Abstract
Several studies have examined associations between particulate matter with aerodynamic diameter of 2.5 µm or less (PM2.5) and preterm birth, but it is uncertain whether results were affected by individual predispositions (e.g., genetic factors, social conditions) that might vary considerably between women. We tested the hypothesis that a woman is at greater risk of preterm delivery when she has had elevated exposure to ambient PM2.5 during a pregnancy than when she has not by comparing pregnancies in the same woman. From 271,204 births, we selected 29,175 women who had vaginal singleton livebirths at least twice in Connecticut in 2000–2006 (n = 61,688 births). Analyses matched pregnancies to the same woman. Adjusted odds ratios per interquartile range (2.33-µg/m3) increase in PM2.5 in the first trimester, second trimester, third trimester, and whole pregnancy were 1.07 (95% confidence interval (CI): 1.00, 1.15), 0.96 (95% CI: 0.90, 1.03), 1.03 (95% CI: 0.97, 1.08), and 1.13 (95% CI: 1.01, 1.28), respectively. Among Hispanic women, the odds ratio per interquartile range increase in whole-pregnancy exposure was 1.31 (95% CI: 1.00, 1.73). Pregnancies with elevated PM2.5 exposure were more likely to result in preterm birth than were other pregnancies to the same woman at lower exposure. Associations were most pronounced in the first trimester and among Hispanic women.
Keywords: air pollution, environmental pollution, longitudinal studies, particulate matter, pregnancy, pregnancy outcomes, preterm birth
Preterm birth is the leading cause of perinatal morbidity and mortality in developed countries (1), occurring among almost 13% of births in the United States (2). Despite the increasing incidence of cesarean deliveries, the iatrogenic fraction of preterm births (30%–35%) remains small compared with idiopathic preterm births (65%–70%) that occur spontaneously without medical indication (2). Although the pathologies that lead to spontaneous preterm birth are not well understood, the etiology of preterm birth is thought to be multifactorial, involving pathways mediated by inflammation or infection and other immunologically mediated processes (3, 4).
Exposure to ambient air pollution has been suggested as a risk factor for adverse birth outcomes (5, 6). Notably, fine particulate matter with aerodynamic diameter less than 2.5 µm (PM2.5) can elicit a wide range of biological responses. It has been causally linked to other adverse outcomes (7) and has been associated with preterm birth (8–11). The majority of studies have investigated the effects of PM2.5 on fetal growth rather than on preterm birth (12). Studies in the United States have been conducted predominantly on the West Coast (in California) (12), which differs from the northeastern United States in seasonality and the composition of PM2.5 (13). Most importantly, it is uncertain whether the results of past studies were affected by individual predispositions, such as genetic factors (14) or social conditions (1) that might vary considerably between women. Because the incidence of preterm birth varies by race (1), our recent observation that race is associated with exposure to PM2.5 (15) highlights a potential pathway by which between-women comparisons might interfere with observed effect estimates.
We addressed this uncertainty by using a more confirmatory ascertainment of risk. Past studies assessed whether women who have elevated exposures to particulate matter are at greater risk than other women who have lower exposures, comparing pregnancies between women. We tested the hypothesis that a woman is more at risk of preterm delivery when she has elevated exposure to ambient PM2.5 during pregnancy, comparing pregnancies in the same woman.
MATERIALS AND METHODS
Study design and population
This was a longitudinal study of women's exposure to ambient airborne PM2.5 and preterm birth across successive pregnancies in Connecticut from 2000 to 2006. From a population of 271,204 singleton livebirths without congenital anomalies, we sequentially excluded 157 birth records (0.1%) with missing gestational age; 1,846 records (0.9%) that could not be geocoded; 1,505 birth records (0.6%) of women who resided farther than 40 km from a PM2.5 monitoring station; 1,443 records (0.5%) with missing data on parity; and 72,362 births (27.2%) by cesarean delivery. The remaining reference population consisted of birth records for 193,891 neonates born to 152,934 women. The reference population was used to calculate the prevalence of preterm birth by race.
For the longitudinal analyses, this reference population was restricted to records in which at least 75% of the weekly means of 24-hour averaged PM2.5 measurements were available in each trimester and for the whole pregnancy; 24,732 birth records (12.8%) were excluded. The population was then restricted to women who gave birth at least twice during the study period, resulting in the longitudinal study population of 61,688 neonates born to 29,175 women.
Data sources and variables
Birth records were obtained from the Connecticut Department of Health for all registered births in Connecticut from January 1, 2000, to December 31, 2006. Each observation contained variables for the residential location at birth, pregnancy-related risk factors (average number of cigarettes smoked per day during pregnancy, birth order), and sociodemographic risk factors (race/ethnicity, maternal age).
