Abstract
Background
Iron (Fe) deficiency in crops is a worldwide agricultural problem. Plants have evolved several strategies to enhance Fe acquisition, but increasing evidence has shown that the intrinsic plant-based strategies alone are insufficient to avoid Fe deficiency in Fe-limited soils. Soil micro-organisms also play a critical role in plant Fe acquisition; however, the mechanisms behind their promotion of Fe acquisition remain largely unknown.
Scope
This review focuses on the possible mechanisms underlying the promotion of plant Fe acquisition by soil micro-organisms.
Conclusions
Fe-deficiency-induced root exudates alter the microbial community in the rhizosphere by modifying the physicochemical properties of soil, and/or by their antimicrobial and/or growth-promoting effects. The altered microbial community may in turn benefit plant Fe acquisition via production of siderophores and protons, both of which improve Fe bioavailability in soil, and via hormone generation that triggers the enhancement of Fe uptake capacity in plants. In addition, symbiotic interactions between micro-organisms and host plants could also enhance plant Fe acquisition, possibly including: rhizobium nodulation enhancing plant Fe uptake capacity and mycorrhizal fungal infection enhancing root length and the nutrient acquisition area of the root system, as well as increasing the production of Fe3+ chelators and protons.
Keywords: Hormones, iron deficiency, microbial community structure, siderophore, symbiosis
INTRODUCTION
Iron (Fe) is an essential nutrient for plants, and it serves as a cofactor for a wide variety of cellular processes, such as oxygen transport, cellular respiration, chlorophyll biosynthesis, thylakoid biogenesis and chloroplast development (Nishio et al., 1985; Kobayashi and Nishizawa, 2012). However, the Fe bioavailability in well-aerated soils is often severely limited, particularly in calcareous soils, which occupy 30 % of the Earth's surface. Hence, Fe-deficiency-induced chlorosis is a serious problem leading to yield loss and reduced quality in agricultural production (Kim and Guerinot, 2007; Zheng, 2010). Fe deficiency in plants is also closely related to the prevalence of Fe-deficiency-induced anaemia in humans (Murgia et al., 2012). Plants have evolved at least two mechanisms favouring efficient acquisition of Fe. Strategy I, which occurs in non-graminaceous plants, relies on acidification of the rhizosphere to increase the solubility of ferric Fe compounds through proton extrusion, trans-plasma membrane electron transfer to reduce Fe to its more soluble ferrous form via ferric chelate reductase (FRO2) and transport of Fe into root cells by iron-regulated transporter 1 (IRT1) (Marschner et al., 1995; Santi and Schmidt, 2009; Ivanov et al., 2012; Kobayashi and Nishizawa, 2012). Strategy II, which is utilized by the Gramineae, relies on extrusion of mugineic acid family phytosiderophores (MAs) via efflux transporter of MAs (e.g. TOM1) to solubilize Fe in the rhizosphere, and subsequent transport of the Fe(III)–phytosiderophore complex across the plasma membrane of the root epidermal cell via yellow stripe1 transporter 1 (YS1) (Curie et al., 2001, 2009; Nozoye et al., 2011; Kobayashi and Nishizawa, 2012).
These strategies have been thought to ensure normal growth for many so-called ‘Fe-efficient’ plants under Fe-limited conditions. In the last decade, however, several lines of evidence have shown that these strategies alone are insufficient to prevent plants from suffering Fe deficiency in Fe-limited soils. For example, sunflower (Helianthus annuus) plants grown in sterile soil show severe chlorosis, poor growth and lower tissue Fe levels than those grown in non-sterile soil (Masalha et al., 2000). Similarly, Fe acquisition and growth of rape and red clover plants also significantly decrease when the plants are grown in sterile soils, but normal growth can be quickly restored by adding Fe–EDDHA to the sterile soil or spraying Fe–EDTA on the leaves (Rroco et al., 2003; Jin et al., 2006). These results provide evidence that soil microbial activity probably plays a critical role in plant Fe acquisition. Further supporting this, de Santiago et al. (2009, 2011, 2013) observed that Fe acquisition in wheat, white lupin and cucumber plants increases when their calcareous media are inoculated with the soil fungus Trichoderma asperellum strain T34. How soil micro-organisms promote plant Fe acquisition is still largely unknown. Nevertheless, researchers have made great efforts to uncover this interesting and important underground mechanism in recent decades and have obtained many valuable clues. Based on these clues, this present prospective review discusses the possible mechanisms for soil micro-organism promotion of plant Fe acquisition.
MICROBIAL COMMUNITY STRUCTURE IN THE RHIZOSPHERE DEPENDS ON THE FE STATUS OF PLANTS
Soils have highly distinct microbial communities, which are thought to result from many different selection factors, including the texture, nutrient and organic matter content and pH of the soil (Berg and Smalla, 2009; Liang et al., 2012). Environmental factors such as climate, soil aeration and moisture content could also affect the make up of microbial communities in soils (Gelsomino et al., 1999; Carelli et al., 2000; Marschner et al., 2004). The rhizosphere is regarded as a component of the soil system, but it can be markedly different from the bulk soil in terms of the above-mentioned factors, because most of these factors vary due to the activity of plant roots, such as the consumption of nutrients and exudation of protons and organic compounds.
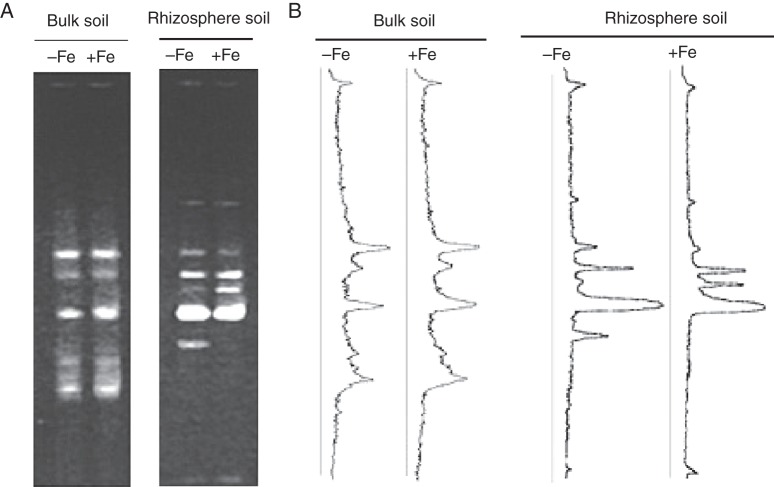
Iron serves as a cofactor for a wide variety of cellular processes in plants, and deficiency of Fe in plants affects root behaviour, particularly the exudation of organic compounds, which are referred to as root exudates (Jin et al., 2006, 2007; Carvalhais et al., 2011). In addition to the exudation of phytosiderophores by Strategy II plants, Fe deficiency often strongly induces the roots of Strategy I plants to release reductants and chelators including phenolic compounds, flavins and organic acids (Curie and Briat, 2003; Hell and Stephan, 2003; Jin et al., 2006, 2007, 2010; Kobayashi and Nishizawa, 2012). As a consequence, Fe deficiency in plants can be predicted to alter the chemical and physical properties of rhizospheric soils, in turn affecting which micro-organisms are found in the rhizosphere and their relative abundance. Indeed, several studies have provided evidence for this. Using PCR–DGGE (polymerase chain reaction–denaturing gradient gel electrophoresis) of rDNA, a culture-independent method, Yang and Crowley (2000) demonstrated that the structure of the microbial community in the barley rhizosphere varies with the plant's Fe nutritional status. In the rhizosphere of a transgenic tobacco plant overaccumulating Fe, Robin et al. (2006, 2007) observed a decrease in Fe availability and shifts in the structure of the microbial community. Recently, we grew red clover plants in an Fe-limited calcareous soil and observed a clearly different PCR–DGGE profile of rhizospheric microbial 16S rDNA, as compared with that from plants grown in the same soil but with foliar application of 100 µm Fe–EDTA solution (Fig. 1).
Fig. 1.
Effect of Fe status on the microbial community in soil. (A) Microbial community 16S rDNA fingerprints of bacteria from rhizosphere soil of Fe-stressed and Fe-sufficient red clover as determined by PCR–DGGE. (B) Line image profiles generated by image analysis of gels shown in A. For the Fe-sufficient treatment, the leaves of red clover plants were sprayed with 100 µM Fe–EDTA solution every other day, while for the Fe-stressed treatment, the leaves were sprayed with deionized water.
The above PCR–DGGE studies demonstrate that the structure of microbial communities in the rhizospheres of Fe-deficient plants can be quite different from that of Fe-sufficient plants, but the exact mechanism underlying this difference remains an open question. To clarify whether the alteration of microbial communities is associated with root exudates, we analysed root exudates from Fe-deficient red clover plants and found that phenolics represent the major component (Jin et al., 2007). Plant phenolic compounds have both antimicrobial and growth-promoting effects (Blum et al., 2000; Dakora and Phillips, 2002; Jin et al., 2006; Medina et al., 2006; Cicerale et al., 2012; Sugiyama and Yazaki, 2012). When a calcareous soil solution was incubated on agar plates containing phenolic root exudates from Fe-deficient red clover, only a few microbial species thrived while growth of the rest was inhibited (Fig. 2; Jin et al., 2006, 2008). Similarly, Masaoka et al. (1997) observed that phenolic exudates collected from Fe-deficient alfalfa plants inhibit the growth of Fusarium oxysporum f. sp. phaseoli, but promote the growth of Rhizobium meliloti. Further, we recently found that the siderophore secretion profile of micro-organisms from soil containing added phenolic root exudates is similar to that found in the rhizosphere soil of Fe-deficient plants (Jin et al., 2010). These studies attest to a possible relationship between Fe-deficiency-induced exudation of phenolics and changes in the composition of the microbial community in the rhizosphere. However, more direct evidence is still necessary to confirm such a relationship. In addition, as mentioned previously, Fe deficiency also induces exudation of phytosiderophores, flavins or organic acids, depending on the plant species (Kobayashi and Nishizawa, 2012), but to date there is no information on whether and how these root exudates link to changes in rhizosphere microbial communities.
