Abstract
Aims
The number of procedures involving upgrade or revision of cardiac implantable electronic devices (CIEDs) is increasing and the risks of adding additional leads are significant. Central venous occlusion in patients with pre-existing devices is often asymptomatic and optimal management of such patients in need of device revision/upgrade is not clear. We sought to assess our use of laser lead extraction in overcoming venous obstruction.
Methods and results
Patients in need of device upgrade/revision underwent pre-procedure venography to assess venous patency. In patients with venous occlusion or stenosis severe enough to preclude passage of a hydrophilic guide wire, laser lead extraction with retention of the outer sheath in the vasculature was performed with the aim of maintaining a patent channel through which new leads could be implanted. Data were recorded on a dedicated database and patient outcomes were assessed. Between July 2004 and April 2012, laser lead extractions were performed in 71 patients scheduled for device upgrade/revision who had occluded or functionally obstructed venous anatomy. New leads were successfully implanted across the obstruction in 67 (94%) cases. There were two major complications (infection) and four minor complications with no peri-procedural mortality. Device follow-up was satisfactory in 65 (92%) cases with mean follow-up up to 26 ± 19 months.
Conclusion
Laser lead extraction is a safe and effective option when managing patients with central venous obstruction in need of CIED revision or upgrade.
Keywords: Laser lead extraction, Venous obstruction, Pacemaker, Implantable cardiac defibrillator, Cardiac resynchronization therapy
What's new?
Laser extraction to overcome venous obstruction in cases of cardiac implantable electronic device upgrade/revision has previously been described in small series with single operators.
This is the first report on wider applicability of the technique and highlights relative safety and success of the approach.
Medium-term device follow-up in patients undergoing the procedure is satisfactory in a vast majority of cases.
Introduction
Cardiac implantable electronic device (CIED) implantation continues to grow with permanent pacemaker implantation in the USA increasing by 56% between 1993 and 2009.1 In Europe, there has been a 75 and 115% increase in implantable cardiac defibrillator (ICD) and cardiac resynchronization therapy pacemaker/defibrillator (CRT-P/CRT-D) implants between 2004 and 2008.2 As a result of increasing indications for complex pacing, there is an expanding need for patients with pre-existing devices to undergo system revision due to lead failure or upgrade to allow ICD and/or CRT implant. In a recent European survey, 28% of CRT implants were performed in patients with pre-existing devices,3 and recent lead advisories have necessitated an increase in lead extraction cases.4 The combination of such factors means that the need for system revision and upgrade is likely to increase in the future. A major obstacle to device revision and/or upgrade is the presence of asymptomatic ipsilateral central venous obstruction. Older reports suggested obstruction occurred in up to 50% of cases5–8 with symptoms affecting only 1–3%,9 but more contemporary reports have demonstrated a lower incidence closer to 30%.10,11 This perhaps reflects improvements in lead design over time, but it remains clear that asymptomatic venous obstruction is not infrequently encountered. Various strategies to overcome venous occlusion exist including contralateral lead or device implantation, venoplasty, and surgical epicardial lead implantation. The addition of extra pacemaker and/or ICD leads is not without its drawbacks. In a recent prospective US registry of pacemaker/ICD generator replacements, the need for an additional lead increased the rate of major complications from 4.0 to 15.3%.12 Lead extraction may be an alternative option to overcome this problem,13 but it is not without risk and this should be weighed against the benefits of removing any leads and also the likelihood of symptom recurrence in cases where there are symptoms of venous occlusion. The practice of laser lead extraction to re-canalize venous obstruction has previously been described in a limited number of patients and the suitability of this technique on a larger scale has not been reported.14,15 We describe our experience using laser lead extraction to overcome venous occlusion to enable ipsilateral device revision and/or upgrade.
