Summary
The identification of evidence-based, efficacious drug combinations for each cancer, among thousands of potential permutations, is a daunting task. In this perspective, we propose a systematic approach towards defining such combinations by molecularly benchmarking a drug against a desired state of efficacy using model systems.
Introduction
While targeted therapies have revolutionized cancer treatment, the lack of durable responses have highlighted the need for combination regimens to overcome primary or acquired drug resistance. To date, the rational design of effective drug combinations has relied on knowledge-based assessments, high-throughput screens, or identification of presumed compensatory pathways after single drug administration. More recently, we have reported on a data-driven strategy based on the concept of benchmarking against a desired phenotype. In an inducible NRAS mouse model of melanoma, the genetic extinction of oncogenic NRAS results in complete tumor regression, hence defining a desired “ideal” state. Benchmarking against such a molecular state allowed for identification of a drug combination that more closely simulates the efficacy of genetic NRAS extinction. In this perspective, we discuss the potential of generalizing this methodology to enable data-driven co-targeting therapeutic strategies against un-druggable cancer targets, both oncogenes and tumor suppressors. Further, we speculate on how this approach can be applied clinically to address the challenge of heterogeneity in patient responses: namely, a systematic approach to designing a combination strategy customized to a patient, by benchmarking the patient’s unique response to a drug against a pre-defined molecular state representing the desired efficacy. Such an adaptive approach to personalizing therapeutic combinations has the potential to delineate the clinical paths for durable complete responses in the clinic.
A benchmark-driven approach for the discovery of co-targeting strategies
Systems biology – the data-driven network modeling of complex biological systems – holds promise to identify novel cancer therapies in an unbiased manner. One recent study applied such modeling to predict that the apoptotic response in breast cancer is optimized by the sequential rather than simultaneous application of chemotherapy and EGFR inhibitor (1). In another, computational modeling identified Erbb3 as the most effective therapeutic target across the Erbb-PI3K axis, leading to the development of a novel and effective therapeutic Errb3 antibody (2). Similarly, modeling of EGFR phospho-signaling identified MET plus EGFR inhibition as synergistic (3).
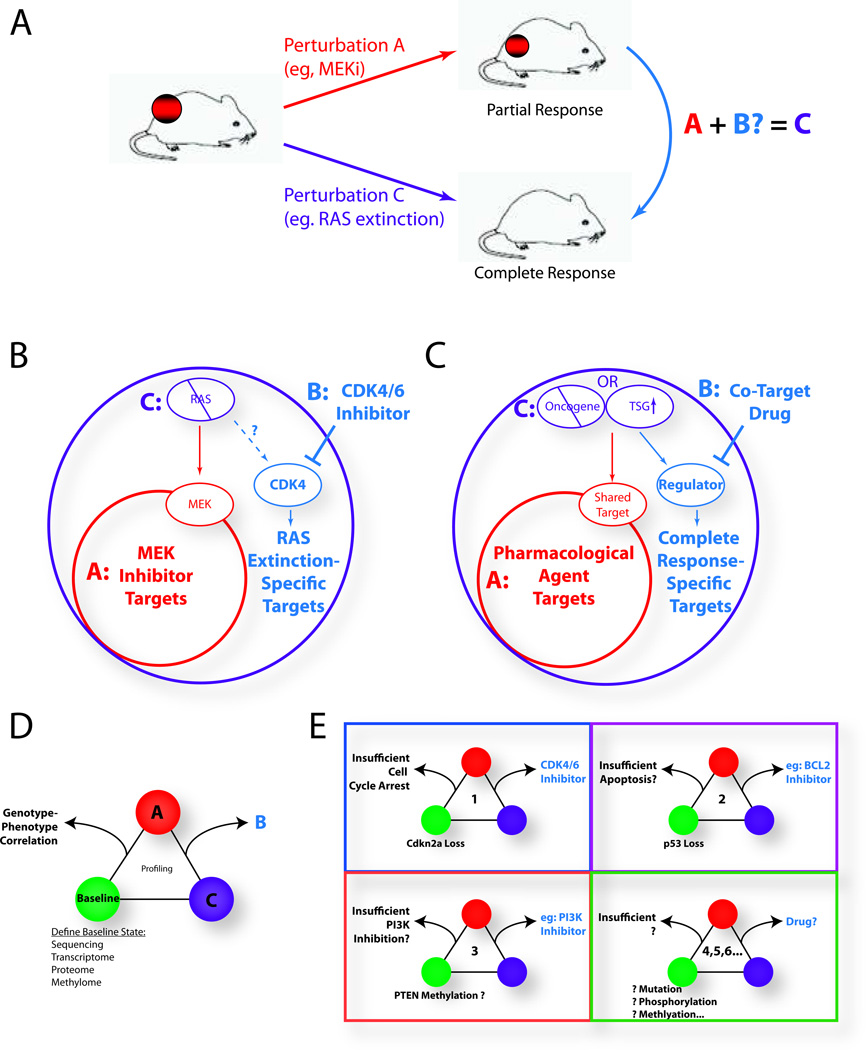
We have recently published (4) another example of a data-driven approach to the development of evidence-based therapeutic combinations (Fig. 1a). This study leveraged a mouse model of melanoma, engineered so that the expression of mutant NRAS can be extinguished via withdrawal of doxycycline; loss of mutant NRAS expression resulted in a rapid and complete tumor regression – the desired state. By contrast, pharmacological inhibition of the RAS downstream effector MEK, given at maximal tolerated doses, was unable to phenocopy this response, failing to induce tumor regression and achieving only transient growth arrest. Global transcriptional and targeted proteomic profiling, validated by tumor histopathological analyses, illuminated the differential molecular effects of genetic NRAS extinction versus pharmacological MEK inhibition. Not surprisingly, mutant NRAS proved to be a tumor-maintenance