Abstract
Background
Arbuscular mycorrhizae (AMs) form a widespread root–fungus symbiosis that improves plant phosphate (Pi) acquisition and modifies the physiology and development of host plants. Increased branching is recognized as a general feature of AM roots, and has been interpreted as a means of increasing suitable sites for colonization. Fungal exudates, which are involved in the dialogue between AM fungi and their host during the pre-colonization phase, play a well-documented role in lateral root (LR) formation. In addition, the increased Pi content of AM plants, in relation to Pi-starved controls, as well as changes in the delivery of carbohydrates to the roots and modulation of phytohormone concentration, transport and sensitivity, are probably involved in increasing root system branching.
Scope
This review discusses the possible causes of increased branching in AM plants. The differential root responses to Pi, sugars and hormones of potential AM host species are also highlighted and discussed in comparison with those of the non-host Arabidopsis thaliana.
Conclusions
Fungal exudates are probably the main compounds regulating AM root morphogenesis during the first colonization steps, while a complex network of interactions governs root development in established AMs. Colonization and high Pi act synergistically to increase root branching, and sugar transport towards the arbusculated cells may contribute to LR formation. In addition, AM colonization and high Pi generally increase auxin and cytokinin and decrease ethylene and strigolactone levels. With the exception of cytokinins, which seem to regulate mainly the root:shoot biomass ratio, these hormones play a leading role in governing root morphogenesis, with strigolactones and ethylene blocking LR formation in the non-colonized, Pi-starved plants, and auxin inducing them in colonized plants, or in plants grown under high Pi conditions.
Keywords: Arbuscular mycorrhizae, root branching, lateral roots, fungal exudates, phosphate, sugars, auxin, cytokinins, ethylene, strigolactones, Arabidopsis thaliana
INTRODUCTION
In almost all natural and agricultural environments, the majority of plant species (perhaps 90 %) form mycorrhizae, with the most common type being represented by arbuscular mycorrhizae (AM; Smith and Read, 2008; Smith and Smith, 2012). To be mycorrhizal can therefore be considered the norm rather than the exception for plants (Hodge et al., 2009). In an AM association, the Glomeromycota fungus inhabits the root cortex tissue, where it obtains sugars from the plant. In turn, the intraradical fungus transfers to the cortical cells mineral nutrients taken up from the soil by the extraradical mycelial network, which extends beyond the root depletion zone (Harrison, 2005; Smith and Smith, 2012). The name of this type of mycorrhiza comes from arbuscules, which are highly dichotomously branched hyphae that develop inside the cortical cells. They are the site in which phosphate (Pi), the most studied mineral nutrient involved in AM symbiosis, is delivered to the root and they contribute, together with intercellular hyphae, to the transfer of carbon compounds to the fungus (Helber et al., 2011). Plants and fungi are both able to detect variations in the resources supplied by their partner, and symbiosis, which is stabilized through ‘reciprocal rewards’, is favoured for the most co-operative symbionts (Kiers et al., 2011).
Phosphorus (P) is one of the most important elements for plants. However, it is also one of the least available of all essential nutrients in the soil. It is normally taken up by roots in the form of Pi. The concentration of Pi in plant cells exceeds by 2000-fold that of soil solutions, which is usually <2 µm (Vance et al., 2003). Phosphate acquisition has a significant impact on plant growth and health, and Pi-starved plants show a range of adaptive responses, including a combination of growth, developmental and metabolic processes (Péret et al., 2011), in order to sustain growth in such a limiting condition. Moreover, Pi availability is a key factor in the establishment of AM symbiosis, which is known to be one of the most prevalent evolutionary adaptations of land plants to P deficiency (Vance et al., 2003; Hodge et al., 2009). In Pi-limiting conditions, intraradical development of the fungus can occur over >80 % of the root length (Harrison, 2005) while high Pi conditions decrease colonization (Balzergue et al., 2011). A wide range of plants, the so-called ‘responsive’ plants, increase their P status and growth upon colonization (Smith and Read, 2008; Smith and Smith, 2012). In addition, plants generally lower their root:shoot biomass ratio (Scannerini et al., 2001; Smith and Read, 2008; Smith and Smith, 2012) because the increased sink strength of the roots induces plants to enlarge their photosynthetic organs, according to both the physiological requirements of the fungal partner and the improved mineral nutrition (Feddermann et al., 2010).
Root system architecture (RSA) is frequently modified following AM interactions (Scannerini et al., 2001; Hodge et al., 2009; Table 1). The total root length may increase, as happens for example in the grape (Vitis vinifera; Schellenbaum et al., 1991), or not increase, as in the tomato (Solanum lycopersicum; Berta et al., 2005), and the number and length of the roots also change according to the different associations, with modifications to the lateral roots (LRs) being more frequent than those to the main roots (Table 1). A common effect of mycorrhization is an increase in LR development, perhaps in order to increase the suitable sites for colonization (Harrison, 2005; Table 1); this gives rise to a more branched root system, which was formerly recognized in colonized leek plants (Berta et al., 1990). Two landmark papers subsequently confirmed the important role of AM symbiosis in LR formation. Paszkowski and Boller (2002) showed that the genetic defect in the lrt1 mutant of maize (Zea mays), which lacks LRs, is partly overcome when AM colonization was established, while Oláh et al. (2005) showed that branching in Medicago truncatula is directly induced by AM germinating spores. Despite the considerable differences in root architecture, increased branching has been shown to occur in monocots and in herbaceous and woody dicots, although differences exist in the order of the roots involved (Berta et al., 1995; Scannerini et al., 2001; Table 1).
Table 1.
Responses of the root system of different plant species to arbuscular mycorrhizal (AM) colonization.
| Plant | AM fungus | Culture condition (d) | Main roots |
First-order LRs |
Second-order LRs |
Third-order LRs |
Total root length | %AMF | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no. | l | rb | no. | l | rb | no. | l | rb | no. | l | rb | ||||||
| Allium porrum | Glomus sp. strain E3 | a (105) | > | < | > | – | < | – | – | – | – | – | – | – | = | 69 | Berta et al. (1990, 1993) |
| Olea europaea | Glomus mosseae | b (180) | > | = | = | > | > | > | > | > | > | > | – | – | > | 29–42 | Citernesi et al. (1998) |
| Oryza sativa | Glomus intraradices | a (42) | = | > | > | >(1, 2) | – | – | >(2) | – | – | – | – | – | – | 30–50 | Gutjahr et al. (2009a) |
| Platanus acerifolia | Glomus fasciculatum | b (77) | = | = | = | = | < | > | > | < | > | > | = | > | > | 79 | Tisserant et al. (1992, 1996) |
| Populus ‘Beaupré’ | Scutellispora calospora | b (115) | – | = | > | – | > | = | – | > | = | – | = | – | = | 8 | Hooker et al. (1992) |
| Glomus sp strain E3; G. caledonium | – | = | = | – | > | > | – | > | > | – | = | – | = | 22; 28 | |||
| Prunus cerasifera | Glomus intraradices | a (75) | – | = | > | – | = | > | – | = | > | – | = | – | > | 80 | Berta et al. (1995) |
| Glomus mosseae | – | = | > | – | = | > | – | = | = | – | = | – | > | 70 | |||
| Vitis vinifera | Glomus fasciculatum | b (56) | = | < | > | > | = | > | > | < | > | > | = | – | > | 90 | Schellenbaum et al. (1991) |
Main roots, primary or adventitious roots; LRs, lateral roots; no., number; l, length; rb, root branching; %AMF, percentage of AM fungal colonization.
Culture conditions: a, sand/nutrient solution; b, soil. The experiment's duration in days is given in parentheses.
(1), large lateral roots; (2), fine lateral roots.
> or <, increased or reduced in relation to the non-mycorrhizal controls; =, not significantly different from the non-mycorrhizal controls; –, not detected.
In this paper, the possible causes of increased branching in AM plants have been reviewed in light of the recent findings on RSA regulation. These causes may be both direct and indirect; the former include the production and action of AM fungal exudates, while the latter are mainly related to increased mineral nutrition and modulation of hormone balance. As reported above, Pi is a key element in AM symbiosis. Moreover, Pi availability has clearly been shown to influence root morphogenesis (Jones and Ljung, 2012; Niu et al., 2013). Therefore, a large part of this review has been focused on the possible involvement of Pi in AM-induced root development. The role of other minerals, including nitrogen (N), in AM symbiosis is still unclear. Although it is widely recognized that AM fungi are involved in plant N uptake, the quantitative contribution of the colonization to the plant N levels is still controversial as it has been demonstrated in some plants but not in others (Smith and Smith, 2011). Therefore, despite the well-known effect of N on root development (Jones and Ljung, 2012), the role of N in AM root morphogenesis is still impossible to assess and, for this reason, has not been covered in this review. The mechanisms that could be responsible for root morphogenesis in mycorrhizal plants and the responses to Pi in AM-host species have been discussed and compared with those of the non-host arabidopsis (Arabidopsis thaliana). This plant has been the subject of an enormous amount of research on the molecular mechanisms that govern RSA and it therefore represents an invaluable starting point and term of comparison for all studies on root morphogenesis, although the results on arabidopsis cannot be transferred directly to AM host plants.
FUNGAL EXUDATES
The AM fungal exudates directly modify root system development. The establishment of AM depends on a co-ordinated exchange of signals between symbiotic fungi and their hosts, and it has recently been demonstrated that AM germinating spores or mycorrhized roots release active symbiotic signals, often called Myc-factors, which are perceived by the host plants (Maillet et al., 2011; Mukherjee and Ané, 2011). These active molecules are released, even in the absence of the host, and are not only symbiotic signals that stimulate mycorrhiza formation, but also plant growth regulators, that are able to modify root development, as has been demonstrated for different plant species (Maillet et al., 2011; Mukherjee and Ané, 2011).
Germinating spores of Gigaspora margarita, G. rosea and Glomus intraradices (recently reassigned to Rhizophagus irregularis) as well as exudates from germinating spores (GSEs) of G. intraradices have been demonstrated to stimulate LR formation significantly and increase the total length of the root system in M. truncatula (Oláh et al., 2005; Mukherjee and Ané, 2011). This stimulation is neither associated with the inhibition of primary root (PR) elongation nor with a change in root geotropism, as happens following auxin administration (Oláh et al., 2005). Furthermore, GSE from G. intraradices increases the number of large LRs (the preferred sites for AM colonization) in rice (Oryza sativa) and the total number of LRs in maize, thus pointing to an effect of these exudates not only on the dicots, but also on the monocots (Mukherjee and Ané, 2011).
Recently, Myc-factors have been purified from exudates of carrot (Daucus carota) roots colonized by G. intraradices and from germinated spores of the same AM fungus, and have been characterized as a mixture of simple sulfated and non-sulfated lipochito-oligosaccharides (LCOs) composed of four or five glucosamine residues, with a strong structural similarity to rhizobial Nod-factors, even though simpler in structure (Maillet et al., 2011). Synthetic LCOs, obtained via bacterial genetic engineering, have been shown to stimulate AM colonization in plant species of diverse families (Fabaceae, Asteraceae and Umbelliferae) (Maillet et al., 2011).
The comprehension of the molecular processes required for AM signalling has mostly been derived from genetic studies of mutants defective in rhizobium–legume symbiosis. ‘Common’ symbiotic (Sym) genes, which control the Nod-factor signalling pathway that leads to nodulation, but which are also required for the formation of mycorrhizae, have been identified in the model legume M. truncatula (Catoira et al., 2000). Two components of this common Sym pathway, DMI1 and DMI2, are also involved in the LR formation induced by GSE in M. truncatula (Oláh et al., 2005; Mukherjee and Ané, 2011). Non-sulfated and sulfated Myc-LCOs have been shown to elicit LR formation in the same plant by a Myc and a Nod pathway, respectively. However, using the nsp1 (nodulation signaling pathway1) mutant to allow branching induction exclusively through the Myc pathway, it has been observed that the required concentrations of both sulfated and non-sulfated Myc-LCO were about 100-fold higher than those required to elicit the same response by the Nod pathway (Maillet et al., 2011). Moreover, GSE-induced restructuring of the root architecture in rice does not require CASTOR or POLLUX (DMI1 orthologues), thus pointing to another uncharacterized pathway that is independent of the Sym pathway (Gutjahr et al., 2009a; Mukherjee and Ané, 2011).
Therefore, although AM fungal exudates have been shown to increase the production of LRs in both monocots and dicots, some aspects of the response have not yet been fully clarified. It is possible that the common Sym pathway elicited by Myc-LCOs may only be active in plants that form both nodules and AMs. An additional or alternative pathway, which mediates AM signalling in a Sym-independent manner, could exist (Mukherjee and Ané, 2011; Ortu et al., 2012). It is also likely that signals of fungal origin other than LCOs may be involved in eliciting LR development (Bonfante and Requena, 2011; Genre et al., 2013).
