Abstract
An effective immune response has the potential for breast cancer sterilization with marked reduction in the potential for disease relapse. Adaptive Type I immune cells uniquely have the capability of (1) cytotoxic T-cell activation and proliferation until all antigen expressing cells are eradicated, (2) the ability to traverse endothelial barriers to penetrate tumor deposits wherever they occur, and (3) immunologic memory which allows the persistence of destructive immunity over the years it may take for breast cancer micrometastases to become clinically evident. Numerous recent investigations suggest that some breast cancers stimulate the type of immunity that results in a decreased risk of relapse. Moreover, the endogenous Type I tumor microenvironment or Type I immunity induced by drugs or biologic agents, may improve response to standard therapies further lowering the probability of disease recurrence.
Keywords: adaptive immunity, Type I, immune modulation, immune signatures, breast cancer, micrometastases
Introduction
The immune microenvironment in most cancers is a balance of immune cells mediating tissue destruction and immune cells working to prevent that destruction. Adaptive immunity, which defines an immune response that requires antigen specific recognition of the tumor, is the primary mode by which cancer can be identified and destroyed by the immune system. A tissue destructive environment is supported by Type I immunity; CD4+ T-cells that secrete cytokines such as interferon (IFN)-gamma (g), tumor necrosis factor (TNF)- alpha (a), and interleukin (IL)-2 and CD8+ cytotoxic T-cells (CTL) that are potentiated by those cytokines (reviewed in (1)). Tissue destructive inflammation must be modulated. A Type II immune environment consisting of CD4+ T-cells that secrete cytokines such as IL-4, IL-6, and IL-10 limits the acute inflammatory response and prevents the elaboration of CTL. These cytokines enhance the proliferation of B-cells and a subsequent antibody response (1). Cytokines secreted by CD4+ T-cells and antigen presenting cells (APC) mediate a profound effect on the functioning of all immune cells in the tumor microenvironment. T-cells recognize antigen that has processed and presented by APC. APC present in a Type I environment are poised to give a “danger” signal to the T-cells activating them in a manner that would elicit CTL while the same cells in a Type II environment would present antigen in with minimal co-stimulation resulting in a limited immune response.
Breast cancers have been shown to be infiltrated with diverse populations of immune system cells, as assessed by either genomic signatures or immunohistochemistry, and these infiltrates appear to be associated with disease outcomes. For example, one group of investigators, evaluating over 100 breast cancers, observed that a signature which favored genes encoding proteins supporting a Th1/CTL phenotype identified patients with favorable outcomes whereas Th2/B-cell related genes were more likely to occur in patients with HER-/ER- disease (2). In a recent analysis of over 1200 breast cancer cases, high levels of CTL (CD8+ T-cells) and low levels of T-regulatory cells (Treg), known to secrete IL-10 and TGF-beta (b) which support a Type II environment, defined the less aggressive molecular subtypes of breast cancer (3). In contrast, high levels of Treg in the tumor with a paucity of CD8+ T-cells was significantly more likely to occur in HER2+ or basal-like rather than in luminal breast cancers. The understanding that the endogenous T-cell infiltration of a tumor that occurs during breast cancer pathogenesis can impact survival sets the stage for the question; can immunity to breast cancer eliminate residual micrometastases?
The immune microenvironment in breast cancer may predict clinical outcome and enhance the anti-tumor effects of certain chemotherapies
Using techniques that are described elsewhere in this issue (4), several prognostic gene signatures have been identified in breast cancer and many of them combine elements of both immunity and cell proliferation (5–7). Although a composite of both inflammation and proliferation, the immune signature component is often a statistically dominant element in predicting favorable prognosis. A recently published analysis of a data set of almost 2000 breast cancers identified 3 distinct immune related gene groups which all predicted metastasis-free survival; a T-cell/natural killer cell (NK) cluster, an antigen presenting cell (APC) cluster, and a B-cell cluster (7). All of these genes are associated with the adaptive immune response suggesting immune recognition of antigenic proteins expressed by the tumor. In essence, these signatures developing only in certain patients may represent self-immunization by exposure to antigens in the tumor in an immune microenvironment which would support an adaptive immune response. This analysis demonstrated other key findings; prognosis was dependent on the interplay of the immune clusters and cell proliferation and the most significant clinical benefit was found in the minority of tumors that demonstrated expression of all 3 adaptive immune clusters (7). While there appear to be distinct differences in immune infiltrates between breast cancer subtypes, the etiology of these differences is unknown. Further, the methods of assessing immune infiltrates in breast cancer are quite varied and due to these differences individual studies are not comparable to each other. Several groups are in the process of validating an “immune score” in a number of tissue types. There are a well-defined group of immune cells (e.g. CD8+ cytotoxic T-cells (CTL), memory T-cells (Type I immunity)) and chemokines (e.g. CXCL9, CCL2) whose presence, number, density, and location in some tumors have been shown to be an independent predictor of prognosis (8).
