Abstract
Background and Aims
Formation of seed banks and dormancy cycling are well known in annual species, but not in woody species. In this study it was hypothesized that the long-lived halophytic cold desert shrub Kalidium gracile has a seed bank and dormancy cycling, which help restrict germination to a favourable time for seedling survival.
Methods
Fresh seeds were buried in November 2009 and exhumed and tested for germination monthly from May 2010 to December 2011 over a range of temperatures and salinities. Germination recovery and viability were determined after exposure to salinity and water stress. Seedling emergence and dynamics of the soil seed bank were investigated in the field.
Key Results
Seeds of K. gracile had a soil seed bank of 7030 seeds m−2 at the beginning of the growing season. About 72 % of the seeds were depleted from the soil seed bank during a growing season, and only 1·4 % of them gave rise to seedlings that germinated early enough to reach a stage of growth at which they could survive to overwinter. About 28 % of the seeds became part of a persistent soil seed bank. Buried seeds exhibited an annual non-dormancy/conditional dormancy (ND/CD) cycle, and germination varied in sensitivity to salinity during the cycle. Dormancy cycling is coordinated with seasonal environmental conditions in such a way that the seeds germinate in summer, when there is sufficient precipitation for seedling establishment.
Conclusions
Kalidium gracile has three life history traits that help ensure persistence at a site: a polycarpic perennial life cycle, a persistent seed bank and dormancy cycling. The annual ND/CD cycle in seeds of K. gracile contributes to seedling establishment of this species in the unpredictable desert environment and to maintenance of a persistent soil seed bank. This is the first report of a seed dormancy cycle in a cold desert shrub.
Keywords: Cold desert habitat, dormancy cycling, halophyte, Kalidium gracile, salt tolerance, seed germination, seedling recruitment, soil seed bank
INTRODUCTION
Survival and regeneration of a species depend on adaptation to its habitat throughout the life cycle. Persistence of soil seed banks is an important adaptive characteristic of plants for avoiding local extinction (Stöcklin and Fischer, 1999; Thompson, 2000), and dormancy is considered to be an effective mechanism for seeds to persist in the soil (Grime, 1981; Pons, 1991; Dalling et al., 2011). Seeds of many temperate-zone herbaceous species exhibit dormancy cycles when buried in soil (Schütz, 1997a, b; Baskin and Baskin, 1998; Carter and Ungar, 2003). When coming out of dormancy, the seeds gain the ability to germinate over the full range of conditions possible for the population, at which time they are non-dormant (Baskin and Baskin, 1998). There is a series of transitional states between dormancy and non-dormancy, called conditional dormancy, during which seeds germinate over a narrower range of conditions than do non-dormant seeds (Baskin and Baskin, 1998). Non-dormant seeds of some species may re-enter dormancy via conditional dormancy and annually cycle between non-dormancy and dormancy; thus, seeds of some species may exhibit an annual cycle between non-dormancy and conditional dormancy.
Dormancy cycling is an endogenous mechanism that helps regulate germination timing and thus plays a role in timing of seedling establishment of the species under seasonally varying environments (Baskin et al., 1993; Baskin and Baskin, 1996). Dormancy cycling coordinates the time of seedling emergence with favourable seasons, which increases the chance of seedling survival in seasonally varying environments (Baskin and Baskin, 1980, 1985).
In addition to seed dormancy, the soil seed bank is crucial in the adaptation of plants to deserts, since environmental conditions, especially the amount of precipitation, are highly variable (Archibold, 1995). Many herbaceous plants in arid regions have soil seed banks that allow them to survive in harsh and unpredictable environments (Facelli et al., 2005; Dreber and Esler, 2011). For seedlings to become established, it is important for seeds of desert plants to germinate when the very scarce rainfall events occur (Gutterman, 1993, 2000).
It is sometimes assumed that seeds of desert species are ready to germinate as soon as temperature and soil moisture become favourable for them to do so (Went, 1949; Tevis, 1958). However, information is accumulating on the importance of dormancy as a mechanism for delay of germination in seeds of desert species (Clauss and Venable, 2000; Adondakis and Venable, 2004; Huang et al., 2004). One aspect of dormancy in seeds of desert plants that has received very little attention is dormancy cycling. We are aware of only two studies on dormancy cycling in seeds of desert annuals (Baskin et al., 1993; Cao et al., 2012), and to our knowledge there are no reports on dormancy cycling in herbaceous perennials, shrubs or trees in deserts.
In arid environments, it is well established that formation of a seed bank is important for the persistence of annuals, whereas it has been assumed that long-lived shrubs do not need a seed bank to persist at the site (Pake and Venable, 1996). If such a species has a persistent seed bank, does dormancy cycling occur, or do the seeds remain non-dormant (Baskin and Baskin, 1998; Thompson et al., 2003; Fenner and Thompson, 2005)? Some trees (Marks, 1974; Marquis, 1975) and shrubs (Hassan and West, 1986) form a persistent seed bank, but little is known about dormancy cycling in woody plants. However, Alnus glutinosa, a small tree of relatively mesic northern temperate regions, especially Europe, is an exception. Seeds of this species buried in the field at a depth of 7 cm and exhumed and tested for germination in light and darkness at 15 °C at various intervals for 34 months exhibited reduced germination in light in September and October (autumn) and peaks of germination from January to August; few seeds germinated in darkness (Schütz, 1998). These results indicate the presence of a dormancy cycle, but since only one test temperature was used the type of cycle cannot be determined.