Daily (24-hour) average PM2.5 measurements (taken every third day) were obtained from the closest Environmental Protection Agency (EPA) PM2.5 monitor (16) within 40 km of each woman's residence at the time of birth, including monitors outside Connecticut (n = 14 sites). Similarly, the closest monitoring stations (also within 40 km) were used to obtain daily measurements of carbon monoxide (n = 11 sites), nitrogen dioxide (n = 12 sites), and sulfur dioxide (n = 17 sites). Daily apparent temperature was calculated (17) by using daily measurements for the closest monitor from the National Climatic Data Center (in 2006). Copollutant and temperature exposures were generated by using the same procedure described for PM2.5 exposure.
Outcome assessment
Preterm birth was defined as birth before 37 weeks’ completed gestation. Period of gestation was obtained from birth certificate records. This was the best clinical estimate of gestational age, based on ultrasonography data or on last menstrual period if ultrasonography data were not available. First and second trimesters were defined as weeks 1–13 and 14–26, respectively. The third trimester was defined as commencing at week 27 and ending at the end of week 36 or at birth, whichever was earlier.
Exposure assessment
Daily PM2.5 measurements from the closest EPA monitor within 40 km of a subject's residence were assigned to each woman. The 40-km distance was selected as the initial maximum distance to minimize exposure misclassification arising from more distant sources (e.g., distant cities). It was chosen to include almost all women in the study (99.4%) and because preliminary research indicated that PM2.5 total mass measurements from pairs of monitors had high correlation (r ≥ 0.9) within this distance. This distance is also comparable with that of 50 km used in a recent study evaluating exposure assessment among pregnant women (18). Weekly mean PM2.5 levels were calculated as 7-day averages for each monitor over the 1999–2006 period. Only monitors for which there were at least 75% of these weekly measurements available for the period 1999–2006 were included. This minimized the number of monitoring sites used to assign exposure to any particular woman and thereby also reduced the influence of local monitoring site characteristics on exposure estimates. For example, had this restriction not been applied and had a monitor been missing all measurements for a woman's third trimester, whole-pregnancy exposure would be nonestimable or would require measurements from another nearby monitor. Mean exposures were computed for each week of gestation and were then used to compute exposure for each trimester and for the whole pregnancy. This avoids bias due to changes in the frequency of PM2.5 measurements (19). Only measurements to week 36 were included in third-trimester and whole-pregnancy analyses.
Statistical methods and analyses
To examine the potential for confounding by secular trends caused by factors unrelated to PM2.5, the rate of preterm birth was calculated (by race) for the reference population by year of conception and was compared (by site) with the temporal trend in mean PM2.5.
Spatiotemporal variation in exposure was partitioned by using random intercepts models fit with Proc Mixed in SAS, version 9.3, software (SAS Institute, Inc., Cary, North Carolina). Pregnancies were matched by mother, and statistical associations were investigated with conditional logistic regression by using odds ratios, 95% confidence intervals, and 2-sided P values. Separate models were fitted for each trimester and for whole-pregnancy exposure. Adjustment was made for the average number of cigarettes smoked per day (none, 1–9, 10–20, or >20), maternal age in years (<20, 20–24, 25–29, 30–34, 35–39, or ≥40), and parity (0, 1, 2, or ≥3 children). Adjustment was made for these factors because of their potential to change considerably between pregnancies and because they are strong independent risk factors for preterm birth (1, 2).
RESULTS
Prevalence of preterm birth in the reference population
The prevalence of preterm birth by vaginal delivery differed considerably by race, with a small upward trend over the period (Figure 1). The prevalence rates of preterm birth to black women, Hispanic women, and white women were 9.44%, 7.0%, and 5.3%, respectively. Preterm birth occurred among 6.2% of vaginal singleton livebirths.
Figure 1.
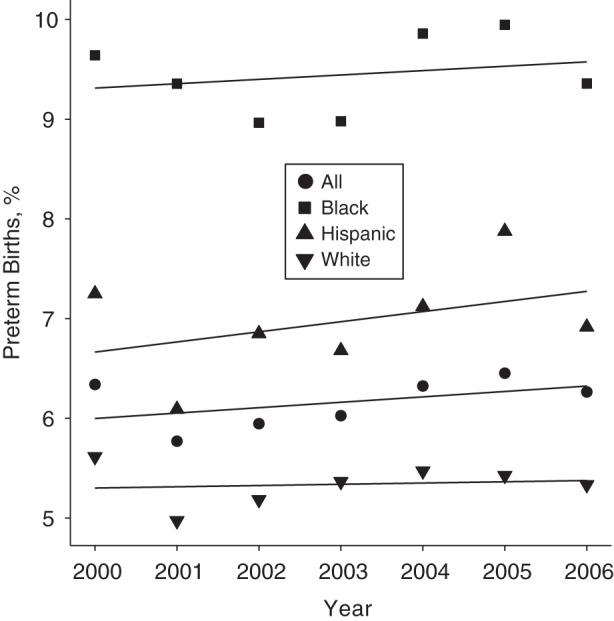
Rates of preterm birth by race (non-Hispanic white, non-Hispanic black, Hispanic) with annual trend for the reference population (n = 193,891 neonates) in Connecticut, 2000–2006.