There is evidence that the presence of a Type I immune microenvironment may improve response to standard therapies (reviewed in (9)). One of the largest studies evaluated over 1000 breast cancer patients receiving neoadjuvant chemotherapy in the multicenter, randomized Phase III GeparDuo trial designed to evaluate pathologic complete response (pCR), a surrogate for clinical outcome, in patients receiving two different chemotherapy regimens (10). The overall pCR in the trial was about 13%, however, those patients whose tumors contained significant numbers of intratumoral lymphocytes, deemed “lymphocyte predominant”, achieved a pCR rate of greater than 40% in both training and validation cohorts (p<0.01). In contrast, those patients with low or no infiltrating lymphocytes had the lowest pCR, less than 10%. The presence of high density intratumoral lymphocytes was an independent predictor of pCR regardless of breast cancer subtype, grade, or chemotherapy regimen. A detailed subset analysis of 134 tumors demonstrated that the lymphocyte infiltration was of a Type I phenotype with significant levels of T-cell activation markers and chemokine gene expression, e.g. CXCL9, by PCR (10). Why would pre-existing Type I immunity influence response to chemotherapy? The rationale is multi-factorial. First, the dominant cytokines released in the Type I environment, IFN-g and TNF-a, have long been known to have direct anti-tumor effects inducing both apoptosis and growth arrest potentially making chemotherapy more effective (11). In addition, it has been shown that certain chemotherapies may have potent immune stimulating effects further enhancing the endogenous immune response, e.g. cyclophosphamide induced reduction of Treg (12), doxorubicin mediated down-regulation of PD-L1 on breast tumors (13), or taxane-mediated enhancement of T-cell egress into the tumor, just to name but a few examples (14) (Table 1). Further, all these elements occur while cancer cell death induced by both CTL and chemotherapy is resulting in increased shed antigen further driving the destructive immune response.
Table 1.
Immune effects of chemotherapy
The understanding that standard chemotherapies have the potential to stimulate the immune system is an area of recent investigation. Evidence suggests that the doses used, administration schedule employed, and combination of therapies used can significantly impact immune effects. For example, lower doses of cyclophosphamide and methotrexate have been associated with immune stimulation rather than the higher does currently used in standard treatment (9, 12). Moreover, many commonly used prevention agents such as aromatase inhibitors and bisphosphonates have also been shown to have beneficial effects on immune stimulation (15). Efforts to define how to best harness the immune impact of common chemotherapies used for the treatment of breast cancer need to be explored further in the clinic. Optimal doses and schedules may work to further augment or modulate the existing immune environment in breast cancer to enhance the elimination of micrometastasis.
Monoclonal antibody therapy both modulates the immune microenvironment and actively immunizes breast cancer patients
Monoclonal antibodies (mAb) are biologic agents that are uniquely poised to both modulate the tumor microenvironment to Type I as well as facilitate active immunization (16). In breast cancer, although the anti-tumor and adjuvant effects of the most commonly used mAb, trastuzumab, have been associated with the agent’s inhibition of signaling pathways, increasing evidence underscores immune effects as a dominant mechanism of action (Fig. 1). Mab have multiple modes of stimulating immunity dependent on the isotype of the antibody (16). Through engagement of the Fc receptor, mAb may activate the complement cascade of enzymes resulting in direct cell death by lysis (Fig. 1A). A more common function of many antibodies used for cancer therapy is activation of antibody dependent cell mediated cytotoxicity via stimulation of NK or other immune system cells (Fig. 1B). NK cells secrete high levels of IFN-g and have the capability of directly killing tumors that have escaped the immune system via down-regulation of MHC molecules on their cell surface preventing immune recognition. Fc receptor ligation by APC recruits the cells to the tumor. The IFN-g secreted by the mAb activated NK cells enhances the function of APC (Fig. 1C) increasing local antigen processing and presentation of antigen to T-cells resulting in self-vaccination of the patient with endogenous tumor antigens.