Since halophytic shrubs grow in very harsh environments and since persistent seed banks and dormancy cycling are known to occur in some halophytic herbaceous species (Carter and Ungar, 2003; Cao et al., 2012), we hypothesized that seeds of the cold desert halophytic shrub Kalidium gracile (Amaranthaceae) would form a persistent seed bank and exhibit dormancy cycling.
MATERIALS AND METHODS
Species and seed source
Kalidium gracile is a small shrub about 20–50 cm in height that grows on alkaline plains and salt-lake shores in northwest China and Mongolia (Zhu et al., 2003). It flowers and fruits from July to September (Zhu et al., 2003). Freshly matured seeds of K. gracile were collected on 20 November 2009 and 15 November 2010 from several hundred plants growing in a saline desert on the Ordos Plateau (38° 14′ N, 107° 29′ E, 1311 m a.s.l.) in Inner Mongolia, northern China. This area has a typical continental semi-arid climate with a mean annual precipitation of 250–490 mm, about 60 % of which occurs from June to August. Mean annual temperature is about 6·0–9·0°C. Seeds collected in 2009 were air-dried and stored at room temperature (10–18°C, 17–32 % relative humidity) until initiation of germination tests on fresh seeds and burial of seeds in soil. After air-drying at room temperature and hand separation, seeds collected in 2010 were stored dry at –20°C until used.
Seed morphology and mass
The lengths and widths of ten seeds were measured using a Nikon 80i (Tokyo, Japan) microscope. The 1000-seed mass was determined by weighing five replicates of 1000 seeds to the nearest 0·1 mg.
Germination tests of fresh seeds
Freshly collected seeds of K. gracile were tested for germination in Petri dishes (diameter 5 cm) on two layers of Whatman No. 1 filter paper moistened with 3 ml of distilled water at 15/5, 20/5, 25/15 and 30/15°C (12/12 h) in light (12 h photoperiod, ∼100 μmol m−2 s−1, cool white fluorescent light) and in total darkness. All Petri dishes were wrapped in plastic film to reduce evaporation, and seeds incubated in darkness were placed inside light-tight black bags. The higher temperature coincided with a 12 h light period and the lower temperature with a 12 h dark period. The alternating temperature regimes represented the approximate mean daily maximum and minimum air temperatures for each month during the growing season on the Ordos Plateau: April and October, 15/5°C; May and September, 20/5°C; June and August 25/15°C; and July, 30/15°C (Cao et al., 2012). Four replicates of 25 seeds each were used for each test condition. Germination was monitored daily for seeds incubated in light, and additional water was added as necessary. Final germination percentages were determined after 20 days. Emergence of the radicle (or cotyledons, which sometimes emerge from the seed coat before the radicle) was the criterion for germination in this and all other germination experiments.
Following all germination tests, non-germinated seeds were examined under a dissecting microscope to determine whether the embryo was firm and white, indicating that it was viable, or soft and grey, indicating that it was non-viable. Tetrazolium tests confirmed that the firm, white embryos were viable and the soft, grey ones non-viable. Only viable seeds were used in calculating germination percentages. More than 90 % of the seeds were viable.
Dormancy breaking
Fresh seeds of K. gracile germinated to very low percentages at 15/5°C, but buried seeds germinated to >80 % under this temperature regime in spring (see Results), indicating that fresh K. gracile seeds have primary dormancy. Thus, we tested the effects of cold stratification, gibberellic acid (GA3) and scarification of the seed coat on germination of fresh seeds collected in November 2010 to determine the kind of dormancy in seeds of this species and the factor(s) that break it.
Cold stratification. Freshly collected seeds were arranged uniformly on two layers of Whatman No. 1 filter paper over moist sand (10–13 % water content) in a metal box (20 cm length×10 cm width×10 cm depth). The metal box was covered with a metal lid and placed in a refrigerator at 5°C. After 0 (control), 5, 10, 15 and 20 days of cold stratification, four dishes (replicates) each of 25 seeds were arbitrarily chosen to test germination in light at 15/5, 20/5, 25/15 and 30/15°C (12 h/12 h) as described above. Final germination percentages were determined after 20 days.
GA3 treatments. Four replicates of 25 freshly collected seeds each were tested for germination in a 0·1 mmol L−1 GA3 solution in light at 15/5°C.
Seed coat scarification. Seed coats of four replicates of 25 freshly collected seeds were scarified with a scalpel, being careful not to injure the embryos, and tested for germination in light at 15/5°C.
Dormancy cycling
On 30 November 2009, about 1000 seeds of K. gracile were placed in each of 20 nylon bags. Each bag was buried at a depth of 2 cm in plastic pots (diameter 20 cm, height 50 cm, with drainage holes) filled with soil collected from the habitat and buried on nearly flat ground at the Ordos Sandland Ecological Research Station of the Chinese Academy of Sciences (OSES) so that the tops were level with the soil surface. Mean daily maximum and minimum monthly air temperatures for each month during the study were obtained from OSES. A bag of buried seeds was exhumed on the first day of each month, beginning on 1 May 2010 and ending on 1 December 2011. Two tests were carried out using the fresh and exhumed seeds.
Test 1: germination of seeds under seasonal temperature regimes. Seeds were placed on filter paper moistened with distilled water in Petri dishes 5 cm in diameter and incubated at 15/5, 20/5, 25/15 and 30/15°C (12/12 h) in light (as described above). Four replicates of 25 seeds each were used in each test condition. Final germination percentages were determined after 20 days.