target as its activities were required for both growth and survival of an established tumor and its genetic extinction resulted in complete tumor regression. However, a potent and specific Mek inhibitor (hereafter MEKi) was only able to block the survival signal by mutant NRAS, consequently activating apoptosis, but failed to inhibit the proliferation signal. Therefore, drug(s) that can inhibit proliferation represented a possible rational combination with MEKi against mutant NRAS. To that end, network modeling was applied to discover key regulators underpinning these molecular differences in an unbiased manner. Here, we utilized TRAP (4), a network model built upon thousands of published transcriptomic profiles in mouse to establish key regulators of transcriptomic features. TRAP identified Cdk4 as the top regulator responsible for the differential molecular states between genetic extinction of NRAS and pharmacological inhibition of Mek in the iNRAS system (Fig. 1b). Indeed, combination of Mek inhibitor with a CDK4/6 inhibitor resulted in tumor regression in both mouse models and human xenografts. The synergy arose from a complementary induction of both apoptosis (via MEKi) and cell cycle arrest (via CDK4/6i). Importantly, CDK4/6i monotherapy induced only cell cycle arrest and not apoptosis – unlike the majority of targeted anti-proliferative drugs (5) – suggesting that systems biology approaches can potentially molecularly distinguish between related drug targets. Finally, in contrast to MEKi alone, transcriptomic analysis confirmed that the drug combination more closely mimicked NRAS extinction in terms of the target gene modulation (LNK and LC, unpublished data).
Figure 1. Overview of the benchmark-driven analysis and the creation of co-target databases.
(A) Schematic: comparison of a pharmacological agent showing a partial response (Perturbation A, red) against a genetically-engineered model designed to generate a complete response (Perturbation C, purple). Identifying what is missed by A allows for the identification of a co-target drug B (blue) that, together with drug A, approximates the complete response seen in C. (B) Specific results of the iNRAS study (4). The large circles represent information obtained from expression and protein arrays. The RAS extinction space (purple) identified RAS-specific targets (blue) that were “missed” by the MEK inhibitor space (red). The TRAP network algorithm identified CDK4 as a co-target regulator. The MEK plus CDK4/6 inhibitor combination thus complementarily targets the RAS extinction space. (C) A generalized schematic. Oncogene extinction or tumor suppressor gene reactivation (i.e. Perturbation C, purple) generates a complete response. A pharmacological agent (i.e. Perturbation A, red) is compared, and a key regulator(s) of pathways “missed” by A is identified via a systems biology approach. A co-target drug B against this regulator (blue), when combined with A, would target the full space (purple). (D) Triplets of information are generated by the comparative analysis. Comprehensive characterization of the baseline tumor allows for correlations to be drawn between key baseline elements (e.g. mutations and epimutations) and the observed treatment responses. (E) A hypothetical database of genotype-phenotype correlations, matching baseline tumor characteristics to preclinically optimized drug combinations. Such databases would inform clinical trial designs and serve as a reference for genotyped patient biopsies to rapidly predict co-targets. NRAS-mutant melanoma is depicted here as an example.