Characteristics of the longitudinal study population at study entry
The requirement of at least 2 births for inclusion in the study resulted in few women (0.6%) over age 40 years (n = 174) and more women (11.4%) aged less than 20 years (n = 3,312) (Table 1). Fewer women (14.3%) had less than 12 years of education (n = 4,121). The majority of women (68.9%) were married (n = 20,097), white (67.1%) (n= 19,394), nonsmokers (94.2%) (n = 27,377), and had no other children (64.8%) (n = 18,912).
Table 1.
Characteristics and Birth Outcomes at Study Entry for Women (n = 29,175) Residing in Connecticut Who Delivered at Least 2 Singleton Neonates Without Congenital Anomaly by Vaginal Delivery, 2000–2006
| Characteristic | No. of Women | % |
|---|---|---|
| Age of mother, years | ||
| <20 | 3,312 | 11.4 |
| 20–24 | 5,768 | 19.8 |
| 25–29 | 8,507 | 29.2 |
| 30–34 | 8,906 | 30.5 |
| 35–39 | 2,508 | 8.6 |
| ≥40 | 174 | 0.6 |
| Educational level, years | ||
| <12 | 4,121 | 14.3 |
| 12 | 6,696 | 23.2 |
| 13–15 | 5,714 | 19.8 |
| 16 | 6,681 | 23.2 |
| >16 | 5,609 | 19.5 |
| Marital status | ||
| Not married | 9,073 | 31.1 |
| Married | 20,097 | 68.9 |
| Race/ethnicity | ||
| White (non-Hispanic) | 19,394 | 67.1 |
| Black (non-Hispanic) | 2,960 | 10.3 |
| Asian | 1,033 | 3.6 |
| Hispanic | 4,940 | 17.1 |
| Other | 557 | 1.9 |
| Mother's parity | ||
| No children | 18,912 | 64.8 |
| 1 Child | 6,617 | 22.7 |
| 2 Children | 2,405 | 8.2 |
| ≥3 Children | 1,241 | 4.3 |
| Smoking, cigarettes per day | ||
| None | 27,377 | 94.2 |
| 1–9 | 801 | 2.8 |
| 10–20 | 860 | 3.0 |
| >20 | 31 | 0.1 |
| Birth outcomes during the study period | ||
| Delivered only preterm neonates | 349 | 1.2 |
| Delivered only term neonates | 26,269 | 90.0 |
| Delivered preterm and term neonates | 2,557 | 8.8 |
Preterm birth outcomes during the study period
A small proportion (1.2%) of women delivered only preterm neonates (n = 349) (Table 1). There were 2,557 women (8.8%) who delivered both preterm and term neonates.
Amount of variation in gestational age explained by individual factors
Gestational age differed more between women than between pregnancies to the same woman, indicating the importance of less understood factors such as genetics, social environmental factors, and recurrent health-related behaviors. For gestational age (in weeks), 66.0% of the variation occurred between women, with the remaining 34.1% due to variation between pregnancies to the same woman.
Changes in characteristics of the longitudinal study population between first and last births
Less than half (45.7%) of the women remained within the same 5-year age category between their first and last births in the study period (n = 13,327). Although 5,553 women (19.4%) changed educational categories during the study period, the reported number of years of education decreased among 2,151 women (7.5%), which implied poor reliability of this variable for indicating change in educational level. There were 2,378 women (8.2%) who changed their marital status, 1,998 of whom (6.9%) changed from unmarried to married. There were 1,955 women (6.7%) who changed level of smoking between their first and last births in the study period, with an increase in level of smoking among 1,057 women (3.6%). Almost half (45.4%) of the women changed residential location between their first and last births in the study period (n = 13,240). The median distance moved was 4.60 km (25th percentile = 1.91 km, 75th percentile = 12.88 km). Among women who moved, approximately half (55.5%) moved farther away from a PM2.5 monitor. Among women who moved farther away from a monitor, the median distance-to-monitor increase was 2.23 km (25th percentile = 0.70 km, 75th percentile = 5.95 km).
Exposure to ambient PM2.5 mass concentration
Levels of ambient PM2.5 varied by monitoring site, with a secular decrease during the study period (Web Figure 1, available at http://aje.oxfordjournals.org/). The majority of variation in daily measurements of PM2.5 was due to temporal factors rather than spatial factors, with 7.1% of variation explained by site and 92.9% explained by within-site variation (day of measurement).
However, because a pregnancy spans multiple seasons, 78.5% of the variation in mean pregnancy exposure to PM2.5 was explained by site, and 21.5% was explained by within-site variation (timing of pregnancy). More variation was explained by time of pregnancy for trimester-specific exposure than for whole-pregnancy exposure. Timing of pregnancy explained 53.5% and 52.5% of the variation in first- and second-trimester PM2.5 exposures, respectively. Timing of pregnancy explained more variation (63.3%) in third-trimester exposure because the levels are averaged over a shorter period of time in the third trimester for preterm births and are therefore more likely to incorporate seasonal variation in emissions and meteorological factors that affect pollutant levels.
