Abstract
High body mass index (BMI) (calculated as weight (kg)/height (m)2) is associated with increased asthma risk, but uncertainty persists about the role of physical activity. We estimated the independent and joint associations of hypothetical interventions on BMI and physical activity with the risk of adult-onset asthma in 76,470 asthma-free women from the Nurses’ Health Study who were followed between 1988 and 1998. Information about asthma, BMI, physical activity, and other factors was updated every 2 years. We used the parametric g-formula to estimate the 10-year asthma risk in the following 4 scenarios: no intervention, 5% BMI reduction in a 2-year period for those who were overweight or obese, at least 2.5 hours/week of moderate-to-vigorous physical activity, and both of the previous 2 interventions. At baseline, women had a mean age of 55 (standard deviation, 7) years and a mean BMI of 25.4 (standard deviation, 4.8). Median time spent in physical activity was 0.7 hours/week. During follow-up, 1,146 women developed asthma. The 10-year asthma risk under no intervention was 1.5%. Compared with no intervention, the population risk ratios were 0.96 (95% confidence interval (CI): 0.93, 0.99) under the BMI intervention, 0.96 (95% CI: 0.81, 1.10) under the physical activity intervention, and 0.92 (95% CI: 0.78, 1.06) under the joint intervention. Interventions on BMI and physical activity may have a modest impact on the risk of adult-onset asthma in this population of US women.
Keywords: asthma, body mass index, g-formula, hypothetical interventions, physical activity
No prevention strategies are known to reduce the incidence of adult-onset asthma (1–3). Most risk factors for adult-onset asthma—female sex, history of parental asthma, early-life infections, atopy, and allergic diseases—are not modifiable (3), and traditional asthma prevention measures such as reducing allergic sensitization or avoiding tobacco exposure in utero are unlikely to affect adult-onset asthma (1). High body mass index (BMI) (weight (kg)/height (m)2) is associated with an increased risk of adult-onset asthma (4, 5). This may be due to an effect of obesity through lung mechanics, an inflammatory state, or sex hormones (6), or it may be the result of inadequate control of confounders such as smoking, physical activity, and diet (7). Low physical activity levels have also been found to be associated with an increased risk of asthma exacerbation (8), which makes it conceivable that physical inactivity may play a role in the development of adult-onset asthma.
Estimation of the effect of the time-varying exposures of BMI and physical activity on asthma risk requires appropriate adjustment for the time-varying confounders that are affected by prior values of the exposure. For example, when we are interested in the effect of physical activity on asthma risk, BMI is a time-dependent confounder because BMI is a risk factor for asthma (3) that may lead to reduced physical activity (9) and is also affected by prior physical activity levels (9).
We used observational data from the Nurses’ Health Study to estimate the 10-year asthma risk under several hypothetical interventions on BMI and physical activity after adjustment for lifestyle measures that had not been adjusted for in previous studies. We used the parametric g-formula, because conventional statistical techniques cannot appropriately adjust for time-dependent confounders that are affected by prior components of the exposure (10–12).
MATERIALS AND METHODS
Study population
The Nurses’ Health Study started in 1976, when 121,701 female nurses aged 30–55 years who were living in 11 US states answered a detailed questionnaire. Follow-up questionnaires have been sent every 2 years (13). The institutional review boards of Partners Health System and the Harvard School of Public Health (both in Boston, Massachusetts) approved the Nurses’ Health Study protocols, and informed consent was obtained from all subjects. The information collected in the questionnaires includes age; smoking status (never, former, current); smoking intensity (in cigarettes/day); BMI; physical activity level; menopausal status; hormone replacement therapy; employment status (homemaker, retired, employed part-time, employed full-time); BMI at age 18 years (assessed in 1980); race/ethnicity; nurse's and spouse's educational attainments (assessed in 1992); type of nursing (education, administration, operating room, inpatient, outpatient, “other,” or nonnursing, assessed in 1992); body silhouettes at ages 5, 10, 20, 30, and 40 years (assessed in 1988); physical activity level at age 18–22 years (assessed in 1988); “prudent” or “Western” dietary pattern (estimated through a food frequency questionnaire in 1986) (14); acetaminophen use (assessed in 1990 and in all subsequent questionnaires); and oral contraceptive use prior to 1980 (assessed in 1980).