Test 2: germination of seeds under various salinities. Seeds were tested at salinities of 0, 0·2, 0·4 and 0·6 mol L−1 NaCl. Seeds were placed in Petri dishes on two layers of filter paper moistened with 3 ml of test solution and wrapped with plastic film to reduce evaporation. Four replicates of 25 seeds each were used. The seeds were incubated in light at 30/15°C. Final germination percentages were determined after 20 days.
Germination recovery
Germination recovery was tested for seeds of K. gracile that had been dry stored at –20°C for 5 months. Seeds were incubated in 1·0 mol L−1 NaCl and isotonic polyethylene glycol 8000 (PEG-8000) solutions in April 2011.The osmotic potential of 1·0 mol L−1 NaCl solution was determined with the Van 't Hoff equation, π=cRT, where c is osmolality in mol L−1, R is the gas constant (8·31 J K−1 mol−1) and T is temperature (K). The isotonic PEG-8000 solution was prepared according to Michel (1983). For both 1·0 mol L−1 NaCl and isotonic PEG-8000 pretreatments, 25 seeds were placed on two layers of filter paper in each of 12 dishes moistened with 3 ml NaCl or PEG-8000 solution. The dishes were placed in an incubator at a constant temperature of 20°C. This temperature was chosen to represent the average temperature of early summer (before the beginning of the rainy season), when seeds were most likely to experience stress from salinity and drought. A constant temperature rather than alternating temperatures was used to avoid changes in the osmotic potential of PEG-8000 solutions.
After 20, 40 and 60 days of pretreatment in the NaCl and PEG-8000 solutions, four dishes of seeds were arbitrarily chosen and rinsed twice with distilled water and transferred to filter paper moistened with distilled water. The seeds transferred to distilled water were incubated in light at 30/15°C for 20 days and germination percentages determined. Following the recovery germination tests, non-germinated seeds were tested for viability as described above. No seeds germinated in the NaCl or PEG-8000 solution. Recovery germination percentage (GR%) was determined as [a/(a + b)] × 100, where a is the number of seeds germinated during the recovery germination test and b the number of viable but non-germinated seeds. Viability (V%) of pretreated seeds was calculated as [(a + b)/c] × 100, where c is the total number of seeds in a dish (i.e. 25). The control consisted of 16 dishes of 25 seeds each without pretreatment with NaCl or PEG-8000 solution stored dry in the 20°C incubator. Four dishes of seeds were incubated in distilled water at 30/15°C after 0, 20, 40 and 60 days of dry storage at 20°C. Final germination percentages and viability of non-germinated seeds were determined after 20 days of incubation.
Dynamics of soil seed bank
The soil seed bank of K. gracile was monitored monthly from April to November 2011. Ten soil cores (5 cm diameter×10 cm depth) were collected from arbitrarily chosen places in a monospecific stand of K. gracile; sampling plots were about 50 m apart. The soil cores were subdivided into three layers: 0–2, 2–5 and 5–10 cm. The soil samples were washed with water through a 0·5 mm mesh sieve, and seeds were hand-sorted from the residue (roots and other vegetative parts). The number of viable seeds was determined under a dissecting microscope as described above. Only viable seeds were used in calculating seed density in the soil seed bank.
Field emergence of seedlings
Five 4 m×4 m plots about 50 m apart were arbitrarily established in a monospecific stand of K. gracile in April 2011. Newly emerged seedlings of K. gracile were marked and recorded monthly in four 20 cm×20 cm subplots in each of the five plots from April to September 2011. Newly emerged seedlings were monitored twice each month (on the 15th and last days), and the number of emerged seedlings was totalled for each month. Seedlings were labelled and the date was recorded when they were first found. The number of seedlings that survived until the end of the experiment was determined. This experiment was terminated on 30 September 2011, when the plants had turned red, indicating the end of the growing season. On this date, the number of living seedlings that had emerged in each month was recorded.
Soil water content, soil salinity, precipitation and temperature were monitored. Eight soil cores, 5 cm in diameter, 0–5 cm deep and about 50 m apart, were collected arbitrarily within the study population of K. gracile on the 15th day of each month from April to September 2011. The moisture content of each soil sample was determined gravimetrically; soil samples were oven-dried at 105°C for 48 h. Salinity was determined by the residue drying quality method (Bao, 2000). Mean monthly air temperature and monthly precipitation were obtained from a national weather station 10 km from the field site.
Data analyses
One-way ANOVA was used to estimate differences in germination percentages and viability of pretreated seeds after different durations of salinity and drought stress pretreatment. Fisher's LSD test was performed for multiple comparisons when significant differences were found. The independent-samples t-test was used to determine significant differences (or not) between germination percentages and viability of seeds after the same period of exposure to NaCl and PEG-8000 solutions. Data were arcsine square root transformed when necessary to meet assumptions of ANOVA for normality and homogeneity of variance. Statistical analyses were performed using SPSS Version 17·0 for windows (SPSS, Chicago, IL, USA).
RESULTS
Seed morphology and mass
Length and width of K. gracile seeds were 1·03 ± 0·02 mm (mean ± s.e.) and 0·80 ± 0·01 mm, respectively, and 1000-seed mass was 155·8 ± 1·5 mg. The seed coat was brown and rough.
Germination tests of fresh seeds
Fresh seeds of K. gracile germinated to <20·0 % at 15/5°C and to <40 % at 20/5°C in light but to 95–100 % at 25/15 and 30/15°C (Fig. 1). Darkness inhibited germination at 15/5 and 20/5°C (P < 0·05) but not at 25/15 and 30/15°C (P > 0·05).
Fig. 1.