In summary, by benchmarking against the “ideal or desired” state (i.e. complete tumor regression) achieved through genetic extinction of an un-druggable tumor maintenance target (i.e. mutant NRAS) in a model system, this data-driven approach identified two drugs (MEK and CDK4 inhibitors) that synergize in combination to approximate the therapeutic efficacy of NRAS inhibition. This general paradigm of benchmarking against a desired state to delineate potential synergistic combinations can be applied broadly (Fig. 1c). Indeed, various genetically engineered mouse models (GEMM) of oncogene addiction have already been established (6). The proof of concept example described above provides a potential path to define novel therapeutic strategies to target other "un-druggable" oncogenes (e.g. Myc). Taken a step further, one could envisage using a similar approach to inform on strategies to reactivate tumor suppressor functions; for example, multiple laboratories have generated mouse models (7) in which restoration of wild-type p53 can induce tumor regression. In addition, Scott Lowe and colleagues (8) have described a novel in vivo shRNA system capable of systematically generating such reactivation mice, using reversible knockdown of endogenous tumor suppressor genes to model robust tumor regression. These models can be used to define the “ideal states” against which rational drugs such as Prima-1 can be benchmarked against to discover co-targets/therapies.
Data-driven approaches are complementary
As a prime example of an undruggable target, the exact signaling circuits and functions of RAS - 30 years after its discovery as an oncogene – are coming into focus but much remains enigmatic. Pathway diagrams usually involve an elaborate network of various molecules, with only the Raf-Mek-Erk and PI3K-Akt-mTor arms generally remaining constant. Often included are pathways regulated by the small GTPases Ral, Rac, and Rho. Additional complexity is observed further downstream of the MAPK and PI3K pathways with respect to downstream effectors and their own complicated networks. The difficulties are compounded by the emergent recognition of differences between K-, N-, and H-RAS, by cell-type and developmental stage-specific activities, and by mutations in other genes that can directly or indirectly impact on RAS activity. Since clinically effective pharmacological approaches to directly target RAS have so far been unsuccessful, comprehensive analyses of Ras/MAPK signaling are required to define vulnerabilities that are shared across tumor types.
Here, we highlight three recent studies that exemplify data-driven approaches to rationally designing anti-MAPK drug combinations. First, David Stern and colleagues conducted a large-scale combinatorial screen, testing over 7000 pairwise combinations of 40 drugs in 19 melanoma cell lines (9). Among a number of context-specific synergistic combinations, they identified simvastatin (an HMG-CoA reductase inhibitor) plus flavopiridol (a pan-CDK inhibitor) as highly efficacious in NRAS mutant lines both in vitro and in vivo. Second, Jefferey Engelman and colleagues performed an shRNA screen to identify genes that, when inhibited, could cooperate with MEKi to target KRAS mutant cell lines (10). They validated BCL-XL knockdown and its associated inhibitor, the BH3 mimetic ABT263, as synergistic with MEKi in vivo. Finally, Gary Johnson and colleagues described a multi-platform approach to study kinome reprogramming of breast cancers in response to MEK inhibition (11). Multiple oncogenic kinases including Axl, and Erbb and Pdgf family members were upregulated due to feedback mechanisms, and combination of MEKi with the multi-kinase inhibitor sorafenib was synergistic in vivo.
From a clinical perspective, this wealth of novel potential combination therapies represents a potential boon, and the next step is to thoroughly and systematically evaluate them pre-clinically to bring forward into the clinic the most promising ones, linked to strong science. Indeed, the complexity and diversity of the results highlight the challenges in identifying and pursuing the most optimal strategies. In vivo data reveal that signaling downstream of pharmacological MEK inhibition is highly complex: even with a conservative cutoff, significant changes are seen in over 1500 genes (4), many of which are known oncogenes or tumor suppressors. Even smaller-scale targeted assays such as phospho-RTK or pathway-specific protein arrays identify multiple potential co-targeting/combination drug targets, as described above and elsewhere (11, 12). A popular analogy is that of a game of "Whack-a-Mole," where hitting one oncogenic target causes others to pop up and compensate (12). For example, in colorectal cancer, BRAF inhibitor monotherapy is rendered ineffective in part through compensatory activation of EGFR (13). But with hundreds of potential moles popping up after MEK inhibition, the options are either to pick the most likely targets based on prior knowledge combined with functional screening (e.g. EGFR, Akt), or selecting the most statistically significant targets for screening and validation. The strength of the benchmarking approach is that the tumor regression phenotype offers the “ideal” state against which these otherwise noisy expression and post-translational adaptive changes can be compared, in an unbiased manner. This approach informs whether a given gene may be a likely co-target or not and prioritizes the most promising strategies for pre-clinical validation.