Of the 103,614 women who were alive at baseline (in 1988) and who returned the 1988 questionnaire, we excluded those with prevalent asthma (defined below) or chronic obstructive pulmonary disease (COPD) (n = 9,278). We also excluded women with missing baseline data on asthma (n = 680) or on main covariates (age, BMI, smoking, physical activity, menopausal status, or hormone replacement therapy) (n = 17,186). For each woman, follow-up started at the 1988 questionnaire (baseline) and finished at asthma diagnosis, death, first skipped questionnaire (incomplete follow-up), or in 1998, whichever occurred first.
Asthma ascertainment
A question about a physician diagnosis of asthma, emphysema, or chronic bronchitis was first included in the 1988 questionnaire and then in all subsequent biennial questionnaires. Supplemental questionnaires about asthma and COPD were sent in 1998 and 2000 to all living women who had reported a physician diagnosis of asthma, emphysema, or chronic bronchitis. In the 1998 and/or 2000 supplemental asthma and COPD questionnaires, we defined asthma as a self-reported physician diagnosis of asthma plus the use of an asthma medication within the past 12 months. This definition was previously validated in a similar cohort of nurses (Nurses’ Health Study II) by using medical records, with confirmation of 95% of self-reported asthma cases (4).
To reduce potential confounding by preclinical asthma (also referred to as “reverse causation”), the date of asthma onset for our analyses was set to be 2 years before 1) the year of return of the questionnaire when the subject first answered “yes” to the asthma question, or 2) the self-reported year of asthma onset in the 1998 or 2000 questionnaire, whichever came first. Therefore, although data on asthma onset were available until 2000, the follow-up for our analysis ended in 1998 (2 years before the last report of asthma diagnosis).
Ascertainment of BMI and physical activity
Height was assessed in the 1976 questionnaire. Weight was assessed on each follow-up questionnaire. The validity of self-reported weight measurements was examined among 140 women, aged 40–65 years, participating in the Nurses’ Health Study (15). Self-reported and measured weights were highly correlated (Pearson correlation = 0.97; mean difference between self-reported and measured weights = −1.5 kg), such that women were, on average, underreporting weight (15).
Physical activity was assessed in each survey (except in 1990) with the questions previously validated against a 7-day activity diary (16). The time spent per week at a variety of leisure-time physical activities (walking (at ≥3 miles/hour) (1 mile = 1.61 km) or hiking outdoors, jogging (slower than 10 minutes/mile), running (10 minutes/mile or faster), cycling (including on a stationary machine), swimming, tennis, or calisthenics/aerobics/aerobic dance/rowing machine) was added to calculate weekly hours of moderate-to-vigorous physical activity.
Hypothetical interventions
All hypothetical interventions started at baseline in 1988 and continued until the end of follow-up in 1998. We considered the BMI intervention (to “reduce BMI by 5% every 2 years if overweight or obese (defined as BMI >25)”) to estimate the effects of maintaining “normal” weight. We considered the physical activity intervention (to “engage in at least 2.5 hours per week of moderate-to-vigorous physical activity”) to estimate the effects of following the American College of Sports Medicine/American Heart Association recommendations, which state that older adults “should perform moderate-intensity aerobic (endurance) physical activity for a minimum of 30 minutes on five days each week” (17). We also considered a joint intervention, which combined both of the interventions.
For comparison purposes, we also performed a secondary analysis that considered more extreme interventions for weight loss (reduce BMI by 5% every 2 years if BMI is >23) and moderate-to-vigorous physical activity (at least 30, 45, or 60 minutes per day).