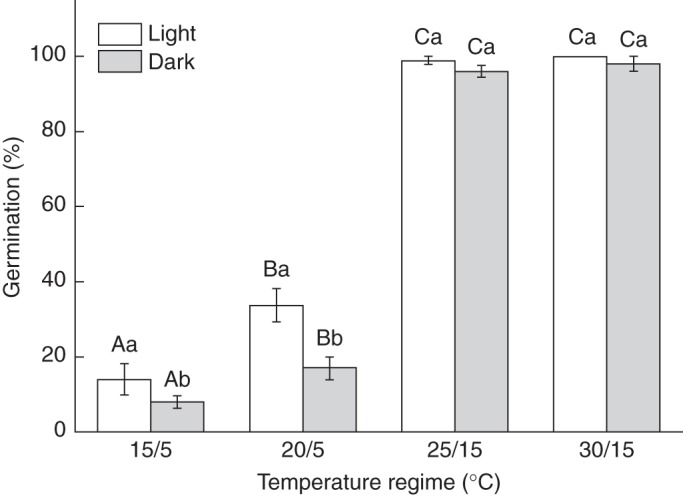
Germination percentages (mean ± s.e.) of fresh Kalidium gracile seeds incubated at various temperature regimes in light (12 h photoperiod) and continuous darkness. Bars with the same upper case letter are not significantly different (P > 0·05) across all temperature regimes in light or darkness. The same lower case letter indicates no significant difference (P > 0·05) between germination percentages in light and in continuous darkness for the same thermoperiod. Error bars are means ± s.e.
Dormancy breaking
Cold stratification significantly (P < 0·05) increased germination percentages at 15/5 and 20/5°C (Fig. 2). Thus, seeds of K. gracile stratified for 20 days germinated to 84·8 ± 7·2 and 90·4 ± 4·8 % at 15/5 and 20/5°C, respectively. Treatment with GA3 and scarification of the seed coat also significantly (P < 0·05) increased germination of seeds at 15/5°C to 94·0 ± 2·6 and 66·0 ± 6·6 %, respectively.
Fig. 2.
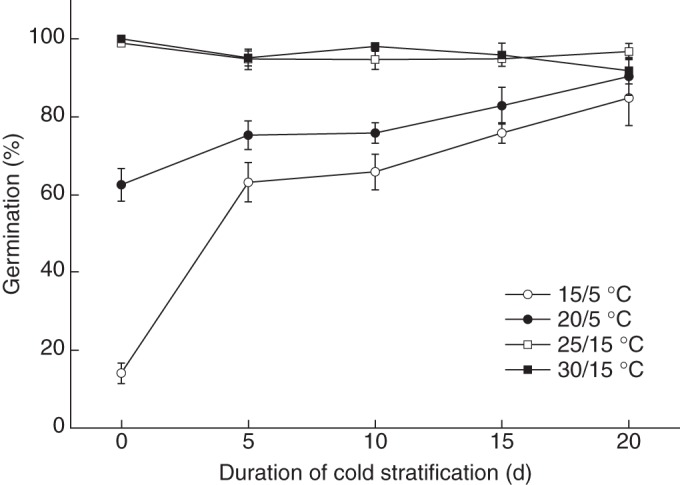
Germination percentages of Kalidium gracile seeds incubated at various alternating temperature regimes in a 12 h photoperiod after 0, 5, 15 and 20 days of cold stratification at 5°C in continuous darkness. Error bars are means ± s.e.
Dormancy cycling
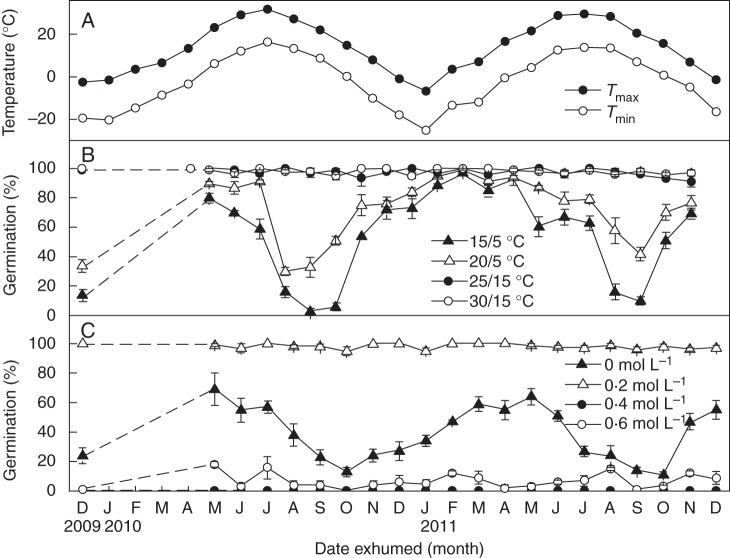
Buried seeds of K. gracile maintained the ability to germinate to >90 % at 25/15 and 30/15°C throughout the 24-month period of burial (Fig. 3B). However, they germinated to only 14·0–33·3, 3·0–33·0 and 10·0–42·0 % at 15/5 and 20/5°C in autumn of 2009 (fresh seeds), 2010 and 2011, but to high percentages at these two temperature regimes in the following springs (Fig. 3B). Thus, germination percentages increased at 15/5 and 20/5°C after exposure to cold stratification in the field in the winters of 2009–2010 and 2010–2011 and decreased at these two temperatures after they were exposed to high temperature in the field in summer (Fig. 3A, B).
Fig. 3.
