Abstract
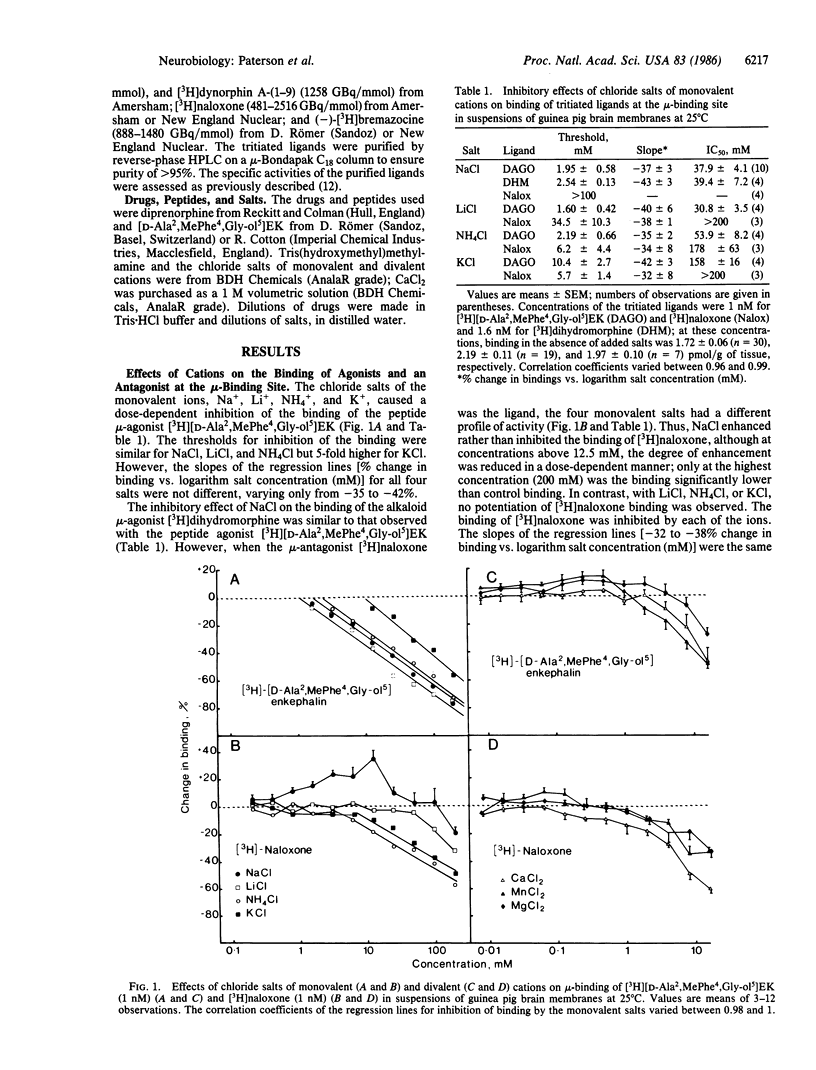
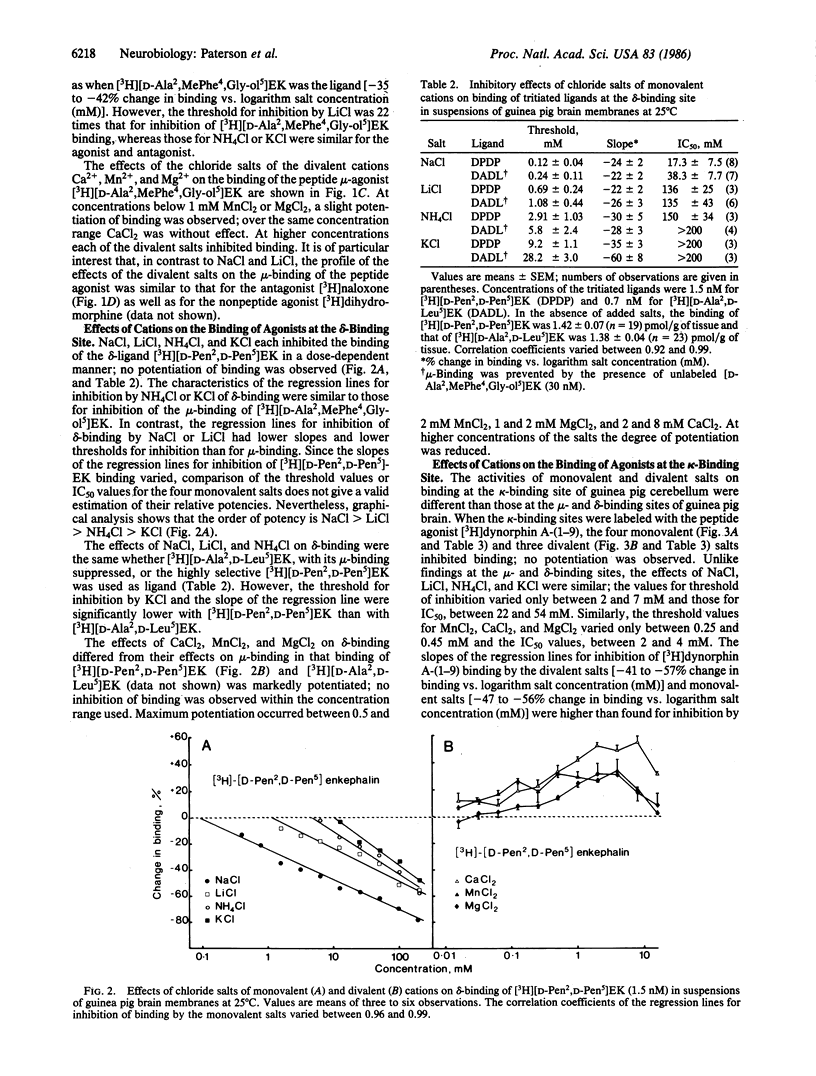
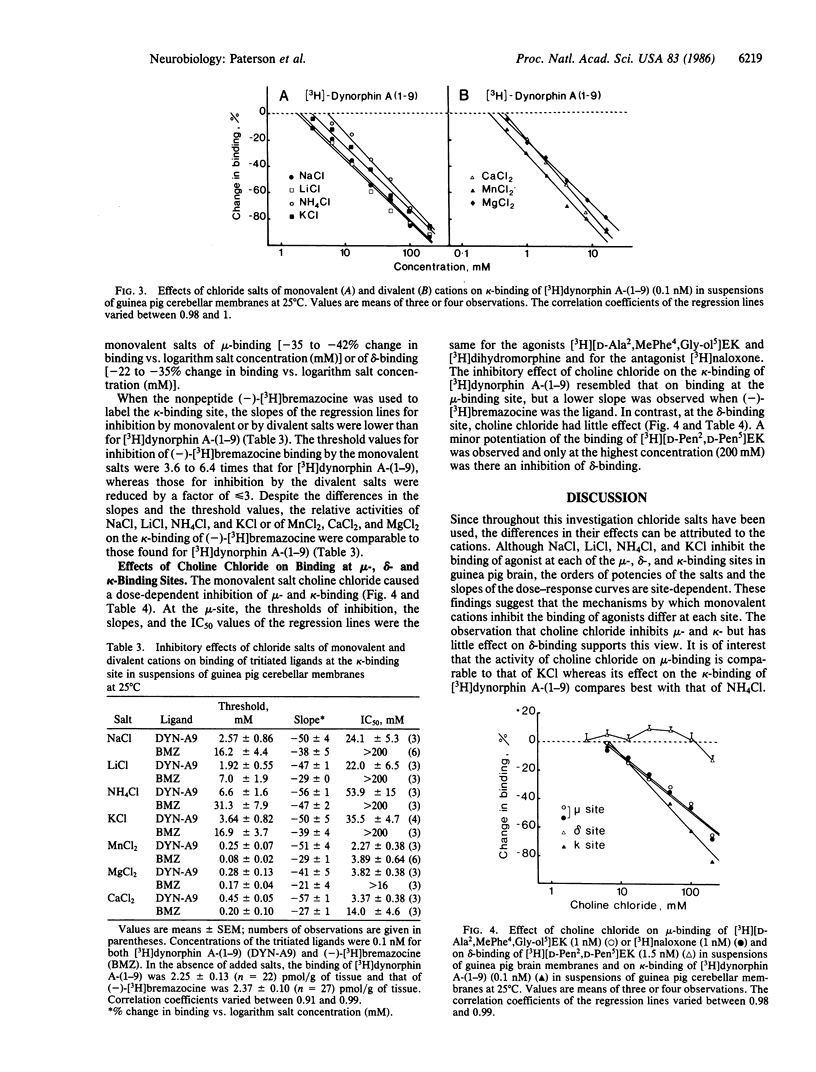
In membrane suspensions from guinea pig brain or cerebellum, NaCl, LiCl, NH4Cl, and KCl inhibit the equilibrium binding at 25 degrees C of the selective mu-agonist [3H][2-D-alanine,4-methylphenylalanine,5-glycinol]enkephalin ([D-Ala2,MePhe4,Gly-ol5]EK), the selective delta-agonist [3H][2-D-penicillamine,5-D-penicillamine]enkephalin ([D-Pen2,D-Pen5]-EK), and the selective kappa-agonist [3H]dynorphin A-(1-9). Choline chloride inhibits mu- and kappa-binding but not delta-binding. The relative activities of these monovalent salts and the slopes of the dose-response curves are site-dependent. Binding at the kappa-binding site is also inhibited by CaCl2, MnCl2, and MgCl2. On the other hand, these divalent salts potentiate delta-binding, and MnCl2 and MgCl2 have both potentiating and inhibitory effects on mu-binding; CaCl2 inhibits but does not potentiate mu-binding. Thus, the mechanisms by which monovalent cations inhibit opioid binding differ from those of divalent cations, and the mechanisms of action of both monovalent and divalent cations may differ at each site. When the antagonist [3H]naloxone, rather than the agonist [3H][D-Ala2,MePhe4,Gly-ol5]EK, is used to label the mu-binding site, the main effect of NaCl is to potentiate binding; a 22-fold higher concentration of LiCl is required to inhibit binding. The effects of NH4Cl, KCl, MnCl2, MgCl2, CaCl2, and choline chloride are little changed when [3H]naloxone is the ligand.