PHOSPHATE AVAILABILITY
Arbuscular mycorrhizal colonization is generally studied in plants grown in low Pi media, because this condition favours colonization (Harrison, 2005; Balzergue et al., 2011). As a consequence, the non-colonized control plants frequently have lower tissue Pi concentrations than the colonized counterparts (Smith and Read, 2008) and are subjected to Pi starvation. Therefore, besides the direct effect of exudates on branching, the increased Pi tissue content of AM plants, which follows colonization, may be involved in modifying RSA.
Influence of Pi availability on the root system architecture
Morphogenetic root adaptation to the low-Pi environment includes an increase in the root:shoot biomass ratio (Table 2), because of an increased proportion of photosynthates being allocated to the roots (Hermans et al., 2006; Karthikeyan et al., 2007), and the development of a specific RSA to maximize the acquisition of external Pi (see, for example, Vance et al., 2003; Hermans et al., 2006; Hammond and White, 2008).
Table 2.
Responses of the root system of different plant species to phosphate (Pi) deprivation.
| Plant species | Culture conditions (d) | Main root length | Main/lateral root number | Lateral root length | Main root branching | Root:shoot ratio | References |
|---|---|---|---|---|---|---|---|
| Allium porrum. | a (105) | >(3) | <(3) | – | < | – | Trotta et al. (1991) |
| Arabidopsis thaliana Col-0 | b (17) | <(1) | >(5) | – | > | > | López-Bucio et al. (2002) |
| Brassica cultivars | a (21) | <(1) | – | > | – | – | Akhtar et al. (2009) |
| Gossipium hirsutum | a (20) | =(1) | <(5) | < | – | > | Price et al. (1989) |
| Hordeum vulgare | a (21) | =(2) | – | < | < | > | Drew (1975) |
| Lepidium sativum | c (5) | =(1) | <(5) | – | – | – | Wiersum (1958) |
| Linum usitatissimum | c (5) | >(1) | >(5) | – | – | – | Wiersum (1958) |
| Nicotiana tabacum | b (28) | >(4) | <(5) | – | < | > | A. Fusconi (unpubl.) |
| Phaseolus vulgaris | a (35) | =(6) | <(5) | = | < | > | Borch et al. (1999) |
| Raphanus sativus | c (5) | =(1) | =(5) | – | – | – | Wiersum (1958) |
| Trifolium repens | d (19) | >(3) | >(5) | > | – | – | Dinh et al. (2012) |
| Triticum aestivum | d (14) | =(2, 3) | <(5) | = | – | > | Aðalsteinsson and Jensén (1989, 1990) |
| Zea mays | d (16) | =(3) | <(3) | < | = | > | Mollier and Pellerin (1999) |
Culture conditions: a, sand/nutrient solution; b, agarized medium; c, moistened filter paper; d, hydroponic. The experiment's duration in days is given in parentheses.
Type of root: (1), primary; (2), seminal; (3), adventitious; (4), basal; (5), lateral roots; (6), not specified.
> or <, increased or reduced in relation to the Pi-sufficient plants, =, not significantly different from the Pi-sufficient plants; –, not detected.
The effects of Pi starvation on root development in arabidopsis, which like other Brassicaceae (DeMars and Boerner, 1996) is unable to form functional AM associations, have been studied in detail over the last 10 years. In this species, PR growth is reduced remarkably in response to a low Pi condition (Table 2), because of the inhibition of cell elongation and progressive differentiation of the apical cells which lose meristematic status (Sánchez-Calderón et al., 2005). The LR density generally increases (see, for example, Williamson et al., 2001; López-Bucio et al., 2002; Jiang et al., 2007; Mayzlish-Gati et al., 2012), although a reduction in the number of LRs per plant has sometimes been reported (Devaiah et al., 2009; Mayzlish-Gati et al., 2012). Elongation of the LRs is, in contrast, controversial, as longer (Williamson et al., 2001) or shorter (Nacry et al., 2005; Sánchez-Calderón et al., 2005) LRs have been observed. The reprogramming of root development under Pi deprivation in arabidopsis leads to a shallow and superficial root system, and this model of the root system is recognized as an important adaptation strategy to optimize the absorption of Pi. The highest Pi concentration in the soil, in fact, is usually found near the soil surface, and a superficial and shallow phenotype allows plants to forage for the available Pi in the topsoil (Vance et al., 2003; Hammond and White, 2008).
However, these changes are not universal, and vary from plant to plant and from genotype to genotype. Many plant species, including many of the potential hosts of AM fungi belonging to both mono- and dicots, do not exhibit an arabidopsis-like response (Forde and Lorenzo, 2001; Ramaekers et al., 2010). Primary root elongation increases under Pi starvation in many dicots, including horse gram (Macrotyloma uniflorum; Anurada and Narayan, 1991), Chinese milk vetch (Astragalus sinicus), alfalfa (Medicago sativa), lettuce (Lactuca sativa), marigold (Tagetes patula), tomato (Yoneyama et al., 2012) and some of the dicot species listed in Table 2. The same occurs for the adventitious roots of leek (Allium porrum) and rice monocots (Trotta et al., 1991; J. Zhou et al., 2008; Arite et al., 2012). These modifications probably facilitate soil exploration for these plants, because a sustained root growth allows plants to encounter areas of higher Pi availability (Berta et al., 1993; Borch et al., 1999; Ramaekers et al., 2010). However, the PR length is not influenced to any extent by Pi availability in other species (Li et al., 2012; Table 2). On the contrary, the total root length frequently decreases (Drew, 1975; Trotta et al., 1991; Borch et al., 1999) and, unlike arabidopsis, plants grown in low Pi media frequently show a low degree of branching, although there are some exceptions (Table 2). The opposite occurs when the plants grow in Pi-rich soils or become colonized with AM fungi. In the latter case, increased branching frequently coincides with an enhancement of Pi acquisition by AM plants (see, for example, Tisserant et al., 1996). A high Pi content and AM colonization therefore seem to act synergistically to increase root branching in most plant–fungus associations, thus pointing to a role for Pi signalling in root response to colonization.
Pi perception and response
Plants can detect and respond to both the local variations in the external Pi concentration and the endogenous Pi status (Thibaud et al., 2010; Chiou and Lin, 2011; Hammond and White, 2011).
Local signalling is involved in the increased density of LRs in regions of the soil with high Pi availability and the reduced activity of the PR meristem of arabidopsis (Hammond and White, 2011). The latter seems to rely on the combined activity of PDR2 (Phosphate Deficiency Response 2), a P5-type ATPase, and the multicopper oxidases LPR1/LPR2 (Low Phosphate Root 1/2) in the root tip, once changes in external Pi have been sensed (Ticconi et al., 2009; Chiou and Lin, 2011). It is not likely that a mechanism for sensing the Pi concentration around the root is involved in the difference between the root morphogenesis of AM and non-AM plants, because these plants grow in the same medium under experimental conditions. Moreover, since Pi in functional AM symbiosis is directly delivered to the cortical tissue by the fungus, bypassing the epidermis (Grace et al., 2009; Smith and Smith, 2011, 2012), the external and internal Pi status are uncoupled.
Systemic signalling regulates many plant responses to Pi starvation, as has been demonstrated through experiments in split-root systems with high and low Pi (Branscheid et al., 2010; Hammond and White, 2011). A growing number of transcription factors that participate in the plant Pi deficiency signalling cascade have been described in arabidopsis and cereals, and some of them (such as MYB62, WRKY75, ZAT6 and AtBHLH32 of arabidopsis, MYB2P-1 of rice, and PTF1 of rice and maize) have been shown to be involved in changes in root growth (Chen et al., 2007; Rouached et al., 2011; Dai et al., 2012; Li et al., 2012).
A central role in the systemic signalling of Pi in arabidopsis is played by the MYB transcription factor PHR1, a key transcriptional activator, which binds to the P1BS element (PHR1 Binding Sequence) present in the promoter region of a sub-set of Pi starvation-inducible genes (Rubio et al., 2001; Hammond and White, 2011; Smith et al., 2011). MicroRNAs of the 399 family (miR399) are induced by PHR1 in arabidopsis, and function as signalling molecules transported from the shoot to the roots; they suppress PHO2 expression, leading to activation of Pi uptake and translocation (Pant et al., 2008; Chiou and Lin, 2011). However, the transcription of PHR1 is not directly influenced by Pi starvation, and the activity of PHR1 is regulated post-translationally through sumoylation by SIZ1, a Small Ubiquitin-like Modifier (SUMO) E3 ligase (Miura et al., 2005). The PHR1–miR399–PHO2 pathway in arabidopsis is not involved in the remodelling of RSA under Pi deprivation, which is instead regulated, independently of PHR1, by SIZ1, which acts as a negative regulator of Pi starvation-dependent signalling through the control of auxin patterning and the regulation of auxin-responsive genes (Miura et al., 2011).
Components of the Pi starvation signalling pathway in arabidopsis are conserved in AM host species (Smith et al., 2011). Two homologous genes of AtPHR1, OsPHR1 and OsPHR2, have been isolated in rice; both are involved in the Pi starvation signalling pathway (J. Zhou et al., 2008). The overexpression of OsPHR2 increases sensitivity to Pi starvation, and causes enhanced root elongation, a typical trait stimulated by Pi starvation in rice under flooding conditions, suggesting, unlike in arabidopsis, a direct involvement of OsPHR2 in Pi-dependent RSA remodelling (J. Zhou et al., 2008). Moreover, PHR2 does not seem to be the only regulator of miR399 in rice. The level of the latter depends to a great extent on the plant Pi status and not on PHR2 expression, and PHO2 does not seem to be the target of miR399 (J. Zhou et al., 2008), thus showing further differences in relation to arabidopsis.
The PHR1–miR399–PHO2 pathway has not been explored to any great extent in AM-colonized plants. It has been shown that the level of miR399 is up-regulated in Pi-depleted tissues (Chiou and Lin, 2011) and, consistently, in tobacco and M. truncatula, higher levels are found in non-colonized Pi-starved plants than in Pi-sufficient plants. However, surprisingly, AM-colonized roots that grow under low Pi display levels of miR399 similar to, or higher than, non-AM controls, despite the increased tissue Pi concentration that occurs following fungus uptake (Branscheid et al., 2010). It has been hypothesized that an unknown mycorrhizal signal leads to the increased synthesis of miR399 in the shoots, which upon phloem transport accumulates as a mature molecule in the mycorrhizal roots. MicroR399 should keep the expression of PHO2 in the roots low; otherwise, the increased level of PHO2 in response to symbiotic Pi uptake would lead to the suppression of AM-induced Pi transporter genes (Branscheid et al., 2010; Smith et al., 2011).
The above data suggest that differences exist in the Pi signalling pathway of AM-host species in relation to arabidopsis. However, at present, little is known about the molecular components that are involved, especially in relation to root morphogenesis. In this respect, a possible breakthrough is represented by the recent identification and characterization of LjMAMI (Lotus japonicus Meristem and Arbuscular Mycorrhiza Induced; Volpe et al., 2012). This is a transcription factor that is phylogenetically related to PHR1, which is upregulated to a great extent in arbusculated root cells and in root apices. Its downregulation, in RNA interference transgenic hairy roots lines, has been shown to cause an important reduction in branching under low Pi. Interestingly, the wild-type phenotype is restored by AM colonization (Volpe et al., 2012, 2013). Hence, unravelling the pathway involved in the LjMAMI action would shed light on the relationship between AM symbiosis, Pi assimilation and root development.
Apart from Pi itself, the role of which has been questioned (Chiou and Lin, 2011), and miRNAs, there may be other signals involved in the modification of RSA in response to low Pi and AM colonization. These include changes in the delivery of carbohydrates, mainly sucrose, to the roots and modulation of the phytohormone concentration, transport and sensitivity.
SUGAR SIGNALLING
Sugars, including both sucrose and hexoses, play an important role in root system morphogenesis, and act as both a metabolite and a signalling molecule by regulating the expression of Pi starvation-induced genes and RSA. They are required for Pi starvation responses, and influence the root morphology of arabidopsis (reviewed by Hammond and White, 2008, 2011; Rouached et al., 2010; Puig et al., 2012) and the formation of cluster roots in the non-mycorrhizal species Lupinus albus (K. Zhou et al., 2008).