Statistical analysis
We used the parametric g-formula (18), the generalization of standardization for time-varying exposures and confounders, to estimate the 10-year risk of asthma under the selected hypothetical interventions (Figure 1). The parametric g-formula has been used previously to estimate the effect of multiple lifestyle interventions on the risks of coronary heart disease and diabetes (19–21). If all time-varying confounders have been correctly measured and modeled at all time-points, the g-formula can be used to consistently estimate the standardized risk of asthma under hypothetical interventions. The standardized risk is a weighted average of the risks of asthma conditional on the specified intervention values and the observed confounder history. The weights are the probability density functions of the time-varying confounders, which are estimated via parametric regression models. We approximated the weighted average by using a Monte Carlo simulation of 10,000 individuals with the baseline values of covariates sampled from their empirical distribution. The values of time-varying covariates for each 2-year interval were drawn from the distribution estimated via the regression models after setting the values of lifestyle factors to those specified by the intervention(s).
Figure 1.
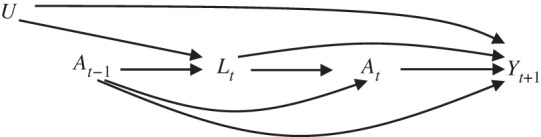
Causal directed acyclic graph to depict the relationship between time-varying exposure A (e.g., body mass index, physical activity, or smoking), outcome Y (e.g., asthma incidence), and time-dependent confounders L affected by prior exposure. U stands for unmeasured common determinants of the time-varying confounders and the outcome.
We included the following variables as potential confounders at baseline (in 1988): age, type of nursing, BMI at age 18 years, smoking history, smoking intensity, menopausal status, and postmenopausal hormone use. We also included in all models baseline values of the intervention variables (i.e., physical activity and BMI). We modeled the distribution of the following time-varying covariates: BMI, smoking intensity, physical activity level, menopausal status, and postmenopausal hormone use. Information from the 1984 and 1986 surveys was used as lag data for time-varying variables. Web Table 1, available at http://aje.oxfordjournals.org/, shows details on covariates and models.
We compared the estimated asthma risks under various interventions with the risk under no intervention to calculate the population risk ratio and population risk difference. To estimate the 95% confidence intervals, we used nonparametric bootstrapping with 500 samples (22). Increasing the number of samples (e.g., to 5,000 samples) greatly increased the computation time but did not materially change the width of the confidence intervals in our primary analysis. We also computed the proportion of individuals who experienced an intervention in any period and the average proportion of individuals who experienced an intervention in each 2-year period. The latter measures adherence to the hypothetical intervention among those following the intervention up until the previous period. To assess effect modification by smoking (23), we stratified analyses according to baseline smoking status (never, former, current).
We performed several analyses to assess the sensitivity of our estimates to varying assumptions regarding selection bias, information bias, confounding, and model misspecification. First, we excluded women who ever reported COPD, chronic bronchitis, or emphysema during follow-up (n = 2,758) and who could have been misclassified as having asthma. Second, we adjusted for variables that could not be considered in the main analysis because of a large proportion (>10%) of missing values, including time-varying factors (employment status and acetaminophen use) and fixed factors (race/ethnicity; education; employment status at baseline; BMI in 1980; body silhouettes at ages 5, 10, 20, 30, and 40 years; and, in 1988, physical activity at age 18–22 years; oral contraceptive use prior to 1980; and “prudent” or “Western” dietary pattern). Third, to evaluate the possibility of “reverse causation” bias, we studied a hypothetical intervention of smoking cessation. (Because the onset of asthma is expected to result in smoking cessation, estimating an apparently protective effect of smoking on asthma incidence would suggest confouding by unmeasured disease.) Finally, we considered alternative modeling strategies, including using only 1 lag (instead of 2 lags) of time-varying covariates when modeling physical activity and altering the arbitrary ordering of concurrently measured time-varying covariates. All analyses were conducted with SAS, version 9.2, software (SAS Institute, Inc., Cary, North Carolina) by using an SAS macro previously developed (19) and available at http://www.hsph.harvard.edu/causal/software. Details on the GFORMULA macro are included in Web Appendix 1.
RESULTS
A total of 76,470 women were followed up for a mean of 9.8 (standard deviation, 1.2) years. Their baseline characteristics are described in Table 1. The mean age was 55 years and mean BMI was 25.4; 29% were overweight and 15% were obese. The median time spent in moderate-to-vigorous physical activity was 0.7 hours/week. During the follow-up period, 1,146 women (1.5%) developed asthma, and 3,897 (5%) died.