For example, we and others have noted that phospho-Akt is enhanced upon MEK inhibition (14). Given the known oncogenic role of Akt, especially in Ras signaling, it is reasonable to assume that this activation might represent a rational point of co-extinction. Indeed, Mek plus Akt/Pi3k inhibition has been shown to be effective pre-clinically (15). However, in the iNRAS model system, extinguishing NRAS genetically also results in an increase in Akt phosphorylation after four days, despite the full engagement of tumor regression (4). This observation suggests that Akt activation within days of MEK inhibition may not necessarily be compensatory, at least in this short-term experimental time frame. Thus, one must be cautious in interpreting the upregulation of oncogenes in response to therapy as necessarily compensatory; monitoring tumor responses provides one way to help sort the true compensatory mechanisms (i.e.: those seen after pharmacological treatment, but not in the regression state) from red herrings (i.e.: those that are seen in both states, but which, by definition, do not rescue the regression state).
We note that immune therapies might also be amenable to such an approach. Data from several laboratories attest to the human relevance of GEMM immune reactions to therapy, including the influx of CD8+ T-cells seen in both a Braf;Pten melanoma model undergoing BRAF inhibition (16) and in human patients treated with BRAF inhibitors (17). Thus, one could envision benchmarking various immune therapies (e.g. anti-CTLA4, anti-PD-1) and determining whether mechanisms of immune suppression can be overcome via co-targeting approaches. Indeed, the concept of ICOS induction as boosting anti-CTLA4 therapy illustrates the types of synergistic pathways that might be uncovered (18). Overall, the different data-driven methods of therapy design complement one another, with the strength of the benchmarking approach lying in its ability to highlight unanticipated pathways most relevant to the complete regression phenotype.
Signaling Activity is not binary
One other aspect of the iNRAS study supports the perspective that oncogene activity is not binary, but rather acts like a rheostat to activate various phenotypes at different thresholds of activity (4, 19). Incomplete extinction of NRAS, at an intermediate timepoint of doxycycline withdrawal, recapitulates MEK inhibition in both the quantitative activity of ERK effectors (e.g.: Fosl1, Maff) and in the phenotypic outputs of activated apoptosis but not cell cycle arrest. In other words, one reason that MEK inhibitors are inefficient in the setting of NRAS-mutant melanoma is that it does not completely or adequately inhibit the flux through downstream signaling pathways, likely due to its biophysical characteristics as a small molecular inhibitor with inherent affinity, PK/PD etc. issues. Insights from other mouse models also weigh in on this aspect of RAS biology. Mariano Barbacid and colleagues have demonstrated that Mek1/2 and Erk1/2 pairs are each necessary for Ras-induced cancer, by genetically ablating each kinase pair specifically in KRAS-positive cells of a mouse model of lung cancer (20). These data imply that, if Mek and/or Erk inhibitors could be specifically targeted to a cancer and made sufficiently potent to completely inhibit the activities of their respective targets, they might be as effective as genetic ablation. Practically speaking, this level of specificity is challenging in the face of likely toxicities incurred by over-inhibiting a single pathway, although one hopes that it could be achieved by disruptive delivery methods such as nano-targeted delivery of highly potent and efficient inhibitors. In the current context, the rheostat nature of oncogene signaling could be taken advantage of, as the de-coupling of therapy-pertinent phenotypes (e.g., apoptosis and proliferation) provides an opportunity to orthogonally target non-redundant pathways. Such complementary drug combinations would therefore avoid over-inhibiting a single pathway (e.g., RAS-MEK-ERK) and might spare a patient the associated toxicity.