Fig. 2.
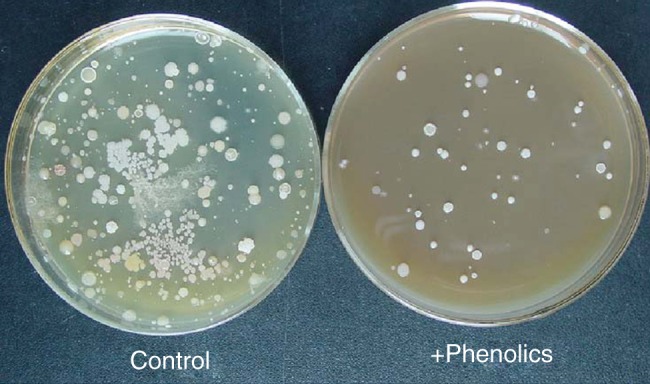
Effect of the phenolics from Fe-deficient root exudates on the growth of soil micro-organisms. A 100 µL aliquot of soil solution (10−4 dilution) was spread on broth peptone agar plates with or without phenolics (0·6 µmol mL−1), and then incubated at 30 °C for 2 d. The phenolics were collected from root exudates of Fe-deficient red clover.
The previously mentioned sterilization experiments and PCR–DGGE analyses provide evidence that soil micro-organisms play an important role in plant Fe acquisition, and micro-organism community structure in the rhizosphere changes along with the Fe status of plants, prompting the question of whether the plant-induced rhizosphere behaviour is beneficial for the plant itself. The ratio of siderophore-producing micro-organisms isolated from soil solution incubated with phenolic root exudates of Fe-deficient red clover is much higher than that isolated from phenolic-free control soil solution (Jin et al., 2006, 2008). Furthermore, micro-organisms from the rhizosphere of Fe-deficient plants have a greater siderophore secretion capacity than those from Fe-sufficient plants (Jin et al., 2010). Due to their strong Fe-chelating ability, secretion of siderophores is expected to increase Fe solubility in rhizosphere soil (Jin et al., 2008). The detailed functions of siderophore compounds will be discussed below. Together, these results suggest that the plant Fe-deficiency-induced changes in microbial community structure in the rhizosphere should favour the Fe uptake of the plant itself. Nevertheless, more direct evidence is also necessary to confirm this.
MECHANISMS OF MICROBIAL ACTIVITY IN IMPROVING THE FE NUTRITION OF PLANTS
Siderophore production
Siderophores are low molecular weight organic chelators with very high and specific affinity for Fe(III). In aerobic calcareous soil, many micro-organisms synthesize and release siderophores to overcome low Fe availability resulting from the minimal solubility of Fe hydroxides (Schalk et al., 2011). Siderophores increase Fe solubility by chelation to form siderophore–Fe complexes (Lemanceau et al., 2009; Saha et al., 2012). There are almost 500 compounds identified as siderophores, which can be classified as hydroxamate, catecholate and/or hydoxycarboxylic acid siderophores based on their ligand architecture (Boukhalfa and Crumbliss, 2002; Bonnefoy and Holmes, 2012). Figure 3 shows some representative siderophore structures with hydroxamic acid and catechol moieties. Because of their solubilizing effect on Fe hydroxides, the production of siderophores in the rhizosphere has been proposed to be the key microbial activity that benefits plant Fe acquisition (Masalha et al. 2000; Yehuda et al., 2000; Jin et al., 2006, 2010; Desai and Archana, 2011; Hayat et al., 2012). In a soil cultivation study, Carrillo-Castañeda et al. (2005) observed that Fe acquisition of common bean plants was increased by siderophore-producing micro-organisms. Furthermore, using hydroponic culture, various microbial Fe–siderophore chelates have been demonstrated to serve as good sources of Fe for plants, for example Fe–ferrioxamine for oat (Crowley et al., 1991), Fe–pyoverdine for arabidopsis (Vansuyt et al., 2007), Fe–erobactin for soybean and oat (Chen et al., 1998, 2000), and Fe–rhizoferrin for tomato, barley and corn (Yehuda et al., 2000). In addition, the siderophore secreted by a Pseudomonas isolated from a soil solution incubated with phenolic root exudates of Fe-deficient red clover plants can dissolve Fe from an insoluble Fe source, and the dissolved Fe can be easily utilized by plants (Jin et al., 2010). Interestingly, the Fe levels in plants supplied with Fe in the form of Fe–siderophore are much higher than those in plants supplied with the same Fe concentration in the form of Fe–EDTA (Jin et al., 2010). A similar phenomenon was observed by Vansuyt et al. (2007), wherein the Fe concentration in arabidopsis plants fed with Fe–pyoverdine was higher than in those fed with Fe–EDTA. These data indicate that Fe–siderophores may be incorporated into the roots of Strategy I plants more efficiently than other Fe sources. The redox potential E(mV) of siderophore–Fe3+ chelates is generally low, within the range of –330 to –750 mV (Boukhalfa and Crumbliss, 2002). In plants, the redox potential of most biological reductants is higher than this; for example, E = –320 mV for NADPH, which is suggested to be the electron donor for Fe(III) reduction by ferric chelate reductase in Strategy I plants (Robinson et al., 1999). Bienfait et al. (1983) demonstrated that there is little reduction of Fe(III)–ferrioxamine B and the Fe(III)–aerobactin complexes. Therefore, it seems that Fe(III)–siderophore chelates are probably acquired via a reduction-independent pathway in Strategy I plants. In Strategy II plants, although Fe acquisition does not depend on a reduction mechanism, the YS1 transporter works efficiently for Fe(III)–phytosiderophore but not for other Fe(III) chelates such as microbial Fe(III)–siderophores (Römheld and Marschner, 1986; Curie et al., 2001). Accordingly, acquisition of Fe(III)–siderophore chelates by the roots of Strategy II plants may also‡ be achieved via an as yet unidentified plasmalemma pathway. It is possible that endocytosis is involved in Fe–siderophore incorporation into root cells (Lemanceau et al., 2009).
Fig. 3.
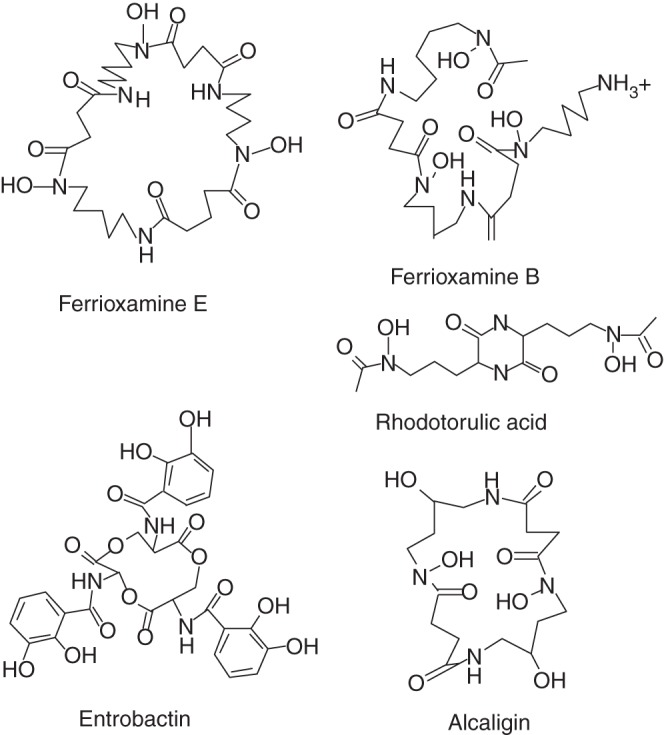
Representative siderophore structures with hydroxamic acid and catechol moieties (Boukhalfa and Crumbliss, 2002). Ferrioxamine E, ferrioxamine B, rhodotorulic acid and alcaligin are shown for the hydroxamic acid moiety, and entrobactin represents the catechol Fe(III)-chelating moiety.
Generation of protons
Theoretically, the solubility of Fe decreases up to 1000-fold for each unit increase in pH (Guerinot and Yi, 1994). Hence, acidification of the rhizosphere can have an enormous impact on Fe solubility in the vicinity of the roots. The two most frequently used nitrogen fertilizers in crop production are urea and ammonium. Upon the application of urea to soil, it is rapidly hydrolysed to ammonia by urease enzymes (Singh et al., 2013). In well-aerated soils, ammonia and ammonium are converted to nitrate via nitrification, which is accompanied by release of protons (H+) (Van Miegroet and Cole, 1984). It should be noted that uptake of nitrogen as ammonium in plants also releases protons (Xu et al., 2012). Therefore, both nitrification and ammonium uptake can lead to soil acidification. However, the key process causing soil acidification due to ammonium-based fertilizers is nitrification, which is catalysed by ammonia-oxidizing prokaryotes (Jetten et al., 1997). Accordingly, in calcareous soils, the micro-organisms associated with nitrification can be expected to increase Fe solubility via H+ generation, and thereby facilitate plant Fe acquisition. Supporting this conclusion, Malhi et al. (1998) found in a 27 year field experiment that both soil acidification and the DTPA-extractable Fe concentration increase significantly with the rate of ammonium nitrate fertilization. Nevertheless, a contribution of plant ammonium uptake to the increase of extractable Fe in soil cannot be excluded.
In calcareous soils, many phosphate-solubilizing bacteria (PSB) also excrete H+. For instance, both Penicillium bilaji and Penicillium cf. fuscum significantly lower the soil pH in the presence of ammonium (Asea et al., 1988), and similar results are observed for Penicillium aurantiogriseum and Penicillium simplicissimum (Illmer and Schinner, 1995; Illmer et al., 1995; Ahuja et al., 2007). Thus, in calcareous soils, the presence of H+-excreting PSB may also help to increase Fe solubility. However, little information is available regarding the effect of H+-excreting PSB on plant Fe uptake. Besides PSB, mycorrhizae also excrete H+. The role of mycorrhizae in plant Fe acquisition will be discussed below.