Methods
Guy's and St Thomas' Hospital is a quaternary referral centre for CIED extraction. All patients undergoing device extractions are prospectively entered into a computer database recording patient demographics, comorbidities, device and lead type, reason for extraction, procedural success, and complications. Complications are classified according to those recommended in the Heart Rhythm Society Consensus Report on Transvenous Lead Extraction.13 Deaths were adjudicated by senior cardiologists within our department, none of whom had any input in the current study. Patients from this database were included in the present study if the indication for lead extraction was to upgrade or revise an existing device in the presence of ipsilateral venous obstruction. In all cases, venous obstruction was identified on the basis of venography performed (either in the radiology department at least 1 day prior to the procedure or in the cardiac catheter laboratory on the day of the procedure) prior to each procedure to ascertain the patency of the venous system (Figure 1). All patients provided written informed consent and all procedures were performed in our cardiac catheter laboratory under general anaesthesia. In patients with ipsilateral venous occlusion, or stenoses severe enough such that a hydrophilic guide wire would not cross the obstruction, laser lead extraction with retention of the outer sheath in the vascular tree was performed. In cases of device upgrade, any non-functional leads were removed; if there was no non-functional lead (for example, upgrade from dual-chamber pacing to CRT-P), then the atrial lead was extracted with an attempt to preserve the existing right ventricular (RV) pacing lead. Any redundant leads were also extracted. In cases of device upgrade, where there was also a failed lead in need of extraction, the failed lead was extracted and two hydrophilic wires passed through the outer sheath of the laser. This allowed the passage of two introducer sheaths. The process of laser extraction has previously been described.16 After opening the existing generator pocket and disconnecting the leads from the generator, the suture sleeve of the lead being extracted was released. The proximal end of the lead was cut and a locking stylet (Liberator Beacon, Cook Medical Inc. or LLD EZ lead locking device; Spectranetics) was advanced as distally as possible and locked in place. A silk suture was then tied to the lead to aid traction and this was fed through the laser sheath (SLS II Excimer Laser Sheaths; Spectranetics) with the outer sheath also in position. Both sheaths were advanced over the lead and the inner sheath advanced until resistance was met at which point laser energy was applied in short pulses to free the lead body from the surrounding vessel wall or cardiac musculature (CVX-300 Excimer Laser System; Spectranetics). Lasing was performed as necessary, up to the final 1 cm proximal to the distal electrode, and the lead was freed with counter-traction using the outer sheath. The lead and inner sheath were removed in their entirety leaving the outer sheath in place thereby maintaining vascular access. A venogram was performed to ensure that the sheath remained in the vascular/cardiac space and a hydrophilic wire (Terumo) passed through the outer sheath when intravascular position was confirmed. A long haemostatic sheath(s) was then placed to allow lead implantation in a standard fashion (see Figure 2).
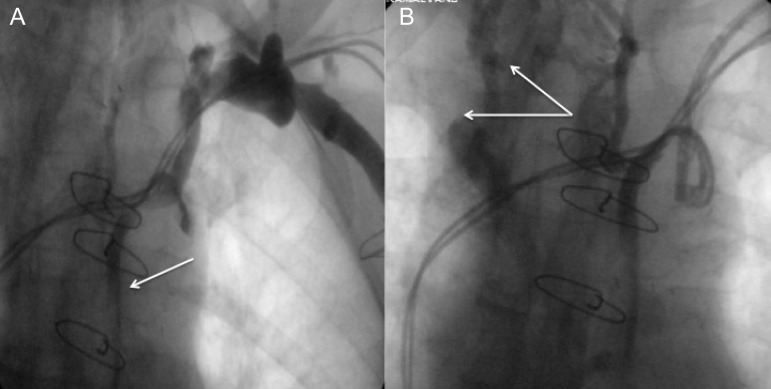
Figure 1.
Venogram of a representative patient with complete occlusion. (A) Suggestive of collateral formation (white arrow), which is confirmed with more medial panning during the venogram (white arrows; B).