Arabidopsis seedlings are generally cultivated on growth media supplemented with sucrose (see, for example, López-Bucio et al., 2002; Pérez-Torres et al., 2008; Richter et al., 2009), and the growth of seedlings on sucrose-free medium greatly suppresses the development of LRs; the addition of sugar, in contrast, increases LR density (Jain et al., 2007; Karthikeyan et al., 2007). Moreover, direct contact between the aerial tissues and sucrose in the growth media has been shown to promote the emergence of LR primordia (MacGregor et al., 2008). The use of the mutant hps1 (hypersensitive to phosphate starvation1) of arabidopsis, which overexpresses the SUC2 (Sucrose Transporter2) gene and shows a high sucrose accumulation in the plant tissues because of enhanced sucrose uptake, has also shown that an elevated sucrose level alone is sufficient to enhance LR formation (Lei et al., 2011). Some studies have suggested that sucrose may be involved in the transport of auxin from the shoot to the root, which is critical for LR formation, and in increasing the responsiveness of the root system to auxin (Jain et al., 2007).
Although photosynthetic carbon assimilation is reduced under Pi deficiency, increased sucrose biosynthesis has been witnessed in the leaves of some plants, such as arabidopsis, common bean (Phaseolus vulgaris), barley (Hordeum vulgare) and soybean (Glycine max) (Hammond and White, 2008). Additionally, a sustained and, in some cases, an increased translocation of mobile carbohydrates, primarily sucrose, has been observed via the phloem to the roots (Hammond and White, 2008). This increased sucrose flux has been related to the changes in root phenotype because the two events occur close to each other in time (Rouached et al., 2010). However, the sucrose concentration increases in the roots of some, but not all, plant species. It remains unchanged in arabidopsis roots (Ciereszko et al., 2001) while it increases in common bean, especially in the meristematic and elongation root zones (Ciereszko et al., 1998). In the latter plant, unlike in arabidopsis, the PR length is similar in both Pi-starved and Pi-sufficient plants, and branching decreases under Pi starvation (Borch et al., 1999). The high sugar level in the root apical zone possibly sustains the PR meristem activity and elongation, despite the unfavourable, low Pi conditions. The different sucrose distribution observed between the common bean and arabidopsis may be related to the different and specific redistribution of root growth in these two species, in response to Pi-limiting conditions.
When plants are colonized by AM fungi, they have to pay the price of sugars for Pi (Smith and Read, 2008). An increased import of sucrose into roots has in fact been reported for mycorrhizal plants, and this is induced, at least in part, by signals released from the fungus (Gutjahr et al., 2009b; Helber et al., 2011). It has been demonstrated, over a range of herbaceous and woody plants, that up to 20 % more photosynthate is transported to the roots of AM plants than to the non-AM control roots (Smith and Read, 2008).
The exchange of carbon and Pi in the roots is closely correlated, with the carbon allocation being controlled locally in relation to the Pi homeostasis of the cell (Fitter, 2006; Kiers et al., 2011). The mechanism of Pi transfer in arbusculated cells has been studied extensively, while the reciprocal carbon transfer process is less known. A fungal high-affinity Monosaccharide Transporter 2 (MST2) from Glomus sp., which has been shown to be required for colonization functionality and arbuscular development, has recently been characterized (Helber et al., 2011). Expression analysis has shown that the activity of MST2 is closely correlated with that of the plant Pi transporter PT4, which is located in the periarbuscular membrane; both proteins are downregulated by sufficient Pi availability. These results made Helber et al. (2011) suggest that the arbuscule interface is the main site where the Pi/carbon exchange is modulated.
These data together indicate that, following AM colonization, in addition to the increased transport of sucrose to the root, a change in the route of photosynthates also occurs, with sugars being diverted towards the arbusculated cortex cells. Because sugar has proven to induce LR formation, it is tempting to speculate that the changed sugar partitioning and pathway that occur in AM roots could be involved in increased branching. However, the data on sugar distribution are still limited to just a few species. Further studies on the effects of exogenous sugar on root branching, on the sugar root distribution and on the expression and localization of the genes involved in the sugar signalling cascade (see Hammond and White, 2011) are therefore needed for potential AM-hosting plants under different levels of Pi nutrition, and in colonized vs. non-colonized plants.
PHYTOHORMONES
Plant hormone levels have been reported to change during AM development, and almost all hormones have been proposed as important regulators of the symbiosis (Hause et al., 2007; Ludwig-Müller, 2010; Foo et al., 2013). Moreover, many of these hormones have been shown to be involved in root morphogenesis under Pi starvation (reviewed by Rouached et al., 2010; Chiou and Lin, 2011; Hammond and White, 2011; Sato and Miura, 2011; Niu et al., 2013). Therefore, hormonal regulation of RSA following AM colonization is to be expected. However, the data on the changes in hormonal concentration, following AM colonization, are often contradictory. There is very little literature on the correlation between the altered hormonal levels in AM plants and root morphogenesis, and the molecular mechanisms involved are almost unknown.
The possible involvement of auxin, which is recognized as essential for LR formation, and that of cytokinin (CK) and ethylene (ET), the effects of which are well documented on RSA, are dealt in this section, taking into account the interactions of these hormones with Pi starvation. The role played by strigolactones (SLs), a novel class of hormones, is also discussed in relation to the regulation of AM root morphogenesis and the plant responses to Pi.
Auxin
Auxin is a major regulator of plant growth and developmental processes. In the roots, it positively regulates the size of the root apical meristem by promoting cell division antagonistically to CK, and it is involved in the regulation of cell elongation with ET (Muday et al., 2012; Vanstraelen and Benková, 2012). Moreover, it is the main regulator of each LR formation step (Fukaki and Tasaka, 2009; De Smet, 2011). Elevated levels of auxin, either due to exogenous application or to enhanced biosynthesis, are sufficient to increase LR formation, while mutations that reduce auxin signalling, such as solitary root1 of arabidopsis, cause a strong reduction in LR formation (revised by Ivanchenko et al., 2008). Since AM colonization increases root branching, the involvement of auxin in the RSA regulation of mycorrhizal plants has been suggested (Ludwig-Müller, 2010; Hanlon and Coenen, 2011; Sukumar et al., 2013).
Auxin is involved in the AM host–fungus interaction. The addition of auxin has been shown to increase spore germination and hyphal growth, and to influence the infection rate and percentage of colonization (Ludwig-Müller, 2010). Moreover, auxin was shown to be required within the host roots for the early stages of AM formation, e.g. during pre-symbiotic signal exchange (Hanlon and Coenen, 2011), in part through the control of the SL levels (Foo et al., 2013).
The auxin level in plant tissues increases in different plant–fungus associations (Ludwig-Müller, 2010), probably independently of fungus production (Jentschel et al., 2007; Ludwig-Müller, 2010). The concentration of indole-3-acetic acid (IAA), the major endogenous auxin, has been observed to increase in the AM roots of leeks with colonization and applied Pi (Torelli et al., 2000). In both situations, this high IAA concentration is closely related to the observed RSA modifications, which consist of more numerous, more branched and shorter adventitious roots (Berta et al., 1990; Trotta et al., 1991). However, colonization does not increase IAA systemically. In fact, in soybean roots grown in a split-root system, IAA only accumulated in the roots growing on the inoculated side, and remained low on the other side as in controls (Meixner et al., 2005).
Arbuscular mycorrhizal colonization in maize (Kaldorf and Ludwig-Müller, 2000; Fitze et al., 2005) and in M. truncatula (Ludwig-Müller and Güther, 2007) increases the IBA (indole-3-butyric acid) concentration. When maize is inoculated with G. intraradices, the IBA synthesis increases, as does the free IBA, and this occurs along with a significant increase in the percentage of fine LRs (reviewed by Kaldorf and Ludwig-Müller, 2000). The hormone IBA is known to contribute to the regulation of RSA (Overvoorde et al., 2010) and is recognized as an important regulator of auxin activity. It acts as a storage form of IAA and may be converted to IAA, thus contributing to the formation of IAA gradients that are required for root development (reviewed by Simon and Petrášek, 2011). Moreover, the root phenotype of AM plants could be mimicked through the application of exogenous IBA (Kaldorf and Ludwig-Müller, 2000). Therefore, an increased IBA concentration might be involved in AM root morphogenesis.
An important auxin homeostasis mechanism involves the formation of auxin conjugates, as free IAA comprises only up to 25 % of the total amount of IAA, depending on the tissue and plant species (Ludwig-Müller, 2011). In most cases, IAA can be converted to ester conjugates with sugars or to amide conjugates with amino acids, and a fraction of these conjugates may be hydrolysed back to free IAA (Ludwig-Müller, 2011). The levels of amide conjugates of IAA and IBA have been shown to increase in the roots of maize inoculated with G. intraradices (Fitze et al., 2005), and the increased formation of these conjugates is in line with the accumulation of transcripts for a putative IAA-amido synthetase and an auxin-responsive GH3-like protein in tomato mycorrhizal roots, mainly in arbuscule-containing cells (Fiorilli et al., 2009). However, the function of auxin conjugates in AM roots is currently unclear. They are possibly involved in the development of colonization and the control of fungus morphogenesis, as suggested by Fiorilli et al. (2009), while their involvement in root morphogenesis is unclear, as they play a negative role in root branching (Quint et al. 2009).
The proper transport of auxin, which leads to the formation of concentration gradients, is required for the regulation of the sequential steps of LR formation, which include priming of pericycle cells, acquisition of founder cell identity, cell cycle reactivation and primordium development (Dubrovsky et al., 2011). Indole-3-acetic acid moves passively through the vascular tissues and actively, in a polar manner, across plant cells, depending on specific influx and efflux protein carrier proteins. Among these proteins, PIN efflux proteins are the main regulators of polar auxin transport in the root apical zone (Finet and Jaillais, 2012). Efflux PIN3 and PIN7 proteins have been shown to be involved in the correct positioning and extension of the competent pericycle zone for LR initiation (Dubrovsky et al., 2011), while the rearrangement of PIN1 polarity, mediated by endocytic recycling of the PIN1 protein, redirects the auxin flux into the developing LR (Ruyter-Spira et al., 2011). Once bound to its receptor, auxin promotes the degradation of AUX/IAA transcription repressors. This allows ARFs (Auxin Response Factors) to activate the transcription of genes related to LR initiation and development (for reviews, see, for example, Fukaki and Tasaka, 2009; Péret et al., 2009; Overvoorde et al., 2010; Finet and Jaillais, 2012). This mechanism is conserved between dicot and monocot plants (Dubrovsky et al., 2011; Smith and De Smet, 2012), and is therefore operative in the potential hosts of AM fungi.
Phosphate starvation interferes with auxin gradient formation and sensitivity. Studies on arabidopsis have shown that during Pi starvation response, auxin accumulates in the PR meristem, and this is connected with the cessation of PR elongation. Coincidentally, auxin accumulates in the LR primordia and this is followed by LR elongation, with SIZ1 involved in the negative regulation of Pi starvation-induced RSA remodelling through the modifications of auxin accumulation (Miura et al., 2011). In addition, enhanced auxin sensitivity has been detected in Pi-deprived arabidopsis plants. This has been correlated to a higher expression of the auxin receptor gene TIR1. A higher TIR1 level may thus activate LR formation, although the free auxin content in Pi-deprived seedlings is quite similar to that present in seedlings grown on high Pi (Pérez-Torres et al., 2008; Chiou and Lin, 2011).
The above-reported data indicate that, in addition to the variations in auxin concentration which have been found in a number of colonized AM plants, the regulation of auxin transport and sensitivity to auxin may be equally important for AM root morphogenesis. Different signal molecules, such as sucrose (Jain et al., 2007; Hammond and White, 2011) and hormones including ET, CKs and SLs (see below), gibberellins (Gou et al., 2010), jasmonate (Sun et al., 2009; 2011) and abscisic acid (ABA; Shkolnik-Inbar and Bar-Zvi, 2010), and other substances, such as nitric oxide (Calcagno et al., 2012; Chen and Kao, 2012) and flavonoids (Harrison and Dixon, 1994; Abdel-Lateif et al., 2012), could influence mycorrhizal RSA by altering the auxin and PIN protein synthesis and/or distribution. However, there is still no evidence in favour of a changed sensitivity/response to auxin in relation to both Pi starvation and AM colonization in AM-hosts. Differential expression of genes involved in auxin signalling has been shown between Pi-starved and Pi-sufficient maize plants (Li et al., 2012), and the induction of putative ARFs has been found during AM symbiosis in maize, rice and M. truncatula, but not in L. japonicus (reviewed by Formey et al., 2013). However, comparative transcriptomic analysis among these plant species has not detected any common orthologous auxin-specific genes involved in root development of AM-colonized plants (Formey et al., 2013).
All these data point to the probable involvement of auxin in AM root branching. Furthermore, they could also indicate the existence of different regulations of auxin homeostasis and response pathways, possibly on the basis of the plant species, as suggested by Formey et al. (2013).
