Abstract
Aims
We sought to describe the management of patients with atrial fibrillation (AF) in Europe after the release of the 2010 AF Guidelines of the European Society of Cardiology.
Methods and results
The PREFER in AF registry enrolled consecutive patients with AF from January 2012 to January 2013 in 461 centres in seven European countries. Seven thousand two hundred and forty-three evaluable patients were enrolled, aged 71.5 ± 11 years, 60.1% male, CHA2DS2VASc score 3.4 ± 1.8 (mean ± standard deviation). Thirty per cent patients had paroxysmal, 24.0% had persistent, 7.2% had long-standing persistent, and 38.8% had permanent AF. Oral anticoagulation was used in the majority of patients: 4799 patients (66.3%) received a vitamin K antagonist (VKA) as mono-therapy, 720 patients a combination of VKA and antiplatelet agents (9.9%), 442 patients (6.1%) a new oral anticoagulant drugs (NOAC). Antiplatelet agents alone were given to 808 patients (11.2%), no antithrombotic therapy to 474 patients (6.5%). Of 7034 evaluable patients, 5530 (78.6%) patients were adequately rate controlled (mean heart rate 60–100 bpm). Half of the patients (50.7%) received rhythm control therapy by electrical cardioversion (18.1%), pharmacological cardioversion (19.5%), antiarrhythmic drugs (amiodarone 24.1%, flecainide or propafenone 13.5%, sotalol 5.5%, dronedarone 4.0%), and catheter ablation (5.0%).
Conclusion
The management of AF patients in 2012 has adapted to recent evidence and guideline recommendations. Oral anticoagulant therapy with VKA (majority) or NOACs is given to over 80% of eligible patients, including those at risk for bleeding. Rate is often adequately controlled, and rhythm control therapy is widely used.
Keywords: Atrial fibrillation, Management, Registry, Anticoagulation, Stroke, Rhythm control, Catheter ablation, Antiarrhythmic drugs, Rate control, Guidelines, Adherence to guidelines
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, and its prevalence is likely to increase markedly in the next decades.1,2 Atrial fibrillation is a common cause of stroke, heart failure, hospitalizations, and death in affected patients.3 The management of AF has seen marked changes in recent years, such as the introduction of new anticoagulants, new antiarrhythmic drugs, and the wider availability of catheter ablation for AF.3 These changes resulted in new or updated management guidelines published in Europe, Canada, and the US.4 Clinical guidelines are not always fully implemented into practice,5–7 even though most recommendations for the management of AF are based on sound evidence, resulting in overlapping recommendations between different guidelines.
We therefore sought to describe the management of patients with AF in Europe after the publication of the guidelines of the European Society of Cardiology in 2010.8
Methods
The PREFER in AF registry (Prevention of thromboembolic events – European Registry in Atrial Fibrillation) was designed as a prospective observational study with a baseline visit at the time of patient enrolment (cross-sectional part) and a 1 year follow-up visit (prospective part). The baseline visit has been collected for all patients and the results of this part of the registry are presented in this manuscript, whereas the conduct of follow-up visits is still ongoing.
The aim of the registry was to gain detailed insight on the characteristics and management of patients with AF with focus on prevention of thromboembolic events, in particular stroke.
In the cross-sectional part, the specific objectives were the description of characteristics of AF patients in terms of key (socio-)demographic data, risk factors, method of diagnosis, treatment modalities, as well as the retrospective documentation of events related to AF and anticoagulation therapy within a 1 year period prior to inclusion. Furthermore, patient data on quality of life and treatment satisfaction were collected, which are not described in this manuscript.
We collected baseline data from patients in seven representative European countries (Austria, France, Germany, Italy, Spain, Switzerland, and the UK). For regional comparisons, Austria, Switzerland, and Germany were combined into one pre-specified region. Patients were included if they were at least 18 years of age, gave written informed consent for participation in the registry, and had a history of AF documented by electrocardiography or by an implanted pacemaker or defibrillator within the preceding 12 months. No explicit exclusion criteria were defined to avoid biased selection of patients and achieve a cohort close to ‘real life’. Furthermore, consecutive patients were included at each site in order to reduce selection bias. All data were captured through an electronic case report form including a wide range of plausibility checks for the entered variables. In addition, on-site source data verification was done or is currently conducted in approximately 5% of the sites. The study management was executed by Daiichi Sankyo Europe GmbH, Munich as sponsor via a contract research organization (SSS International Clinical Research GmbH, Munich, Germany). The study management was overseen by a scientific steering committee.
