249–254. doi: 10.1016/j.thromres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Ahmadi-Abhari S, Luben R, Wareham NJ, et al. Distribution and determinants of C-reactive protein in the older adult population: European Prospective Investigation Into Cancer-Norfolk Study. Eur J Clin Invest. 2013;43(9):899–911. doi: 10.1111/eci.12116. [DOI] [PubMed] [Google Scholar]
- 21.De Martinis M, Franceschi C, Monti D, et al. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80(3):219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 22.De Martinis M, Franceschi C, Monti D, et al. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579(10):2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 23.Goto M. Inflammaging (inflammation + aging): a driving force for human aging based on an evolutionarily antagonistic pleiotropy theory? Biosci Trends. 2008;2(6):218–230. [PubMed] [Google Scholar]
- 24.MacNee W. Aging, inflammation, and emphysema. Am J Respir Crit Care Med. 2011;184(12):1327–1329. doi: 10.1164/rccm.201110-1764ED. [DOI] [PubMed] [Google Scholar]
- 25.Tuder RM, Kern JA, Miller YE. Senescence in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2012;9(2):62–63. doi: 10.1513/pats.201201-012MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clapp BR, Hingorani AD, Kharbanda RK, et al. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64(1):172–178. doi: 10.1016/j.cardiores.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Ramage L, Proudfoot L, Guy K. Expression of C-reactive protein in human lung epithelial cells and upregulation by cytokines and carbon particles. Inhal Toxicol. 2004;16(9):607–613. doi: 10.1080/08958370490464599. [DOI] [PubMed] [Google Scholar]
- 28.Quay JL, Reed W, Samet J, et al. Air pollution particles induce IL-6 gene expression in human airway epithelial cells via NF-kappaB activation. Am J Respir Cell Mol Biol. 1998;19(1):98–106. doi: 10.1165/ajrcmb.19.1.3132. [DOI] [PubMed] [Google Scholar]
- 29.He Z, Chen Y, Chen P, et al. Local inflammation occurs before systemic inflammation in patients with COPD. Respirology. 2010;15(3):478–484. doi: 10.1111/j.1440-1843.2010.01709.x. [DOI] [PubMed] [Google Scholar]
- 30.Kido T, Tamagawa E, Bai N, et al. Particulate matter induces translocation of IL-6 from the lung to the systemic circulation. Am J Respir Cell Mol Biol. 2011;44(2):197–204. doi: 10.1165/rcmb.2009-0427OC. [DOI] [PubMed] [Google Scholar]
- 31.Takabatake N, Nakamura H, Abe S, et al. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(4):1179–1184. doi: 10.1164/ajrccm.161.4.9903022. [DOI] [PubMed] [Google Scholar]
- 32.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45(10):1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 33.Sawyer K, Mundandhara S, Ghio AJ, et al. The effects of ambient particulate matter on human alveolar macrophage oxidative and inflammatory responses. J Toxicol Environ Health A. 2010;73(1):41–57. doi: 10.1080/15287390903248901. [DOI] [PubMed] [Google Scholar]