(A) Mean monthly maximum and minimum air temperatures at the seed burial site. (B, C) Germination percentages (mean ± s.e.) of Kalidium gracile seeds incubated (B) in distilled water at four temperature regimes in a 12 h photoperiod and (C) in 0, 0·2, 0·4 and 0·6 mol L−1 NaCl solutions at 30/15°C in a 12 h photoperiod at time 0 (fresh seeds) and following 5–24 months of burial in soil.
The pattern of germination in 0·2 mol L−1 NaCl at 30/15°C in light was almost identical to that in distilled water at 15/5 and 20/10°C. Thus, germination percentages in 0·2 mol L−1 NaCl were highest in spring and lowest in autumn. Fewer than 20 % of the seeds germinated in 0·4 mol L−1 NaCl throughout the 24-month burial period, and none germinated at 0·6 mol L−1 NaCl (Fig. 3C).
Recovery germination
Non-pretreated seeds of K. gracile had a viability of 96·0 ± 2·3 % and germinated to 95·0 ± 1·9 %. During 0–60 days of immersion in 1·0 mol L−1 NaCl or isotonic PEG-8000 solution, seeds did not change significantly in recovery germination at 30/15°C or in viability (P > 0·05 ). They had recovery germination percentages of 94·0 ± 1·2 and 97·0 ± 1·0 after 60 days of pretreatment in the solutions of NaCl and PEG, respectively.
Dynamics of soil seed bank
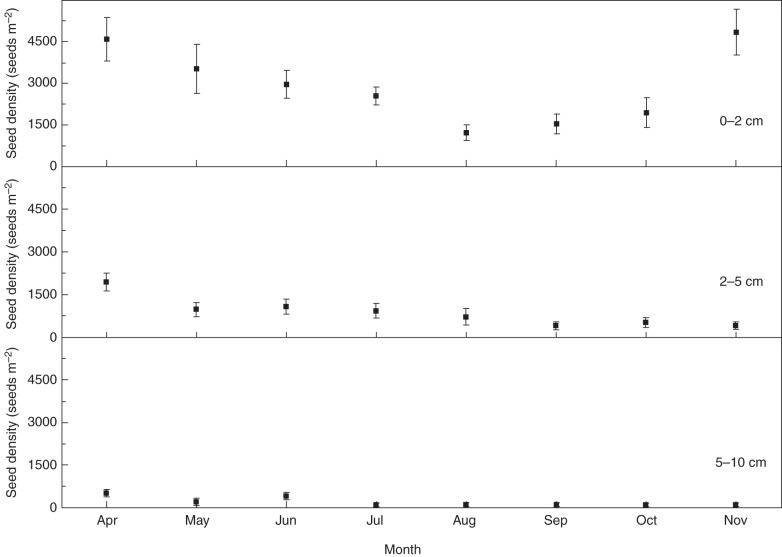
Seeds of K. gracile were most abundant in the 0–2 cm level of soil, and seed density decreased with increasing soil depth (Fig. 4). Seed density in the 0–2 cm level of soil decreased in the growing season (April to September) and increased in autumn (October and November). More than 70 % of the seeds were depleted from the soil seed bank (0–10 cm) during the growing season, decreasing gradually from 7030 ± 1131 seeds per m2 in April to 1987 ± 433 seeds per m2 in September.
Fig. 4.
Density of Kalidium gracile seeds at three soil depths in the natural habitat from April to November 2011. Error bars are means ± s.e.
Field emergence of seedlings
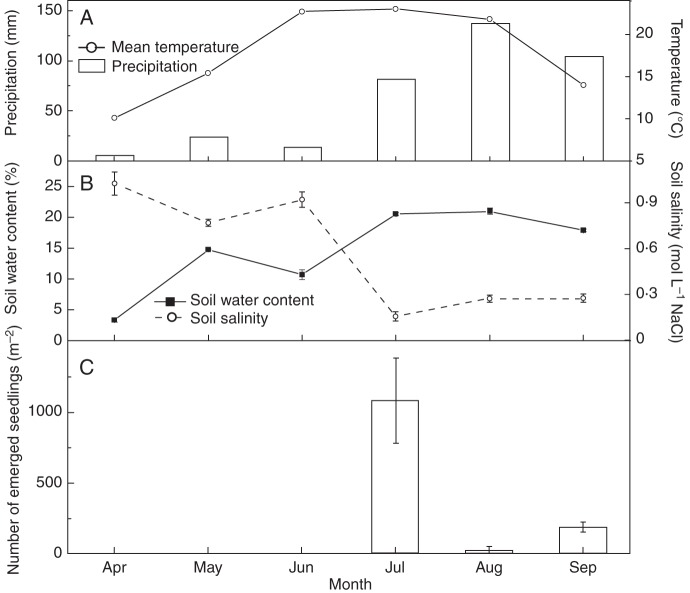
Seedlings emerged in the field in July, August and September, when soil salinity was relatively low and soil water content relatively high (Fig. 5). The rate of emergence was 1300 seedlings m−2, and 83 % of the seedlings that emerged did so in July, concurrently with the first major precipitation event, lowest soil salinity and highest mean monthly temperature of the growing season (Fig. 5). Only 9·7 % (105/1083) of the seedlings that emerged in July were alive at the end of the growing season. The mean emergence rate was 28 and 190 seedlings m−2 in August and September, respectively, but none of the seedlings survived until the end of the growing season. Thus, 1301 of the 7030 (18·5 %) seeds m−2 in the soil seed bank at the beginning of the growing season (April) produced seedlings, of which only 1·4 % (105/7300) survived until the end of the first growing season.