Production of hormonal compounds
Enhancing Fe-deficiency-inducible responses can be predicted to increase plant acquisition of Fe from Fe-limited soils. Indeed, Zhang et al. (2009) reported that the soil bacterium Bacillus subtilis GB03 could enhance Fe acquisition of arabidopsis plants by activating the Fe-deficiency-inducible responses, suggesting that soil micro-organisms could regulate plant Fe acquisition via a signalling process. In the last decade, plant physiologists have made a great effort to uncover the signals responsible for triggering Fe deficiency responses in plant roots, and several hormonal compounds have been identified as signalling elements (Hindt and Guerinot, 2012; Ivanov et al., 2012; Kobayashi and Nishizawa, 2012). These phytohormones include auxin (Jin et al., 2008; Chen et al., 2010), nitric oxide (NO) (Graziano and Lamattina, 2007), ethylene (Garcia et al., 2011), cytokinin (Séguéla et al., 2008) and brassinosteroids (Wang et al., 2012). Among these, auxin, NO and ethylene are particularly interesting with regard to revealing potential interactions between soil micro-organisms and Fe uptake of plants since these compounds can be generated by soil micro-organisms.
Production of auxin-like compounds
Indole-3-acetic acid (IAA) is the most active auxin in plants, and it is known to stimulate both rapid (e.g. increases in cell elongation) and long-term (e.g. cell division and differentiation) responses (Zhao et al., 2010). The majority of micro-organisms isolated from rhizosphere soil can produce IAA (Patten et al., 2012). In addition to IAA, micro-organisms such as Paenibacillus polymyxa, Azospirilla and Klebsiella pneumoniae also release other auxin-like compounds, such as indole-3-butyric acid (IBA), indole-3-ethanol (TOL), indole-3-carboxylic acid and indole-3-aldehyde (Hayat et al., 2010). Some examples of soil micro-organisms known to produce auxin compounds are listed in Table 1. The ratio of auxin-producing micro-organisms in soil solution incubated with phenolic root exudates of Fe-deficient red clover is higher than that in phenolic-free control soil solution (Jin et al., 2006, 2008), suggesting that Fe deficiency in plants may lead to beneficial effects on the growth of auxin-producing micro-organisms in the rhizosphere. Nevertheless, this needs to be confirmed by in situ comparisons between rhizosphere soils of Fe-sufficient and Fe-deficient plants.
Table 1.
Production of auxin-like compounds by soil micro-organisms
| Micro-organisms | Auxin compounds | References |
|---|---|---|
| Paenibacillus polymyxa | Indole-3-acetic acid; indole-3-butyric acid; indole-3-ethanol; indole-3-carboxylic acid; indole-3-aldehyde | Lebuhn et al. (1997) |
| Azospirillum brasilense | Indole-3-acetic acid; indole-3-ethanol | El-Khawas and Adachi (1999) |
| Klebsiella pneumoniae | Indole-3-acetic acid; indole-3-ethanol; indole-3-pyruvic acid; indole-3-acetaldehyde | El-Khawas and Adachi (1999) |
| Rhizobium leguminosarum | Indole-3-acetic acid | Dazzo et al. (2000); Bhattacharjee et al. (2012) |
| Azotobacter sp. | Indole-3-acetic acid | Zahir et al. (2000); Ahmad et al. (2005) |
| Rhizobacteria (unidentified) | Indole-3-acetic acid | Asghar et al. (2002) |
| Pseudomonas fluorescens | Indole-3-acetic acid | Dey et al. (2004) |
| Rhizobacteria (unidentified) | Indole-3-acetic acid | Khalid et al. (2004) |
| Azospirillum brasilenseA3, A4, A7, A10, CDJA; Bacillus circulans P2; Bacillus sp. P3; Bacillus magaterium P5; Bacillus. Sp. Psd7; Streptomyces anthocysnicus; Pseudomonas aeruginosa Psd5; Pseudomonas pieketti Psd6; Pseudomonas fluorescens MTCC103 | Indole-3-acetic acid | Thakuria et al. (2004) |
| Pseudomonas sp. | Indole-3-acetic acid | Roesti et al. (2006) |
| Soil bacterial isolates | Auxin (unidentified) | Jin et al. (2006) |
| Rhizobium leguminosarum b. Trifolii ACCC18002 | Indole-3-acetic acid | Jin et al. (2006) |
| Bacillus cereus RC18; Bacillus licheniformis RC08; Bacillus megaterium RC07; Bacillus subtilis RC11; Bacillus. OSU-142; Bacillus M-13; Pseudomonas putida RC06; Paenibacillus polymyxa RC05 and RC14 | Indole-3-acetic acid | Çakmakçi et al. (2007) |
| Mesorhizobium loti MP6 | Indole-3-acetic acid | Chandra et al. (2007) |
| Pseudomonas tolaasii ACC23; Pseudomonas fluorescens ACC9; Alcaligenes sp. ZN4; Mycobacterium sp. ACC14 | Indole-3-acetic acid | Dell'Amico et al. (2008) |
| Bacillus sp; Paenibacillus sp. | Indole-3-acetic acid | Beneduzi et al. (2008) |
| Enterobacter aerogenes sp. NII-0907; Enterobacter aerogenes sp. NII-0929; Enterobacter cloacae subsp. cloacae sp. NII-0931; Enterobacter asburiae sp. NII-0934 | Indole-3-acetic acid | Deepa et al. (2010) |
| Streptomyces strains C | Indolyl-3-acetic acid | Sadeghi et al. (2012) |
| Enterobacter; Klebsiella | Indolyl-3-acetic acid | de Santi Ferrara et al. (2013) |
Auxin has been demonstrated to be an important chemical signal enhancing Fe-deficiency-inducible responses. Exogenous addition of synthetic auxin, either IAA or α-naphthaleneacetic acid, enhances Fe-deficiency-induced reduction of ferric Fe, expression of FRO2 and IRT1, and development of root hairs and lateral roots to increase the surface area for Fe uptake (Jin et al., 2008; Chen et al., 2010; Wu et al., 2012). Accordingly, production of auxin-like compounds by soil micro-organisms can be considered a beneficial microbial activity for plant Fe uptake under Fe-limited conditions. In support of this, auxins produced by a microbe that was isolated from soil mixed with phenolics secreted from Fe-deficient red clover plants markedly enhance the activity of ferric chelate reductase in roots of Fe-deficient plants (Jin et al., 2006).
Production of NO gas
Nitric oxide is a small, highly reactive and membrane-permeable gaseous molecule. In the last decade, NO has been recognized as a plant hormone due to its signalling functions in numerous cellular and physiological processes (Shapiro, 2005; Palmieri et al., 2008; Baudoin, 2011). In addition to eukaryotic organisms such as plants, soil micro-organisms also produce NO. The processes of nitrification and denitrification have been identified as the most important sources of NO generation in soil micro-organisms (Gasche and Papen, 2002; Kitzler et al., 2006; Kim et al., 2012). The critical factor affecting generation of NO by soil micro-organisms (Davidson et al., 2000; Kim et al., 2012) is the soil water content, since it controls the oxygen content of the soil, which in turn determines whether nitrification or denitrification is the dominant process in the soil. Therefore, in dry, well-aerated soils, the oxidative process of nitrification dominates, and the more oxidized gas, NO, is the most common nitrogen oxide produced by soil micro-organisms (Davidson et al., 2000; Stange et al., 2013). NO production in soils also depends on soil N availability (Kitzler et al., 2006). The mean level of NO can reach 250 ppb in forest soils (Rudolph and Conrad, 1996), whereas it is probably much higher in agricultural soils due to N fertilizer input during crop cultivation. Like auxin, NO acts as an enhancer signal in the regulation of Fe-deficiency-inducible responses. Exogenous addition of the NO donor S-nitrosoglutathione (GNSO) promotes the Fe-deficiency-induced reduction of ferric Fe, expression of FRO2 and IRT1, and development of root hairs and lateral roots (Graziano and Lamattina, 2007; Chen et al., 2010; Jin et al., 2011; Meiser et al., 2011; Zhang et al., 2012). Therefore, it is reasonable to assume that NO generation by soil micro-organisms may enhance Fe acquisition of plants grown in Fe-limited soils. Experimental evidence from soil culture is still necessary to test this, however.
Production of ethylene gas
Ethylene is also a small, membrane-permeable gaseous signal molecule in plants. Ethylene concentrations as low as 10 nL L−1 can evoke plant responses. Interestingly, ethylene is a common constituent of the soil atmosphere and may accumulate to 10 µL L−1 in soil when conditions favour ethylene production or inhibit ethylene degradation (Smith, 1976). Therefore the ethylene concentration in most soil conditions is high enough to be biologically active for plants. Up to now, all evidence points to micro-organisms as the major source of ethylene in soil (Jäckel et al., 2004), and bacteria, fungi and yeast may all produce ethylene. Accumulation of ethylene in the soil greatly depends on the oxygen level in the soil, with low oxygen concentrations favouring ethylene accumulation (Xu and Inubushi, 2007). Thus, aerobic conditions in Fe-limited soil seem to be disadvantageous for ethylene generation by micro-organisms. However, López-Millán et al. (2000) reported that the oxygen consumption rate in root tips of Fe-deficient plants is about 3-fold more than that of Fe-sufficient plants. Therefore, it is reasonable to assume that there is less oxygen in the rhizosphere soil of Fe-deficient plants; in other words, the growth of Fe-deficient plants may favour ethylene generation in rhizosphere soil. Moreover, a study by Arshad and Frankenberger (1991) revealed that ethylene generation is inhibited in the presence of Fe(III) >10 mg kg−1 soil, which suggests that the low level of available Fe in Fe-limited soils should also favour ethylene production. Like auxin and NO, ethylene acts as an enhancer of Fe-deficiency-inducible responses. Exogenous addition of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) significantly increases the Fe-deficiency-induced reduction of ferric Fe, expression of FRO2 and IRT1, and development of root hairs (García et al., 2011; Wu et al., 2011). Therefore, generation of ethylene may be another microbial activity that improves plant Fe acquisition in Fe-limited soils.