Table 1.
Baseline Sociodemographic and Clinical Characteristics of 76,470 Women in the Nurses’ Health Study, United States, 1988
| Characteristic | No. of Women | % |
|---|---|---|
| Age, years | 55 (7)a | |
| Type of nursing (more than 1 allowed)b | ||
| Education | 2,381 | 3 |
| Operating room | 1,537 | 2 |
| Inpatient or emergency room | 5,486 | 7 |
| Outpatient or community | 4,613 | 6 |
| Administration | 4,341 | 6 |
| Other | 16,387 | 21 |
| Nonnursing | 42,452 | 55 |
| Body mass indexc | 25.4 (4.8)a | |
| Body mass index category | ||
| <18.0 | 5,881 | 1 |
| 18.0–21.9 | 18,076 | 24 |
| 22.0–24.9 | 23,849 | 31 |
| 25.0–29.9 | 22,518 | 29 |
| 30.0–34.9 | 7,907 | 10 |
| ≥35.0 | 3,532 | 5 |
| Smoking status | ||
| Current (or active) smoking | 16,073 | 21 |
| Ever smoker | 42,444 | 56 |
| Smoking intensity, cigarettes/day | ||
| 0 | 60,397 | 79 |
| 1–4 | 1,430 | 2 |
| 5–14 | 3,625 | 5 |
| 15–29 | 6,528 | 8 |
| 30–39 | 2,968 | 4 |
| ≥40 | 1,522 | 2 |
| Moderate-intense physical activity, hours/week | 0.7 (0–2.5)d | |
| Moderate-intense physical activity category, hours/week | ||
| 0–0.49 | 35,495 | 46 |
| 0.5–1.49 | 14,915 | 20 |
| 1.5–2.49 | 9,776 | 13 |
| 2.5–3.49 | 3,313 | 4 |
| 3.5–4.49 | 1,546 | 2 |
| ≥4.5 | 11,425 | 15 |
| Menopausal | 43,173 | 56 |
| Postmenopausal hormone use | 12,207 | 16 |
a Reported as mean (standard deviation).
b Reported in 1992.
c Weight (kg)/height (m)2.
d Reported as median (25th–75th percentile).
The estimated 10-year asthma risk under no intervention was 1.5% (95% confidence interval (CI): 1.4, 1.7). Table 2 shows the estimated risks, population risk ratios, and population risk differences under the hypothetical interventions. Compared with no intervention, the risk ratios were 0.96 (95% CI: 0.93, 0.99) for the BMI intervention, 0.96 (95% CI: 0.81, 1.10) for the physical activity intervention, and 0.92 (95% CI: 0.78, 1.06) for the joint intervention. Of all participants, 33% maintained a BMI of 25, 3% were physically active at least 2.5 hours/week, and 2% did both for the entire follow-up period.
Table 2.
Ten-Year Risk of Asthmaa Under Hypothetical Interventions on BMIb and Moderate-to-Vigorous Physical Activity in Womenc in the Nurses’ Health Study, United States, 1988–1998
| Intervention | Asthma Risk, % | 95% CI | Population RR | 95% CI | Population Risk Difference, % | 95% CI | Women Who Received Intervention |
|
|---|---|---|---|---|---|---|---|---|
| % of Total | % in Each 2-Year Period | |||||||
| Natural course (no intervention) | 1.5 | 1.4, 1.7 | 1 | Referent | 0 | 0 | 0 | |
| Primary interventions | ||||||||
| Lose 5% of BMI if BMI >25 | 1.5 | 1.4, 1.6 | 0.96 | 0.93, 0.99 | −0.1 | −0.1, −0.01 | 67 | 56 |
| Physical activity at least 2.5 hours/weekd | 1.5 | 1.3, 1.7 | 0.96 | 0.81, 1.10 | −0.1 | −0.3, 0.2 | 97 | 67 |
| Joint intervention | 1.4 | 1.2, 1.6 | 0.92 | 0.78, 1.06 | −0.1 | −0.3, 0.1 | 98 | 77 |
| Secondary interventions | ||||||||
| Lose 5% of BMI if BMI >23 | 1.4 | 1.3, 1.6 | 0.94 | 0.89, 1.00 | −0.1 | −0.2, −0.01 | 85 | 69 |
| Physical activity at least 3.5 hours/weekd | 1.4 | 1.0, 1.8 | 0.90 | 0.65, 1.15 | −0.2 | −0.6, 0.2 | 98 | 71 |
| Joint intervention | 1.3 | 0.9, 1.7 | 0.85 | 0.62, 1.08 | −0.2 | −0.6, 0.1 | 99 | 85 |
Abbreviations: BMI, body mass index; CI, confidence interval; RR, risk ratio.