Cytokinins
Cytokinins play a crucial role in regulating the proliferation and differentiation of plant cells, and also control many developmental processes. They are recognized as essential regulators of the plant root system, as they are involved, antagonistically to auxin, in the control of the size of the root apical meristem, and in the rate of root growth and LR organogenesis (Sakakibara, 2006; Werner et al., 2010; Marhavý et al., 2011). They can redirect assimilates and induce invertases, thus contributing directly to the plant carbon redistribution (Ludwig-Müller, 2010). Cytokinin receptors are essential for the establishment of symbiosis with rhizobial bacteria (Gonzalez-Rizzo et al., 2006), and CKs are thought to be involved, like auxin, in the repression of defence responses of the host during the establishment of symbiosis (Ludwig-Müller, 2010). However, recent studies have suggested that CKs might not be involved to any great extent in the regulation of mycorrhizal development (see Foo et al., 2013).
A number of AM plants accumulate more CKs than non-mycorrhizal plants in both the shoots and the roots (Allen et al., 1980; Drüge and Schönbeck, 1992; van Rhijn et al., 1997; Torelli et al., 2000; Shaul-Keinan et al., 2002). However, the CK concentration in AM-colonized maize plants has been shown to increase only during the late plant growth phase, in relation to non-mycorrhizal controls (Danneberg et al., 1992). Since the main sites of CK synthesis include the root tips (Aloni et al., 2006), the high CK level found may be, in part, a consequence of increased root branching. Cytokinin-like substances have been shown to be produced by axenically grown mycelium of G. mosseae (Barea and Azcon-Aguilar, 1982). However, the possible contribution of AM fungi to the regulation of the host CK level is unclear (Barker and Tagu, 2000).
The higher CK content in AM plants is in line with the reduction in the root:shoot biomass ratio, which occurs when colonization is established. In fact, CK functions as a repressor of root development. Larger root systems have been observed in plants that show a reduction in the CK status, such as mutants for genes encoding CK biosynthetic enzymes, transgenic arabidopsis and tobacco plants with enhanced root-specific degradation of CK, or plants treated with anti-CKs (Arata et al., 2010). A low root:shoot biomass ratio is also one of the plant responses to a high Pi status, and a direct correlation has been found between CK concentration and Pi availability/tissue content in different plant species. The CK level decreases in arabidopsis under Pi starvation, along with a decrease in the expression of CRE1, a CK receptor gene (Franco-Zorrilla et al., 2002). The CK and Pi contents are also directly related in some potential AM fungal hosts, such as sunflower (Helianthus annuus; Salama and Wareing, 1979), Plantago major (Baas and Kuiper, 1989) and leek (Torelli et al., 2000).
Numerous studies have shown that CK acts as a negative regulator of LR initiation (e.g. Fukaki and Tasaka, 2009). Both exogenous CK and the overproduction of CK have been shown to inhibit LR initiation in arabidopsis (López-Bucio et al., 2002; Laplaze et al., 2007). Conversely, mutants in CK receptors or signal transduction and transgenic plants with reduced levels of CK, caused by the overexpression of CK Oxidase/Dehydrogenase, which encodes a CK-degrading enzyme, exhibit an increased number of LRs (Laplaze et al., 2007; Bielach et al., 2012).
An important part of the CK-mediated regulation of development involves interaction with the auxin pathway. Thus, an accurate balance between opposing auxin and CK effects is crucial for proper developmental output (Marhavý et al., 2011). Recent results have shown that CK and auxin response maxima barely overlap and are complementary in the root, where LR organogenesis takes place. The zone in which the priming and initiation of LRs occur displays elevated levels of biologically active CKs but a repressed CK response, while enhanced CK responses occur in the pericycle cells between existing LR primordia, perhaps in order to block additional primordia formation (Bielach et al., 2012).
Enhanced CK levels perturb the expression of PIN genes in LR founder cells (Laplaze et al. 2007), prevent PIN1 recycling and promote the lytic degradation of PIN1 in vacuoles (Marhavy et al., 2011). This CK action thus prevents the auxin gradient required for LR initiation, but it does not repress the further development of LR primordia (Laplaze et al., 2007). According to Bielach et al. (2012), this phase-dependent effect of CK could rely on the robustness and stability of the auxin gradient.
In agreement with the negative role of CK in LR formation, root branching decreases in arabidopsis plants grown under high Pi (and therefore with a high CK content) (López-Bucio et al., 2002; Laplaze et al., 2007). However, the opposite occurs in many plant species, including several AM host plants, which instead exhibit decreased branching when grown under low Pi conditions (Table 2). Nevertheless, a reduction in LR formation, induced by CK, has been documented in different plants. The inhibition of LR primordia formation has been observed after exogenous CK administration in rice (Debi et al., 2005), and RNA interference of the CK receptor MtCRE1 has been shown to increase the number of LRs in M. truncatula (Gonzalez-Rizzo et al., 2006).
Taken together, these literature data would seem to point to a primary role for CKs in the regulation of the root:shoot biomass ratio in AM plants. The contradiction between high CK content and high branching found in some potential AM-host plants grown under high Pi or colonized by AM fungi is unclear, as there are very few data on the root distribution of auxin and CK in plants other than arabidopsis, or on the sensitivity to CK and/or the CK–auxin balance.
Ethylene
Ethylene plays an important role in co-ordinating internal and external signals, as well as in several stress responses and interaction of plants with other organisms (Lei et al., 2010; López-Ráez et al., 2010). In AM symbiosis, ET and salicylic acid function as negative regulators of mycorrhizal intensity (Gamalero et al., 2008; Ludwig-Müller, 2010). In fact, a strong ET inhibitory effect has been observed on early symbiotic gene expression, on fungus entry into roots (Mukherjee and Ané, 2011) and on intraradical fungal spread (Martín-Rodríguez et al., 2011). The ET content is increased by a deficiency of ABA, which is in contrast necessary for arbuscule formation and is positively correlated to mycorrhizal establishment (Ludwig-Müller, 2010; Martín-Rodríguez et al., 2011). Accordingly, most papers indicate that ET production is diminished in AM-infected plants (McArthur and Knowles, 1992; Besmer and Koide, 1999; López-Ráez et al., 2010), although a few contradictory results have also been reported (Dugassa et al., 1996).
Ethylene, like auxin and CK, is an important regulator of root morphogenesis. It inhibits root elongation by reducing cell elongation synergistically with auxin (reviewed by Muday et al., 2012). However, it also acts antagonistically to auxin by inhibiting LR formation in the earliest stages of LR initiation, as has been shown through treatments with ET or with the ET precursor 1-aminocyclopropane carboxylic acid (ACC), and in the recent genetic studies on arabidopsis and tomato (reviewed by Fukaki and Tasaka, 2009; Lewis et al., 2011; Muday et al., 2012).
The regulation of ET–auxin interactions plays an important role in root morphogenesis: it has, in fact, been shown that ET and auxin can reciprocally influence and regulate their biosynthesis and response pathway (Stepanova et al., 2007; Vanstraelen and Benková, 2012). The precursor ACC has been found to reduce free IAA and to decrease auxin-induced gene expression in regions where LRs form (Negi et al., 2010; Lewis et al., 2011). In addition, high ET levels increase PIN3 and PIN7 expression, and this increase results in elevated auxin transport, which prevents the localized accumulation of the auxin needed to drive LR formation (Lewis et al., 2011). However, the effects of ET have been shown to depend on its concentration (Pierik et al., 2006). Treatments with low concentrations of ACC have been shown to promote the initiation of new LR primordia by increasing tryptophan-dependent auxin synthesis. Higher doses have, in contrast, been shown to inhibit initiation to a great extent, as reported above, but also to promote the emergence of existing primordia in arabidopsis (Ivanchenko et al., 2008; Fukaki and Tasaka, 2009).
The reduced level of ET generally found in AM plants is therefore in agreement with the increased branching of the colonized roots. It has also been shown that exogenous ACC has a strong inhibitory effect on LR formation in response to germinating spore exudates, in M. truncatula and rice (Mukherjee and Ané, 2011). Moreover, a reduced ET level has frequently been shown to occur under high Pi (Borch et al., 1999; Lynch and Brown, 2001; Li et al., 2009).
Ethylene is involved in root development in response to low Pi availability, as has been shown in different plants (Borch et al., 1999; López-Bucio et al., 2002; Ma et al., 2003; Dinh et al., 2012; Niu et al., 2013) including arabidopsis and common bean. Ethylene has shown an opposite effect on the primary/main root length in low and high Pi conditions in these two species. The use of ET inhibitors or mutants has shown that endogenous ET limits PR lengthening in Pi-sufficient conditions, as reported above, while the opposite happens in low Pi conditions, with ET promoting root extension (Borch et al., 1999; Ma et al., 2003). This happens although Pi-deficient roots of common bean produce twice as much ET g−1 dry weight as roots of Pi-sufficient plants (Borch et al., 1999), and increased transcript levels for ET biosynthetic genes have been found in arabidopsis (reviewed by Nagarajan and Smith, 2012). Moreover, in the common bean, endogenous ET decreases LR density in low Pi conditions and increases it in Pi-sufficient conditions (Borch et al., 1999). The use of some ET signalling mutants (such as etr1, ein2 and ein3) in arabidopsis has shown that endogenous ET also decreased the LR number and density under low Pi (López-Bucio et al., 2002). A different root sensitivity to ET has thus been considered in relation to Pi availability (Borch et al., 1999; Ma et al., 2003).
Despite showing similar responses to ET, the morphogenesis of the root system of the common bean and arabidopsis under low Pi is quite different (Borch et al., 1999; López-Bucio et al., 2002), thus showing a different responsiveness to ET also from species to species. Transcriptomic analyses, in agreement, have shown both up- and downregulation of ET Response Factor genes in a variety of plant species on the basis of the Pi availability (reviewed by Nagarajan and Smith, 2012). In arabidopsis, according to López-Bucio et al. (2002), ET is not involved in the LR response to low Pi. When auxin is applied simultaneously with ACC, the latter is unable to prevent auxin stimulation of LR formation in arabidopsis (Ivanchenko et al., 2008), which is consistent with a dominant role for auxin on ET. In contrast, root morphogenesis of the common bean under low Pi is probably under the main control of ET. This plant, in these conditions, decreases the number of LRs without any significant change in the main root length and therefore reduces root branching, as happens in many non-colonized potential AM hosts. This leads to the hypothesis that the different degree of branching found for non-colonized and colonized AM-host plants depends on a switch from one state dominated by ET and found in Pi-starved, non-colonized plants to another one that is controlled by auxin, when colonization has been established.
Strigolactones
Among the hormones that can affect RSA, SLs have been the subject of a great deal of interest in recent years, although their effects have only been analysed in a few species. Strigolactones are terpenoid lactones (for a review, see Seto et al., 2012) which play different roles in plants. They act as stimulants for the germination of seeds of root parasitic plants, such as Orobanche spp. and Striga spp. (Cook et al., 1966), and hence play a negative role on the plant that exudes them. At the same time, they are rhizosphere signals that induce hyphal branching (Akiyama et al., 2005) and spore germination of some AM fungi (Besserer et al., 2006); inside the root, they seem to promote AM colonization, thus favouring the establishment of symbiosis with AM fungi (reviewed by Foo et al., 2013). This may be related to an SL-induced fungal production of short chain chitin oligomers, which, after perception, have been shown to activate the Sym-dependent signalling pathway involved in the initial stages of fungal root colonization in M. truncatula (Genre et al., 2013). Besides their role in plant interactions, SLs act as phytohormones: they are thought to be synthesized mainly in the lower parts of the stem and in the roots and move acropetally towards the shoot apex (Kohlen et al., 2011). They have been shown to inhibit shoot branching and to regulate root development and its architecture (Ruyter-Spira et al., 2011; Seto et al., 2012; Brewer et al., 2013). Several genes, isolated from both mono- and dicots, are involved in the synthesis, starting from carotenoids, or the signalling of SLs. The biosynthetic genes include MAX1 (More Axillary Growth1), MAX3 and MAX4 of arabidopsis, RMS1 (Ramosus1) and RMS5 of pea (Pisum sativum), D10 (Dwarf10), D17 and D27 of rice, and DAD1 (Decreased Apical Dominance1) and DAD3 of petunia. The only SL signalling genes described so far are MAX2/D3/RMS4 and AtD14/OsD14/DAD2 (reviewed by Arite et al., 2009; Janssen and Snowden, 2012; Seto et al., 2012; Waters et al., 2012; Yoshida et al., 2012). It has recently been suggested that the binding of DAD2 to SLs allows an interaction with MAX2, which leads to ubiquination and degradation of downstream signalling proteins (Janssen and Snowden, 2012).