Micro-organisms enhance plant Fe uptake via symbiotic interactions with plants
Rhizobium–legume symbiosis
Rhizobium nodulation is ubiquitous in leguminous plants. The most important function of nodules is symbiotic N2 fixation, a process in which Fe-containing proteins play very important roles (Terpolilli et al., 2012). Nodulated legumes thus have an increased need for Fe as compared with non-nodulated plants. In order to meet this increased Fe demand, legumes have developed a mechanism to enhance Fe-deficiency-induced responses in the roots upon nodulation. For example, secretion of both protons and reductants in Fe-deficient roots of peanut plants is significantly increased by rhizobium nodulation (Terry et al., 1988). Soerensen et al. (1988) obtained similar results in soybean plants. Rhizobium nodulation also clearly enhances the activity of ferric chelate reductase in roots of Fe-deficient red clover plants (Deryl and Skorupska, 1992; Jin et al., 2007). In a split-root experiment, Soerensen et al. (1989) found more Fe(III)–EDTA reduction activity on the Bradyrhizobium-infected side of soybean roots than on the non-inoculated side, and the reduction activity on the infected side was greater on the root below the nodule clusters. Furthermore, in a recent study by Slatni et al. (2012), overaccumulation of H+-ATPase and IRT1 proteins was observed especially around the cortex cells of nodules from Fe-deficient common bean plants. These results suggest that the positive effects of rhizobium nodulation on Fe-deficiency-induced responses could be locally controlled, probably by a signal derived from nodules.
The question arises of whether the enhancement of Fe-deficiency-induced responses in nodulated roots merely meets the increased Fe requirement of the nodules, or systemically improves the Fe nutrition of whole plants. Using the radioactive isotope 55Fe, Deryl and Skorupska (1992) found that rhizobium nodulation promotes Fe transport from roots to shoots in red clover. In addition, we observed that Fe acquisition and growth of red clover significantly decrease when the plants are grown in sterilized calcareous soil, but are restored by nodulation with Rhizobium leguminosarum bv. trifolii ACCC18002 (Jin et al., 2006). Most recently, Mishra et al. (2011, 2012) demonstrated that nodulation of roots by R. leguminosarum-PR1 elevates the Fe content in pea (Pisum sativum L.) and lentil (Lens culinaris L.) plants. All of these results suggest that rhizobium nodulation can systemically improve the Fe nutrition of plants through enhancing Fe-deficiency-induced responses. However, the mechanism through which rhizobium nodulation enhances Fe-deficiency-induced responses still remains largely unknown. It appears to occur independently of N2 fixation, as nodules that are null for N2 fixation still enhance the activity of ferric chelate reductase in roots (Deryl and Skorupska, 1992).
It is interesting to note that leguminous plants secrete more phenolics under Fe-deficient conditions. Several phenolic compounds can stimulate the growth of nodulating rhizobia, and also act as signal molecules to induce rhizobial nod gene expression, both of which enhance rhizobium nodulation (Hassan and Mathesius, 2012). Therefore, the Fe-deficiency-induced secretion of phenolics may favour the growth of nodulating rhizobia in leguminous plants. Indeed, phenolics secreted by Fe-deficient alfalfa significantly stimulate the growth of Rhizobium meliloti (Masaoka et al., 1997). Thus, phenolics secreted by Fe-deficient legumes may improve Fe acquisition of the legumes themselves via a mechanism involving phenolic-induced rhizobium nodulation.
It is also worth noting that the nodulation of legume roots can be improved by inoculation of siderophore-producing rhizobial strains under Fe-deficient conditions (e.g. Tang et al., 1991; Arora et al., 2001). The underlying mechanism may be that siderophore production facilitates the Fe acquisition of nodules, and ultimately promotes the synthesis of Fe-containing proteins, which figure prominently in nodules (Terpolilli et al., 2012). Considering the resulting availability of Fe–siderophores for plant growth and the effect of rhizobium nodulation on Fe-deficiency-induced responses of plant roots, inoculation with siderophore-producing rhizobia can be predicted to enhance Fe nutrition of leguminous plants more than inoculation with rhizobia that do not produce siderophores.
Mycorrhizal symbiosis
The mycorrhizal symbioses formed between plant roots and mycorrhizal fungi are of great interest to ecologists and plant nutritionists because of their potential influence on ecosystem processes, their role in determining plant diversity in natural communities and the ability of mycorrhizae to enhance nutrient acquisition of host roots (Wagg et al., 2011; Smith and Smith, 2012). The majority of plants growing under natural conditions have associated mycorrhizae (Smith and Reed, 1997; Harrison, 2012). It is generally accepted that morphological effects are the most important mechanism by which mycorrhizae increase nutrient acquisition of plants (Clark and Zeto, 2000). For example, in ectomycorrhiza-infected pine seedlings, the total hypha length is >100-fold greater than the total root length (Rousseau et al., 1994; Smith and Reed, 1997), and similar findings have been reported for ectomycorrhiza-infected willow seedlings (Jones et al., 1990). In addition to increases in length, the available surface area for absorbing nutrients is also increased remarkably by the formation of mycorrhizal hyphae (Smith and Reed, 1997). Accordingly, although plants with mycorrhizal roots have access to pools of soil nutrients similar to those of non-mycorrhizal plants, the hyphal network allows considerably larger volumes of soil to be explored. These morphological effects should theoretically enhance plant acquisition of all essential mineral elements, including Fe.
Mycorrhizae also excrete H+ and low molecular weight organic chelating compounds, such as citric acid, oxalic acid and siderophores (Li et al., 1991; Winkelmann, 2007; Bharadwaj et al., 2012), which should facilitate mobilization of sparingly available Fe in the rhizosphere soil, and therefore also theoretically promote plant Fe acquisition.
Several studies have investigated the effect of mycorrhizal symbiosis on plant Fe uptake. Some of these studies, however, found that acquisition of Fe was enhanced in mycorrhizae-infected plants (Cress et al., 1986; Raju et al., 1990; Treeby, 1992; Medeiros et al., 1993; Clark and Zeto, 1996; El-Ghandour et al., 1996; Al-Karaki and Clark, 1998; Al-Karaki et al., 1998; Caris et al., 1998; Purakayastha et al., 1998; Pirazzi et al, 1999; Wang et al., 2007; Suri et al., 2011; Amanullah et al., 2012; Labidi et al., 2012), whereas others found it to be decreased (Pacovsky and Fuller, 1988; Kothari et al., 1990, 1991; Clark et al., 1999). This discrepancy is probably due to differences in soil properties and growth environments for plant culture among these studies. For instance, Treeby (1992) and Medeiros et al. (1993, 1994) found that mycorrhizal plants grown at low pH had higher Fe acquisition than those grown at high pH. In addition, Rajue (1990) observed that Glomus macrocarpum (a species of vesicular-arbuscular mycorrhizal fungi)-colonized plants grown at 25 or 30 °C had 10-fold more Fe than those grown at 20 °C. Furthermore, both the plant species and the mycorrhiza species affect the contribution of mycorrhizal symbiosis to plant Fe uptake (Clark and Zeto, 2000). Overall, the majority of the above studies provide evidence that mycorrhizal symbiosis can beneficially affect plant Fe acquisition. However, further investigation is necessary to explore the effects of soil/growth conditions so that the contradictory results can be reconciled.
CONCLUSIONS AND OUTLOOK
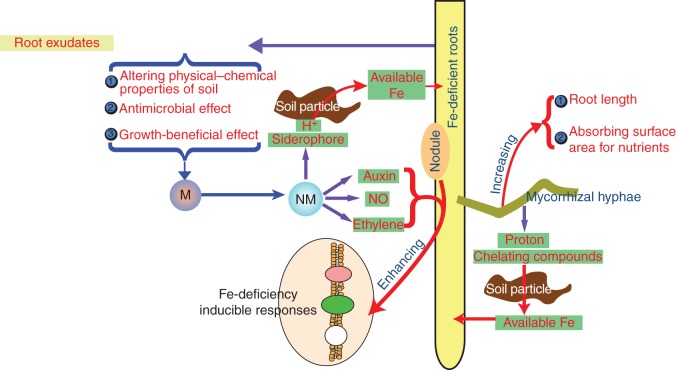
Based on the results discussed herein, we have developed a model for how rhizospheric micro-organisms enhance plant Fe acquisition (Fig. 4). When the plant suffers Fe deficiency stress, organic exudates are secreted from roots and accumulate in the rhizosphere. Then, the root exudates selectively alter the microbial community in the rhizosphere by altering the physical–chemical properties of soil, and/or via their antimicrobial and/or growth-beneficial effects. Favoured micro-organisms then produce siderophores and protons, both of which increase Fe bioavailability, and hormones that enhance plant Fe-deficiency-inducible responses. In addition, plant Fe acquisition could also be promoted by symbioses between some micro-organisms and plants, possibly including: rhizobium nodulation (enhancing plant Fe-deficiency-inducible responses) and mycorrhizal symbiosis (increasing root length and nutrient acquisition area of the root system and increasing Fe availability).
Fig. 4.
Proposed model for microbially enhanced plant Fe acquisition. The ‘M’ in the small circle denotes the original microbial community in the rhizosphere. The ‘NM’ in the small circle denotes the newly formed microbial community.