Figure 1. Mab both increase Type I cells in the breast cancer microenvironment and “auto vaccinate”.
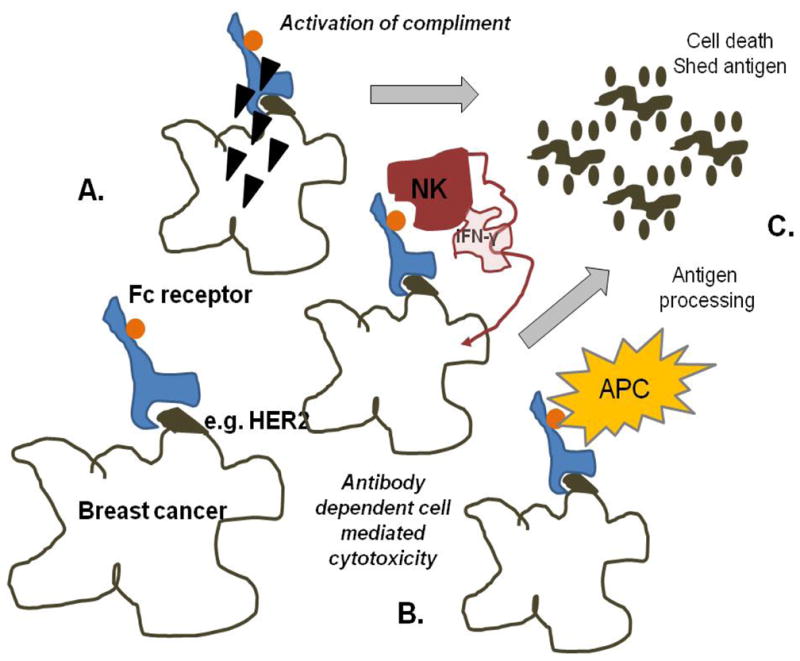
(A) Dependent on the antibody isotype, mAb can fix complement resulting in direct lysis of cells to which the antibody binds. (B) Many mAb mediate ADCC, the recruitment of NK and other immune system cells to the tumor via Fc receptor binding. NK cells are a major source of secreted IFN-g which activates APC. (C) Antigen shed by tumor lysis is taken up by the activated APC and presented to T-cells, stimulating Th1 and CTL in the IFN-g rich microenvironment.
Estimating the relative contribution of either signaling inhibition or immune activation to the anti-tumor response of breast cancer patients who have received trastuzumab is difficult. In animal models, however, the therapeutic effect of the antibody is clearly dependent on the generation of an endogenous immune response (17). Studies in an immune competent rodent model demonstrated a trastuzumab-like antibody could induce tumor regression, but regression was dependent on the presence of CD8+ T-cells. In addition, after successful treatment, not only did animals develop high levels of tumor specific Th1, the rodents could resist a tumor challenge and remain disease free even after injection of a large bolus of viable breast cancer cells. This last observation indicates that mAb treatment induced immunologic memory; the type of adaptive immunity needed for control of micrometastases (17).