Median exposure to PM2.5 in pregnancy was 12.38 µg/m3 (Table 2). At each period of pregnancy, exposure to PM2.5 was positively correlated with carbon monoxide and sulfur dioxide levels, negligibly correlated with temperature, and negatively correlated with nitrogen dioxide levels (Web Table 1). After adjustment for clustering in PM2.5 exposure at the individual-mother level, there were negative associations among exposures for adjacent trimesters and strong positive associations between first- and third-trimester exposures to PM2.5 (Web Table 2). The associations among trimester exposures varied by race. Among Hispanic women, second-trimester exposure decreased by 10% of the observed increase in first-trimester exposure, whereas there was insufficient evidence of such an association among white women.
Table 2.
Exposure to PM2.5, Copollutants and Ambient Temperature for the Longitudinal Study Population Residing in Connecticut and Giving Birth at Least Twice During 2000–2006
| Exposure | First Trimester |
Second Trimester |
Third Trimester |
Whole Pregnancy |
||||
|---|---|---|---|---|---|---|---|---|
| Median (IQR) | 25th–75th Percentile | Median (IQR) | 25th–75th Percentile | Median (IQR) | 25th–75th Percentile | Median (IQR) | 25th–75th Percentile | |
| PM2.5, µg/m3 | 12.41 (3.49) | 10.69–14.18 | 12.30 (3.41) | 10.65–14.06 | 12.13 (3.96) | 10.42–14.38 | 12.38 (2.33) | 11.26–13.59 |
| Carbon monoxide, ppb | 496.42 (278.19) | 374.03–652.22 | 491.40 (274.78) | 376.09–650.86 | 478.41 (279.71) | 363.30–643.00 | 483.24 (261.80) | 380.73–642.53 |
| Nitrogen dioxide, ppb | 20.06 (7.22) | 16.33–23.56 | 19.99 (7.44) | 16.01–23.44 | 19.65 (7.79) | 15.35–23.14 | 19.92 (6.74) | 16.83–23.57 |
| Sulfur dioxide, ppb | 4.05 (3.41) | 2.65–6.06 | 4.12 (3.51) | 2.65–6.17 | 3.95 (3.64) | 2.38–6.03 | 4.48 (2.14) | 3.39–5.53 |
| Temperature, maximum °C | 14.85 (16.16) | 6.99–23.15 | 14.01 (15.90) | 6.71–22.61 | 15.17 (16.79) | 6.53–23.32 | 14.37 (6.82) | 11.25–18.07 |
Abbreviations: IQR, interquartile range; PM2.5, particulate matter with aerodynamic diameter of 2.5 µm or less; ppb, parts per billion.
Change in PM2.5 mass concentration exposure between births
We calculated the change in PM2.5 mass concentration exposure between the first 2 births from study entry for different outcome combinations. Exposure was more likely to decrease over time because of the decreasing trend in PM2.5 mass concentration. The mean whole-pregnancy exposures for the first and second births to each woman in the study period were 12.69 µg/m3 and 12.36 µg/m3, respectively (P < 0.001). The proportion of women with a decrease in exposure from first to second birth was calculated for women with 2 term births, those with a term birth followed by a preterm birth, those with a preterm birth followed by a term birth, and those with 2 preterm births. Compared with women who had 2 term births, these proportions were 2.5% lower (P = 0.097) and 0.8% higher (P = 0.588) for women with a term birth followed by a preterm birth and those with a preterm birth followed by a term birth, respectively. Compared with women who had 2 preterm births, these proportions were 1.5% lower (P = 0.617) and 1.8% higher (P = 0.531) for women who had a term birth followed by a preterm birth and for those who had a preterm birth followed by a term birth, respectively.
Association between PM2.5 mass concentration and preterm birth by exposure period
We observed the strongest evidence for an association of preterm birth with PM2.5 exposure in the first trimester and weaker evidence for third-trimester and whole-pregnancy exposures (Table 3). The odds of preterm birth increased by a factor of 1.10 (95% CI: 1.03, 1.17) per interquartile range increase in first-trimester PM2.5. There was weaker evidence for an association in the opposite direction for second-trimester exposure. The odds of preterm birth decreased by a factor of 0.93 (95% CI: 0.87, 0.99) per interquartile range increase in second-trimester PM2.5. Because of the autocorrelation in PM2.5 exposures, we assessed the independent associations of each trimester conditional on exposure in other trimesters (Table 3). We observed stronger evidence for first-trimester PM2.5 exposure (odds ratio = 1.07, 95% CI: 1.00, 1.15).
Table 3.