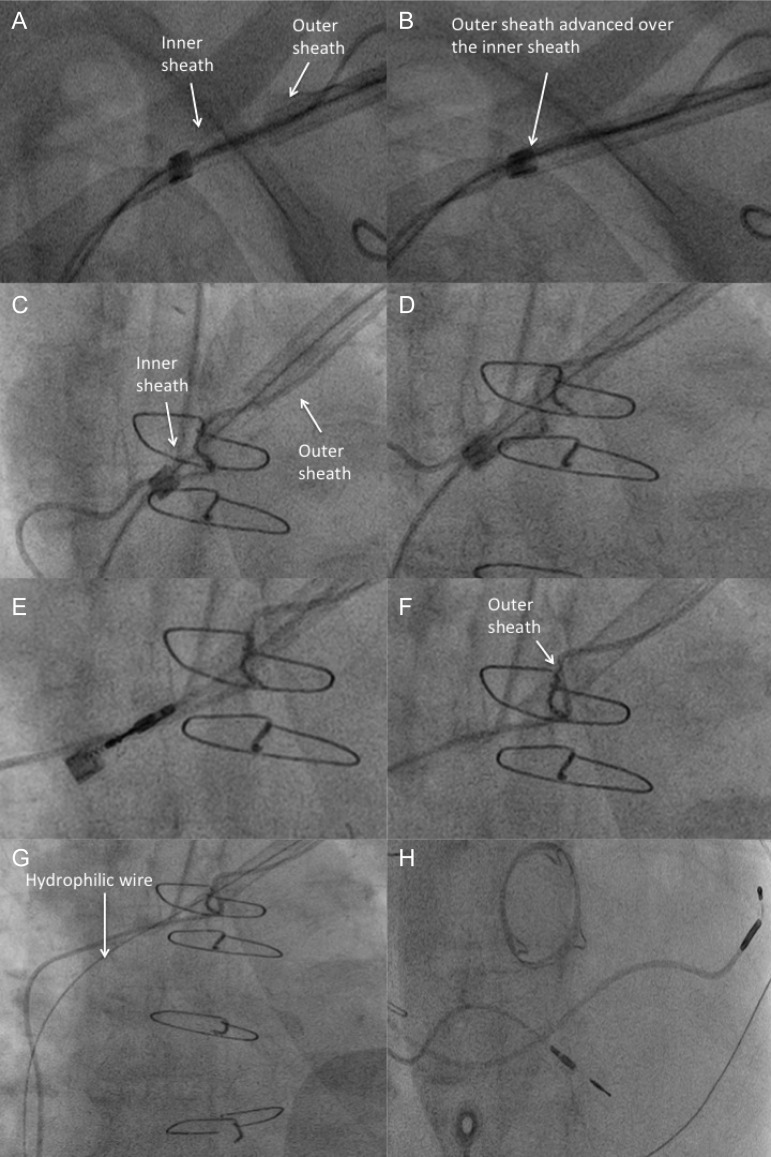
Figure 2.
Sequential images of the steps taken to successfully extract an atrial pace/sense (patient in atrial fibrillation and in need of upgrade to a biventricular pacemaker), with maintenance of venous access across the level of occlusion. (A) The inner sheath is advanced with the outer sheath trailing. As demonstrated in this case, the laser is often required to overcome fibrosis at the clavicular level before the outer sheath can be advanced (B). (C) Further lasing upto and beyond the point of occlusion with passage of the outer sheath beyond the occlusion aided by rotational torque (D). (E) The atrial lead is successfully extracted in its entirety using a combination of forward pressure on the outer sheath and manual traction on a locking stylet. (F) The inner sheath and lead are removed leaving the outer sheath in the vascular space just beyond the level of occlusion. (G) A hydrophilic wire is then passed through the outer sheath allowing passage of an introducer sheath and subsequent LV lead placement (H).
Results
Between July 2004 and April 2012, 242 upgrade/revision procedures were performed and of these 71 (29%) were performed in patients with occluded or severely stenosed venous anatomy. Complete ipsilateral occlusion was present in 52 of 71 patients (73%) in this series. The remainder had severe stensoses that did not allow passage of hydrophilic guide wires and/or introducer sheaths and this was taken as indicating functional obstruction. The vast majority of obstructions were identified in the subclavian vein (67 of 71) with the remainder being at the junction of the subclavian vein with the superior vena cava (SVC) (see Figure 1).
Patient characteristics
Baseline patient characteristics are shown in Table 1. The mean age was 62 ± 15 years and 78% of patients were male. Twenty-nine (41%) patients had a history of ischaemic heart disease and 19 (27%) had prior cardiac surgery (coronary artery bypass surgery or valve surgery). The mean left ventricular (LV) ejection fraction was 38% (derived from 2D echocardiography using Simpson's biplane method). Three patients (4%) had symptoms of venous occlusion (arm swelling and pain on the side of device implant).
Table 1.