Although studies have indicated that plant root exudates may be responsible for the alterations of microbial community structure in the rhizosphere that occur in response to plant Fe status, the direct evidence for this is limited. This is probably because the components of root exudates and the composition of soil micro-organism communities are both quite complicated. However, recent advances in chemical analysis and molecular biotechnologies may help to elucidate the above relationship. For example, secondary ion mass spectrometry (SIMS) can be used to trace the uptake of 13C and 15N from labelled compounds by cells (Cliff et al., 2002; Herrman et al., 2007; Clode et al., 2009). The SIMS technology has been used successfully to visualize and measure nutrient absorption by roots and rhizosphere micro-organisms at the nicromolar and nanomolar scale (Herrman et al., 2007; Clode et al., 2009). Hence, this technology can be used to study the root exudate-mediated root–microbe interactions between individual root cells and individual microbes in situ. In addition, the recent development of high-throughput tag-encoded FLX amplicon pyrosequencing (Acosta-Martinez et al., 2008; Su et al., 2012) may also facilitate diversity analyses and species identification of micro-organisms inhabiting the rhizospheric soil. This method generates massive amounts of 16S rRNA gene sequences from hitherto uncultured bacterial groups (Uroz, et al., 2010). For a more in-depth discussion of the technologies that could be used to study root–microbe interactions, the reader is referred to a recent review by Marschner et al. (2011). Besides the above technologies, screening plant mutants failing to exude specific organic compounds may help to clarify which kinds of root exudates are responsible for the Fe-deficiency-induced alteration of microbial community structure in the rhizosphere.
Soil microbial activity clearly plays a central role in plant Fe uptake, but the mechanism underlying this process remains largely unknown. It is likely to involve siderophore and proton production, both of which improve Fe bioavailability in the rhizosphere, and hormone generation, which triggers plant development of increased Fe uptake capacity (Fig. 4). Screening micro-organism mutants that fail to produce siderophores or hormones, and analysis of the effects of such mutants on plant Fe uptake in soil cultivation systems, will help to test this directly. In addition, the mechanism of Fe–siderophore acquisition by plants and their subsequent utilization within plants also remains unknown. Iron–siderophores may be directly acquired by root cells via an unidentified component that is independent of the IRT1 transporters in Strategy I plants, and also independent of the YS1 transporter in Strategy II plants. Screening mutant plants by using Fe–siderophores as the sole Fe source may help to uncover the unidentified component(s) used for Fe–siderophore uptake. In addition, the SIMS technology mentioned above may facilitate elucidation of the mechanism of Fe–siderophore uptake by plant roots.
Rhizobial and mycorrhizal symbioses might also improve the Fe nutrition of host plants (Fig. 4). However, the beneficial effects of mycorrhizal symbiosis on plant Fe nutrition may depend on the soil properties and growth environment, as well as plant and mycorrhiza species. Therefore, it is still necessary to determine what soil proprieties, growth environments, plant species and mycorrhiza species co-operate to improve plant Fe nutrition, information that may help to promote crop production in calcareous soils.
ACKNOWLEDGEMENTS
This work was supported by the Natural Science Foundation of China (31270041), the Zhejiang Provincial Natural Science Foundation (LR13C130001), the Innovative Research Team (IRT1185) and the Fundamental Research Funds for Central Universities.
LITERATURE CITED
- Acosta-Martinez V, Dowd S, Sun Y, Allen V. Tag-encoded pyrosequencing analysis of bacterial diversity in a single soil type as affected by management and land use. Soil Biology and Biochemistry. 2008;40:2762–2770. [Google Scholar]
- Ahmad F, Ahmad I, Khan MS. Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turkish Journal of Biology. 2005;29:29–34. [Google Scholar]
- Ahuja A, Ghosh SB, D'Souza SF. Isolation of a starch utilizing, phosphate solubilizing fungus on buffered medium and its characterization. Bioresource Technology. 2007;98:3408–3411. doi: 10.1016/j.biortech.2006.10.041. [DOI] [PubMed] [Google Scholar]
- Al-Karaki GN, Clark RB. Growth, mineral acquisition, and water use by mycorrhizal wheat grown under water stress. Journal of Plant Nutrition. 1998;21:263–276. [Google Scholar]
- Al-Karaki GN, Al-Raddad A, Clark RB. Water stress and mycorrhizal-isolate effects on growth and nutrient acquisition of wheat. Journal of Plant Nutrition. 1998;21:891–902. [Google Scholar]
- Amanullah MM, Archana J, Manoharan S, Subramanian KS. Influence of iron and AM inoculation on metabolically active iron, chlorophyll content and yield of hybrid maize in calcareous soil. Journal of Agronomy. 2012;11:27–30. [Google Scholar]
- Arora NK, Kang SC, Maheswari DK. Isolation of siderophore-producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Current Science. 2001;81:673–677. [Google Scholar]
- Arshad M, Frankenberger WT., Jr Effects of soil properties and trace elements on ethylene production in soils. Soil Science. 1991;151:377–386. [Google Scholar]
- Asea PEA, Kucey RMN, Stewart JWB. Inorganic phosphate solubilization by two Penicillium species in solution culture and soil. Soil Biology and Biochemistry. 1998;20:459–464. [Google Scholar]
- Asghar HN, Zahir ZA, Arshad M, Khaliq A. Relationship between in vitro production of auxins by rhizobacteria and their growth-promoting activities in Brassica juncea L. Biology and Fertility of Soils. 2002;35:1–237. [Google Scholar]
- Baudoin E. The language of nitric oxide signaling. Plant Biology. 2011;13:233–242. doi: 10.1111/j.1438-8677.2010.00403.x. [DOI] [PubMed] [Google Scholar]
- Beneduzi A, Peres D, Vargas LK, Bodanese-Zanettini MH, Passaglia LMP. Evaluation of genetic diversity and plant growth promoting activities of nitrogen-fixing Bacilli isolated from rice fields in South Brazil. Applied Soil Ecology. 2008;39:311–320. [Google Scholar]
- Berg G, Smalla K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiology Ecology. 2009;68:1–13. doi: 10.1111/j.1574-6941.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- Bharadwaj DP, Alström S, Lundquist PO. Interactions among Glomus irregulare, arbuscular mycorrhizal spore-associated bacteria, and plant pathogens under in vitro conditions. Mycorrhiza. 2012;22:437–447. doi: 10.1007/s00572-011-0418-7. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee RB, Jourand P, Chaintreuil C, Dreyfus B, Singh A, Mukhopadhyay SN. Indole acetic acid and ACC deaminase-producing Rhizobium leguminosarum bv. trifolii SN10 promote rice growth, and in the process undergo colonization and chemotaxis. Biology and Fertility of Soils. 2012;48:173–182. [Google Scholar]
- Bienfait HF, Bino AM, Bliek JF, Duivenvoorden JF, Fontains JM. Characterization of ferric reducing activity in roots of Fe-deficient Phaseolus vulgaris. Physiologia Plantarum. 1983;59:196–202. [Google Scholar]
- Blum U, Staman KL, Flint LJ, Shafer SR. Induction and/or selection of phenolic acid-utilizing bulk-soil and rhizosphere bacteria and their influence on phenolic acid phytotoxicity. Journal of Chemical Ecology. 2000;26:2059–2078. [Google Scholar]
- Bonnefoy V, Holmes DS. Genomic insights into microbial iron oxidation and iron uptake strategies in extremely acidic environments. Environmental Microbiology. 2012;14:1597–1611. doi: 10.1111/j.1462-2920.2011.02626.x. [DOI] [PubMed] [Google Scholar]
- Boukhalfa H, Crumbliss AL. Chemical aspects of siderophore mediated iron transport. Biometals. 2002;15:325–339. doi: 10.1023/a:1020218608266. [DOI] [PubMed] [Google Scholar]
- Çakmakçi R, Erat M, Erdoğan ÜG, Dönmez MF. The influence of PGPR on growth parameters, antioxidant and pentose phosphate oxidative cycle enzymes in wheat and spinach plants. Journal of Plant Nutrition and Soil Science. 2007;170:288–295. [Google Scholar]
- Carelli M, Gnocchi S, Fancelli S, et al. Genetic diversity and dynamics of Sinorhizobium meliloti populations nodulating different alfalfa cultivars in Italian soils. Applied and Environmental Microbiology. 2000;66:4785–4789. doi: 10.1128/aem.66.11.4785-4789.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caris C, Hördt W, Hawkins HJ, Römheld V, George E. Studies of iron transport by arbuscular mycorrhizal hyphae from soil to peanut and sorghum plants. Mycorrhiza. 1998;8:35–39. [Google Scholar]
- Carrillo-Castañeda G, Muñoz JJ, Peralta-Videa JR, Gomez E, Gardea-Torresdey JL. Modulation of uptake and translocation of iron and copper from root to shoot in common bean by siderophore-producing microorganisms. Journal of Plant Nutrition. 2005;28:1853–1865. [Google Scholar]
- Carvalhais LC, Dennis PG, Fedoseyenko D, Hajirezaei MR, Borriss R, von Wirén N. Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. Journal of Plant Nutrition and Soil Science. 2011;174:3–11. [Google Scholar]
- Chandra S, Choure K, Dubey RC, Maheshwari DK. Rhizosphere competent Mesorhizobium loti MP6 induces root hair curling, inhibits Sclerotinia sclerotiorum and enhances growth of Indian mustard (Brassica campestris) Brazilian Journal of Microbiology. 2007;38:124–130. [Google Scholar]