Analysis of SL-deficient and signalling mutants and the use of the synthetic SL analogue GR24 have shown that endogenous SLs have little impact on PR length in rice (Arite et al., 2012) and tomato (Koltai et al., 2010), as well as in arabidopsis under optimal growth conditions (Ruyter-Spira et al., 2011). Nevertheless, SLs stimulate PR lengthening in arabidopsis under carbohydrate starvation, because of an increased meristem cell number and size of the transition zone (Ruyter-Spira et al., 2011). Endogenous SLs increase the lengthening of the crown roots of rice, as demonstrated by the shorter crown roots of the d10-1 (max4) synthesis mutant and the d14 signalling mutant and the rescuing of the defect in the d10-1 mutant but not in d14 with application of GR24 (Arite et al., 2012), thus pointing to a general role for SLs on root lengthening. In addition, SLs negatively regulate LR density in arabidopsis by affecting both LR initiation and elongation (Kapulnik et al., 2011a; Ruyter-Spira et al., 2011).
The morphological responses of the root to SLs involve a reduction in auxin transport, through changes in the regulation of the auxin efflux, which may affect the auxin optimum required for LR formation (Koltai et al., 2010; Ruyter-Spira et al., 2011). An enhanced expression of PIN1 has in fact been found in stems of arabidopsis max mutants (Bennett et al., 2006), while, in the same plant, a GR24 treatment has been shown to cause a reduction in PIN1/3/7–green fluorescent protein intensities in the provascular tissue of the PR (Ruyter-Spira et al., 2011).
The SL content increases under low Pi. Increased SL levels in M. truncatula in this condition have been shown to be related to an important upregulation of the Mt-D27 synthetic gene (Liu et al., 2011). An inverse correlation between SL synthesis and Pi supply has been demonstrated in different plants, including pea, tomato, wheat (Triticum aestivum) and arabidopsis (Balzergue et al., 2011; Kohlen et al., 2011; Liu et al., 2011; Yoneyama et al., 2012). Nevertheless, the amount of SLs in the latter, a non-host for AM fungi, is low compared with that of plants forming AMs (Westwood, 2000). In agreement with the decreased SL levels observed in high Pi conditions, fully established AM colonization lowers SL production in mono- and dicots (López-Ráez et al., 2011), although the contribution of SLs to the regulation of AM symbiosis by Pi is still poorly understood (Balzergue et al., 2011).
The enhanced crown root elongation observed under Pi starvation in rice is in line with the enhanced production of SLs in these conditions, and is supported by a lack of crown root elongation in d10-1 and d14-1 mutant seedlings (Arite et al., 2012). The effects of SLs under low Pi on root elongation are thus similar to those of ET in rice. Unfortunately there are no data on the effect of SLs on branching in this plant.
In arabidopsis, unlike in rice, the PR length and the branched RSA do not seem to be affected much by SLs. In fact, increased root branching has also been found in max mutants in low Pi conditions (Mayzlish-Gati et al., 2012). It is known that the responses to low Pi in arabidopsis are associated with induction of the transcription of the auxin receptor TIR1 (Pérez-Torres et al., 2008). It has recently been shown that such an induction does not occur in the SL signalling mutant max2-1 and is reduced in the synthetic max4-1 mutants relative to the wild type (Mayzlish-Gati et al., 2012). Although this indicates the involvement of SLs in the increased sensitivity to auxin in low Pi conditions, differences between max2-1 and the wild type in terms of RSA are moderate under Pi starvation. Thus, according to the authors, the possibility exists that as yet unknown factors, such as MAX2-independent auxin responses, may dominate the root morphogenesis of arabidopsis in some stages of development (Mayzlish-Gati et al., 2012).
According to these data, the involvement of SLs in the responses of the roots to low Pi seems to be greater in rice than in arabidopsis, and in rice it is synergistic with that of ET. Cross-talk between SLs and ET has been described during root-hair elongation in arabidopsis (Kapulnik et al., 2011b) and during the germination of seeds of Striga hermonthica (Sugimoto et al., 2003). In both cases, an SL effect through ET biosynthesis and signalling has been suggested, and a more general effect of SLs on plant growth mediated by ET has been proposed (Kapulnik et al., 2011b; Koltai, 2013). The root morphogenesis of plants under low Pi, characterized by an extension of the main roots and reduced branching, as in many non-colonized AM hosts, may thus be controlled by SLs through the ET pathway. In contrast, the branched root growth of arabidopsis in low Pi conditions, which is only in part mediated by SLs (Mayzlish-Gati et al., 2012) and which has been considered to be independent of ET (López-Bucio et al., 2002), may be mainly directed by auxin. The decreased SL level found in AM-colonized plants (López-Ráez et al., 2011) could negatively influence the ET pathway, and this could in part explain the increased branching of AM-colonized plants.
CONCLUSIONS
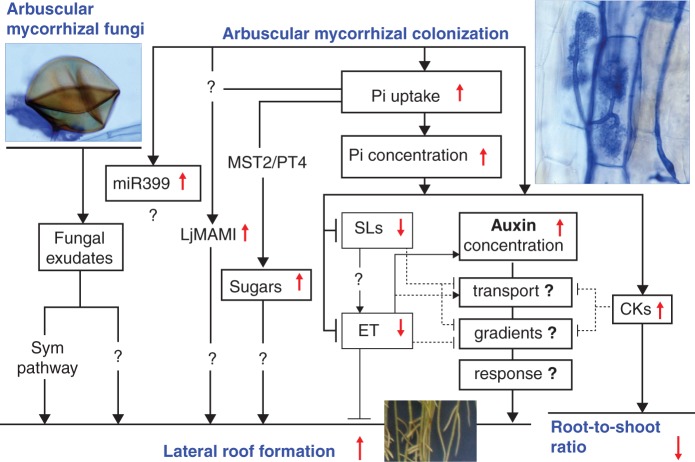
Plant responses to AM colonization involve physiological, molecular and morphological mechanisms, including a change in RSA which becomes more branched in relation to the non-colonized controls. In this paper, an overview of the possible mechanisms implicated in AM root morphogenesis and a model of root growth regulation in which fungal exudates, sugars and hormones are the main players in the regulation of mycorrhizal root growth, are provided (Fig. 1).
Fig. 1.
Schematic drawing of the possible signalling events that lead to increased root branching in arbuscular mycorrhizal (AM) plants. In the first stage of colonization, fungal exudates induce lateral root (LR) formation through the common Sym pathway (Medicago truncatula; Oláh et al., 2005) and/or another as yet unknown pathway (Oryza sativa; Gutjahr et al., 2009a). Increased phosphate (Pi) uptake may change the root architecture through different, integrated mechanisms and probably plays a central role when colonization is established. MicroR399 increases in AM plants (Branscheid et al., 2010); however, the PHR1–miRNA–PHO2 pathway has not been explored to any extent in relation to LR formation. The recently discovered Lotus japonicus Meristem and Arbuscular Mycorrhiza Induced (LjMAMI) transcription factor could link the AM symbiosis to Pi nutrition and branching (Volpe et al., 2012). The increased import of sugars into the AM roots and the flux towards the arbusculated cells, sustained by C/Pi exchange (high-affinity Monosaccharide Transporter 2, MST2/Pi transporter, PT4; Helber et al., 2011) could also favour LR induction and growth. Arbuscular mycorrhizal colonization and the resulting increased Pi tissue content act together with hormone homeostasis and signalling. An increased auxin and cytokinin (CK) concentration and reduction in strigolactones (SLs) and ethylene (ET) generally have been found in both AM and Pi-sufficient plants, in relation to Pi-starved, non-colonized, plants (see text). A dominant role for auxin on SL and ET signalling in AM root morphogenesis is thus suspected, with CKs probably being involved in the reduction of the root:shoot ratio, which generally occurs following AM colonization and high Pi nutrition.
Fungal exudates induce LR formation in the first stages of plant–fungus interaction (Oláh et al., 2005; Mukherjee and Ané, 2011), possibly to increase the potential sites of colonization. However, questions about the nature of the bioactive molecules and the pathways involved in LR formation are still unclear, particularly for non-legume plants, where a Sym-independent pathway seems to exist (Gutjahr et al., 2009a; Mukherjee and Ané, 2011). Fungal exudates may also influence root morphogenesis at later stages of colonization, when, however, others factors probably are the main regulators.
Colonized plants generally show a higher Pi tissue level than the non-colonized, Pi-starved controls. Thus, mycorrhizal RSA relies on one hand on the suppression of the responses to Pi starvation and, on the other hand, on the effects of higher Pi levels, both being mainly mediated by hormonal regulation. Levels of ET and SL frequently increase under low Pi, whereas they decrease in AM-colonized or Pi-sufficient plants. Since they have been shown to reduce root branching, at least in some species, their effects are probably correlated to the loss of the Pi-starved condition. Auxin and CKs, in contrast, tend to increase in mycorrhizal plants. Auxin is recognized to be essential for LR formation. Although regulation of auxin homeostasis and response pathways is still little understood in AM plants and seems to change from plant to plant (Formey et al., 2013), a pre-eminent role for auxin in AM root morphogenesis is likely. High CK levels are possibly involved in decreasing the root:shoot ratio in response to high Pi and colonization, while a possible influence of CKs on branching is unclear, due to their suppressive effects on LR formation.
Apart from hormones, in this review it has been proposed that, in established mycorrhizae, the symbiotic carbon/Pi exchange itself, which occurs mainly in the arbusculated cells (Helber et al., 2011), may regulate AM root morphogenesis. When plants are colonized by AM fungi an increased transport of photosynthates, which are directed towards the fungal sink zones of the root cortex, occurs. Since a relationship exists between elevated sugar levels and enhanced LR formation (Lei et al., 2011), the flux of sugars towards the colonized root cortex may stimulate LR formation. However, further research is required to confirm this hypothesis, as well as for a more detailed understanding of the role of hormones in AM root morphogenesis. There is still limited knowledge on the distribution of hormones and other morphogens, as well as of their complex network of interactions, in AM roots. As far as auxin is concerned, it has been shown that a large number of factors, hormonal or not, converge on the regulation of its synthesis, transport and the downstream signalling pathway. It would not be surprising to find that fungal exudates may also influence AM root morphogenesis through interaction with auxin, in analogy with the Nod-factor during nodule formation (see Kuppusamy et al., 2009). Moreover, among hormones, a possible role in AM root morphogenesis may be played by gibberellins. These latter, in addition to auxin, CKs, ET and SLs, are involved in the root morphogenesis in response to Pi availability (Jiang et al., 2007; Devaiah et al., 2009); however, their behaviour and functions are still unclear in AM plants (Ludwig-Müller, 2010; Foo et al., 2013).
Thus, many issues still have to be clarified in order to confirm (or refute) the assumptions presented herein. Moreover, to gain an overall picture of AM root morphogenesis, efforts should be focused on the search for the genetic determinants that act at the crossroads between mycorrhization and root development. Despite the large amount of molecular data available on mycorrhizae, only a few clues have been found on this topic. Understanding the mechanisms involved in the regulation of miR399 (Branscheid et al., 2010) and the signalling pathway related to the action of LjMAMI (Volpe et al., 2012, 2013) could be instrumental in deciphering the complex network that underlies the AM colonization and the morphogenetic processes. In-depth knowledge of the regulation of AM root morphogenesis could also shed new light on the role of RSA in the physiology of mycorrhizae and in the protection of AM colonization from biotic and abiotic stresses.
ACKNOWLEDGEMENTS
The author thanks L. Lanfranco, M. Mucciarelli and the anonymous reviewers for their constructive comments and suggestions.