What is the evidence that trastuzumab vaccinates breast cancer patients? In a study of 27 HER2+ breast cancer patients treated with trastuzumab, 29% had evidence of HER2 specific antibody immunity prior to treatment (18). After therapy, 56% of patients developed or augmented HER2 specific antibodies (p<0.001). Similarly, 60% of patients tested demonstrated significant increases in HER2 specific Th1 in the peripheral blood with trastuzumab treatment. T-cell immunity persisted even after trastuzumab therapy had ended. There is also evidence that trastuzumab significantly modulates systemic immune suppression and the tumor microenvironment. Evaluating a limited number of HER2+ patients undergoing trastuzumab monotherapy or trastuzumab in combination with chemotherapy, either for metastatic disease or in the adjuvant setting, treatment significantly decreased the number of circulating peripheral blood Treg compared to pre-treatment levels (p=0.04) (19). A small neoadjuvant clinical trial in HER2+ patients treated with trastuzumab and chemotherapy demonstrated a significant increase in the numbers of NK cells and T-cells expressing the activation marker Granzyme B in the patients’ breast cancers as compared with tumors derived from a HER2- control population receiving similar chemotherapy without trastuzumab (20). Granzyme B is associated with cell lytic capability for both NK cells and T-cells. These data suggest that trastuzumab, via NK activation, can elicit and sustain adaptive anti-HER2 immunity which has the potential to persist long after therapy has ended.
A provocative study indicates the possibility of the manipulation of the immune score by trastuzumab treatment. As described elsewhere in this issue, the neoadjuvant setting provides an excellent venue for studying specific drug effects on the tumor (21). In over 100 consecutively treated HER2+ patients receiving neoadjuvant therapy, with anthracyclines (in cases pre-dating trastuzumab) or trastuzumab and a taxane, investigators noted that Th1 tumor infiltrates could be elicited with treatment (22). The investigators measured the number of T-bet+ T-cells, which define Th1, in the tumors of patients before and after therapy by immunohistochemical staining. Before treatment, no matter what the group, T-bet+ positive cells in the tumor were rare. After treatment 50% of patients who received a trastuzumab containing regimen demonstrated infiltrating T-bet+ T-cells as compared to only 16% of patients treated with anthracyclins alone (p<0.01). Only in the trastuzumab treated patients was the induction of T-bet+ tumor infiltrating T-cells after neoadjuvant chemotherapy independently associated with an improved relapse free survival (p=0.04, HR 4.76, CI: 1.07-20). Studies such as these beg the question as to whether treatment regimens optimized for both cytotoxic and immune stimulatory effects could significantly alter outcome in patients whose immune score was not optimal at the time of diagnosis.
Antigen specific vaccination to induce a Type I microenvironment
Investigations described above suggest that in a minority of breast cancer patients, at the time of diagnosis, the immune system is already actively attempting to control further disease progression and that combination chemotherapy may be most effective in these patients. Furthermore, agents that are designed as immune modulators have the potential to improve the patient’s tumor immune score to one that is also more responsive to treatment resulting in a decreased risk of relapse. Active immunization with antigen specific vaccines attempts to bypass the immune score and directly stimulate CD4+ Th1 and or CD8+ tumor specific T-cells which will be primed outside the immune suppressive tumor microenvironment (Fig. 2A). The vaccine induced antigen specific T-cells would then traffic to tumor, secreting the appropriate Type I cytokines, to begin to reverse a potential Th2 environmental phenotype to Th1 while lysing tumor (Fig. 2B). The ability to achieve this reversal would be dependent on the potency of the vaccine to elicit high levels of Type I T-cells capable of homing to the cancer.
Figure 2. Vaccine induced tumor T-cell infiltrates.