a Estimated risk in the population using the parametric g-formula to standardize for time-varying covariates (smoking intensity, BMI, menopausal status, postmenopausal hormone use, physical activity), and baseline covariates (age, smoking history prior to 1988, type of nursing, BMI, BMI at 18 years of age, smoking intensity, menopausal status, postmenopausal hormone use, physical activity).
b Weight (kg)/height (m)2.
c Total of 76,470 women and 1,146 asthma cases representing 746,096 person-years.
d Equates to 30 minutes/day on 5 days/week.
Table 2 also shows that the corresponding risk ratios for more extreme interventions were 0.94 (95% CI: 0.89, 1.00) for reduction of BMI by 5% every 2 years until BMI was under 23, 0.90 (95% CI: 0.65, 1.15) for engaging in moderate-to-vigorous activity at least 3.5 hours/week, and 0.85 (95% CI: 0.62, 1.08) for the corresponding joint intervention. An even more extreme intervention on physical activity, engaging in moderate-to-vigorous activity for at least 7 hours/week, yielded a risk ratio of 1.07 (95% CI: 0.93, 1.25), but this estimate relies on model extrapolation because 0% of studied women maintained that activity level throughout the entire follow-up period.
Table 3 shows the estimated risk ratios and risk differences under the hypothetical interventions across groups defined by smoking status at baseline. The risk ratios for the primary joint intervention were 1.04 (95% CI: 0.79, 1.30), 0.91 (95% CI: 0.71, 1.16), and 0.67 (95% CI: 0.44, 0.98) among never, former, and current smokers, respectively. All sensitivity analyses yielded similar results (Web Tables 2 and 3). The risk ratio was 1.03 (95% CI: 0.99, 1.06) for smoking cessation versus no intervention on smoking (Web Table 4).
Table 3.
Ten-Year Risk of Asthmaa Under Hypothetical Interventions on BMIb and Moderate-to-Vigorous Physical Activity by Smoking Status at Baseline in Women in the Nurses’ Health Study, United States, 1988–1998
| Intervention by Smoking Status | No. | Asthma Risk, % | 95% CI | Population RR | 95% CI | Population Risk Difference, % | 95% CI |
|---|---|---|---|---|---|---|---|
| Never smokersc | 34,026 | ||||||
| Lose 5% of BMI if BMI >25 | 1.4 | 1.3, 1.6 | 0.98d | 0.94, 1.03 | −0.03e | −0.1, 0.04 | |
| Physical activity ≥2.5 hours/week | 1.5 | 1.2, 1.9 | 1.05f | 0.82, 1.32 | 0.1g | −0.3, 0.5 | |
| Joint intervention | 1.5 | 1.2, 1.9 | 1.04h | 0.79, 1.30 | 0.1i | −0.3, 0.5 | |
| Former smokersj | 26,371 | ||||||
| Lose 5% of BMI if BMI >25 | 1.5 | 1.4, 1.7 | 0.95 | 0.90, 1.00 | −0.1 | −0.2, 0 | |
| Physical activity ≥2.5 hours/week | 1.6 | 1.2, 2.0 | 0.96 | 0.76, 1.21 | −0.1 | −0.4, 0.3 | |
| Joint intervention | 1.5 | 1.2, 1.9 | 0.91 | 0.71, 1.16 | −0.2 | −0.5, 0.03 | |
| Active smokersk | 16,073 | ||||||
| Lose 5% of BMI if BMI >25 | 1.5 | 1.3, 1.7 | 0.94 | 0.89, 1.01 | −0.1 | −0.2, 0.01 | |
| Physical activity ≥2.5 hours/week | 1.1 | 0.7, 1.6 | 0.71 | 0.47, 1.02 | −0.5 | −0.9, 0.3 | |
| Joint intervention | 1.0 | 0.7, 1.5 | 0.67 | 0.44, 0.98 | −0.5 | −0.9, 0.04 |
Abbreviations: BMI, body mass index; CI, confidence interval; RR, risk ratio.