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang K. J., Blanchard S. G., Cuatrecasas P. Unmasking of magnesium-dependent high-affinity binding sites for [dAla2, dLeu5]enkephalin after pretreatment of brain membranes with guanine nucleotides. Proc Natl Acad Sci U S A. 1983 Feb;80(4):940–944. doi: 10.1073/pnas.80.4.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frances B., Moisand C., Meunier J. C. Na+ ions and Gpp(NH)p selectively inhibit agonist interactions at mu- and kappa-opioid receptor sites in rabbit and guinea-pig cerebellum membranes. Eur J Pharmacol. 1985 Nov 5;117(2):223–232. doi: 10.1016/0014-2999(85)90607-7. [DOI] [PubMed] [Google Scholar]
- Gillan M. G., Kosterlitz H. W. Spectrum of the mu, delta- and kappa-binding sites in homogenates of rat brain. Br J Pharmacol. 1982 Nov;77(3):461–469. doi: 10.1111/j.1476-5381.1982.tb09319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan M. G., Robson L. E., McKnight A. T., Kosterlitz H. W. Kappa-binding and degradation of [3H]dynorphin A (1-8) and [3H]dynorphin A (1-9) in suspensions of guinea pig brain membranes. J Neurochem. 1985 Oct;45(4):1034–1042. doi: 10.1111/j.1471-4159.1985.tb05520.x. [DOI] [PubMed] [Google Scholar]
- Kouakou Y., Zajac J. M., Moisand C., Meunier J. C. The opiate receptor-binding interactions of opiate alkaloids and of an opioid peptide in rat brain membranes. Selection by manganese ions and by cholic acid (sodium salt) and minimalization of cross-reaction in vitro. Mol Pharmacol. 1982 May;21(3):564–569. [PubMed] [Google Scholar]
- Pert C. B., Pasternak G., Snyder S. H. Opiate agonists and antagonists discriminated by receptor binding in brain. Science. 1973 Dec 28;182(4119):1359–1361. doi: 10.1126/science.182.4119.1359. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A., Herz A. Enhancement of delta- but not mu-opiate agonist binding by calcium. Naunyn Schmiedebergs Arch Pharmacol. 1982 May;319(2):147–153. doi: 10.1007/BF00503929. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A., Sadée W., Herz A. Differential regulation of the mu-, delta-, and kappa-opiate receptor subtypes by guanyl nucleotides and metal ions. J Neurosci. 1982 Jul;2(7):912–917. doi: 10.1523/JNEUROSCI.02-07-00912.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson L. E., Foote R. W., Maurer R., Kosterlitz H. W. Opioid binding sites of the kappa-type in guinea-pig cerebellum. Neuroscience. 1984 Jun;12(2):621–627. doi: 10.1016/0306-4522(84)90077-0. [DOI] [PubMed] [Google Scholar]
- Robson L. E., Gillan M. G., Kosterlitz H. W. Species differences in the concentrations and distributions of opioid binding sites. Eur J Pharmacol. 1985 May 28;112(1):65–71. doi: 10.1016/0014-2999(85)90239-0. [DOI] [PubMed] [Google Scholar]
- Simon E. J., Hiller J. M., Edelman I. Stereospecific binding of the potent narcotic analgesic (3H) Etorphine to rat-brain homogenate. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1947–1949. doi: 10.1073/pnas.70.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. J., Hiller J. M., Groth J., Edelman I. Further properties of stereospecific opiate binding sites in rat brain: on the nature of the sodium effect. J Pharmacol Exp Ther. 1975 Mar;192(3):531–537. [PubMed] [Google Scholar]
- Zajac J. M., Roques B. P. Differences in binding properties of mu and delta opioid receptor subtypes from rat brain: kinetic analysis and effects of ions and nucleotides. J Neurochem. 1985 May;44(5):1605–1614. doi: 10.1111/j.1471-4159.1985.tb08802.x. [DOI] [PubMed] [Google Scholar]