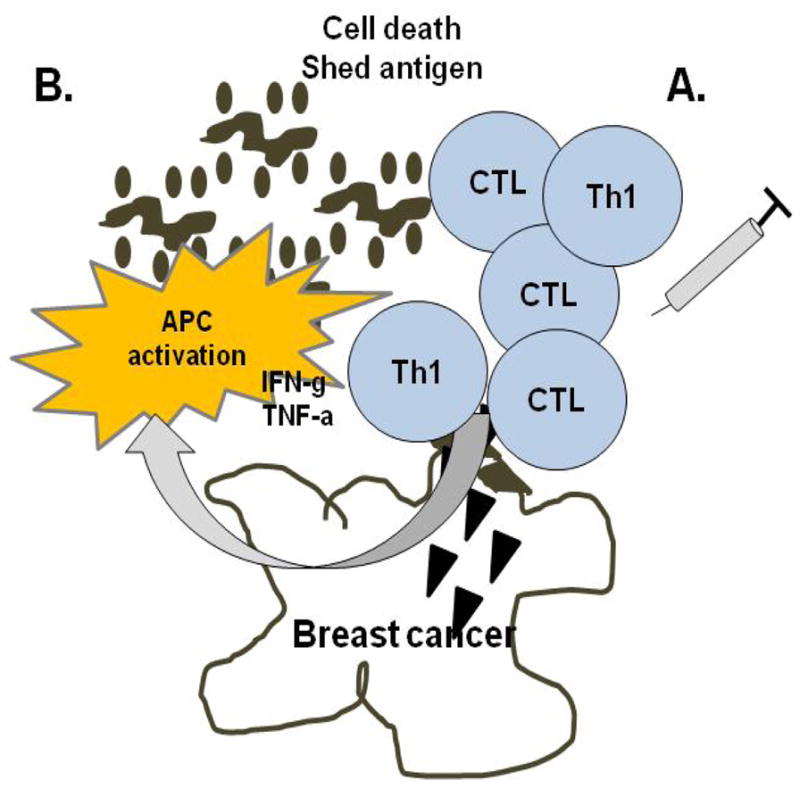
(A) Active antigen specific immunization aims to induce Type I adaptive immunity outside the immune suppressive tumor microenvironment. (B) T-cells, homing to tumor and secreting Type I cytokines, both modulate the tumor microenvironment by activating APC and directly lysing tumor cells. The resultant shed antigen, now in a Type I environment, further drives destructive immunity.
The development of a breast cancer vaccine to prevent disease recurrence though the elimination of micrometastases has been impeded by (1) a lack of well-defined immunogenic proteins expressed in breast cancer that could be targeted by vaccination, (2) the need for immunogenic adjuvants to use in combination with vaccination to elicit Type I immunity, and (3) the focus of testing vaccines first in metastatic disease to attempt to induce tumor regression as a proof of vaccine efficacy. The most clinically advanced breast cancer vaccines are those that target the HER2 protein in patients with HER2+ breast lesions. Although potentially useful in only a subpopulation of breast cancer patients, immunization against HER2 in a variety of settings has allowed intensive investigation as to the utility of active immunization alone or in combination with standard therapies in controlling micrometastases. HER2 vaccination in Stage IV breast cancer patients with minimal residual disease has been shown to modulate the tumor microenvironment by increasing CTL and decreasing serum levels of TGF-beta (23). Immunity persisted after immunization for as long as a year of follow-up which suggested memory responses were generated (23).
The majority of breast cancer vaccine studies have been performed in advanced stage patients, however, increasingly vaccine trials are moving to the adjuvant setting with the goal of preventing disease recurrence. Several Phase I and II studies have been performed in the adjuvant setting in high risk Stage I and II breast cancer patients using a HER2 specific synthetic peptide vaccine designed to elicit CTL. A recently published pooled analysis of several studies made a series of observations (24); clinical benefit was more likely in patients with HER2 1 or 2+ vs. 3+ disease, in patients with lower grade tumors, and in patients who received optimal doses of the vaccine rather than any dose reduction. The finding that HER2low expressers may have more benefit than HER2high expressers is in contradiction to mAb therapy where benefit is associated with higher levels of expression. Perhaps those with HER2 3+ disease are already optimally self-immunized. A study of endogenous HER2 immunity in over 100 HER2+ breast cancer patients demonstrated that the level of overexpression of the protein was an independent predictor of immunity (p=0.016) (25). The incidence and magnitude of both HER2 specific antibodies and T-cells was significantly greater in patients whose tumors were 3+ overexpressors than 1–2+. Alternatively, HER2 may be such a potent antigen that mechanisms of self-regulation dampen immunity in the presence of excessive antigen inducing functional anergy. Potentially, a combination of vaccination with checkpoint blockade antibodies such as anti-PD-1 could allow the development of effective immunity which would prevent disease relapse.