Odds Ratios by Exposure Period and Race for Preterm Births per Interquartile Range Increase in Whole-Pregnancy PM2.5, Comparing Each Woman's Preterm Pregnancy With Her Term Pregnancy, Connecticut, 2000–2006
| Exposure Period by Race | No. of Births | No. of Womena | Stratab |
Unadjusted |
Adjustedc |
Fully Adjustedd |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | OR | 95% CI | P Valuee | OR | 95% CI | P Value | OR | 95% CI | P Value | |||
| All women | ||||||||||||
| First trimester | 61,688 | 29,175 | 8.8 | 1.11 | 1.05, 1.19 | 0.001 | 1.10 | 1.03, 1.17 | 0.005 | 1.07 | 1.00, 1.15 | 0.065 |
| Second trimester | 61,688 | 29,175 | 8.8 | 0.94 | 0.89, 1.01 | 0.070 | 0.93 | 0.87, 0.99 | 0.026 | 0.96 | 0.90, 1.03 | 0.292 |
| Third trimester | 61,688 | 29,175 | 8.8 | 1.06 | 1.01, 1.12 | 0.020 | 1.06 | 1.00, 1.11 | 0.037 | 1.03 | 0.97, 1.08 | 0.390 |
| Whole pregnancy | 61,688 | 29,175 | 8.8 | 1.18 | 1.05, 1.33 | 0.005 | 1.13 | 1.00, 1.28 | 0.043 | |||
| White | ||||||||||||
| First trimester | 41,118 | 19,939 | 7.2 | 1.05 | 0.96, 1.14 | 0.273 | 1.02 | 0.94, 1.11 | 0.626 | 1.00 | 0.91, 1.09 | 0.913 |
| Second trimester | 41,118 | 19,939 | 7.2 | 0.98 | 0.90, 1.07 | 0.669 | 0.94 | 0.86, 1.03 | 0.190 | 0.95 | 0.87, 1.05 | 0.310 |
| Third trimester | 41,118 | 19,939 | 7.2 | 1.05 | 0.98, 1.12 | 0.148 | 1.04 | 0.97, 1.11 | 0.280 | 1.03 | 0.95, 1.11 | 0.488 |
| Whole pregnancy | 41,118 | 19,939 | 7.2 | 1.11 | 0.96, 1.29 | 0.154 | 1.02 | 0.88, 1.20 | 0.768 | |||
| Black | ||||||||||||
| First trimester | 6,378 | 3,117 | 11.7 | 1.16 | 0.98, 1.37 | 0.076 | 1.17 | 0.98, 1.38 | 0.079 | 1.13 | 0.93, 1.38 | 0.217 |
| Second trimester | 6,378 | 3,117 | 11.7 | 0.93 | 0.79, 1.10 | 0.411 | 0.93 | 0.78, 1.10 | 0.391 | 1.03 | 0.84, 1.26 | 0.814 |
| Third trimester | 6,378 | 3,117 | 11.7 | 1.10 | 0.97, 1.26 | 0.136 | 1.12 | 0.98, 1.27 | 0.107 | 1.08 | 0.93, 1.26 | 0.300 |
| Whole pregnancy | 6,378 | 3,117 | 11.7 | 1.35 | 0.98, 1.88 | 0.068 | 1.39 | 0.99, 1.96 | 0.056 | |||
| Hispanic | ||||||||||||
| First trimester | 10,656 | 5,241 | 10.1 | 1.23 | 1.07, 1.41 | 0.003 | 1.25 | 1.08, 1.44 | 0.003 | 1.18 | 1.00, 1.39 | 0.045 |
| Second trimester | 10,656 | 5,241 | 10.1 | 0.88 | 0.77, 1.00 | 0.054 | 0.85 | 0.74, 0.98 | 0.022 | 0.93 | 0.80, 1.08 | 0.345 |
| Third trimester | 10,656 | 5,241 | 10.1 | 1.12 | 1.01, 1.26 | 0.040 | 1.13 | 1.01, 1.27 | 0.039 | 1.06 | 0.93, 1.20 | 0.394 |
| Whole pregnancy | 10,656 | 5,241 | 10.1 | 1.34 | 1.03, 1.75 | 0.031 | 1.31 | 1.00, 1.73 | 0.054 | |||
Abbreviations: CI, confidence interval; OR, odds ratio; PM2.5, particulate matter with aerodynamic diameter of 2.5 µm or less.
a Numbers of women may not equal those in Table 1. Counts in Table 1 were determined at the time of first birth (at study entry). Table 3 counts reflect women who ever identified with the particular race/ethnicity during the study period.
b Informative strata is the number of women with both preterm and term births during the study period.
c Adjusted for parity, maternal age, and smoking tobacco during pregnancy.
d Adjusted for parity, maternal age, smoking tobacco during pregnancy, and exposure to PM2.5 in both of the other trimesters.
e P values are 2-sided from Wald χ2 test statistics.