Statistical analysis
All variables collected in the eCRF at baseline and all derived parameters were used in the statistical analysis. For the analysis of the baseline data, only patients fulfilling the inclusion criteria were taken into account. Binary, categorical, and ordinal parameters were summarized by means of absolute and percentage numbers within the various categories. Numerical data were summarized by means of standard statistics (i.e. number of available data, number of missing data, mean, standard deviation, minimum, median, maximum, lower and upper quartile). For all analyses the term ‘Germany’ includes data from Austria and Switzerland. No formal statistical tests were performed. The statistical analysis was performed using SAS v. 9.2.
Results
Patient characteristics
Between January 2012 and January 2013, we enrolled 7243 evaluable patients (age 71.5 ± 11 years, 60.1% male) in 461 centres in France, Germany, Austria, Switzerland, Italy, Spain, and UK. Forty-two per cent of the patients were enrolled by office-based outpatient centres and 53% by hospital-based physicians, 89% of the patients were enrolled by cardiologists. Stroke risk was high (mean CHA2DS2VASc score of 3.4 ± 1.8, Table 1). Only 318 patients (4.8%) had none of the CHA2DS2VASc stroke risk factors. About one-third of the patients (30.0%) were enrolled with paroxysmal AF, one-third in persistent or long-standing persistent AF (24.0% persistent, 7.2% long-standing persistent), and 38.8% in permanent AF. The proportion of patients in permanent AF was higher in patients at higher stroke risk (Figure 1), while patients without concomitant disease presented more often in paroxysmal AF.3
Table 1.
Clinical characteristics of the study population
| Total | France | Germanya | Italy | Spain | UK | |
|---|---|---|---|---|---|---|
| (N = 7243) | (N = 1532) | (N = 1771) | (N = 1888) | (N = 858) | (N = 1194) | |
| Age (years) (mean) | 71.5 | 72.9 | 71.9 | 70.9 | 70.5 | 70.7 |
| Height (cm) (mean) | 169.2 | 169.1 | 171.7 | 167.3 | 165.5 | 171.5 |
| Male (%) | 60.1 | 59.3 | 63.0 | 57.0 | 56.0 | 64.5 |
| Valvular AF (%) | 4.2 | 5.0 | 3.3 | 5.4 | 5.0 | 1.9 |
| CHA2DS2VASc score (mean) | 3.4 | 3.3 | 3.7 | 3.3 | 3.3 | 3.2 |
| Points 1 (%) | 10.1 | 9.2 | 7.1 | 11.3 | 11.7 | 12.8 |
| Points 2+ (%) | 84.1 | 83.0 | 89.6 | 83.4 | 81.8 | 80.2 |
| Congestive heart failure (%)b | 29.0 | 25.9 | 36.5 | 27.6 | 28.0 | 24.1 |
| Hypertension (%)b | 71.8 | 62.9 | 81.4 | 75.4 | 70.9 | 62.7 |