Patient characteristics
| Characteristic | |
|---|---|
| Age (years) | 62 ± 15 |
| Gender, n (%) | Male 55 (77) |
| Female 16 (23) | |
| Ejection fraction (%) | 38 ± 15 |
| Comorbidities | |
| IHD, n (%) | 29 (41) |
| Cardiac surgery, n (%) | 19 (27) |
| Diabetes, n (%) | 5 (7) |
| Hypertension, n (%) | 16 (23) |
| PVD, n (%) | 3 (4) |
| Stroke, n (%) | 5 (7) |
| COPD, n (%) | 14 (20) |
| CKD, n (%) | 12 (17) |
IHD, ischaemic heart disease; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease.
Device and lead characteristics
Most extractions were performed in patients with existing dual-chamber pacemakers, ICDs, or CRT-Ds (24, 37, and 27%, respectively). In total, 129 leads were extracted from 71 patients (see Table 2). Of these, 40 (31%) were passive fixation right atrial (RA) or RV pacemaker leads, 33 (26%) were active fixation RA or RV pacing leads, 41 (31%) were single- or dual-coil defibrillator leads, and 15 (12%) were coronary sinus LV pacing leads, which were extracted due to sub-optimal function (phrenic nerve capture, or sub-optimal lead positioning). The mean duration of lead implant was 80 ± 62 months. The commonest indications for extraction were lead malfunction (56%) and need for device upgrade (40%). The remaining extractions were performed for symptomatic venous occlusion (4%).
Table 2.
Device characteristics
| Characteristic | |
|---|---|
| Device, n (%) | |
| VVI | 2 (3) |
| DDD | 17 (24) |
| ICD | 26 (37) |
| CRT-P | 7 (9) |
| CRT-D | 19 (27) |
| Indication for extraction, n (%) | |
| Lead failure | 40 (56) |
| Upgrade | 28 (40) |
| Symptoms | 3 (4) |
| Nature of upgrade, n (%) | |
| PPM to ICD | 8 (29) |
| PPM to CRT-P | 9 (32) |
| ICD to CRT-D | 11 (39) |
| Number of leads extracted, n (%) | |
| Total | 129 |
| Passive A/V | 40 (31) |
| Active A/V | 33 (26) |
| ICD | 41 (31) |
| CS | 15 (12) |
| Mean duration of lead implant (months) | 80 ± 62 |
VVI, single-chamber pacemaker (lead in RV); DDD, dual-chamber pacemaker; ICD, implantable cardioverter defibrillator; CRT-P, cardiac resynchronization therapy-pacemaker; CRT-D, cardiac resynchronization therapy-defibrillator; PPM, permanent pacemaker; passive A/V, passive fixation atrial/ventricular leads; active A/V, active fixation atrial/ventricular leads; and CS, coronary sinus.
Procedural characteristics
All 129 leads were successfully extracted in their entirety. It was necessary to snare leads from the femoral venous approach in two cases following unsuccessful laser extraction when the lead fragmented despite the obstruction being crossed. New leads were successfully re-implanted via the laser sheath across the venous obstruction/stenosis in 67 (94%) cases. In four cases, the laser sheath was unable to pass the obstruction due to intense fibrosis/calcification, and in three of these cases a subclavian vein puncture medial to the venous occlusion was performed to obtain venous access and successfully place the lead. In one case, a transvenous lead could not be placed and an epicardial LV pacing lead was surgically implanted during a later procedure. Mean procedure time was 116 ± 32 min. Mean fluoroscopic screening time was 16 ± 13 min and mean radiation dose was 837 ± 1269 cGycm2. There were two major complications (3%) that were both cases of infection of a previously sterile site, and four (6%) minor complications (ipsilateral pneumothorax, phrenic nerve palsy, acute renal failure, and pocket haematoma). There were no peri-procedural deaths (Table 3).
Table 3.