- Chen LM, Dick WA, Streeter JG, Hoitink HAJ. Fe chelates from compost microorganisms improve Fe nutrition of soybean and oat. Plant and Soil. 1998;200:139–147. [Google Scholar]
- Chen LM, Dick WA, Streeter JG. Production of aerobactin by microorganisms from a compost enrichment and soybean utilization. Journal of Plant Nutrition. 2000;23:2047–2060. [Google Scholar]
- Chen WW, Yang JL, Qin C, et al. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiology. 2010;154:810–819. doi: 10.1104/pp.110.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerale S, Lucas LJ, Keast RSJ. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Current Opinion in Biotechnology. 2012;23:129–135. doi: 10.1016/j.copbio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Clark RB, Zeto SK. Iron acquisition by mycorrhizal maize grown on alkaline soil. Journal of Plant Nutrition. 1996;19:247–264. [Google Scholar]
- Clark RB, Zeto SK. Mineral acquisition by arbuscular mycorrhizal plants. Journal of Plant Nutrition. 2000;23:867–902. [Google Scholar]
- Clark RB, Zobel RW, Zeto SK. Effects of mycorrhizal fungus isolate on mineral acquisition by Panicum virgatum in acidic soil. Mycorrhiza. 1999;9:167–176. [Google Scholar]
- Cliff JB, Gaspar DJ, Bottomley PJ, Myrold DD. Exploration of inorganic C and N assimilation by soil microbes with time-of-flight secondary ion mass spectrometry. Applied and Environmental Microbiology. 2002;68:4067–4073. doi: 10.1128/AEM.68.8.4067-4073.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clode PL, Kilburn MR, Jones DL, et al. In situ mapping of nutrient uptake in the rhizosphere using nanoscale secondary ion mass spectrometry. Plant Physiology. 2009;151:1751–1757. doi: 10.1104/pp.109.141499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress WA, Johnson GV, Barton LL. The role of endomycorrhizal fungi in iron uptake by Hillaria jamesii. Journal of Plant Nutrition. 1986;9:547–556. [Google Scholar]
- Crowley DE, Wang YC, Reid CPP, Szaniszlo PJ. Mechanisms of iron acquisition from siderophores by microorganisms and plants Plant and Soil. 1991;130:179–198. [Google Scholar]
- Curie C, Briat JF. Iron transport and signaling in plants. Annual Review of Plant Biology. 2003;54:183–206. doi: 10.1146/annurev.arplant.54.031902.135018. [DOI] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature. 2001;409:346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- Curie C, Cassin G, Couch D, et al. Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Annals of Botany. 2009;103:1–11. doi: 10.1093/aob/mcn207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakora FD, Phillips DA. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant and Soil. 2002;245:35–47. [Google Scholar]
- Davidson EY, Keller M, Erickson HE, Verchot LV, Veldkamp E. Testing a conceptual model of soil emissions of nitrous and nitric oxides. BioScience. 2000;50:667–680. [Google Scholar]
- Dazzo FB, Yanni YG, Rizk R, et al. Progress in multinational collaborative studies on the beneficial association between Rhizobium leguminosarum bv. trifolii and rice. In: Ladha JK, Reddy PM, editors. The quest for nitrogen fixation in rice. Los Banos, The Philippines: IRRI; 2000. pp. 167–189. [Google Scholar]
- Deepa CK, Dastager SG, Pandey A. Isolation and characterization of plant growth promoting bacteria from non-rhizospheric soil and their effect on cowpea (Vigna unguiculata (L.) Walp.) seedling growth. World Journal of Microbiology and Biotechnology. 2010;26:1233–1240. doi: 10.1007/s11274-009-0293-y. [DOI] [PubMed] [Google Scholar]
- Dell'Amico E, Cavalca L, Andreoni V. Improvement of Brassica napus growth under cadmium stress by cadmium-resistant rhizobacteria. Soil Biology and Biochemistry. 2008;40:74–84. [Google Scholar]
- Deryl M, Skorupska A. Rhizobial siderophore as an iron source for clover. Physiologia Plantarum. 1992;85:549–553. [Google Scholar]
- Desai A, Archana G. Role of siderophores in crop improvement. In: Maheshwari DK, editor. Bacteria in agrobiology: plant nutrient management. Berlin: Springer; 2011. pp. 109–139. [Google Scholar]
- Dey R, Pal KK, Bhatt DM, Chauhan SM. Growth promotion and yield enhancement of peanut (Arachis hypogaea L) by application of plant growth promoting rhizobacteria. Microbiological Research. 2004;159:371–394. doi: 10.1016/j.micres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- El-Ghandour IA, El-Sharawy MAO, Abdel-Moniem EM. Impact of vesicular arbuscular mycorrhizal fungi and Rhizobium on the growth and P, N, and Fe uptake by faba-bean. Fertilizers and Environment. 1996;43:43–48. [Google Scholar]
- El-Khawas H, Adachi K. Identification and quantification of auxins in culture media of Azospirillum and Klebsiella and their effect on rice roots. Biology and Fertility of Soils. 1999;28:377–381. [Google Scholar]
- García MJ, Suárez V, Romera FJ, Alcántara E, Pérez-Vicente R. A new model involving ethylene, nitric oxide and Fe to explain the regulation of Fe-acquisition genes in Strategy I plants. Plant Physiology and Biochemistry. 2011;49:537–544. doi: 10.1016/j.plaphy.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Gasche R, Papen H. Spatial variability of NO and NO2 flux rates from soil of spruce and beech forest ecosystems. Plant and Soil. 2002;240:67–76. [Google Scholar]
- Gelsomino A, Keijzer-Wolters AC, Cacco G, van Elsas JD. Assessment of bacterial community structure in soil by polymerase chain reaction and denaturing gradient gel electrophoresis. Journal of Microbiological Methods. 1999;38:1–15. doi: 10.1016/s0167-7012(99)00054-8. [DOI] [PubMed] [Google Scholar]
- Graziano M, Lamattina L. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. The Plant Journal. 2007;52:949–960. doi: 10.1111/j.1365-313X.2007.03283.x. [DOI] [PubMed] [Google Scholar]
- Guerinot ML, Yi Y. Iron: nutritious, noxious, and not readily available. Plant Physiology. 1994;104:815–820. doi: 10.1104/pp.104.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MJ. Cellular programs for arbuscular mycorrhizal symbiosis. Current Opinion in Plant Biology. 2012;15:691–698. doi: 10.1016/j.pbi.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Hassan S, Mathesius U. The role of flavonoids in root–rhizosphere signalling: opportunities and challenges for improving plant–microorganism interactions. Journal of Experimental Botany. 2012;63:3429–3444. doi: 10.1093/jxb/err430. [DOI] [PubMed] [Google Scholar]
- Hayat R, Ali S, Amara U, Khalid R, Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Annals of Microbiology. 2010;60:579–598. [Google Scholar]
- Hayat R, Ahmed I, Sheirdil RI. An overview of plant growth promoting rhizobacteria (PGPR) for sustainable agriculture. In: Ashraf M, Öztürk M, Ahmad MS, Aksoy A, editors. Crop production for agricultural improvement, part 3. Dordrecht: Springer; 2012. pp. 557–579. [Google Scholar]
- Hell R, Stephan UW. Iron uptake, trafficking and homeostasis in plants. Planta. 2003;216:541–551. doi: 10.1007/s00425-002-0920-4. [DOI] [PubMed] [Google Scholar]
- Herrmann AM, Ritz K, Nunan N, et al. Nano-scale secondary ion mass spectrometry – a new analytical tool in biogeochemistry and soil ecology. Soil Biology and Biochemistry. 2007;39:1835–1850. [Google Scholar]
- Hindt MN, Guerinot ML. Getting a sense for signals: regulation of the plant iron deficiency response. Biochimica et Biophysica Acta. 2012;1823:1521–1530. doi: 10.1016/j.bbamcr.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illmer P, Schinner F. Solubilization of inorganic calcium phosphates – solubilization mechanisms. Soil Biology and Biochemistry. 1995;27:257–263. [Google Scholar]
- Illmer P, Barbato A, Schinner F. Solubilization of hardly-soluble AlPO4 with P-solubilizing microorganisms. Soil Biology and Biochemistry. 1995;27:265–270. [Google Scholar]
- Ivanov R, Brumbarova T, Bauer P. Fitting into the harsh reality: regulation of iron-deficiency responses in dicotyledonous plants. Molecular Plant. 2012;5:27–42. doi: 10.1093/mp/ssr065. [DOI] [PubMed] [Google Scholar]
- Jäckel U, Schnell S, Conrad R. Microbial ethylene production and inhibition of methanotrophic activity in a deciduous forest soil. Soil Biology and Biochemistry. 2004;36:835–840. [Google Scholar]
- Jetten MSM, Logemann S, Muyzer G, et al. Novel principles in the microbial conversion of nitrogen compounds. Antonie van Leeuwenhoek International Journal of General and Molecular Microbiology. 1997;71:75–93. doi: 10.1023/a:1000150219937. [DOI] [PubMed] [Google Scholar]
- Jin CW, He YF, Tang CX, Wu P, Zheng SJ. Mechanisms of microbially enhanced iron uptake in red clover. Plant, Cell and Environment. 2006;29:888–897. doi: 10.1111/j.1365-3040.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- Jin CW, You GY, Tang CX, Wu P, Zheng SJ. Iron-deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover (Trifolium pratense L.) Plant Physiology. 2007;144:278–285. doi: 10.1104/pp.107.095794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, You GY, Zheng SJ. The iron deficiency-induced phenolics secretion plays multiple important roles in plant iron acquisition underground. Plant Signaling and Behavior. 2008;3:60–61. doi: 10.4161/psb.3.1.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, Li GX, Yu XH, Zheng SJ. Plant Fe status affects the composition of siderophore-secreting microorganisms in the rhizosphere. Annals of Botany. 2010;105:835–841. doi: 10.1093/aob/mcq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, Du ST, Shamsi IH, Luo BF, Lin XY. NO synthase-generated NO acts downstream of auxin in regulating Fe-deficiency-induced root branching that enhances Fe-deficiency tolerance in tomato plants. Journal of Experimental Botany. 2011;62:3875–3884. doi: 10.1093/jxb/err078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MD, Durall DM, Tinker PB. Phosphorus relationship and production of extrametrical hyphae by two types of willow ectomycorrhizas at different soil phosphorus levels. New Phytologist. 1990;115:259–267. doi: 10.1111/j.1469-8137.1990.tb00451.x. [DOI] [PubMed] [Google Scholar]