Bridging to the Clinic
In the data-driven example above, the desired phenotype and associated molecular state is provided by a tractable, genetically-engineered mouse system that approximates, but does not fully capture the complexities of human cancer. How then can we build on this initial step forward to developing a systematic approach that benchmarks against a desired molecular state for designing clinically effective combinations? We suggest here that a comprehensive, coordinated (i.e., consortium-type) effort to apply the benchmark-driven paradigm to an array of preclinical models of cancer can serve as bridges towards the clinic. Such an effort would enlist not only inducible GEMM mouse models, but would also employ patient-derived, in vitro/ex vivo engineered xenograft benchmarks to identify optimal drug combinations across a wide array of tumors and their diverse genotypes. Such a preclinical knowledge base of optimized drug combinations would provide a valuable resource for launching rationally designed clinical trials that employ target engagement biomarkers and real time genomic profiles before and during treatment.
In achieving the goal of establishing such a knowledge base, several important aspects must be taken into consideration. First is accounting for the broad mutational heterogeneity within each cancer subtype. For example, even among the 25% of melanomas driven by NRAS mutations, other significant and often mutually exclusive mutations, such as in CDKN2A, p53, RAC1, PPP6C, and NF1 (21), could differentially determine MEKi co-targets for a given tumor. Indeed, preliminary data suggests that CDKN2A and p53 mutants may respond differently to MEK and/or CDK4/6 inhibition in their ability to induce cell cycle arrest (LNK, unpublished). Fortunately, the benchmark approach precisely allows one the flexibility to adapt the design of combinations to address such mutational heterogeneity. Specifically, the output consists of unbiased triplets of information that compare tumor characteristics across baseline, treatment, and regression (desired phenotype) cohorts (Fig. 1d). Importantly, characterization of the baseline tumor, including whole-exome and RNA transcriptome sequencing as well as proteome analyses, establishes a way to model mutational heterogeneity in the laboratory, allowing for matching baseline genotypes to optimal drug combinations (Fig. 1e).
To further enhance human relevance in this effort, we propose that systems involving human cell lines and ex vivo patient-derived xenografts be included: by carefully selecting a diverse set of cell lines and patient-derived cultures to represent genetic heterogeneity, a first view of potential broad drug combination categories can be defined. These can then be expanded for refinement, iteratively. To model oncogene extinction or tumor suppressor reactivation, inducible shRNA or expression plasmids could be used to define the molecular state corresponding to a desired efficacy, such as tumor regression. Ultimately, correlations between the known baseline genotypes of tumors and their optimal drug combination will allow us to generate a database of therapies tailored to specific mutational profiles both within and across tumor types (Fig. 2b).
A second aspect to bridging towards the clinic is in anticipating how to consistently model complete regression in the laboratory. For example, not all KRAS-mutant cancers rely on KRAS: when KRAS expression is extinguished by potent shRNAs in human lung and pancreatic cell lines, nearly half of the lines tested were found to be KRAS-independent, as shRNA knockdown failed to induce apoptosis (22), reflecting a differential dependence on mutant KRAS signaling to maintain growth. This spectrum of responses may be generally true for most if not all oncogenes and tumor suppressors. In such cases, it will be necessary to identify and overcome the underlying independence-conferring mechanism(s) to properly generate complete regression phenotypes. In the above referenced KRAS study, EMT was identified as a pervasive signature throughout the KRAS-independent cell lines (22). Indeed, many recent publications have highlighted a similar correlation between EMT status and a wide range of drug resistances. However, it is likely that this represents only one of many mechanisms of oncogene independence. Overall, resensitizing agents would be necessary in oncogene-independent samples for generating the tumor regression state, additionally acting as a co-targeting agent.
In summary, accounting for mutational heterogeneity and consistent benchmarking will require a large-scale investment that a consortial effort can create, with the capacity to potentially provide a clinic-ready, biologically significant knowledge base of tumor genotypes and their matched, optimized drug combination therapies.