Procedural characteristics and outcome
| Characteristic | |
|---|---|
| Obstruction crossed, n (%) | 67 (94) |
| Lead successfully extracted via laser sheath, n (%) | 69 (97) |
| Transvenous lead successfully sited | 70 (99) |
| Procedural time (min) | 116 ± 32 |
| Fluoroscopy time (min) | 16 ± 13 |
| Radiation dose (cGycm2) | 837 ± 1269 |
| Complications | |
| Major, n (%) | 2 (3) |
| Minor, n (%) | 4 (5) |
| 30-day mortality, n (%) | 2 (3) |
Device follow-up
Medium- to long-term device follow-up was available in 65 of 71 (92%) cases with a mean follow-up of 26 ± 19 months. Two patients died within 30 days of the procedure. One patient developed non-procedure-related sepsis (urinary sepsis following urethral instrumentation for urinary retention) while recuperating from their procedure. The second death occurred in a patient with severe heart failure (New York Heart Association IV) with a pre-existing CRT-D that had a non-functioning LV pacing lead. The patient had refractory hypotension and hyponatraemia limiting the use of medical therapy and a decision was made to attempt revision of the CRT-D device as a procedure of last resort. The patient died in hospital from end-stage heart failure despite successful lead extraction. Both deaths were adjudicated by senior physicians within our department, who were blinded to the interventions in the current study. It was necessary to further revise CIED implants following the index procedure in four (6%) cases. Two cases required re-intervention for defibrillator leads with diminished sensing, one patient developed phrenic nerve capture from their LV pacing lead, and one patient deteriorated with a sub-optimal LV lead position that required revision.
Discussion
We have described our experience using laser lead extraction to overcome ipsilateral venous obstruction in patients undergoing device revision and/or upgrade. To date, this is the largest series of cases, where laser lead extraction has been used to overcome venous obstruction thereby allowing ipsilateral lead revision or device upgrade. We have shown that the technique is feasible in the vast majority of cases. In 100% of cases, the targeted lead(s) were completely extracted, and in 94% re-canalization of the obstructed vein allowed successful lead implantation via the laser sheath. In the remaining patients transvenous lead implantation was successful on the ipsilateral side with a medial puncture and in only one case did the patient need a further procedure (surgical LV lead implant) to achieve implant success. Our complication rate is low even in a cohort of patients with reduced LV systolic function and significant comorbidities.
The Heart Rhythm Society (HRS) consensus statement on lead extraction in relation to venous obstruction states that lead removal is a class I recommendation in patients with ipsilateral venous occlusion preventing additional lead placement when there is a contraindication for using the contralateral side (e.g. contralateral atrioventricular fistula, shunt or vascular access port, and mastectomy). Lead removal in patients with ipsilateral venous occlusion and no contraindication to using the contralateral side is a class IIa indication.13 Obstruction and thrombosis of the access vein after implantation of permanent pacing and defibrillator leads are well described.10 The reported incidence of asymptomatic cases is up to 50% in older series and up to 30% in more contemporary series. Symptomatic occlusion occurs in 1–3%, thus highlighting the importance of developing strategies for overcoming such obstacles at the time of device upgrade or lead revision. In our study, the finding of venous obstruction precluding device revision or upgrade was 29% in keeping with recent reports. In such cases, it may be possible to obtain venous access with a de novo puncture, but it is often not possible to advance introducer sheaths across a very tight stenosis and there is the added risk of increased lead–lead interaction. These issues are avoided by using the technique described in this report and we have demonstrated that the procedure can be performed safely.