LITERATURE CITED
- Abdel-Lateif K, Bogusz D, Hocher V. The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and Frankia bacteria. Plant Signaling and Behavior. 2012;7:1–6. doi: 10.4161/psb.20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar MS, Oki Y, Adachi T. Mobilization and acquisition of sparingly soluble P-sources by Brassica cultivars under P-starved environment. II. Rhizospheric pH changes, redesigned root architecture and Pi-uptake kinetics. Journal of Integrative Plant Biology. 2009;51:1024–1039. doi: 10.1111/j.1744-7909.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- Allen MF, Moore TS, Jr, Christensen M. Phytohormone changes in Bouteloua gracilis infected by vesicular-arbuscular mycorrhizae. I. Cytokinin increases in the host plant. Canadian Journal of Botany. 1980;58:371–374. [Google Scholar]
- Aloni R, Aloni E, Langhans M, Ullrich CI. Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Annals of Botany. 2006;97:883–893. doi: 10.1093/aob/mcl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aðalsteinsson S, Jensén P. Modifications of root geometry in winter wheat by phosphorus deprivation. Journal of Plant Physiology. 1989;135:513–517. [Google Scholar]
- Aðalsteinsson S, Jensén P. Influence of temperature on root development and phosphate influx in winter wheat grown at different P levels. Physiologia Plantarum. 1990;80:69–74. [Google Scholar]
- Anurada M, Narayanan A. Promotion of root elongation by phosphorus deficiency. Plant and Soil. 1991;136:273–275. [Google Scholar]
- Arata Y, Nagasawa-Iida A, Uneme H, Nakajima H, Kakimoto T, Sato R. The phenylquinazoline compound S-4893 a non-competitive cytokinin antagonist that targets Arabidopsis cytokinin receptor CRE1 and promotes root growth in Arabidopsis and rice. Plant and Cell Physiology. 2010;51:2047–2059. doi: 10.1093/pcp/pcq163. [DOI] [PubMed] [Google Scholar]
- Arite T, Umehara M, Ishikawa S, et al. d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant and Cell Physiology. 2009;50:1416–1424. doi: 10.1093/pcp/pcp091. [DOI] [PubMed] [Google Scholar]
- Arite T, Kameoka H, Kyozuka J. Strigolactone positively controls crown root elongation in rice. Journal of Plant Growth Regulation. 2012;31:165–172. [Google Scholar]
- Baas R, Kuiper D. Effects of vesicular-arbuscular mycorrhizal infection and phosphate on Plantago major ssp. pleiosperma in relation to internal cytokinin concentration. Physiologia Plantarum. 1989;76:211–215. [Google Scholar]
- Balzergue C, Puech-Pagès V, Bécard G, Rochange SF. The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signaling events. Journal of Experimental Botany. 2011;62:1049–1060. doi: 10.1093/jxb/erq335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barea JM, Azcón-Aguilar C. Production of plant growth-regulating substances by the vesicular-arbuscular mycorrhizal fungus Glomus mosseae. Applied and Environmental Microbiology. 1982;43:810–813. doi: 10.1128/aem.43.4.810-813.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker SJ, Tagu D. The roles of auxins and cytokinins in mycorrhizal symbioses. Journal of Plant Growth Regulation. 2000;19:144–154. doi: 10.1007/s003440000021. [DOI] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Current Biology. 2006;16:553–563. doi: 10.1016/j.cub.2006.01.058. [DOI] [PubMed] [Google Scholar]
- Berta G, Fusconi A, Trotta A, Scannerini S. Morphogenetic modifications induced by the mycorrhizal fungus Glomus strain E3 in the root system of Allium porrum L. New Phytologist. 1990;114:207–215. [Google Scholar]
- Berta G, Fusconi A, Trotta A. VA mycorrhizal infection and the morphology and function of root systems. Environmental and Experimental Botany. 1993;33:159–173. [Google Scholar]
- Berta G, Trotta A, Fusconi A, et al. Arbuscular mycorrhizal induced changes to plant growth and root system morphology in Prunus cerasifera. Tree Physiology. 1995;15:281–293. doi: 10.1093/treephys/15.5.281. [DOI] [PubMed] [Google Scholar]
- Berta G, Sampò S, Gamalero E, Massa N, Lemanceau P. Suppression of Rhizoctonia root-rot of tomato by Glomus mossae BEG12 and Pseudomonas fluorescens A6RI is associated with their effect on the pathogen growth and on the root morphogenesis. European Journal of Plant Pathology. 2005;111:279–288. [Google Scholar]
- Besmer YL, Koide RT. Effect of mycorrhizal colonization and phosphorous on ethylene production by snapdragon (Anthirrhinum majus L.) flowers. Mycorrhiza. 1999;9:161–166. [Google Scholar]
- Besserer A, Puech-Pagès V, Kiefer P, et al. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biology. 2006;4 doi: 10.1371/journal.pbio.0040226. e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielach A, Podlešáková K, Marhavý P, et al. Spatiotemporal regulation of lateral root organogenesis in Arabidopsis by cytokinin. The Plant Cell. 2012;24:3967–3981. doi: 10.1105/tpc.112.103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante P, Requena N. Dating in the dark: how roots respond to fungal signals to establish arbuscular mycorrhizal symbiosis. Current Opinion in Plant Biology. 2011;14:451–457. doi: 10.1016/j.pbi.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Borch K, Bouma TJ, Lynch JP, Brown KM. Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant, Cell and Environment. 1999;22:425–431. [Google Scholar]
- Branscheid A, Sieh D, Pant BD, et al. Expression pattern suggests a role of MiR399 in the regulation of the cellular response to local Pi increase during arbuscular mycorrhizal symbiosis. Molecular Plant–Microbe Interactions. 2010;23:915–926. doi: 10.1094/MPMI-23-7-0915. [DOI] [PubMed] [Google Scholar]
- Brewer PB, Koltai H, Beveridge CA. Diverse roles of strigolactones in plant development. Molecular Plant. 2013;6:18–28. doi: 10.1093/mp/sss130. [DOI] [PubMed] [Google Scholar]
- Calcagno C, Novero M, Genre A, Bonfante P, Lanfranco L. The exudate from an arbuscular mycorrhizal fungus induces nitric oxide accumulation in Medicago truncatula roots. Mycorrhiza. 2012;22:259–269. doi: 10.1007/s00572-011-0400-4. [DOI] [PubMed] [Google Scholar]
- Catoira R, Galera C, de Billy F, et al. Four genes of Medicago truncatula controlling components of a Nod-factor transduction pathway. The Plant Cell. 2000;12:1647–1665. doi: 10.1105/tpc.12.9.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Kao CH. Calcium is involved in oxide- and auxin-induced lateral root formation in rice. Protoplasma. 2012;249:187–195. doi: 10.1007/s00709-011-0277-2. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Nimmo GA, Jenkins GI, Nimmo HG. BHLH32 modulates several biochemical and morphological processes that respond to Pi starvation in Arabidopsis. Biochemical Journal. 2007;405:191–198. doi: 10.1042/BJ20070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Lin SI. Signaling network in sensing phosphate availability in plants. Annual Review of Plant Biology. 2011;62:185–206. doi: 10.1146/annurev-arplant-042110-103849. [DOI] [PubMed] [Google Scholar]
- Ciereszko I, Zambryzcka A, Rychter A. Sucrose hydrolysis in bean roots (Phaseolus vulgaris L.) under phosphate deficiency. Plant Science. 1998;133:139–144. [Google Scholar]
- Ciereszko I, Johansson H, Hurry V, Kleczkowski LA. Phosphate status affects the gene expression, protein content and enzymatic activity of UDP-glucose pyrophosphorylase in wildtype and pho mutants of Arabidopsis. Planta. 2001;212:598–605. doi: 10.1007/s004250000424. [DOI] [PubMed] [Google Scholar]
- Citernesi AS, Vitagliano C, Giovannetti M. Plant growth and root system morphology of Olea europaea L. rooted cuttings as influenced by arbuscular mycorrhizas. Journal of Horticultural Science and Biotechnology. 1998;73:647–654. [Google Scholar]
- Cook CE, Whichard LP, Turner B, Wall ME, Grant H. Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science. 1966;154:1189–1190. doi: 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- Dai X, Wang Y, Yang A, Zhang WH. OsMYB2P-1, an R2R3 MYB transcription factor, is involved in the regulation of phosphate-starvation responses and root architecture in rice. Plant Physiology. 2012;159:169–183. doi: 10.1104/pp.112.194217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danneberg G, Latus C, Zimmer W, Hundeshagen B, Schneider-Poetsch H, Bothe H. Influence of vesicular-arbuscular mycorrhiza on phytohormone balance in maize (Zea mays L.) Journal of Plant Physiology. 1992;141:33–39. [Google Scholar]
- Debi BR, Taketa S, Ichii M. Cytokinin inhibits lateral root initiation but stimulates lateral root elongation in rice (Oryza sativa) Journal of Plant Physiology. 2005;162:507–515. doi: 10.1016/j.jplph.2004.08.007. [DOI] [PubMed] [Google Scholar]
- DeMars BG, Boerner EJ. Vesicular arbuscular mycorrhizal development in the Brassicaceae in relation to plant life span. Flora. 1996;191:179–189. [Google Scholar]
- De Smet I. Lateral root initiation: one step at a time. New Phytologist. 2011;193:867–873. doi: 10.1111/j.1469-8137.2011.03996.x. [DOI] [PubMed] [Google Scholar]
- Devaiah BN, Madhuvanthi R, Karthikeyan AS, Raghothama KG. Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Molecular Plant. 2009;2:43–58. doi: 10.1093/mp/ssn081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh PTY, Roldan M, Leung S, McManus MT. Regulation of root growth by auxin and ethylene is influenced by phosphate supply in white clover (Trifolium repens L.) Plant Growth Regulation. 2012;66:179–190. [Google Scholar]
- Drew MC. Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytologist. 1975;75:479–490. [Google Scholar]
- Drüge U, Schönbeck F. Effects of vesicular-arbuscular mycorrhizal infection on transpiration, photosynthesis and growth of flax (Linum usitatissimum L.) in relation to cytokinin levels. Journal of Plant Physiology. 1992;141:40–48. [Google Scholar]
- Dubrovsky JG, Napsucialy-Mendivil S, Duclercq J, et al. Auxin minimum defines a developmental window for lateral root initiation. New Phytologist. 2011;191:970–983. doi: 10.1111/j.1469-8137.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- Dugassa GD, von Alten H, Schönbeck F. Effects of arbuscular mycorrhiza (AM) on health of Linum usitatissimum L. infected by fungal pathogens. Plant and Soil. 1996;185:173–182. [Google Scholar]
- Feddermann N, Finlay R, Boller T, Elfstrand M. Functional diversity in arbuscular mycorrhiza – the role of gene expression, phosphorus nutrition and symbiotic efficiency. Fungal Ecology. 2010;3:1–8. [Google Scholar]
- Finet C, Jaillais Y. Auxology: when auxin meets plant evo-devo. Developmental Biology. 2012;369:19–31. doi: 10.1016/j.ydbio.2012.05.039. [DOI] [PubMed] [Google Scholar]
- Fiorilli V, Catoni M, Mozzi L, Novero M, Accotto GP, Lanfranco L. Global and cell-type gene expression profiles in tomato plants colonized by an arbuscular mycorrhizal fungus. New Phytologist. 2009;184:975–987. doi: 10.1111/j.1469-8137.2009.03031.x. [DOI] [PubMed] [Google Scholar]
- Fitter AH. What is the link between carbon and phosphorus fluxes in arbuscular mycorrhizas? A null hypothesis for symbiotic function. New Phytologist. 2006;172:3–6. doi: 10.1111/j.1469-8137.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- Fitze D, Wiepninga A, Kaldorf M, Ludwig-Müller J. Auxins in the development of an arbuscular mycorrhizal symbiosis in maize. Journal of Plant Physiology. 2005;162:1210–1219. doi: 10.1016/j.jplph.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Foo E, Ross JJ, Jones WT, Reid JB. Plant hormones in arbuscular mycorrhizal symbioses: an emerging role for gibberellins. Annals of Botany. 2013;111:769–779. doi: 10.1093/aob/mct041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B, Lorenzo H. The nutritional control of root development. Plant and Soil. 2001;232:51–68. [Google Scholar]
- Formey D, Jourda C, Roux C, Delaux PM. What the genomics of arbuscular mycorrhizal symbiosis teaches us about root development. In: Crespi M, editor. Root genomics and soil interactions. Oxford: Blackwell; 2013. pp. 171–188. [Google Scholar]
- Franco-Zorrilla JM, Martin AC, Solano R, Rubio V, Leyva A, Paz-Ares J. Mutations at CRE1 impair cytokinin-induced repression of phosphate starvation responses in Arabidopsis. The Plant Journal. 2002;32:353–60. doi: 10.1046/j.1365-313x.2002.01431.x. [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tasaka M. Hormone interactions during lateral root formation. Plant Molecular Biology. 2009;69:437–449. doi: 10.1007/s11103-008-9417-2. [DOI] [PubMed] [Google Scholar]
- Gamalero E, Berta G, Massa N, Glick BR, Lingua G. Synergistic interactions between the ACC deaminase-producing bacterium Pseudomonas putida UW4 and the AM fungus Gigaspora rosea positively affect cucumber plant growth. FEMS Microbiology Ecology. 2008;64:459–467. doi: 10.1111/j.1574-6941.2008.00485.x. [DOI] [PubMed] [Google Scholar]