| Age ≥ 75 years (%)b | 44.7 | 54.8 | 42.5 | 42.1 | 42.5 | 41.5 |
| Diabetes mellitus (%)b | 22.7 | 17.1 | 31.6 | 19.8 | 25.7 | 18.4 |
| Prior stroke/TIA/thromboembolic event (%)b | 15.5 | 13.7 | 19.1 | 12.4 | 12.8 | 19.0 |
| Vascular disease (%)b | 22.6 | 21.5 | 25.6 | 22.7 | 21.6 | 20.0 |
| Age 65–74 years (%)b | 32.9 | 25.4 | 38.8 | 34.4 | 29.4 | 33.5 |
| Female gender (%)b | 39.8 | 40.9 | 36.8 | 42.6 | 43.5 | 35.7 |
| Heart failure (%) | 21.3 | 18.2 | 28.4 | 19.4 | 24.4 | 15.4 |
| Ejection fraction (mean) | 56.5 | 59.8 | 57.0 | 53.6 | 58.8 | 51.1 |
| Hypertension (%) | 72.0 | 63.8 | 81.9 | 75.3 | 72.7 | 62.1 |
| Diabetes mellitus (%) | 22.4 | 16.8 | 31.2 | 19.2 | 26.4 | 18.8 |
| Prior stroke (%) | 8.4 | 8.9 | 10.7 | 6.5 | 7.7 | 8.0 |
| Coronary artery disease (%) | 23.4 | 18.2 | 29.6 | 20.6 | 21.6 | 26.6 |
| Prior stent (%) | 10.2 | 8.2 | 14.1 | 8.9 | 11.2 | 8.2 |
| Prior myocardial infarction (%) | 10.7 | 8.0 | 10.5 | 11.3 | 11.2 | 13.0 |
| Peripheral or aortic artery disease (%) | 4.4 | 5.9 | 5.0 | 3.4 | 4.3 | 3.4 |
| Chronic kidney disease (%) | 12.9 | 10.1 | 14.9 | 12.5 | 12.7 | 14.0 |
| Stage 2 (GFR 60–89 mL/min/1.73 m²) (%) | 2.3 | 1.6 | 3.2 | 2.4 | 2.0 | 2.0 |
| Stage 3 (GFR 30–59 mL/min/1.73 m²) (%) | 8.3 | 6.3 | 9.7 | 7.0 | 8.3 | 10.5 |
| Stage 4 (GFR 15–29 mL/min/1.73 m²) (%) | 1.5 | 1.6 | 1.0 | 2.0 | 2.1 | 1.1 |
| Stage 5 (GFR <15 mL/min/1.73 m²) (%) | 0.2 | 0.1 | 0.1 | 0.3 | 0.2 | 0.1 |
| Systole/diastole blood pressure (mmHg) at baseline (mean) | 132/78 | 134/78 | 133/80 | 129/77 | 131/76 | 131/76 |
| Alcohol abuse (%) | 2.5 | 3.6 | 2.0 | 1.2 | 2.6 | 3.9 |
| Concomitant antiplatelet therapy (%) | 22.1 | 16.9 | 17.2 | 27.0 | 18.7 | 30.7 |
| Prior bleeding event (%) | 7.3 | 4.1 | 5.1 | 7.5 | 8.7 | 13.1 |
| Chronic hepatic disease (%) | 2.1 | 1.3 | 2.2 | 3.6 | 1.6 | 0.7 |
| HASBLED score (mean) | 2.0 | 1.9 | 2.1 | 2.1 | 2.0 | 2.0 |
| Labile INRs (%)c | 13.5 | 15.3 | 6.6 | 16.4 | 18.5 | 12.1 |
| Elderly (age >65) (%)c | 75.0 | 78.4 | 79.0 | 73.5 | 70.7 | 71.2 |
| Drugs (such as antiplatelet agents, NSAIDs) (%)c | 27.3 | 13.8 | 24.9 | 32.9 | 25.0 | 39.7 |
| Alcohol (alcohol abuse) (%)c | 2.5 | 3.4 | 2.3 | 1.1 | 2.9 | 3.9 |
NSAID, nonsteroidal anti-inflammatory drug; INR, international normalized ratio; GFR, glomerular filtration rate; HASBLED is an acronym for factors associated with bleeding.10
aIncludes Austria and Switzerland.
bRisk factors reported in correlation with CHA2DS2VASC score.
cRisk factors reported in correlation with HASBLED score.
Figure 1.