a Estimated risk in the population using the parametric g-formula to standardize for time-varying covariates (smoking intensity, BMI, menopausal status, postmenopausal hormone use, physical activity), and baseline covariates (age, smoking history prior to 1988, type of nursing, BMI, BMI at 18 years of age, smoking intensity, menopausal status, postmenopausal hormone use, physical activity).
b Weight (kg)/height (m)2.
c There were 485 asthma cases among never smokers.
d P for heterogeneity across smoking groups = 0.512.
e P for heterogeneity across smoking groups = 0.395.
f P for heterogeneity across smoking groups = 0.183.
g P for heterogeneity across smoking groups = 0.264.
h P for heterogeneity across smoking groups = 0.142.
i P for heterogeneity across smoking groups = 0.126.
j There were 417 asthma cases among former smokers.
k There were 244 asthma cases among current smokers.
DISCUSSION
Overall, we estimated that interventions on BMI and physical activity may have a modest impact on the risk of adult-onset asthma in this population of US women. Our primary intervention on BMI resulted in an estimated 4% reduction in asthma risk; the physical activity and joint interventions resulted in comparable reductions, but the 95% confidence intervals were wide. The impact of these interventions might be greater for smokers, and more extreme interventions might reduce asthma risk by 15% overall.
No previous randomized trials have studied the long-term effects of weight loss and physical activity on adult-onset asthma. A systematic review identified 15 studies of various weight loss interventions and asthma-related outcomes (e.g., symptoms, health care utilization); all of them found better outcomes after weight loss (24). A meta-analysis of observational studies found that those who are overweight/obese have a 51% greater risk of adult-onset asthma than those who are of normal weight (4). However, previous observational studies (4, 5) might not have appropriately adjusted for physical activity and other measured time-dependent confounders (7), and they did not attempt to estimate the effect of weight loss interventions (even if only hypothetical) because they did not compare changes in BMI during follow-up.
Previous observational studies of physical activity have provided mixed results. A Finnish study of 10,597 adult twins followed for 9 years found an association of baseline physical activity with lower asthma risk in men and higher asthma risk in women (25). A Danish study of 6,090 adult twins followed for 8 years found an association of baseline physical activity with lower asthma risk in monozygotic twins and higher asthma risk in dizygotic twins (26). A cohort study of 51,080 French women followed for 10 years found no association between baseline physical activity and adult-onset asthma incidence (27). None of these studies considered changes in physical activity during follow-up.
Our estimates may not be generalizable to other populations with different distributions of risk factors, because the g-formula standardizes the risk to the distribution of risk factors in the particular population under study. For example, we estimated little reduction in risk of asthma if all women had maintained a BMI of 25 or less throughout the follow-up period, but 33% of the women in our study did maintain a BMI of 25 or less. In populations with a higher proportion of overweight/obesity, the same BMI intervention might have had a stronger effect on asthma incidence.