- Khalid A, Arshad M, Zahir ZA. Screening plant growth promoting rhizobacteria for improving growth and yield of wheat. Journal of Applied Microbiology. 2004;96:473–480. doi: 10.1046/j.1365-2672.2003.02161.x. [DOI] [PubMed] [Google Scholar]
- Kim SA, Guerinot ML. Mining iron: iron uptake and transport in plants. FEBS Letters. 2007;581:2273–2280. doi: 10.1016/j.febslet.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Kim DG, Vargas R, Bond-Lamberty B, Turetsky MR. Effects of soil rewetting and thawing on soil gas fluxes: a review of current literature and suggestions for future research. Biogeosciences. 2012;9:2459–2483. [Google Scholar]
- Kitzler B, Zechmeister-Boltenstern S, Holtermann C, Skiba U, Butterbach-Bahl K. Nitrogen oxides emission from two beech forests subjected to different nitrogen loads. Biogeosciences. 2006;3:293–310. [Google Scholar]
- Kobayashi T, Nishizawa NK. Iron uptake, translocation, and regulation in higher plants. Annual Review of Plant Biology. 2012;63:131–152. doi: 10.1146/annurev-arplant-042811-105522. [DOI] [PubMed] [Google Scholar]
- Kothari SK, Marschner H, Römheld V. Direct and indirect effects of VA mycorrhizal fungi and rhizosphere microorganisms on acquisition of mineral nutrients by maize (Zea mays L.) in a calcareous soil. New Phytologist. 1990;116:637–645. [Google Scholar]
- Kothari SK, Marschner H, Römheid V. Effect of a vesicular-arbuscular mycorrhizal fungus and rhizosphere micro-organisms on manganese reduction in the rhizosphere and manganese concentrations in maize (Zea mays L.) New Phytologist. 1991;117:649–655. [Google Scholar]
- Labidi S, Jeddi FB, Tisserant B, et al. Role of arbuscular mycorrhizal symbiosis in root mineral uptake under CaCO3 stress. Mycorrhiza. 2012;22:337–345. doi: 10.1007/s00572-011-0405-z. [DOI] [PubMed] [Google Scholar]
- Lebuhn M, Heulin T, Hartmann A. Production of auxin and other indolic and phenolic compounds by Paenobacillus polymyxa strains isolated from different proximity to plant roots. FEMS Microbiology Ecology. 1997;22:325–334. [Google Scholar]
- Lemanceau P, Bauer P, Kraemer S, Briat JF. Iron dynamics in the rhizosphere as a case study for analyzing interactions between soils, plants and microbes. Plant and Soil. 2009;321:513–535. [Google Scholar]
- Li XL, Geoege E, Marschner H. Phosphorus depletion and pH decrease at the root–soil and hyphae–soil inter-faces of VAM white clover fertilized with ammonium. New Phytologist. 1991;119:397–404. [Google Scholar]
- Liang C, Jesus EC, Duncan DS, Jackson RD, Tiedje JM, Balser TC. Soil microbial communities under model biofuel cropping systems in southern Wisconsin, USA: impact of crop species and soil properties. Applied Soil Ecology. 2012;54:24–31. [Google Scholar]
- López-Millán AF, Morales F, Andaluz S, et al. Responses of sugar beet roots to iron deficiency. Changes in carbon assimilation and oxygen use. Plant Physiology. 2000;124:885–897. doi: 10.1104/pp.124.2.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi SS, Nyborg M, Harapiak JT. Effects of long-term N fertilizer-induced acidification and liming on micronutrients in soil and in bromegrass hay. Soil and Tillage Research. 1998;48:91–101. [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2nd edn. London: Academic Press; 1995. [Google Scholar]
- Marschner P, Crowley D, Yang CH. Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant and Soil. 2004;261:199–208. [Google Scholar]
- Masalha J, Kosegarten H, Elmaci Ö, Mengel K. The central role of microbial activity for iron acquisition in maize and sunflower. Biology and Fertility of Soils. 2000;30:433–439. [Google Scholar]
- Masaoka Y, Koshino H, Arakawa Y, Asanuma S. Growth promoting effect of root exudates of Fe-deficient alfalfa on Rhizobium meliloti. Proceedings of the XIII International Plant Nutrition Colloquium, Tokyo, Japan. 1997:505–506. [Google Scholar]
- Medeiros CAB, Clark RB, Ellis JR. Effects of MES [2(N-morpholino)-ethanesulfonic acid] and pH on mineral nutrient uptake by mycorrhizal and nonmycorrhizal maize. Journal of Plant Nutrition. 1993;16:2255–2272. [Google Scholar]
- Medina E, De Castro A, Romero CM. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: correlation with antimicrobial activity. Journal of Agricultural and Food Chemistry. 2006;54:4954–4961. doi: 10.1021/jf0602267. [DOI] [PubMed] [Google Scholar]
- Meiser J, Lingam S, Bauer P. Posttranslational regulation of the iron deficiency basic helix–loop–helix transcription factor FIT is affected by iron and nitric oxide. Plant Physiology. 2011;157:2154–2166. doi: 10.1104/pp.111.183285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PK, Bisht SC, Ruwari P, et al. Bioassociative effect of cold tolerant Pseudomonas spp. and Rhizobium leguminosarum-PR1 on iron acquisition, nutrient uptake and growth of lentil (Lens culinaris L.) European Journal of Soil Biology. 2011;47:35–43. [Google Scholar]
- Mishra PK, Bisht SC, Mishra S, Selvakumar G, Bisht JK, Gupta HS. Coinoculation of Rhizobium leguminosarum-PR1 with a cold tolerant Pseudomonas SP. improves iron acquisition, nutrient uptake and growth of field pea (Pisum sativum L.) Journal of Plant Nutrition. 2012;35:243–256. [Google Scholar]
- Murgia I, Arosio P, Tarantino D, Soave C. Biofortification for combating ‘hidden hunger’ for iron. Trends in Plant Science. 2012;17:47–55. doi: 10.1016/j.tplants.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Nishio JN, Abadia J, Terry N. Chlorophyll-proteins and electron transport during iron nutrition-mediated chloroplast development. Plant Physiology. 1985;78:296–299. doi: 10.1104/pp.78.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozoye T, Nagasaka S, Kobayashi T, et al. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. Journal of Biological Chemistry. 2011;286:5446–5454. doi: 10.1074/jbc.M110.180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacovsky RS, Fuller G. Mineral and lipid composition of Glycine–Glomus–Bradyrhizobium symbioses. Physiologia Plantarum. 1988;72:733–746. [Google Scholar]
- Palmieri C, Sell S, Huang X, et al. Nitric oxide-responsive genes and promoters in Arabidopsis thaliana: a bioinformatics approach. Journal of Experimental Botany. 2008;59:177–186. doi: 10.1093/jxb/erm345. [DOI] [PubMed] [Google Scholar]
- Patten CL, Blakney AJC, Coulson TJD. Activity, distribution and function of indole-3-acetic acid biosynthetic pathways in bacteria. Critical Reviews in Microbiology. 2012 doi: 10.3109/1040841X.2012.716819. (in press) [DOI] [PubMed] [Google Scholar]
- Pirazzi R, Rea E, Bragaloni M. Improvement of micronurrient uptake of valuable broadleaves in interaction with Glomus mosseae. Geomicrobiology Journal. 1999;16:79–84. [Google Scholar]
- Purakayastha TJ, Singh CS, Chhondar PK. Growth and iron nutrition of broccoli (Brassica oleracea L. var. italica Planck), grown in a Typic Ustochrept, as influenced by vesicular-arbuscular mycorrhizal fungi in the presence of pyrite and farmyard manure. Biology and Fertility of Soils. 1998;27:35–38. [Google Scholar]
- Raju PS, Clark RB, Ellis JR, Maranville JW. Effects of species of VA-mycorrhizal fungi on growth and mineral uptake of sorghum at different temperatures. Plant and Soil. 1990;121:165–170. [Google Scholar]
- Robin A, Mougel C, Siblot S, Vansuyt G, Mazurier S, Lemanceau P. Effect of ferritin overexpression in tobacco on the structure of bacterial and pseudomonad communities associated with the roots. FEMS Microbiology Ecology. 2006;58:492–502. doi: 10.1111/j.1574-6941.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- Robin A, Mazurier S, Mougel C, et al. Diversity of root-associated fluorescent pseudomonads as affected by ferritin overexpression in tobacco. Environmental Microbiology. 2007;9:1724–1737. doi: 10.1111/j.1462-2920.2007.01290.x. [DOI] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- Roesti D, Guar R, Johri BN, et al. Plant growth stage, fertilizer management and bioinoculation of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria affect the rhizobacterial community structure in rain-fed wheat field. Soil Biology and Biochemistry. 2006;38:1111–1120. [Google Scholar]
- Römheld V, Marschner H. Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiology. 1986;80:175–180. doi: 10.1104/pp.80.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau JVD, Sylvia DM, Fox AJ. Contribution of ectomycorrhiza to the potential nutrient absorbing surface of pine in pure culture. Agriculture, Ecosystems and Environment. 1994;28:421–424. [Google Scholar]
- Rroco E, Kosegarten H, Harizaj F, Imani J, Mengel K. The importance of soil microbial activity for the supply of iron to sorghum and rape. European Journal of Agronomy. 2003;19:487–493. [Google Scholar]
- Rudolph J, Conrad R. Flux between soil and atmosphere, vertical concentration profiles in soil, and turnover of nitric oxide: 2. Experiments with naturally layered soil cores. Journal of Atmospheric Chemistry. 1996;23:275–300. [Google Scholar]
- Sadeghi A, Karimi E, Dahaji PA, Javid MG, Dalvand Y, Askari H. Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions. World Journal of Microbiology and Biotechnology. 2012;28:1503–1509. doi: 10.1007/s11274-011-0952-7. [DOI] [PubMed] [Google Scholar]