Personalizing therapeutic combinations in the Clinic
The co-clinical trial concept represents an attractive transition from laboratory to clinic (23). In such a trial, biopsies of a patient’s tumor are passaged through preclinical model systems (e.g. mouse) and the response of the tumor to the same targeted therapy being administered to the patient is monitored. From there, genomic analyses – sequencing and expression profiling – can make several important determinations. First, expansion and drug dosing of patient-derived xenografts will provide models that can be quickly cross-referenced to the preclinical knowledge base to predict rational co-targeting strategies. Second, such a co-clinical trial can provide early predictions of response by allowing for a rapid molecular assessment of response and/or biological biomarkers. Third, the expression assessment can determine whether the patient xenograft is behaving as predicted by the preclinical knowledgebase, or whether novel combinations need to be quickly uncovered. In this last regard, it should also be possible to establish patient-derived cell cultures ex vivo, transfect with the appropriate vector (oncogene knockdown or tumor suppressor expression), and perform xenografting to establish a truly personalized drug combination by benchmarking. Ultimately, the co-clinical information will be routed back to engage the optimal course of treatment for the patients.
The ultimate clinical application of this approach to designing personalized combination therapy is to be able to monitor, non-invasively and in real-time, the response of a patient and his/her tumor to a targeted pharmaceutical compared to a benchmark model. Guided by the preceding preclinical and co-clinical data, physicians would be able make informed decisions about efficacious, rational drug combinations in both predictive and reactive manners. The ability to longitudinally monitor important phenotypes (such as various hallmarks of cancer in a tumor) in response to a treatment would provide the rational basis to customize additional drug(s) in combination for each individual patient. For example, if proliferation and apoptosis could be monitored in a clinically relevant time frame, one can imagine treating an NRAS mutant melanoma patient first with a MEK inhibitor, followed by evidence-based selection of a CDK4 inhibitor as a combination, based on the observation that proliferation was not inhibited; alternatively, it is also possible that, in a separate patient with different genomic make up of a NRAS mutant melanoma, MEK inhibitor is able to shut off proliferation but apoptosis is not activated; in such a patient, the personalized combination could be an AKT or BCL2 inhibitor. The capability to longitudinally monitor a patient’s response as envisioned means a data-driven approach to customized combinations can address both the passive and adaptive mechanisms of drug resistance to initial therapies in a timely manner. Although not yet available today, we believe such real-time clinical monitoring is on the horizon given the rapidly advancing technologies with imaging and non-invasive biomarker discovery.
Conclusions and Prospectus
The advent of targeted therapies has allowed for tailoring clinical care based on driver genetic lesions in a patient’s DNA; for example: vemurafenib in melanoma targets mutant Braf, imatinib mesylate in various cancers target Abl or Pdgfr translocations or Kit amplifications, and trastuzumab in breast cancer targets Her2 amplifications. However, the full promise of such targeted therapy has not yet been realized in part due to the powerful adaptive responses of the tumors. The benchmark-driven paradigm to defining co-targeting strategies has the potential to design optimized combinations that are customized to individual patients. Leveraging model systems to establish benchmarks for tumor regression or other desired states in a preclinical or co-clinical trial context offers a first step toward translating such a paradigm – and associated in vivo systems biology approaches – to clinical application. This will require a coordinated and systematic effort among preclinical modelers and biologists, genomic scientists, and computational modelers, similar to those represented by NCI’s MMHCC (Mouse Model of Human Cancer Consortium), TCGA (The Cancer Genome Atlas) and ICBP (Integrative Cancer Biology Program). Further, such an effort will require a new model of cooperation and collaboration between academia and industry to ensure that these studies are conducted with industry standards and that results are clinically actionable and available as a public resource. Finally, this must be accompanied by educational efforts so that our clinical colleagues can optimize their care of patients and, importantly, feedback to teach and improve these exploratory studies conducted by the research communities.
Acknowledgements
The authors thank Ron DePinho and Carlo Toniatti for critical readings of the manuscript.