The validity of our estimates relies on the following usual assumptions for all analyses of observational data: no unmeasured confounding, no measurement error, and no model misspecification. We adjusted for many potential risk factors of adult-onset asthma, but not for lung function. However, the exclusion of women who had received any diagnosis of COPD, chronic bronchitis, or emphysema did not change the results, and we estimated no beneficial effect of smoking cessation on the risk of adult-onset asthma (Web Table 4), which indirectly supports no “reverse causation” bias. We relied on self-reported information, which is subject to measurement error, but several validation substudies on asthma diagnosis, weight measurement, and physical activity have previously shown very good relationships between self-reported and measured data (4, 15, 16). To minimize potential misclassification in asthma diagnosis, we restricted asthma cases to those who used an asthma medication within the past 12 months and, in a sensitivity analysis, excluded all women who had a concomitant diagnosis of emphysema, chronic bronchitis, or COPD. Finally, the similarity between the predicted 10-year asthma risk of 1.5% and the observed risk of 1.5% is a necessary condition for no model misspecification in the calculation of the g-formula estimates.
Strengths of the current study include its longitudinal design, the use of repeated measurements for risk factors, outcomes, and intermediate variables, and the application of the parametric g-formula. Besides appropriately adjusting for time-varying confounders, the parametric g-formula is especially well suited to estimating the effects of joint interventions (e.g., changes in both BMI and physical activity) and of interventions that depend on the value of evolving time-dependent factors, so the estimates are more directly relevant for public health and clinical decisions (18, 19).
Yet the interpretation of our estimates for weight loss (and, to a certain extent, physical activity) is complicated because its mechanisms of action on asthma risk are unknown, and there are possibly multiple versions of the interventions (28, 29). For example, participants may lose weight by reducing their caloric intakes, by increasing their physical activity levels, or by using weight-loss medications. Our results suggest that physical activity by itself cannot fully explain the estimated beneficial effect of weight loss on asthma risk, and there is little evidence for an association between diet and risk of adult-onset asthma (30). Hence, further research is warranted before our estimates can be directly translated into feasible public health interventions.
In conclusion, we estimated that interventions on BMI and physical activity may have a modest impact on the risk of adult-onset asthma in this population of US women.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Centre for Research in Environmental Epidemiology, Barcelona, Spain (Judith Garcia-Aymerich); Departament de Ciències Experimentals i de la Salut, Universitat Pompeu Fabra, Barcelona, Spain (Judith Garcia-Aymerich); CIBER Epidemiología y Salud Pública, Barcelona, Spain (Judith Garcia-Aymerich); INSERM, CESP Centre for Research in Epidemiology and Population Health, U1018, Respiratory and Environmental Epidemiology Team, Villejuif, France (Raphaëlle Varraso); Université Paris Sud 11, UMRS 1018, Villejuif, France (Raphaëlle Varraso); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Goodarz Danaei, Carlos A. Camargo, Jr., Miguel A. Hernán); Department of Global Health and Population, Harvard School of Public Health, Boston, Massachusetts (Goodarz Danaei); Channing Division of Network Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts (Carlos A. Camargo, Jr.); Department of Emergency Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts (Carlos A. Camargo, Jr.); Department of Biostatistics, Harvard School of Public Health, Boston, Massachusetts (Miguel A. Hernán); and Harvard-MIT Division of Health Sciences and Technology, Boston, Massachusetts (Miguel A Hernán).
This work was supported by the US National Institutes of Health (grants R01 HL080644, CA-87969, HL-63841, and AI-52338); and mobility grants from the “José Castillejo” Spanish Ministry of Science and Innovation (J0C2009-00182), Catalan Society of Pneumology, and Spanish Society of Pneumology (2010/847) to J.G.A.
We thank Jessica Young and Roger Logan for their technical assistance and Gary Chase, Karen Corsano, and Barbara Egan for their assistance with the data.
Conflict of interest: none declared.