Laser technology is increasingly being used to facilitate lead extraction. In the PLEXES trial, use of the laser sheath resulted in complete removal of 94% of leads compared with only 64% where non-laser tools were used (predominantly telescoping sheaths).17 More recently, Bordachar et al.18 showed that laser extraction results in shorter procedures with lower radiation exposure to operators when compared with femoral snare techniques. The LExICon study was an observational retrospective study of 1449 consecutive laser lead extractions in North America and confirmed high success rates and low complication rates, particularly in high volume centres.19 The use of the laser sheath to overcome venous obstruction was first described by Bracke et al.14 in three patients, where the laser was only used up to the point of obstruction and the lead left in situ. The largest previous series by Gula et al.15 included 18 patients, where laser lead extraction was performed to facilitate system upgrade in the presence of central venous occlusion. In both earlier reports, the technique was successful in all cases and there were no procedural complications. Our current study provides an expanded assessment of the technique in a larger number of patients. Procedures were performed by three experienced operators in a single centre, each with extensive experience in laser lead extraction. Our patients tended to have a longer duration of lead implant (80 ± 62 vs. 70.8 ± 43.5 months in the series of Gula et al.). In addition, our cohort of patients is typical of those in whom these procedures are performed, namely depressed ejection fraction and attendant comorbidities. Another key difference between the current report and the work of Gula et al. is the use of the outer sheath. In the earlier report no outer sheath was used. In a minority of cases, tissue build-up at the tip of the laser sheath prevented the withdrawal of the lead tip through the laser sheath. This necessitated extraction of a functional atrial lead to ensure maintenance of venous patency. Also, our study included patients requiring non-functional lead extraction rather than just device upgrade. In such cases, extraction may be preferable to adding extra leads. In our study, all leads were removed in their entirety, but it was necessary to snare two leads from the femoral venous route. Therefore, 98% of leads were removed using the laser sheath alone and in total, venous access was maintained with the outer sheath in 94% of cases. No leads were unintentionally damaged and it was not necessary to extract any extra leads. This is important as it may have implications in reducing the risk of the procedure. There are inherent risks in extracting leads, particularly from the thin-walled RA, and so if extraneous extraction can be avoided this is preferable. In the current study, laser lead extraction was unable to overcome the obstruction in four cases. Leads were successfully extracted in all cases after the laser sheath had successfully overcome fibrosis at the subclavicular level. There were two major procedural complications, both of which were cases of infection of a previously sterile site and this possibly reflects the added risk in performing upgrade or revision procedures. Of the four minor complications, none were specifically related to the use of the laser sheath to overcome the obstruction.
The alternative options available if ipsilateral venous occlusion is present include:
Insertion of new leads via the contralateral subclavian vein with either tunnelling of the leads across the sternum or abandonment of the pre-existing leads;
Venoplasty of the occluded vessels;
Alternative venous access;
Surgical epicardial lead implant.
Each of the techniques listed have their own specific drawbacks. The practice of adding leads is not without risk; in the REPLACE registry, there was a 15.3% major complication rate and a 1.1% 6-month mortality rate in patients undergoing generator change with a planned lead addition or revision.12 Multiple leads traversing the SVC can increase the risk of SVC syndrome.20,21 The HRS lead extraction consensus statement states that lead removal is reasonable in patients if CIED implantation would require more than four leads on one side or more than five leads through the SVC (Class IIA recommendation).13 The presence of redundant leads is also associated with increased risks of infection and erosion.21 Tunnelling across the sternum to the contralateral position of the existing generator potentially avoids the risk of SVC syndrome, but there is a heightened risk of lead erosion, patient discomfort, and bilateral occlusion. Venoplasty has been previously described, where a wire is passed through the occlusion, and balloon venoplasty performed to open a patent channel. The results of this procedure are encouraging with lead implantation possible in 96% of patients in a recent report.22 The technique may not be ideal in cases, where there is an indication for lead revision (such as in defibrillator lead failure), where extraction of the malfunctioning lead may be the preferred option. Lead malfunction was the primary indication for revision in 56% of our cases and the use of the laser sheath to overcome obstruction and remove the malfunctioning lead represents an attractive option in such cases. Use of other venous access sites, such as the internal jugular vein, with subsequent tunnelling of leads has been described; however lead erosion remains an issue.23 Surgical implantation of epicardial leads negates the need for a transvenous approach but requires a thoracotomy and lead failure is not uncommon.24
Study limitations
This study is a single-centre experience with experienced operators and therefore our results may not be widely applicable in less experienced centres. Lead extraction is not without risk and should only be performed in centres with experienced staff and necessary equipment/tools and with access to onsite emergency surgery. This study is retrospective and therefore the results must be viewed with some caution. A prospective study of laser extraction to treat venous obstruction would be necessary to extrapolate our results.
Conclusion
Our results suggest that laser lead extraction to overcome ipsilateral venous obstruction is effective and safe, and therefore represents an attractive approach to deal with device upgrade/lead revision in patients with obstructed or severely stenosed venous anatomy.
Conflict of interest: C.A.R. serves as a consultant to Spectranetics.
Funding
M.O.N. received research funding from St Jude Medical and Biosense Webster; J.G. received research funding from Biosense Webster and St Jude Medical; C.A.R. is a consultant to Spectranetics and received research funding from St Jude Medical, Boston Scientific, and Medtronic; M.S., M.A., A.S., Z.C., M.W., and C.B. have not received any funding.