Fig. 5.
(A) Mean monthly air temperature and precipitation, (B) soil water content and soil salinity and (C) number of Kalidium gracile seedlings that emerged in each month from April to September 2011.
DISCUSSION
Our hypothesis that seeds of K. gracile form a persistent seed bank and have dormancy cycling was supported. Fresh seeds of K. gracile have primary conditional dormancy, and the germination percentage can be increased at low incubation temperatures by a short period of cold stratification, GA3 or scarification of seed coat. Thus, K. gracile seeds have non-deep physiological dormancy (Baskin and Baskin, 1998). Seeds of many herbaceous annual species with non-deep physiological dormancy exhibit dormancy cycles (Baskin and Baskin, 1998), and it has recently been demonstrated that cycling also occurs at the hormonal–molecular level via differential expression of genes in response to environmental cues (Cadman et al., 2006; Footitt et al., 2011). Although seeds of K. gracile maintained high germination at 25/15 and 30/15°C throughout the year, they varied seasonally in germination at 15/5 and 20/5°C. Thus, seeds in the soil had an annual non-dormancy/conditional dormancy (ND/CD) cycle. To our knowledge, this is the first report of seed dormancy cycling in a cold desert shrub.
In addition to being a polycarpic perennial, K. gracile can form a seed bank, and the buried seeds can undergo annual dormancy cycling. These three life history traits help ensure the persistence of K. gracile in its harsh saline habitat. Rainfall not only increases soil water content, but also decreases soil salinity in saline deserts; however, the timing and intensity of these changes is unpredictable in deserts. The ND/CD dormancy cycle in seeds of K. gracile enables them to germinate throughout the growing season whenever there is available rainfall for them to do so. Seeds can only germinate to relatively high percentages at salinities equal to or lower than about 0·2 mol L−1 NaCl, a condition that is especially unpredictable due to the unpredictability of the timing of rainfall. Thus, the ability to maintain high germinability at high temperatures ensures field emergence of this species in the growing season. On the other hand, the decrease in the ability of conditionally dormant seeds to germinate at low temperatures contributes to persistence of the soil seed bank for this species by preventing seed germination in the unfavourable autumn season, when there would be no chance of seedling survival. In this study, many fewer seedlings emerged in August and September than in July. Thus, the inability of most seeds in the soil to germinate in autumn contributes to the persistence of seeds in the soil.
Seeds of K. gracile in the soil seed bank vary in sensitivity to salinity (sensitive↔insensitive) during the year, which was well correlated with the ND/CD cycle. Thus, seeds germinated to their highest percentages in 0·2 mol L−1 NaCl when they were non-dormant and to their lowest percentages when they were conditionally dormant (Fig. 3). The annual cycling at 30/15°C in germination response to 0·2 mol L−1 NaCl shows that cyclic changes are occurring in the seeds' physiological response to stress that are not detectable when seeds are incubated in distilled water (Fig. 3C). In a previous study, an annual cycle of sensitivity to salinity was found in the black seed morph of the seed-dimorphic halophytic summer annual herb Suaeda corniculata subsp. mongolica (Amaranthaceae), which has an annual D (dormancy)↔CD↔ND seed dormancy cycle (Cao et al., 2012) and co-occurs with K. gracile in the saline desert on the Ordos Plateau. Seeds of both species exhibited less tolerance for germination to salinity when they were in conditional dormancy than when they were non-dormant. This characteristic of halophyte seeds may play a role in preventing germination in autumn, which is an unfavourable time for seedling establishment.
Soil seed banks are an important component of the regeneration strategies of many species inhabiting unpredictable environments (Leck and Schutz, 2005; Evans et al., 2007). K. gracile produces large numbers of seeds, and >7000 seeds per m2 were present in the soil seed bank at the beginning of the growing season in 2011. Although 71·7 % of seeds of K. gracile in the soil were depleted in the growing seasons, those that remained after 1 and 2 years of burial were part of a persistent soil seed bank sensu Thompson and Grime (1979). The maintenance of a persistent soil seed bank of this species may be attributed to dormancy cycling (see above) and to the ability to maintain seed viability under salinity stress. The ability to maintain viability under salinity stress is required for a species to persist in its saline habitats (Engels et al., 2011).
Seeds of K. gracile maintain viability under high salinity stress and then germinate to high percentages at favourable temperatures and moisture conditions. The ability of seeds to commence germination when salinity decreases following a precipitation event is an overriding factor in seedling emergence of this species, given that the seeds can germinate to high percentages at prevailing habitat temperatures throughout the growing season. In the natural habitat of K. gracile, the top layer of soil dries quickly after rainfall due to the high evaporation rate. The ability of seeds of K. gracile to commence germination once rainfall decreases soil salinity increases the possibility of seedling establishment before the concentration of salts in the soil becomes inhibitory for germination. The small radicles of K. caspicum are very vulnerable to salinity (Tobe et al., 2000), and this is probably the case with K. gracile. This being so, it is necessary for the species to complete germination and to establish seedlings before salinity at the surface soil increases. In addition, the ability of the seeds to germinate in 0·2 mol L−1 NaCl may play a role in the persistence of this species at the site, since soil salinity was greater than 0·2 mol L−1 NaCl in the field during most of the growing season (Fig. 5). Thus, most seedlings emerged in July when rainfall decreased soil salinity to about 0·2 mol L−1 NaCl.