REFERENCES
- 1.Bateman ED, et al. Cape Town, South Africa: Global Initiative for Asthma (GINA); 2011. Global strategy for asthma management and prevention. (http://www.ginasthma.org/GINA-Report,-Global-Strategy-for-Asthma-Management-and-Prevention. ) (Accessed December 23, 2012) [Google Scholar]
- 2.Basagaña X, Sunyer J, Zock JP, et al. Incidence of asthma and its determinants among adults in Spain. Am J Respir Crit Care Med. 2001;164(7):1133–1137. doi: 10.1164/ajrccm.164.7.2012143. [DOI] [PubMed] [Google Scholar]
- 3.Antó JM, Sunyer J, Basagaña X, et al. Risk factors of new-onset asthma in adults: a population-based international cohort study. Allergy. 2010;65(8):1021–1030. doi: 10.1111/j.1398-9995.2009.02301.x. [DOI] [PubMed] [Google Scholar]
- 4.Camargo CA, Jr, Weiss ST, Zhang S, et al. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159(21):2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 5.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175(7):661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121(5):1087–1093. doi: 10.1016/j.jaci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Sin DD, Sutherland ER. Obesity and the lung: 4. Obesity and asthma. Thorax. 2008;63(11):1018–1023. doi: 10.1136/thx.2007.086819. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Aymerich J, Varraso R, Antó JM, et al. Prospective study of physical activity and risk of asthma exacerbations in older women. Am J Respir Crit Care Med. 2009;179(11):999–1003. doi: 10.1164/rccm.200812-1929OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 10.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 11.Cole SR, Hernán MA. Fallibility in estimating direct effects. Int J Epidemiol. 2002;31(1):163–165. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- 12.Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology. 2003;14(3):300–306. [PubMed] [Google Scholar]
- 13.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 14.Varraso R, Fung TT, Barr RG, et al. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am J Clin Nutr. 2007;86(2):488–495. doi: 10.1093/ajcn/86.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of selfreported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 17.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 18.Robins J. A new approach to causal inference in mortality studies with sustained exposure periods—application to control of the healthy worker survivor effect. Math Model. 1986;7(9-12):1393–1512. [Google Scholar]
- 19.Taubman SL, Robins JM, Mittleman MA, et al. Intervening on risk factors for coronary heart disease: an application of the parametric g-formula. Int J Epidemiol. 2009;38(6):1599–1611. doi: 10.1093/ije/dyp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danaei G, Pan A, Hu FB, et al. Hypothetical midlife interventions in women and risk of type 2 diabetes. Epidemiology. 2013;24(1):122–128. doi: 10.1097/EDE.0b013e318276c98a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lajous M, Willett WC, Robins JM, et al. Changes in fish consumption in midlife and the risk of coronary heart disease in men and women. Am J Epidemiol. 2013;178(3):382–391. doi: 10.1093/aje/kws478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1(1):54–75. [Google Scholar]
- 23.Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175(5):458–463. doi: 10.1164/rccm.200607-896OC. [DOI] [PubMed] [Google Scholar]
- 24.Eneli IU, Skybo T, Camargo CA., Jr Weight loss and asthma: a systematic review. Thorax. 2008;63(8):671–676. doi: 10.1136/thx.2007.086470. [DOI] [PubMed] [Google Scholar]
- 25.Huovinen E, Kaprio J, Koskenvuo M. Factors associated to lifestyle and risk of adult onset asthma. Respir Med. 2003;97(3):273–280. doi: 10.1053/rmed.2003.1419. [DOI] [PubMed] [Google Scholar]
- 26.Thomsen SF, Ulrik CS, Kyvik KO, et al. Risk factors for asthma in young adults: a co-twin control study. Allergy. 2006;61(2):229–233. doi: 10.1111/j.1398-9995.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 27.Benet M, Varraso R, Kauffmann F, et al. The effects of regular physical activity on adult-onset asthma incidence in women. Respir Med. 2011;105(7):1104–1107. doi: 10.1016/j.rmed.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Hernán MA, Taubman SL. Does obesity shorten life? The importance of well-defined interventions to answer causal questions. Int J Obes (Lond) 2008;32(suppl 3):8S–14S. doi: 10.1038/ijo.2008.82. [DOI] [PubMed] [Google Scholar]
- 29.Hernán MA, VanderWeele TJ. Compound treatments and transportability of causal inference. Epidemiology. 2011;22(3):368–377. doi: 10.1097/EDE.0b013e3182109296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varraso R. Nutrition and asthma. Curr Allergy Asthma Rep. 2012;12(3):201–210. doi: 10.1007/s11882-012-0253-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