Association between PM2.5 mass concentration and preterm birth by race
There was negligible evidence of an association between PM2.5 mass concentration and preterm birth for white women and weak evidence for black women. For black women, for each interquartile range increase in whole-pregnancy exposure to PM2.5, the odds of preterm birth increased by a factor of 1.39 (95% CI: 0.99, 1.96). For Hispanic women, the odds of preterm birth increased by factors of 1.25 (95% CI: 1.08, 1.44), 1.13 (95% CI: 1.01, 1.27), and 1.31 (95% CI: 1.00, 1.73) per interquartile range increase in first-trimester, third-trimester, and whole-pregnancy exposures, respectively. A protective effect was observed among these women for second-trimester exposure. However, second-trimester exposure was negatively correlated with exposures in the first trimester (r = −0.02, P = 0.0382) and third trimester (r = −0.09, P < 0.0001). After simultaneous adjustment for exposure in the other trimesters (full adjustment), the association with second-trimester exposure (odds ratio = 0.93, 95% CI: 0.80, 1.08) and third-trimester exposure (odds ratio = 1.06, 95% CI: 0.93, 1.20) became statistically nonsignificant, whereas the adverse association for first-trimester exposure remained (odds ratio = 1.18, 95% CI: 1.00, 1.39) (Table 3).
Robustness of the associations
Odds ratios per interquartile range increase in whole-pregnancy PM2.5 exposure were robust to separate adjustments for carbon monoxide, nitrogen dioxide, sulfur dioxide, ambient maximum temperature, and area-level socioeconomic factors. Adjustment for a propensity score derived from the timing of pregnancy resulted in an increase in the odds ratio estimate, although the 95% confidence intervals overlapped. Analyses were also restricted to women living within 10 km, 20 km, or 30 km of a monitor. At each distance, the associations remained statistically significant. The intervals overlapped considerably, and point estimates increased with restriction to participants who lived closer to a monitor. Details of these sensitivity analyses are provided in Web Table 3 and the Web Appendix.
DISCUSSION
Summary of results
We observed an adverse association with preterm birth for elevated maternal exposure to PM2.5 in the first trimester of pregnancy. Per interquartile range increase in first-trimester exposure to PM2.5, there was weak evidence for a 7% increase in risk of preterm birth among all women and strong evidence for an 18% increase in risk among Hispanic women. Odds ratio estimates for black women were comparable to those for Hispanic women, but interval estimates were wider, possibly because of the smaller cohort size. There was negligible evidence for an association among white women. Odds ratios observed in this study appeared to be robust to adjustment for exposure to ambient air pollutants and temperature. Moreover, estimates were greater after adjustment for unmeasured temporal factors (propensity score), indicating that this score is more likely to be a proxy for risk factors independent of PM2.5 rather than confounders, and that estimates unadjusted for this score were attenuated. It also appeared that odds ratios were attenuated by exposure misclassification due to distance from an EPA monitor. Although the intervals overlapped, the point estimates increased with decreasing exposure misclassification (shorter distance to a monitor). We observed adverse associations among a cohort whose median whole-pregnancy exposure (12.38 µg/m3) was close to the EPA annual standard (12 µg/m3) set under the authority of the Clean Air Act of 1970 as requisite to protect public health with an adequate margin of safety (20). The annual standard recommended by the World Health Organization (Geneva, Switzerland) is 10 µg/m3 (21).
Comparison with past studies
It is difficult to directly compare the risk estimates from this study with those reported in past studies, because this is the first longitudinal study accounting for individual-level risk. Adverse associations with preterm birth have been reported for elevated first-trimester exposure to total suspended particles in the Czech Republic (22); more coarse particulates (particulate matter with aerodynamic diameter less than 10 µm) in California (23), South Korea (24) and Australia (25); and PM2.5 in California (8, 9). However, few studies have reported associations for all trimesters of exposure (12), and therefore the influence of selective reporting is unclear. A recent meta-analysis reported a pooled odds ratio for preterm birth of 1.05 (95% CI: 0.98, 1.13) per 10-µg/m3 increase in third-trimester exposure to ambient PM2.5 (6). We also observed an adverse association with preterm birth for elevated exposure in the third trimester. However, we did not observe sufficient evidence to suggest that the adverse association for third-trimester exposure persists after adjustment for exposure in other trimesters. Our results are also likely to be different from those of the meta-analysis because 1) we investigated individual-level risk rather than its approximation from between-women comparisons, 2) only 4 studies contributed to the pooled estimate, and 3) we included only pregnancies at risk of preterm birth by excluding cesarean deliveries, the majority of which occur without labor (26).