- Genre A, Chabaud M, Balzergue C, et al. Short-chain chitin oligomers from arbuscular mycorrhizal fungi trigger nuclear Ca2+ spiking in Medicago truncatula roots and their production is enhanced by strigolactone. New Phytologist. 2013;198:190–202. doi: 10.1111/nph.12146. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rizzo S, Crespi M, Frugier F. The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. The Plant Cell. 2006;18:2680–2693. doi: 10.1105/tpc.106.043778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou J, Strauss SH, Tsai CJ, et al. Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. The Plant Cell. 2010;22:623–639. doi: 10.1105/tpc.109.073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace EJ, Cotsaftis O, Tester M, Smith FA, Smith SE. Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytologist. 2009;181:938–949. doi: 10.1111/j.1469-8137.2008.02720.x. [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Casieri L, Paszkowski U. Glomus intraradices induces changes in root system architecture of rice independently of common symbiosis signaling. New Phytologist. 2009a;182:829–837. doi: 10.1111/j.1469-8137.2009.02839.x. [DOI] [PubMed] [Google Scholar]
- Gutjahr C, Novero M, Guether M, Montanari O, Udvardi M, Bonfante P. Presymbiotic factors released by the arbuscular mycorrhizal fungus Gigaspora margarita induce starch accumulation in Lotus japonicus roots. New Phytologist. 2009b;183:53–61. doi: 10.1111/j.1469-8137.2009.02871.x. [DOI] [PubMed] [Google Scholar]
- Hammond JP, White PJ. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. Journal of Experimental Botany. 2008;59:93–109. doi: 10.1093/jxb/erm221. [DOI] [PubMed] [Google Scholar]
- Hammond JP, White PJ. Sugar signaling in root responses to low phosphorus availability. Plant Physiology. 2011;156:1033–1040. doi: 10.1104/pp.111.175380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon MT, Coenen C. Genetic evidence for auxin involvement in arbuscular mycorrhiza initiation. New Phytologist. 2011;189:701–709. doi: 10.1111/j.1469-8137.2010.03567.x. [DOI] [PubMed] [Google Scholar]
- Harrison MJ. Signaling in the arbuscular mycorrhizal symbiosis. Annual Review of Microbiology. 2005;59:19–42. doi: 10.1146/annurev.micro.58.030603.123749. [DOI] [PubMed] [Google Scholar]
- Harrison M, Dixon R. Spatial patterns of expression of flavonoid/isoflavonoid pathway genes during interactions between roots of Medicago truncatula and the mycorrhizal fungus Glomus versiforme. The Plant Journal. 1994;6:9–20. [Google Scholar]
- Hause B, Mrosk C, Isayenkov S, Strack D. Jasmonates in arbuscular mycorrhizal interactions. Phytochemistry. 2007;68:101–110. doi: 10.1016/j.phytochem.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Helber N, Wippel K, Sauer N, Schaarschmidt S, Hause B, Requena N. A versatile monosaccharide transporter that operates in the arbuscular mycorrhizal fungus Glomus sp is crucial for the symbiotic relationship with plants. The Plant Cell. 2011;23:3812–3823. doi: 10.1105/tpc.111.089813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans C, Hammond JP, White PJ, Verbruggen N. How do plants respond to nutrient shortage by biomass allocation? Trends in Plant Science. 2006;11:610–617. doi: 10.1016/j.tplants.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Hodge A, Berta G, Doussan C, Merchan F, Crespi M. Plant root growth, architecture and function. Plant and Soil. 2009;321:153–187. [Google Scholar]
- Hooker JE, Munro M, Atkinson D. Vesicular-arbuscular mycorrhizal fungi induced alteration in poplar root system morphology. Plant and Soil. 1992;145:207–214. [Google Scholar]
- Ivanchenko MG, Muday GK, Dubrovsky JG. Ethylene–auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. The Plant Journal. 2008;5:335–347. doi: 10.1111/j.1365-313X.2008.03528.x. [DOI] [PubMed] [Google Scholar]
- Jain A, Poling MD, Karthikeyan AS, et al. Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiology. 2007;144:232–247. doi: 10.1104/pp.106.092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BJ, Snowden KC. Strigolactone and karrikin signal perception: receptors, enzymes, or both? Frontiers in Plant Science/Plant Evolution and Development. 2012;3:1–13. doi: 10.3389/fpls.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentschel K, Thiel D, Rehn F, Ludwig-Müller J. Arbuscular mycorrhiza enhances auxin levels and alters auxin biosynthesis in Tropaeolum majus during early stages of colonization. Physiologia Plantarum. 2007;129:320–333. [Google Scholar]
- Jiang C, Gao X, Liao L, Harberd NP, Fu X. Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin–DELLA signaling pathway in Arabidopsis. Plant Physiology. 2007;145:1460–1470. doi: 10.1104/pp.107.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Ljung K. Subterranean space exploration: the development of root system architecture. Current Opinion in Plant Biology. 2012;15:97–102. doi: 10.1016/j.pbi.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Kaldorf M, Ludwig-Müller J. AM fungi might affect the root morphology of maize by increasing indole-3-butyric acid biosynthesis. Physiologia Plantarum. 2000;109:58–67. [Google Scholar]
- Kapulnik Y, Delaux PM, Resnick N, et al. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta. 2011a;233:209–216. doi: 10.1007/s00425-010-1310-y. [DOI] [PubMed] [Google Scholar]
- Kapulnik Y, Resnick N, Mayzlish-Gati E, et al. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. Journal of Experimental Botany. 2011b;62:2915–2924. doi: 10.1093/jxb/erq464. [DOI] [PubMed] [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Jain A, Held MA, Carpita NC, Raghothama KG. Phosphate starvation responses are mediated by sugar signaling in Arabidopsis. Planta. 2007;225:907–918. doi: 10.1007/s00425-006-0408-8. [DOI] [PubMed] [Google Scholar]
- Kiers ET, Duhamel M, Beesetty Y, et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science. 2011;333:880–882. doi: 10.1126/science.1208473. [DOI] [PubMed] [Google Scholar]
- Kohlen W, Charnikhova T, Liu Q. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiology. 2011;155:974–987. doi: 10.1104/pp.110.164640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltai H, Dor E, Hershenhorn J, et al. Strigolactones’ effect on root growth and root-hair elongation may be mediated by auxin-efflux carriers. Journal of Plant Growth Regulation. 2010;29:129–136. [Google Scholar]
- Koltai H. Strigolactones activate different hormonal pathways for regulation of root development in response to phosphate growth conditions. Annals of Botany. 2013;112:409–415. doi: 10.1093/aob/mcs216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppusamy KT, Ivashuta S, Bucciarelli B, et al. Knockdown of CELL DIVISION CYCLE16 reveals an inverse relationship between lateral root and nodule numbers and a link to auxin in Medicago truncatula. Plant Physiology. 2009;151:1155–1166. doi: 10.1104/pp.109.143024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplaze L, Benkova E, Casimiro I, et al. Cytokinins act directly on lateral root founder cells to inhibit root initiation. The Plant Cell. 2007;19:3889–3900. doi: 10.1105/tpc.107.055863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Zhu C, Liu Y, et al. Ethylene signalling is involved in regulation of phosphate starvation-induced gene expression and production of acid phosphatases and anthocyanin in Arabidopsis. New Phytologist. 2010;189:1084–1095. doi: 10.1111/j.1469-8137.2010.03555.x. [DOI] [PubMed] [Google Scholar]
- Lei M, Liu Y, Zhang B, et al. Genetic and genomic evidence that sucrose is a global regulator of plant responses to phosphate starvation in Arabidopsis. Plant Physiology. 2011;156:1116–1130. doi: 10.1104/pp.110.171736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Negi S, Sukumar P, Muday GK. Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development. 2011;138:3485–3495. doi: 10.1242/dev.065102. [DOI] [PubMed] [Google Scholar]
- Li YS, Mao XT, Tian QY, Li LH, Zhang WH. Phosphorus deficiency-induced reduction in root hydraulic conductivity in Medicago falcata is associated with ethylene production. Environmental and Experimental Botany. 2009;67:172–177. [Google Scholar]
- Li Z, Xu C, Li K, Yan S, Qu X, Zhang J. Phosphate starvation of maize inhibits lateral root formation and alters gene expression in the lateral root primordium zone. BMC Plant Biology. 2012;12:89–105. doi: 10.1186/1471-2229-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Kohlen W, Lillo A. Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. The Plant Cell. 2011;23:3853–3865. doi: 10.1105/tpc.111.089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L. Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiology. 2002;129:244–256. doi: 10.1104/pp.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Ráez JA, Verhage A, Fernández I. Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. Journal of Experimental Botany. 2010;61:2589–2601. doi: 10.1093/jxb/erq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Ráez JA, Charnikhova T, Fernández I, Bouwmeester H, Pozo MJ. Arbuscular mycorrhizal symbiosis decreases strigolactone production in tomato. Journal of Plant Physiology. 2011;168:294–297. doi: 10.1016/j.jplph.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Ludwig-Müller J. Hormonal responses in host plants triggered by arbuscular mycorrhizal fungi. In: Koltai H, Kapulnik Y, editors. Arbuscular mycorrhizas: physiology and function. 2nd edn. Dordrecht: Springer; 2010. pp. 169–190. [Google Scholar]
- Ludwig-Müller J. Auxin conjugates: their role for plant development and in the evolution of land plants. Journal of Experimental Botany. 2011;62:1757–1773. doi: 10.1093/jxb/erq412. [DOI] [PubMed] [Google Scholar]
- Ludwig-Müller J, Güther M. Auxins as signals in arbuscular mycorrhiza formation. Plant Signaling and Behavior. 2007;2:194–196. doi: 10.4161/psb.2.3.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Brown KM. Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant and Soil. 2001;237:225–237. [Google Scholar]
- Ma Z, Baskin TI, Brown KM, Lynch JP. Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiology. 2003;131:1381–1390. doi: 10.1104/pp.012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor DR, Deak KI, Ingram PA, Malamy JE. Root system architecture in Arabidopsis grown in culture is regulated by sucrose uptake in the aerial tissues. The Plant Cell. 2008;20:2643–2660. doi: 10.1105/tpc.107.055475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet F, Poinsot V, André O, et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature. 2011;469:58–63. doi: 10.1038/nature09622. [DOI] [PubMed] [Google Scholar]
- Marhavý P, Bielach A, Abas L, et al. Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Developmental Cell. 2011;21:796–804. doi: 10.1016/j.devcel.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Martín-Rodríguez JA, León-Morcillo R, Vierheilig H, Ocampo JA, Ludwig-Müller J, García-Garrido JM. Ethylene-dependent/ethylene-independent ABA regulation of tomato plants colonized by arbuscular mycorrhiza fungi. New Phytologist. 2011;190:193–205. doi: 10.1111/j.1469-8137.2010.03610.x. [DOI] [PubMed] [Google Scholar]
- Mayzlish-Gati E, De Cuyper C, Goormachtig S, et al. Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiology. 2012;160:1329–1341. doi: 10.1104/pp.112.202358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur DAJ, Knowles NR. Resistance responses of potato to vesicular-arbuscular mycorrhizal fungi under varying abiotic phosphorus levels. Plant Physiology. 1992;100:341–351. doi: 10.1104/pp.100.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meixner C, Ludwig-Müller J, Miersch O, Gresshoff P, Staehelin C, Vierheilig H. Lack of mycorrhizal autoregulation and phytohormonal changes in the supernodulating soybean mutant nts1007. Planta. 2005;222:709–715. doi: 10.1007/s00425-005-0003-4. [DOI] [PubMed] [Google Scholar]
- Miura K, Rus A, Sharkhuu A, et al. The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses. Proceedings of the National Academy of Sciences, USA. 2005;102:7760–7765. doi: 10.1073/pnas.0500778102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Lee J, Gong Q, et al. SIZ1 regulation of phosphate starvation-induced root architecture remodeling involves the control of auxin accumulation. Plant Physiology. 2011;155:1000–1012. doi: 10.1104/pp.110.165191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollier A, Pellerin S. Maize root system growth and development as influenced by phosphorus deficiency. Journal of Experimental Botany. 1999;50:487–497. [Google Scholar]
- Muday GK, Rahman A, Binder BM. Auxin and ethylene: collaborators or competitors? Trends in Plant Science. 2012;17:181–195. doi: 10.1016/j.tplants.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Ané JM. Germinating spore exudates from arbuscular mycorrhizal fungi: molecular and developmental responses in plants and their regulation by ethylene. Molecular Plant-Microbe Interactions. 2011;24:260–270. doi: 10.1094/MPMI-06-10-0146. [DOI] [PubMed] [Google Scholar]
- Nacry P, Canivenc G, Muller B, et al. A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiology. 2005;138:2061–2074. doi: 10.1104/pp.105.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan VK, Smith AP. Ethylene's role in phosphate starvation signaling: more than just a root growth regulator. Plant and Cell Physiology. 2012;53:277–286. doi: 10.1093/pcp/pcr186. [DOI] [PubMed] [Google Scholar]