Grant Support
This review was supported in part by: an American Cancer Society Postdoctoral Fellowship (117842-PF-09-261-01-TBG) for LNK, and a Mouse Models of Human Cancer Consortium UO1 grant (CA141508-05), an NIH RO1 grant (CA93947-10), and a Cancer Prevention & Research Institute of Texas grant (R1204).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Lee Michael J, Ye Albert S, Gardino Alexandra K, Heijink Anne M, Sorger Peter K, MacBeath G, et al. Sequential Application of Anticancer Drugs Enhances Cell Death by Rewiring Apoptotic Signaling Networks. Cell. 2012;149:780–794. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoeberl B, Pace EA, Fitzgerald JB, Harms BD, Xu L, Nie L, et al. Therapeutically Targeting ErbB3: A Key Node in Ligand-Induced Activation of the ErbB Receptor-PI3K Axis. Sci Signal. 2009;2:ra31-. doi: 10.1126/scisignal.2000352. [DOI] [PubMed] [Google Scholar]
- 3.Huang PH, Mukasa A, Bonavia R, Flynn RA, Brewer ZE, Cavenee WK, et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proceedings of the National Academy of Sciences. 2007;104:12867–12872. doi: 10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwong LN, Costello JC, Liu H, Jiang S, Helms TL, Langsdorf AE, et al. Oncogenic NRAS signaling differentially regulates survival and proliferation in melanoma. Nat Med. 2012;18:1503–1510. doi: 10.1038/nm.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10:825–841. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- 6.Felsher DW. Oncogene Addiction versus Oncogene Amnesia: Perhaps More than Just a Bad Habit? Cancer research. 2008;68:3081–3086. doi: 10.1158/0008-5472.CAN-07-5832. [DOI] [PubMed] [Google Scholar]
- 7.Kastan MB. Wild-Type p53: Tumors Can't Stand It. Cell. 2007;128:837–840. doi: 10.1016/j.cell.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Premsrirut PK, Dow LE, Kim SY, Camiolo M, Malone CD, Miething C, et al. A rapid and scalable system for studying gene function in mice using conditional RNA interference. Cell. 2011;145:145–158. doi: 10.1016/j.cell.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Held MA, Langdon CG, Platt JT, Graham-Steed T, Liu Z, Chakraborty A, et al. Genotype-Selective Combination Therapies for Melanoma Identified by High-Throughput Drug Screening. Cancer Discovery. 2012 doi: 10.1158/2159-8290.CD-12-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corcoran RB, Cheng KA, Hata AN, Faber AC, Ebi H, Coffee EM, et al. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer cell. 2013;23:121–128. doi: 10.1016/j.ccr.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan JS, Whittle MC, Nakamura K, Abell AN, Midland AA, Zawistowski JS, et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell. 2012;149:307–321. doi: 10.1016/j.cell.2012.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science (New York, NY. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 13.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 14.Gopal YN, Deng W, Woodman SE, Komurov K, Ram P, Smith PD, et al. Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244 (ARRY-142886) in Braf-mutant human cutaneous melanoma cells. Cancer research. 2010;70:8736–8747. doi: 10.1158/0008-5472.CAN-10-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posch C, Moslehi H, Feeney L, Green GA, Ebaee A, Feichtenschlager V, et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proceedings of the National Academy of Sciences. 2013;110:4015–4020. doi: 10.1073/pnas.1216013110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooijkaas AI, Gadiot J, van der Valk M, Mooi WJ, Blank CU. Targeting BRAFV600E in an Inducible Murine Model of Melanoma. Am J Pathol. 2012;181:785–794. doi: 10.1016/j.ajpath.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF Inhibition Is Associated with Enhanced Melanoma Antigen Expression and a More Favorable Tumor Microenvironment in Patients with Metastatic Melanoma. Clinical Cancer Research. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu T, He Q, Sharma P. The ICOS/ICOSL Pathway Is Required for Optimal Antitumor Responses Mediated by Anti–CTLA-4 Therapy. Cancer research. 2011;71:5445–5454. doi: 10.1158/0008-5472.CAN-11-1138. [DOI] [PubMed] [Google Scholar]
- 19.Albeck John G, Mills Gordon B, Brugge Joan S. Frequency-Modulated Pulses of ERK Activity Transmit Quantitative Proliferation Signals. Mol Cell. 2013;49:249–261. doi: 10.1016/j.molcel.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blasco Rafael B, Francoz S, Santamaría D, Cañamero M, Dubus P, Charron J, et al. c-Raf, but Not B-Raf, Is Essential for Development of K-Ras Oncogene-Driven Non-Small Cell Lung Carcinoma. Cancer cell. 2011;19:652–663. doi: 10.1016/j.ccr.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, et al. A Gene Expression Signature Associated with“K-Ras Addiction” Reveals Regulators of EMT and Tumor Cell Survival. Cancer cell. 2009;15:489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh M, Lima A, Molina R, Hamilton P, Clermont AC, Devasthali V, et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat Biotech. 2010;28:585–593. doi: 10.1038/nbt.1640. [DOI] [PubMed] [Google Scholar]