Limitations
From a large base population (>270,000 births), we obtained a longitudinal population of approximately 61,000 births over a 7-year period. This allowed estimation of risk estimates for larger subpopulations (white, Hispanic), but the smaller cohort size for black women limited statistical power. Similarly, longer periods are needed with residence-to-monitor distances of less than 10 km to study associations with risk of preterm birth. Although we obtained highly temporally resolved measurements of PM2.5 total mass (a 24-hour sample taken at least every third day), spatial resolution was limited by the number and location of EPA monitoring stations. However, exposures were not driven primarily by timing of pregnancy, because pregnancies are distributed throughout the year. In fact, the timing of pregnancy explained as much of the variation in trimester exposures as did residential location. We did not identify the specific source or the chemical composition of the PM2.5 associated with preterm birth. However, PM2.5 is still regulated on the basis of total mass, not chemical composition, and therefore epidemiologic studies of total mass are necessary to inform the setting or revision of these standards. Women excluded on the basis of the exposure criterion were similar in sociodemographic characteristics to the study population (Web Table 4). However, excluded women were slightly more likely to be older, married, and white and to have more education and larger family sizes. Moreover, the proportion of women who delivered both preterm and term neonates (the informative strata) in the excluded population was similar to that of the study population. Finally, we note that future studies would additionally benefit from 1) a large longitudinal (i.e. maternally linked) cohort, 2) focus on more vulnerable populations (such as black and Hispanic women), 3) knowledge of the timing of intermediary precursors of preterm birth, and 4) in the absence of this knowledge, the ability to separately identify cesarean deliveries with labor and those without labor.
Biological plausibility
Inhaled PM2.5 can penetrate the gas exchange region of the lungs and enter the bloodstream, taking with it large amounts of biologically harmful radicals that also cause DNA damage (27), toxic chemicals such as carcinogenic polycyclic aromatic hydrocarbons, and harmful metals (28). Mechanisms resulting in preterm birth might be similar to those of cardiovascular endpoints (29) for which causal associations with PM2.5 have been concluded (7). Although the mechanisms by which PM2.5 may lead to preterm birth are not as well understood (30), elevated exposure to PM2.5 might lead to preterm birth by increasing susceptibility to infection (31, 32), by interfering with placental development (33), or through an abnormal production or early activation of cytokines favoring inflammation, which are part of the body's preparation for parturition (34, 35).
Because almost two-thirds of the variation in gestational age was due to variability between women, this study, comparing multiple pregnancies in the same woman, is less subject to unmeasured factors that vary across women than were previous studies. Socioeconomic status, in particular, is an abstract concept used to reflect the social determinants of health and is difficult to measure (36, 37). It is strongly associated with preterm birth and can exhibit inequitable exposure distributions (15). Perhaps more important are the factors marking individual predisposition that are not only difficult to measure but are yet unidentified. We addressed this uncertainty by using a longitudinal design. In this study, pregnancies with elevated PM2.5 exposure were more likely to result in preterm birth than were other pregnancies to the same women at lower levels of exposure. Associations were more pronounced in the first trimester and among Hispanic women.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Center for Perinatal Pediatric and Environmental Epidemiology, School of Medicine, Yale University, New Haven, Connecticut (Gavin Pereira, Kathleen Belanger, Michelle L. Bell); and School of Forestry and Environmental Studies, Yale University, New Haven, Connecticut (Keita Ebisu, Michelle L. Bell).
This work was supported by the National Institute of Environmental Health Sciences (grants R0101ES016317 and R01ES019587).
Conflict of interest: none declared.
REFERENCES
- 1.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362(6):529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(3 suppl):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas JS, Fuentes-Afflick E, Stewart AL, et al. Prepregnancy health status and the risk of preterm delivery. Arch Pediatr Adolesc Med. 2005;159(1):58–63. doi: 10.1001/archpedi.159.1.58. [DOI] [PubMed] [Google Scholar]
- 5.Shah PS, Balkhair T Knowledge Synthesis Group on Determinants of Preterm/LBW births. Air pollution and birth outcomes: a systematic review. Environ Int. 2011;37(2):498–516. doi: 10.1016/j.envint.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Stieb DM, Chen L, Eshoul M, et al. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117(1):100–111. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 8.Huynh M, Woodruff TJ, Parker JD, et al. Relationships between air pollution and preterm birth in California. Paediatr Perinat Epidemiol. 2006;20(6):454–461. doi: 10.1111/j.1365-3016.2006.00759.x. [DOI] [PubMed] [Google Scholar]
- 9.Ritz B, Wilhelm M, Hoggatt KJ, et al. Ambient air pollution and preterm birth in the environment and pregnancy outcomes study at the University of California, Los Angeles. Am J Epidemiol. 2007;166(9):1045–1052. doi: 10.1093/aje/kwm181. [DOI] [PubMed] [Google Scholar]