A final example is the use of HER vaccination in the treatment of DCIS (26). Immunization of 13 patients with a HER2 peptide vaccine prior to definitive surgery resulted in the generation of high levels of both CD4+ and CD8+ HER2 specific T-cells. Over 60% of evaluable patients demonstrated markedly reduced HER2 expression in their resected surgical specimens suggesting immune eradication of targeted disease. The studies described above, immunizing against the same antigen, using similar vaccine strategies but in very different patient populations indicates there may be utility in the use of vaccination for the prevention of breast cancer relapse at any stage of the disease. A recent search of clinicaltrials.gov using the key words breast cancer vaccine, Phase II, and Phase III resulted in over 60 studies, evaluating a variety of vaccine approaches and antigens, in nearly every stage of breast cancer.
Conclusions
There are many methods available to elicit immunity in breast cancer patients and potentially modulate the tumor immune environment to synergize with standard therapies (Fig. 3). As the immune response is initiated with chemotherapy and environmental immune modulation (e.g. HER2, EGFR, IGFR mAb) further immune boosting might be achieved via polyantigen specific vaccines and immune stimulatory cytokines. Finally, novel checkpoint blockade approaches could be used to ensure the immune response will evolve to complete eradication of tumor. Checkpoint blockade has not been studied as a clinical modality for the treatment of breast cancer, but increasing evidence suggests that it should be. We have all the tools needed to begin to answer the question; can immunity to breast cancer eliminate residual micrometastases?
Figure 3. An approach to immune eradication of residual micrometastases in breast cancer.
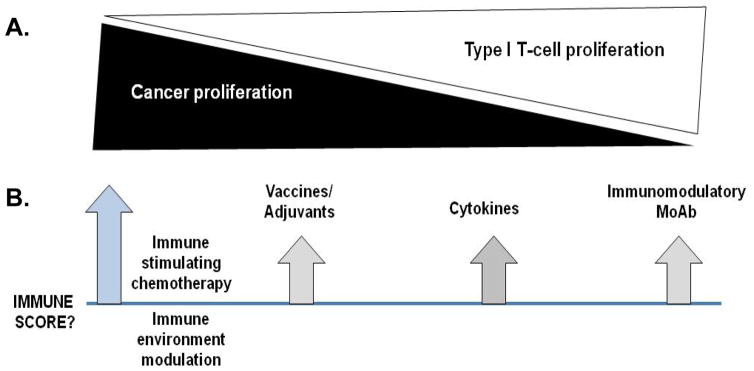
(A) Based on prognostic signatures, consideration must be given to both controlling breast cancer growth as well as increasing Type 1 T-cells in the tumor environment. (B) Based on the initial immune score at the time of diagnosis multi-modality treatment approaches with concurrent and/or sequential strategies may be able to achieve the optimal immune score in all breast cancer patients rather than in just a minority.
Acknowledgments
Grant support: Mary L Disis was supported by the Athena Distinguished Professor of Breast Cancer Research, U01CA141539, and DOD W81XWH-11-1-0760. SES was supported by T32CA009515-28
Footnotes
COI: Mary L Disis is an inventor on patents held by University of Washington that may pertain to data presented in this manuscript.
References
- 1.Disis ML. Immune regulation of cancer. J Clin Oncol. 2010;28:4531–8. doi: 10.1200/JCO.2009.27.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kristensen VN, Vaske CJ, Ursini-Siegel J, Van Loo P, Nordgard SH, Sachidanandam R, et al. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci U S A. 2012;109:2802–7. doi: 10.1073/pnas.1108781108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, et al. CD8(+) cytotoxic T cell and FOXP3(+) regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130:645–55. doi: 10.1007/s10549-011-1647-3. [DOI] [PubMed] [Google Scholar]
- 4.Tabchy A, Ma CX, Bose R, Ellis MJ. Incorporating genomics into breast cancer clinical trials and care. Clin Cancer Res. 2013;19:xx–xx. doi: 10.1158/1078-0432.CCR-13-0837. [DOI] [PubMed] [Google Scholar]
- 5.Rody A, Karn T, Liedtke C, Pusztai L, Ruckhaeberle E, Hanker L, et al. A clinically relevant gene signature in triple negative and basal-like breast cancer. Breast Cancer Research: BCR. 2011;13:R97. doi: 10.1186/bcr3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staaf J, Ringner M, Vallon-Christersson J, Jonsson G, Bendahl PO, Holm K, et al. Identification of subtypes in human epidermal growth factor receptor 2--positive breast cancer reveals a gene signature prognostic of outcome. J Clin Oncol. 2010;28:1813–20. doi: 10.1200/JCO.2009.22.8775. [DOI] [PubMed] [Google Scholar]