- Negi S, Sukumar P, Liu X, Cohen JD, Muday GK. Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. The Plant Journal. 2010;61:3–15. doi: 10.1111/j.1365-313X.2009.04027.x. [DOI] [PubMed] [Google Scholar]
- Niu YF, Chai RS, Jin GL, Wang H, Tang CX, Zhang YS. Responses of root architecture development to low phosphorous availability: a review. Annals of Botany. 2013;112:391–408. doi: 10.1093/aob/mcs285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláh B, Brière C, Bécard G, Dénarié J, Gough C. Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway. The Plant Journal. 2005;44:195–207. doi: 10.1111/j.1365-313X.2005.02522.x. [DOI] [PubMed] [Google Scholar]
- Ortu G, Balestrini R, Pereira PA, Becker J, Küster H, Bonfante P. Plant genes related to gibberellin biosynthesis and signaling are differentially regulated during the early stages of AM fungal interactions. Molecular Plant. 2012;5:951–954. doi: 10.1093/mp/sss027. [DOI] [PubMed] [Google Scholar]
- Overvoorde P, Fukaki H, Beeckman T. Auxin control of root development. Cold Spring Harbor Perspectives in Biology. 2010;2 doi: 10.1101/cshperspect.a001537. a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant BD, Buhtz A, Kehr J, Scheible WR. MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. The Plant Journal. 2008;53:731–738. doi: 10.1111/j.1365-313X.2007.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszkowski U, Boller T. The growth defect of lrt1, a maize mutant lacking lateral roots, can be complemented by symbiotic fungi or high phosphate nutrition. Planta. 2002;214:584–590. doi: 10.1007/s004250100642. [DOI] [PubMed] [Google Scholar]
- Péret B, De Rybel B, Casimiro I, et al. Arabidopsis lateral root development: an emerging story. Trends in Plant Sciences. 2009;14:399–408. doi: 10.1016/j.tplants.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Péret B, Clément M, Nussaume L, Desnos T. Root developmental adaptation to phosphate starvation: better safe than sorry. Trends in Plant Science. 2011;16:442–450. doi: 10.1016/j.tplants.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Pérez-Torres CA, López-Bucio J, Cruz-Ramírez A, et al. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. The Plant Cell. 2008;20:3258–3272. doi: 10.1105/tpc.108.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LACJ. The Janus face of ethylene: growth inhibition and stimulation. Trends in Plant Science. 2006;11:176–183. doi: 10.1016/j.tplants.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Price NS, Roncadori RW, Hussey RS. Cotton root growth as influenced by phosphorus nutrition and vesicular-arbuscular mycorrhizas. New Phytologist. 1989;111:61–66. [Google Scholar]
- Puig J, Pauluzzi G, Guiderdoni E, Gantet P. Regulation of shoot and root development through mutual signalling. Molecular Plant. 2012;5:974–983. doi: 10.1093/mp/sss047. [DOI] [PubMed] [Google Scholar]
- Quint M, Barkawi LS, Fan KT, Cohen JD, Gray WM. Arabidopsis IAR4 modulates auxin response by regulating auxin homeostasis. Plant Physiology. 2009;150:748–758. doi: 10.1104/pp.109.136671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers L, Remans R, Rao IM, Blair MW, Vanderleydena J. Strategies for improving phosphorus acquisition efficiency of crop plants. Field Crops Research. 2010;117:169–176. [Google Scholar]
- Richter GL, Monshausen GB, Krol A, Gilroy S. Mechanical stimuli modulate lateral root organogenesis. Plant Physiology. 2009;151:1855–1866. doi: 10.1104/pp.109.142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouached H, Arpat AB, Poirier Y. Regulation of phosphate starvation responses in plants: signaling players and cross-talks. Molecular Plant. 2010;3:288–299. doi: 10.1093/mp/ssp120. [DOI] [PubMed] [Google Scholar]
- Rouached H, Stefanovic A, Secco D, et al. Uncoupling phosphate deficiency from its major effects on growth and transcriptome via PHO1 expression in Arabidopsis. The Plant Journal. 2011;65:557–570. doi: 10.1111/j.1365-313X.2010.04442.x. [DOI] [PubMed] [Google Scholar]
- Rubio V, Linhares F, Solano R, et al. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes and Development. 2001;15:2122–2133. doi: 10.1101/gad.204401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyter-Spira C, Kohlen W, Charnikhova T, et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiology. 2011;155:721–734. doi: 10.1104/pp.110.166645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H. Cytokinins: activity, biosynthesis, and translocation. Annual Review of Plant Biology. 2006;57:431–449. doi: 10.1146/annurev.arplant.57.032905.105231. [DOI] [PubMed] [Google Scholar]
- Salama AMS, Wareing PF. Effects of mineral nutrition on endogenous cytokinins in plants of sunflower (Helianthus annuus L.) Journal of Experimental Botany. 1979;30:971–981. [Google Scholar]
- Sánchez-Calderón L, López-Bucio J, Chachón-López A, et al. Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant and Cell Physiology. 2005;46:174–184. doi: 10.1093/pcp/pci011. [DOI] [PubMed] [Google Scholar]
- Sato A, Miura K. Root architecture remodeling induced by phosphate starvation. Plant Signaling and Behavior. 2011;6:1122–1126. doi: 10.4161/psb.6.8.15752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannerini S, Fusconi A, Mucciarelli M. The effect of endophytic fungi on host plant morphogenesis. In: Seckback J, editor. Symbiosis: organisms and model systems. Dordrecht: Kluwer; 2001. pp. 427–447. [Google Scholar]
- Schellenbaum L, Berta G, Raviolanirina F, Tisserant B, Gianinazzi S, Fitter AH. Influence of endomycorrhizal infection on root morphology in a micropropagated woody plant species (Vitis vinifera L.) Annals of Botany. 1991;68:135–141. [Google Scholar]
- Seto Y, Kameoka H, Yamaguchi S, Kyozuka J. Recent advances in strigolactone research: chemical and biological aspects. Plant and Cell Physiology. 2012;53:1843–1853. doi: 10.1093/pcp/pcs142. [DOI] [PubMed] [Google Scholar]
- Shaul-Keinan O, Gadkar V, Ginzberg I, et al. Hormone concentrations in tobacco roots change during arbuscular mycorrhizal colonization with Glomus intraradices. New Phytologist. 2002;154:501–507. doi: 10.1046/j.1469-8137.2002.00388.x. [DOI] [PubMed] [Google Scholar]
- Shkolnik-Inbar D, Bar-Zvi D. ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. The Plant Cell. 2010;22:3560–3573. doi: 10.1105/tpc.110.074641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S, Petrášek J. Why plants need more than one type of auxin. Plant Science. 2011;180:454–460. doi: 10.1016/j.plantsci.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Smith S, De Smet I. Root system architecture: insights from Arabidopsis and cereal crops. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367:1441–1452. doi: 10.1098/rstb.2011.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal symbiosis. 3rd edn. London: Academic Press; 2008. [Google Scholar]
- Smith SE, Smith FA. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia. 2012;104:1–13. doi: 10.3852/11-229. [DOI] [PubMed] [Google Scholar]
- Smith SE, Jakobsen I, Grønlund M, Smith FA. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiology. 2011;156:1050–1057. doi: 10.1104/pp.111.174581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Smith FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annual Review of Plant Biology. 2011;62:227–250. doi: 10.1146/annurev-arplant-042110-103846. [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. The Plant Cell. 2007;19:2169–2185. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Ali AM, Yabuta S, Kinoshita H, Inanaga S, Itai A. Germination strategy of Striga hermonthica involves regulation of ethylene biosynthesis. Physiologia Plantarum. 2003;119:137–145. [Google Scholar]
- Sukumar P, Legué V, Vayssières A, et al. Involvement of auxin pathways in modulating root architecture during beneficial plant–microorganism interactions. Plant, Cell and Environment. 2013;36:909–919. doi: 10.1111/pce.12036. [DOI] [PubMed] [Google Scholar]
- Sun J, Xu Y, Ye S, et al. Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. The Plant Cell. 2009;21:1495–1511. doi: 10.1105/tpc.108.064303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Chen Q, Qi L, et al. Jasmonate modulates endocytosis and plasma membrane accumulation of the Arabidopsis PIN2 protein. New Phytologist. 2011;191:360–375. doi: 10.1111/j.1469-8137.2011.03713.x. [DOI] [PubMed] [Google Scholar]
- Thibaud MC, Arrighi JF, Bayle V, et al. Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. The Plant Journal. 2010;64:775–789. doi: 10.1111/j.1365-313X.2010.04375.x. [DOI] [PubMed] [Google Scholar]
- Ticconi CA, Lucero RD, Sakhonwasee S, et al. ER-resident proteins PDR2 and LPR1 mediate the developmental response of root meristems to phosphate availability. Proceedings of the National Academy of Sciences, USA. 2009;106:14174–14179. doi: 10.1073/pnas.0901778106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserant B, Schellenbaum L, Gianinazzi-Pearson V, Gianinazzi S, Berta G. Influence of infection by an endomycorrhizal fungus on root development and architecture in Platanus acerifolia. Allionia. 1992;30:171–181. [Google Scholar]
- Tisserant B, Gianinazzi S, Gianinazzi-Pearson V. Relationships between lateral root order, arbuscular mycorrhiza development, and the physiological state of the symbiotic fungus in Platanus acerifolia. Canadian Journal of Botany. 1996;74:1947–1955. [Google Scholar]
- Torelli A, Trotta A, Acerbi L, Arcidiacono G, Berta G, Branca C. IAA and ZR content in leek (Allium porrum L.) as influenced by P nutrition and arbuscular mycorrhizae, in relation to plant development. Plant and Soil. 2000;226:29–35. [Google Scholar]
- Trotta A, Carminati C, Schellenbaum L, Scannerini S, Fusconi, Berta G. Correlation between root morphogenesis, VA mycorrhizal infection and phosphorus nutrition. In: McMichael BL, Person H, editors. Plant roots and their environment. Amsterdam: Elsevier; 1991. pp. 333–339. [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. Phosphorus acquisition and use: critical adaptation by plants for securing a non-renewable resource. New Phytologist. 2003;157:423–447. doi: 10.1046/j.1469-8137.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- Van Rhijn P, Fang Y, Galili S, et al. Expression of early nodulin genes in alfalfa mycorrhizae indicates that signal transduction pathways used in forming arbuscular mycorrhizae and Rhizobium-induced nodules may be conserved. Proceedings of the National Academy of Sciences, USA. 1997;94:5467–5472. doi: 10.1073/pnas.94.10.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstraelen M, Benková E. Hormonal interactions in the regulation of plant development. Annual Review of Cell and Developmental Biology. 2012;28:463–487. doi: 10.1146/annurev-cellbio-101011-155741. [DOI] [PubMed] [Google Scholar]
- Volpe V, Dell'Aglio E, Giovanetti M, et al. An AM-induced, MYB-family gene of Lotus japonicus (LjMAMI) affects root growth in an AM-independent manner. The Plant Journal. 2012;73:442–455. doi: 10.1111/tpj.12045. [DOI] [PubMed] [Google Scholar]
- Volpe V, Dell'Aglio E, Bonfante P. The Lotus japonicus MAMI gene links root development, arbuscular mycorrhizal symbiosis and phosphate availability. Plant Signaling and Behavior. 2013;8 doi: 10.4161/psb.23414. e23414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Brewer PB, Bussell JD, Smith SM, Beveridge CA. The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant Physiology. 2012;159:1073–1085. doi: 10.1104/pp.112.196253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood JH. Characterization of the Orobanche–Arabidopsis system for studying parasite–host interactions. Weed Science. 2000;48:742–748. [Google Scholar]
- Werner T, Nehnevajova E, Köllmer I, et al. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. The Plant Cell. 2010;22:3905–3920. doi: 10.1105/tpc.109.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersum LK. Density of root branching as affected by substrate and separate ions. Acta Botanica Neerlandica. 1958;7:174–190. [Google Scholar]
- Williamson LC, Ribrioux SPCP, Fitter AH, Leyser HMO. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiology. 2001;126:875–882. doi: 10.1104/pp.126.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama K, Xie X, Kim HI, et al. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta. 2012;235:1197–1207. doi: 10.1007/s00425-011-1568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Kameoka H, Tempo M, et al. The D3 F-box protein is a key component in host strigolactone responses essential for arbuscular mycorrhizal symbiosis. New Phytologist. 2012;196:1208–1216. doi: 10.1111/j.1469-8137.2012.04339.x. [DOI] [PubMed] [Google Scholar]
- Zhou J, Jiao FC, Wu Z, et al. OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiology. 2008;146:1673–1686. doi: 10.1104/pp.107.111443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K, Yamagishi M, Mitsuru Osaki M, Masuda K. Sugar signalling mediates cluster root formation and phosphorus starvation-induced gene expression in white lupin. Journal of Experimental Botany. 2008;59:2749–2756. doi: 10.1093/jxb/ern130. [DOI] [PMC free article] [PubMed] [Google Scholar]