References
- 1.Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J. Am Coll Cardiol. 2012;60:1540–5. doi: 10.1016/j.jacc.2012.07.017. doi:10.1016/j.jacc.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 2.van Veldhuisen DJ, Maass AH, Priori SG, Stolt P, van Gelder IC, Dickstein K, et al. Implementation of device therapy (cardiac resynchronization therapy and implantable cardioverter defibrillator) for patients with heart failure in Europe: changes from 2004 to 2008. Eur J Heart Fail. 2009;11:1143–51. doi: 10.1093/eurjhf/hfp149. doi:10.1093/eurjhf/hfp149. [DOI] [PubMed] [Google Scholar]
- 3.Dickstein K, Bogale N, Priori S, Auricchio A, Cleland JG, Gitt A, et al. The European cardiac resynchronization therapy survey. Eur Heart J. 2009;30:2450–60. doi: 10.1093/eurheartj/ehp359. doi:10.1093/eurheartj/ehp359. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Brumberg G, Rattan R, Jain S, Saba S. Class I recall of defibrillator leads: a comparison of the Sprint Fidelis and Riata families. Heart Rhythm. 2012;9:1251–5. doi: 10.1016/j.hrthm.2012.04.003. doi:10.1016/j.hrthm.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Spittell PC, Hayes DL. Venous complications after insertion of a transvenous pacemaker. Mayo Clin Proc. 1992;67:258–65. doi: 10.1016/s0025-6196(12)60103-7. doi:10.1016/S0025-6196(12)60103-7. [DOI] [PubMed] [Google Scholar]
- 6.Stoney WS, Addlestone RB, Alford WC, Jr, Burrus GR, Frist RA, Thomas CS., Jr The incidence of venous thrombosis following long-term transvenous pacing. Ann Thorac Surg. 1976;22:166–70. doi: 10.1016/s0003-4975(10)63980-x. doi:10.1016/S0003-4975(10)63980-X. [DOI] [PubMed] [Google Scholar]
- 7.Oginosawa Y, Abe H, Nakashima Y. The incidence and risk factors for venous obstruction after implantation of transvenous pacing leads. Pacing Clin Electrophysiol. 2002;25:1605–11. doi: 10.1046/j.1460-9592.2002.01605.x. doi:10.1046/j.1460-9592.2002.01605.x. [DOI] [PubMed] [Google Scholar]
- 8.Lickfett L, Bitzen A, Arepally A, Nasir K, Wolpert C, Jeong KM, et al. Incidence of venous obstruction following insertion of an implantable cardioverter defibrillator. A study of systematic contrast venography on patients presenting for their first elective ICD generator replacement. Europace. 2004;6:25–31. doi: 10.1016/j.eupc.2003.09.001. doi:10.1016/j.eupc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Williams EH, Tyers GF, Shaffer CW. Symptomatic deep venous thrombosis of the arm associated with permanent transvenous pacing electrodes. Chest. 1978;73:613–5. doi: 10.1378/chest.73.5.613. doi:10.1378/chest.73.5.613. [DOI] [PubMed] [Google Scholar]
- 10.Haghjoo M, Nikoo MH, Fazelifar AF, Alizadeh A, Emkanjoo Z, Sadr-Ameli MA. Predictors of venous obstruction following pacemaker or implantable cardioverter-defibrillator implantation: a contrast venographic study on 100 patients admitted for generator change, lead revision, or device upgrade. Europace. 2007;9:328–32. doi: 10.1093/europace/eum019. doi:10.1093/europace/eum019. [DOI] [PubMed] [Google Scholar]
- 11.Bulur S, Vural A, Yazici M, Ertas G, Ozhan H, Ural D. Incidence and predictors of subclavian vein obstruction following biventricular device implantation. J Interv Card Electrophysiol. 2010;29:199–202. doi: 10.1007/s10840-010-9516-2. doi:10.1007/s10840-010-9516-2. [DOI] [PubMed] [Google Scholar]
- 12.Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R, et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122:1553–61. doi: 10.1161/CIRCULATIONAHA.110.976076. doi:10.1161/CIRCULATIONAHA.110.976076. [DOI] [PubMed] [Google Scholar]