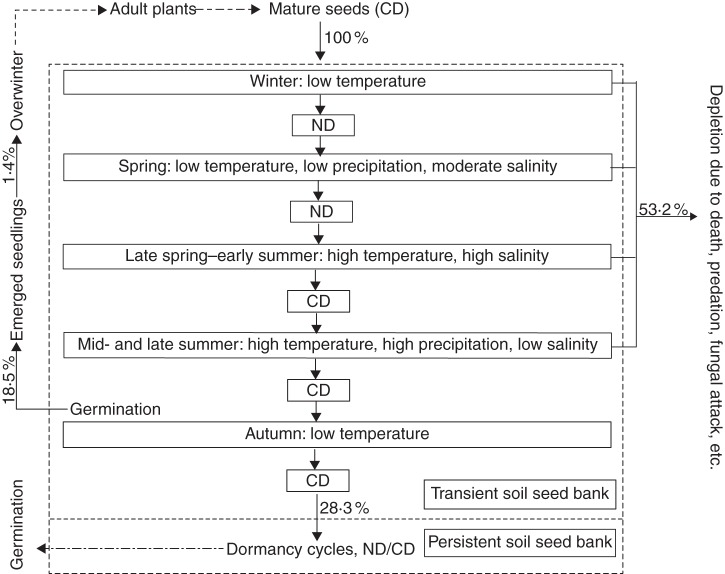
The results of our study on seed and seedling dynamics of K. gracile in the field are summarized in a conceptual model (Fig. 6). Seeds have CD when dispersed at maturity in autumn, and they become ND by exposure to low temperatures in winter. Thus, seeds are ND in spring. However, no seedlings emerged until July, when the concentration of salts in the soil was diluted by rain. Seeds re-entered CD in late spring–early summer, but retained the ability to germinate to 100 % at prevailing habitat temperatures (Fig. 3). Thus, seedling emergence occurred in summer after major precipitation events in July, August and September.
Fig. 6.
Conceptual model of seed and seedling dynamics in a population of Kalidium gracile. The percentages indicate proportions of seeds in a soil seed bank that gave rise to seedlings and of those that entered the persistent soil seed bank. CD, conditional dormancy; D, dormancy; ND, non-dormancy. The dash–dot arrows represent events that may occur.
Although the seeds were ND, they did not germinate during the low-rainfall events in April to June, when soil moisture content was low and soil salinity high. The seed bank present in April 2011 was depleted by 71·7 % during the growing season, and only 1·4 % of the seeds gave rise to seedlings that likely overwintered. This left 28·3 % of the seeds in the seed bank in April that did not germinate, or died before seedling emergence during the 2011 growing season. Presumably these seeds survived another winter and some of them germinated in the following growing season.
ACKNOWLEDGEMENTS
We thank Prof. D. Lawrence Venable, University of Arizona, USA, for an insightful discussion on soil seed bank strategies of shrubs. This work was funded by the Key Basic Research and Development Plan of China (2012BAD16B03, 2010CB951304), the National Natural Science Foundation of P. R. China (30872074) and by the Joint Project of Ministry of Environmental Protection of P.R. China and the Chinese Academy of Sciences (STSN-21-04).
LITERATURE CITED
- Adondakis S, Venable DL. Dormancy and germination in a guild of Sonoran Desert annuals. Ecology. 2004;85:2582–2590. [Google Scholar]
- Archibold OW. Ecology of world vegetation. London: Chapman and Hall; 1995. [Google Scholar]
- Bao SD. Soil and agricultural chemistry analysis. Beijing: China Agriculture Press. (in Chinese); 2000. [Google Scholar]
- Baskin CC, Baskin JM. Role of temperature and light in the germination ecology of buried seeds of weedy species of disturbed forests. II. Erechtites hieracifolia. Canadian Journal of Botany. 1996;74:2002–2005. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press; 1998. [Google Scholar]
- Baskin CC, Chesson PL, Baskin JM. Annual seed dormancy cycles in two desert winter annuals. Journal of Ecology. 1993;81:551–556. [Google Scholar]
- Baskin JM, Baskin CC. Ecophysiology of secondary dormancy in seeds of Ambrosia artemisiifolia. Ecology. 1980;61:475–480. [Google Scholar]
- Baskin JM, Baskin CC. The annual dormancy cycle in buried weed seeds: a continuum. BioScience. 1985;35:492–498. [Google Scholar]
- Cadman CSC, Toorop PE, Hilhorst HWM, Finch-Savage WE. Gene expression profiles of Arabidopsis Cvi seeds during dormancy cycling indicate a common underlying dormancy control mechanism. Plant Journal. 2006;46:805–822. doi: 10.1111/j.1365-313X.2006.02738.x. [DOI] [PubMed] [Google Scholar]
- Cao DC, Baskin CC, Baskin JM, Yang F, Huang ZY. Comparison of germination and seed bank dynamics of dimorphic seeds of the cold desert halophyte Suaeda corniculata subsp. mongolica. Annals of Botany. 2012;110:1545–1558. doi: 10.1093/aob/mcs205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CT, Ungar IA. Germination response of dimorphic seeds of two halophyte species to environmentally controlled and natural conditions. Canadian Journal of Botany. 2003;81:918–926. [Google Scholar]
- Clauss MJ, Venable DL. Seed germination in desert annuals: an empirical test of adaptive bet hedging. American Naturalist. 2000;155:168–186. doi: 10.1086/303314. [DOI] [PubMed] [Google Scholar]
- Dalling JW, Davis AS, Schutte BJ, Arnold AE. Seed survival in soil: interacting effects of predation, dormancy and the soil microbial community. Journal of Ecology. 2011;99:89–95. [Google Scholar]
- Dreber N, Esler KJ. Spatio-temporal variation in soil seed banks under contrasting grazing regimes following low and high seasonal rainfall in arid Namibia. Journal of Arid Environments. 2011;75:174–184. [Google Scholar]
- Engels JG, Rink F, Jensen K. Stress tolerance and biotic interactions determine plant zonation patterns in estuarine marshes during seedling emergence and early establishment. Journal of Ecology. 2011;99:277–287. [Google Scholar]