- 10.Brauer M, Lencar C, Tamburic L, et al. A cohort study of traffic-related air pollution impacts on birth outcomes. Environ Health Perspect. 2008;116(5):680–686. doi: 10.1289/ehp.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang HH, Reich BJ, Miranda ML. Time-to-event analysis of fine particle air pollution and preterm birth: results from North Carolina, 2001–2005. Am J Epidemiol. 2012;175(2):91–98. doi: 10.1093/aje/kwr403. [DOI] [PubMed] [Google Scholar]
- 12.Bosetti C, Nieuwenhuijsen MJ, Gallus S, et al. Ambient particulate matter and preterm birth or birth weight: a review of the literature. Arch Toxicol. 2010;84(6):447–460. doi: 10.1007/s00204-010-0514-z. [DOI] [PubMed] [Google Scholar]
- 13.Bell ML, Dominici F, Ebisu K, et al. Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environ Health Perspect. 2007;115(7):989–995. doi: 10.1289/ehp.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kistka ZA, DeFranco EA, Ligthart L, et al. Heritability of parturition timing: an extended twin design analysis. Am J Obstet Gynecol. 2008;199(1):43.e1–43.e5. doi: 10.1016/j.ajog.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Bell M, Ebisu K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ Health Perspect. 2012;120(12):1699–1704. doi: 10.1289/ehp.1205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Environmental Protection Agency. Washington, DC: Environmental Protection Agency; 2012. Download detailed Air Quality System data. http://www.epa.gov/ttn/airs/airsaqs/detaildata/downloadaqsdata.htm. (Accessed October 1, 2012) [Google Scholar]
- 17.Kalkstein LS, Valimont KM. An evaluation of summer discomfort in the United-States using a relative climatological index. Bull Am Meteorol Soc. 1986;67(7):842–848. [Google Scholar]
- 18.Nethery E, Leckie SE, Teschke K, et al. From measures to models: an evaluation of air pollution exposure assessment for epidemiological studies of pregnant women. Occup Environ Med. 2008;65(9):579–586. doi: 10.1136/oem.2007.035337. [DOI] [PubMed] [Google Scholar]
- 19.Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115(7):1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.1970. US Environmental Protection Agency Clean Air Act. 42 USC §7401.
- 21.World Health Organization. Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 22.Bobak M. Outdoor air pollution, low birth weight, and prematurity. Environ Health Perspect. 2000;108(2):173–176. doi: 10.1289/ehp.00108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritz B, Yu F, Chapa G, et al. Effect of air pollution on preterm birth among children born in Southern California between 1989 and 1993. Epidemiology. 2000;11(5):502–511. doi: 10.1097/00001648-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Leem JH, Kaplan BM, Shim YK, et al. Exposures to air pollutants during pregnancy and preterm delivery. Environ Health Perspect. 2006;114(6):905–910. doi: 10.1289/ehp.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen C, Neller A, Williams G, et al. Maternal exposure to low levels of ambient air pollution and preterm birth in Brisbane, Australia. BJOG. 2006;113(8):935–941. doi: 10.1111/j.1471-0528.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Zeki R, Hilder L, et al. Canberra, Australia: Australian Institute of Health and Welfare, National Perinatal Epidemiology and Statistics Unit; 2012. Perinatal statistics series no. 27. Australia's mothers and babies 2010 http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=60129542372. (Accessed October 1, 2012) [Google Scholar]
- 27.Dellinger B, Pryor WA, Cueto R, et al. Role of free radicals in the toxicity of airborne fine particulate matter. Chem Res Toxicol. 2001;14(10):1371–1377. doi: 10.1021/tx010050x. [DOI] [PubMed] [Google Scholar]
- 28.Sørensen M, Autrup H, Hertel O, et al. Personal exposure to PM2.5 and biomarkers of DNA damage. Cancer Epidemiol Biomarkers Prev. 2003;12(3):191–196. [PubMed] [Google Scholar]
- 29.van Eeden SF, Yeung A, Quinlam K, et al. Systemic response to ambient particulate matter relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(1):61–67. doi: 10.1513/pats.200406-035MS. [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Ding H, Wang X. Acute effects of total suspended particles and sulfur dioxides on preterm delivery: a community-based cohort study. Arch Environ Health. 1995;50(6):407–415. doi: 10.1080/00039896.1995.9935976. [DOI] [PubMed] [Google Scholar]
- 31.Slama R, Darrow L, Parker J, et al. Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect. 2008;116(6):791–798. doi: 10.1289/ehp.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibbs R, Romero R, Hillier S, et al. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166(5):1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 33.Dejmek J, Solanský I, Benes I, et al. The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. Environ Health Perspect. 2000;108(12):1159–1164. doi: 10.1289/ehp.001081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keelan J, Blumenstein M, Helliwell R, et al. Cytokines, prostaglandins and parturition: a review. Placenta. 2003;24:33S–46S. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 35.Engel SA, Erichsen HC, Savitz DA, et al. Risk of spontaneous preterm birth is associated with common proinflammatory cytokine polymorphisms. Epidemiology. 2005;16(4):469–477. doi: 10.1097/01.ede.0000164539.09250.31. [DOI] [PubMed] [Google Scholar]
- 36.Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007;99(9):1013–1023. [PMC free article] [PubMed] [Google Scholar]
- 37.Messer LC, Vinikoor LC, Laraia BA, et al. Socioeconomic domains and associations with preterm birth. Soc Sci Med. 2008;67(8):1247–1257. doi: 10.1016/j.socscimed.2008.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