- 13.Wilkoff BL, Love CJ, Byrd CL, Bongiorni MG, Carrillo RG, Crossley GH, III, et al. Transvenous lead extraction: Heart Rhythm Society Expert Consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA) Heart Rhythm. 2009;6:1085–104. doi: 10.1016/j.hrthm.2009.05.020. doi:10.1016/j.hrthm.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Bracke FA, van Gelder LM, Sreeram N, Meijer A. Exchange of pacing or defibrillator leads following laser sheath extraction of non-functional leads in patients with ipsilateral obstructed venous access. Heart. 2000;83:E12. doi: 10.1136/heart.83.6.e12. doi:10.1136/heart.83.6.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gula LJ, Ames A, Woodburn A, Matkins J, McCormick M, Bell J, et al. Central venous occlusion is not an obstacle to device upgrade with the assistance of laser extraction. Pacing Clin Electrophysiol. 2005;28:661–6. doi: 10.1111/j.1540-8159.2005.00163.x. doi:10.1111/j.1540-8159.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan SC, Epstein LM. Initial experience with a laser sheath to extract chronic transvenous implantable cardioverter-defibrillator leads. Am J Cardiol. 1998;82:1293–5. doi: 10.1016/s0002-9149(98)00620-1. A10 doi:10.1016/S0002-9149(98)00620-1. [DOI] [PubMed] [Google Scholar]
- 17.Wilkoff BL, Byrd CL, Love CJ, Hayes DL, Sellers TD, Schaerf R, et al. Pacemaker lead extraction with the laser sheath: results of the pacing lead extraction with the excimer sheath (PLEXES) trial. J Am Coll Cardiol. 1999;33:1671–6. doi: 10.1016/s0735-1097(99)00074-1. doi:10.1016/S0735-1097(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 18.Bordachar P, Defaye P, Peyrouse E, Boveda S, Mokrani B, Marquie C, et al. Extraction of old pacemaker or cardioverter-defibrillator leads by laser sheath versus femoral approach. Circ Arrhythm Electrophysiol. 2010;3:319–23. doi: 10.1161/CIRCEP.109.933051. doi:10.1161/CIRCEP.109.933051. [DOI] [PubMed] [Google Scholar]
- 19.Wazni O, Epstein LM, Carrillo RG, Love C, Adler SW, Riggio DW, et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol. 2010;55:579–86. doi: 10.1016/j.jacc.2009.08.070. doi:10.1016/j.jacc.2009.08.070. [DOI] [PubMed] [Google Scholar]
- 20.Blackburn T, Dunn M. Pacemaker-induced superior vena cava syndrome: consideration of management. Am Heart J. 1988;116:893–6. doi: 10.1016/0002-8703(88)90361-4. doi:10.1016/0002-8703(88)90361-4. [DOI] [PubMed] [Google Scholar]
- 21.Suga C, Hayes DL, Hyberger LK, Lloyd MA. Is there an adverse outcome from abandoned pacing leads? J Interv Card Electrophysiol. 2000;4:493–9. doi: 10.1023/a:1009860514724. doi:10.1023/A:1009860514724. [DOI] [PubMed] [Google Scholar]
- 22.Maluenda G, Bustos F, Viganego F, Ben-Dor I, Hanna NN, Torguson R, et al. Endovascular recanalization of central venous access to allow for pacemaker implantation or upgrade. Cardiovasc Revasc Med. 2012;13:215–8. doi: 10.1016/j.carrev.2012.04.008. doi:10.1016/j.carrev.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Bosa-Ojeda F, Bethencourt-Munoz M, Vargas-Torres M, Lara-Paoron A, Rodriguez-Gonzalez A, Marrero-Rodriguez F. Upgrade of a pacemaker defibrillator to a biventricular device: the internal jugular vein approach in a case of bilateral subclavian veins occlusion. J Interv Card Electrophysiol. 2007;19:209–11. doi: 10.1007/s10840-007-9149-2. doi:10.1007/s10840-007-9149-2. [DOI] [PubMed] [Google Scholar]
- 24.Odim J, Suckow B, Saedi B, Laks H, Shannon K. Equivalent performance of epicardial versus endocardial permanent pacing in children: a single institution and manufacturer experience. Ann Thorac Surg. 2008;85:1412–6. doi: 10.1016/j.athoracsur.2007.12.075. doi:10.1016/j.athoracsur.2007.12.075. [DOI] [PubMed] [Google Scholar]