- Evans MEK, Ferriere R, Kane MJ, Venable DL. Bet hedging via seed banking in desert evening primroses (Oenothera, Onagraceae): demographic evidence from natural populations. American Naturalist. 2007;169:184–194. doi: 10.1086/510599. [DOI] [PubMed] [Google Scholar]
- Facelli JM, Chesson P, Barnes N. Differences in seed biology of annual plants in arid lands: a key ingredient of the storage effect. Ecology. 2005;86:2998–3006. [Google Scholar]
- Fenner M, Thompson K. The ecology of seeds. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Footitt S, Douterelo-Soler I, Clay H, Finch-Savage WE. Dormancy cycling in Arabidopsis seeds is controlled by seasonally distinct hormone-signaling pathways. Proceedings of the National Academy of Sciences of the USA. 2011;108:20236–20241. doi: 10.1073/pnas.1116325108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grime JP. The role of seed dormancy in vegetation dynamics. Annals of Applied Biology. 1981;98:555–558. [Google Scholar]
- Gutterman Y. Seed germination in desert plants. Berlin: Springer; 1993. [Google Scholar]
- Gutterman Y. Environmental factors and survival strategies of annual plant species in the Negev Desert, Israel. Plant Species Biology. 2000;15:113–125. [Google Scholar]
- Hassan MA, West NE. Dynamics of soil seeds pools in burned and unburned sagebrush semi-deserts. Ecology. 1986;67:269–272. [Google Scholar]
- Huang ZY, Dong M, Gutterman Y. Caryopsis dormancy, germination and seedling emergence in sand, of Leymus racemosus (Poaceae), a perennial sand-dune grass inhabiting the Junggar Basin of Xinjiang, China. Australian Journal of Botany. 2004;52:519–528. [Google Scholar]
- Leck MA, Schutz W. Regeneration of Cyperaceae, with particular reference to seed ecology and seed banks. Perspectives in Plant Ecology, Evolution and Systematics. 2005;7:95–133. [Google Scholar]
- Marks PL. The role of pin cherry (Prunus pensylvanica L.) in the maintenance of stability in northern hardwood ecosystems. Ecological Monographs. 1974;44:73–88. [Google Scholar]
- Marquis DA. Seed storage and germination under northern hardwood forests. Canadian Journal of Forest Research. 1975;5:478–484. [Google Scholar]
- Michel BE. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiology. 1983;72:66–70. doi: 10.1104/pp.72.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pake CE, Venable DL. Seed banks in desert annuals: implications for persistence and coexistence in variable environments. Ecology. 1996;77:1427–1435. [Google Scholar]
- Pons TL. Induction of dark dormancy in seeds: its importance for the seed bank in the soil. Functional Ecology. 1991;5:669–675. [Google Scholar]
- Schütz W. Are germination strategies important for the ability of cespitose wetland sedges (Carex) to grow in forests? Canadian Journal of Botany. 1997a;75:1692–1699. [Google Scholar]
- Schütz W. Primary dormancy and annual dormancy cycles in seeds of six temperate wetland sedges. Aquatic Botany. 1997b;59:75–85. [Google Scholar]
- Schütz W. Bildet Alnus glutinosa eine Samenbank? – Untersuchungen zum Verlauf der Samendormanz und der Überdauerung im Boden. Berichte Institut Landschafts-Pflanzenökologie Universität Hohenheim. 1998;7(109–121) (in German with English abstract) [Google Scholar]
- Stöcklin J, Fischer M. Plants with longer-lived seeds have lower local extinction rates in grassland remnants 1950–1985. Oecologia. 1999;120:539–543. doi: 10.1007/s004420050888. [DOI] [PubMed] [Google Scholar]
- Tevis L. A population of desert ephemerals germinated by less than one inch of rain. Ecology. 1958;39:688–695. [Google Scholar]
- Thompson K. The functional ecology of soil seed banks. In: Fenner M, editor. Seeds: the ecology of regeneration in plant communities. 2nd edn. Wallingford, UK: CABI Publishing; 2000. [Google Scholar]
- Thompson K, Grime JP. Seasonal variation in seed banks of herbaceous species in ten contrasting habitats. Journal of Ecology. 1979;67:893–921. [Google Scholar]
- Thompson K, Ceriani RM, Bakker JP, Bekker RM. Are seed dormancy and persistence in soil related? Seed Science Research. 2003;13:97–100. [Google Scholar]
- Tobe K, Li X, Omasa K. Effects of sodium chloride on seed germination and growth of two Chinese desert shrubs, Haloxylon ammodendron and H. persicum (Chenopodiaceae) Australian Journal of Botany. 2000;48:455–460. [Google Scholar]
- Went FW. Ecology of desert plants. II. The effect of rain and temperature on germination and growth. Ecology. 1949;30:1–13. [Google Scholar]
- Zhu G, Mosyakin SL, Clemants SE. Chenopodiaceae. In: Wu Z, Raven PH, Hong D, editors. Flora of China 5. Beijing and St Louis: Science Press and Missouri Botanical Garden Press; 2003. pp. 351–414. [Google Scholar]