- 7.Nagalla S, Chou JW, Willingham MC, Ruiz J, Vaughn JP, Dubey P, et al. Interactions between immunity, proliferation and molecular subtype in breast cancer prognosis. Genome Biology. 2013;14:R34. doi: 10.1186/gb-2013-14-4-r34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 9.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–33. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 10.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 11.Liu F, Hu X, Zimmerman M, Waller JL, Wu P, Hayes-Jordan A, et al. TNFalpha cooperates with IFN-gamma to repress Bcl-xL expression to sensitize metastatic colon carcinoma cells to TRAIL-mediated apoptosis. PLoS One. 2011;6:e16241. doi: 10.1371/journal.pone.0016241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 2012;72:3439–44. doi: 10.1158/0008-5472.CAN-11-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Molecular Immunology. 2008;45:1470–6. doi: 10.1016/j.molimm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111–24. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzbani E, Inatsuka C, Lu H, Disis ML. The invisible arm of immunity in common cancer chemoprevention agents. Cancer Prev Res (Phila) 2013;6:764–73. doi: 10.1158/1940-6207.CAPR-13-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–9. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–70. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor C, Hershman D, Shah N, Suciu-Foca N, Petrylak DP, Taub R, et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res. 2007;13:5133–43. doi: 10.1158/1078-0432.CCR-07-0507. [DOI] [PubMed] [Google Scholar]
- 19.Horlock C, Stott B, Dyson PJ, Morishita M, Coombes RC, Savage P, et al. The effects of trastuzumab on the CD4+CD25+FoxP3+ and CD4+IL17A+ T-cell axis in patients with breast cancer. Brit J Cancer. 2009;100:1061–7. doi: 10.1038/sj.bjc.6604963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Brit J Cancer. 2006;94:259–67. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bardia A, Baselga J. Neoadjuvant therapy as a platform for drug development and approval in breast cancer. Clin Cancer Res. 2013;19:xx–xx. doi: 10.1158/1078-0432.CCR-13-0916. [DOI] [PubMed] [Google Scholar]
- 22.Ladoire S, Arnould L, Mignot G, Apetoh L, Rebe C, Martin F, et al. T-bet expression in intratumoral lymphoid structures after neoadjuvant trastuzumab plus docetaxel for HER2-overexpressing breast carcinoma predicts survival. Brit J Cancer. 2011;105:366–71. doi: 10.1038/bjc.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, Lu H, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27:4685–92. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittendorf EA, Clifton GT, Holmes JP, Clive KS, Patil R, Benavides LC, et al. Clinical trial results of the HER-2/neu (E75) vaccine to prevent breast cancer recurrence in high-risk patients: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer. 2012;118:2594–602. doi: 10.1002/cncr.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodell V, Waisman J, Salazar LG, de la Rosa C, Link J, Coveler AL, et al. Level of HER-2/neu protein expression in breast cancer may affect the development of endogenous HER-2/neu-specific immunity. Mol Cancer Ther. 2008;7:449–54. doi: 10.1158/1535-7163.MCT-07-0386. [DOI] [PubMed] [Google Scholar]
- 26.Czerniecki BJ, Koski GK, Koldovsky U, Xu S, Cohen PA, Mick R, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67:1842–52. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Rao GS, Groh V, Spies T, Gattuso P, Kaufman HL, et al. Major histocompatibility complex class I-related chain A/B (MICA/B) expression in tumor tissue and serum of pancreatic cancer: role of uric acid accumulation in gemcitabine-induced MICA/B expression. BMC Cancer. 2011;11:194. doi: 10.1186/1471-2407-11-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wehner R, Bitterlich A, Meyer N, Kloß A, Schäkel K, Bachmann M, et al. Impact of chemotherapeutic agents on the immunostimulatory properties of human 6-sulfo LacNAc+ (slan) dendritic cells. Int J Cancer. 2013;132:1351–9. doi: 10.1002/ijc.27786. [DOI] [PubMed] [Google Scholar]