- Saha R, Saha N, Donofrio RS, Bestervelt LL. Microbial siderophores: a mini review. Journal of Basic Microbiology. 2012;52:1–15. doi: 10.1002/jobm.201100552. [DOI] [PubMed] [Google Scholar]
- Santi S, Schmidt W. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytologist. 2009;183:1072–1084. doi: 10.1111/j.1469-8137.2009.02908.x. [DOI] [PubMed] [Google Scholar]
- de Santi Ferrara FI, Oliveira ZM, Gonzales HHS, Floh EIS, Barbosa HR. Endophytic and rhizospheric enterobacteria isolated from sugar cane have different potentials for producing plant growth-promoting substances. Plant and Soil. 2012;353:409–417. [Google Scholar]
- de Santiago A, Quintero JM, Aviles M, Delgado A. Effect of Trichoderma asperellum strain T34 on iron nutrition in white lupin. Soil Biology and Biochemistry. 2009;41:2453–2459. [Google Scholar]
- de Santiago A, Quintero JM, Aviles M, Delgado A. Effect of Trichoderma asperellum strain T34 on iron, copper, manganese, and zinc uptake by wheat grown on a calcareous medium. Plant and Soil. 2011;342:97–104. [Google Scholar]
- de Santiago A, García-López AM, Quintero JM, Avilés M, Delgado A. Effect of Trichoderma asperellum strain T34 and glucose addition on iron nutrition in cucumber grown on calcareous soils. Soil Biology and Biochemistry. 2013;57:598–605. [Google Scholar]
- Schalk IJ, Hannauer M, Braud A. New roles for bacterial siderophores in metal transport and tolerance. Environmental Microbiology. 2011;13:2844–2854. doi: 10.1111/j.1462-2920.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- Séguéla M, Briat JF, Vert G, Curie C. Cytokinins negatively regulate the root iron uptake machinery in Arabidopsis through a growth-dependent pathway. The Plant Journal. 2008;55:289–300. doi: 10.1111/j.1365-313X.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- Shapiro AD. Nitric oxide signaling in plants. Vitamins and Hormones. 2005;72:339–398. doi: 10.1016/S0083-6729(05)72010-0. [DOI] [PubMed] [Google Scholar]
- Singh J, Kunhikrishnan A, Bolan NS, Saggar S. Impact of urease inhibitor on ammonia and nitrous oxide emissions from temperate pasture soil cores receiving urea fertilizer and cattle urine. Science of the Total Environment. 2013;465:56–63. doi: 10.1016/j.scitotenv.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Slatni T, Dell'Orto M, Salab IB, et al. Immunolocalization of H+-ATPase and IRT1 enzymes in N2-fixing common bean nodules subjected to iron deficiency. Journal of Plant Physiology. 2012;169:242–248. doi: 10.1016/j.jplph.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Smith AM. Ethylene in soil biology. Annual Review of Phytopathology. 1976;14:53–73. [Google Scholar]
- Smith KG, Reed DJ. Mycorrhizal symbosis. London: Academic Press; 1997. pp. 589–590. [Google Scholar]
- Smith SE, Smith FA. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia. 2012;104:1–13. doi: 10.3852/11-229. [DOI] [PubMed] [Google Scholar]
- Soerensen KU, Twrry RE, Jolley VD, Brown JC, Vargas ME. The interaction of iron-stress response and root nodules in iron efficient and inefficient soybeans. Journal of Plant Nutrition. 1988;11:853–865. [Google Scholar]
- Soerensen KU, Twrry RE, Jolley VD, Brown JC. Iron-stress response of inoculated and non-inoculated roots of an iron inefficient soybean cultivar in split-root system. Journal of Plant Nutrition. 1989;12:437–447. [Google Scholar]
- Stange CF, Spott O, Arriaga H, Menéndez S, Estavilloe JM, Merinoc P. Use of the inverse abundance approach to identify the sources of NO and N2O release from Spanish forest soils under oxic and hypoxic conditions. Soil Biology and Biochemistry. 2013;57:451–458. [Google Scholar]
- Su C, Lei LP, Duan YQ, Zhang KQ, Yang JK. Culture-independent methods for studying environmental microorganisms: methods, application, and perspective. Applied Microbiology and Biotechnology. 2012;93:993–1003. doi: 10.1007/s00253-011-3800-7. [DOI] [PubMed] [Google Scholar]
- Sugiyama A, Yazaki K. Root exudates of legume plants and their involvement in interactions with soil microorganisms. In: Vivanco J, Baluska F, editors. Secretions and exudates in biological systems. Berlin: Springer; 2012. pp. 27–48. [Google Scholar]
- Suri VK, Choudhary AK, Chander G, Verma TS. Influence of vesicular arbuscular mycorrhizal fungi and applied phosphorus on root colonization in wheat and plant nutrient dynamics in a phosphorus-deficient acid alfisol of western Himalayas. Communications in Soil Science and Plant Analysis. 2011;42:1177–1186. [Google Scholar]
- Tang C, Robson AD, Dilworth MJ. Inadequate iron supply and high bicarbonate impair the symbiosis of peanuts (Arachis hypogaea L.) with different Bradyrhizobium strains. Plant and Soil. 1991;138:159–168. [Google Scholar]
- Terpolilli JJ, Hood GA, Poole PS. What determines the efficiency of N2-fixing Rhizobium–legume symbioses? Advances in Microbial Physiology. 2012;60:325–389. doi: 10.1016/B978-0-12-398264-3.00005-X. [DOI] [PubMed] [Google Scholar]
- Terry RE, Hartzook A, Jolley VD, Brown JC. Interactions of iron nutrition and symbiotic nitrogen fixation in peanuts. Journal of Plant Nutrition. 1988;11:811–820. [Google Scholar]
- Thakuria D, Taleekdar NC, Goswami C, Hazarika S, Boro RC, Khan MR. Characterization and screening of bacteria from rhizosphere of rice grown in acidic soils of Assam. Current Science. 2004;86:978–985. [Google Scholar]
- Treeby MT. The role of mycorrhizal fungi and non-mycorrhizal microorganisms in iron nutrition of citrus. Soil Biology and Biochemistry. 1992;24:857–864. [Google Scholar]
- Uroz S, Buée M, Murat C, Frey-Klett P, Martin F. Pyrosequencing reveals a contrasted bacterial diversity between oak rhizosphere and surrounding soil. Environmental Microbiology Reports. 2010;2:281–288. doi: 10.1111/j.1758-2229.2009.00117.x. [DOI] [PubMed] [Google Scholar]
- Van Miegroet H, Cole DW. The impact of nitrification on soil acidification and cation leaching in red alder ecosystem. Journal of Environmental Quality. 1984;13:586–590. [Google Scholar]
- Vansuyt G, Robin A, Briat JF, Curie C, Lemanceau P. Iron acquisition from Fe-pyoverdine by Arabidopsis thaliana. Molecular Plant-Microbe Interactions. 2007;20:441–447. doi: 10.1094/MPMI-20-4-0441. [DOI] [PubMed] [Google Scholar]
- Wagg C, Jansa J, Stadler M, Schmid B, van der Heijden MGA. Mycorrhizal fungal identity and diversity relaxes plant–plant competition. Ecology. 2011;92:1303–1313. doi: 10.1890/10-1915.1. [DOI] [PubMed] [Google Scholar]
- Wang MY, Xia RX, Hu LM, Dong T, Wu QS. Arbuscular mycorrhizal fungi alleviate iron deficient chlorosis in Poncirus trifoliata L. Raf under calcium bicarbonate stress. Journal of Horticultural Science and Biotechnology. 2007;82:776–780. [Google Scholar]
- Wang BL, Li YS, Zhang WH. Brassinosteroids are involved in response of cucumber (Cucumis sativus) to iron deficiency. Annals of Botany. 2012;110:681–688. doi: 10.1093/aob/mcs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann G. Ecology of siderophores with special reference to the fungi. Biometals. 2007;20:379–392. doi: 10.1007/s10534-006-9076-1. [DOI] [PubMed] [Google Scholar]
- Wu J, Wang C, Zheng L, et al. Ethylene is involved in the regulation of iron homeostasis by regulating the expression of iron-acquisition-related genes in Oryza sativa. Journal of Experimental Botany. 2011;62:667–674. doi: 10.1093/jxb/erq301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Zhang H, Wang Y, et al. Induction of root Fe(III) reductase activity and proton extrusion by iron deficiency is mediated by auxin-based systemic signalling in Malus xiaojinensis. Journal of Experimental Botany. 2012;63:859–870. doi: 10.1093/jxb/err314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GH, Fan X, Miller AJ. Plant nitrogen assimilation and use efficiency. Annual Review of Plant Biology. 2012;63:153–182. doi: 10.1146/annurev-arplant-042811-105532. [DOI] [PubMed] [Google Scholar]
- Xu XK, Inubushi K. Production and consumption of ethylene in temperate volcanic forest surface soils. European Journal of Soil Science. 2007;58:668–679. [Google Scholar]
- Yang CH, Crowley DE. Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Applied and Environmental Microbiology. 2000;66:345–351. doi: 10.1128/aem.66.1.345-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda Z, Shenker M, Hadar Y, Chen YN. Remedy of chlorosis induced by iron deficiency in plants with the fungal siderophore rhizoferrin. Journal of Plant Nutrition. 2000;23:1991–2006. [Google Scholar]
- Zahir ZA, Abbas SA, Khalid M, Arshad M. Substrate dependent microbially derived plant hormones for improving growth of maize seedlings. Pakistan Journal of Biological Sciences. 2000;3:289–291. [Google Scholar]
- Zhang H, Sun Y, Xie X, Kim MS, Dowd SE, Paré PW. A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. The Plant Journal. 2009;58:568–577. doi: 10.1111/j.1365-313X.2009.03803.x. [DOI] [PubMed] [Google Scholar]
- Zhang XW, Dong YJ, Qiu XK, Hu GQ, Wang YH, Wang QH. Exogenous nitric oxide alleviates iron-deficiency chlorosis in peanut growing on calcareous soil. Plant, Soil and Environment. 2012;58:111–120. [Google Scholar]
- Zhao Y. Auxin biosynthesis and its role in plant development. Annual Review of Plant Biology. 2010;61:49–64. doi: 10.1146/annurev-arplant-042809-112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ. Iron homeostasis and iron acquisition in plants: maintenance, functions and consequences. Annals of Botany. 2010;105:799–800. doi: 10.1093/aob/mcq082. [DOI] [PMC free article] [PubMed] [Google Scholar]