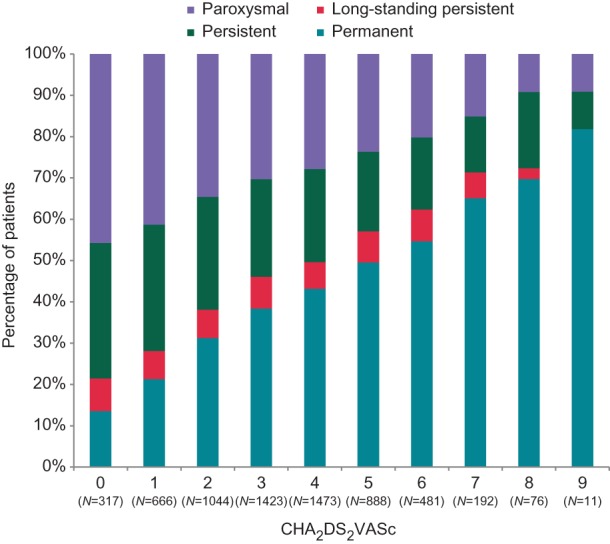
Proportion of patients with a given AF pattern (paroxysmal, persistent, long-standing persistent, or permanent, plotted as percentage, y axis) in the study population plotted by the number of concomitant cardiovascular diseases and age as summarized in the CHA2DS2VASc score (x axis). The proportion of patients with permanent AF increases in each CHA2DS2VASc stratum, while the proportion of patients with paroxysmal AF decreases.
High use of oral anticoagulants
Many patients were on oral anticoagulation, reflecting adequate use of this therapy in the population studied, In patients with a CHA2DS2VASc score ≥2, 85.6% (4793 of 5600) received oral anticoagulants, with a clear tendency towards higher use of oral anticoagulation in those at higher stroke risk (Figure 2). Oral anticoagulation was also used in 70.1% of the patients with a CHA2DS2VASc score of 1 (468 of 668 patients). 62.5% Of the patients without any CHA2DS2VASc stroke risk factor received oral anticoagulation (199 of 318 patients).
Figure 2.
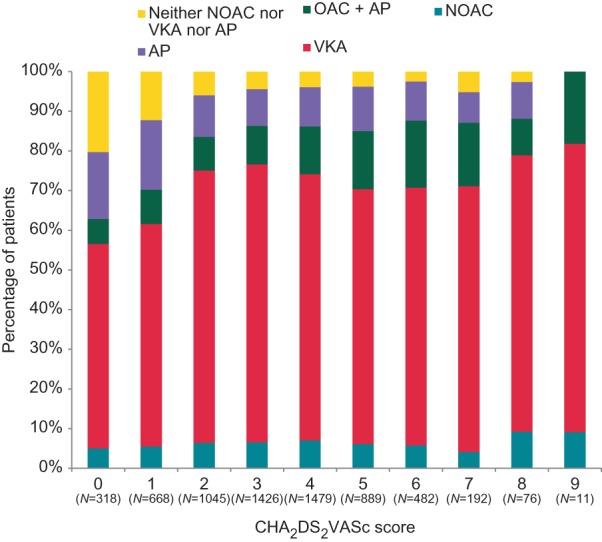
Use of antithrombotic therapy by stroke risk. Most patients with a high stroke risk received adequate anticoagulation, mainly delivered as vitamin K antagonist therapy, antiplatelet agent. VKA vitamin K antagonist, NOAC new oral anticoagulant, OAC oral anticoagulation (either VKA or NOAC).
Use of different vitamin K antagonists and new oral anticoagulants
Several vitamin K antagonists (VKAs) were used in the PREFER in AF population. Warfarin was often used in Italy and in the UK, fluindione in France, phenprocoumon in Germany/Austria/Switzerland, acenocoumarol in Spain (Table 2). Either of these VKAs allowed adequate anticoagulation in the short-term (Figure 3). Patients receiving phenprocoumon or fluindione had numerically a higher proportion of international normalized ratio (INR) values in the therapeutic range. New oral anticoagulant drugs were used in younger patients than VKA at either high or low stroke risk (Figure 2).
Table 2.
Therapy of the study population
| Total | France | Germanya | Italy | Spain | UK | |
|---|---|---|---|---|---|---|
| (n = 7243) | (n = 1532) | (n = 1771) | (n = 1888) | (n = 858) | (n = 1194) | |
| Pacemaker/defibrillator, % (n) | 9.0 (651) | 8.4 (126) | 9.6 (169) | 11.8 (223) | 6.5 (56) | 6.5 (77) |
| Antithrombotic therapy (i.e. all OACs), % (n) | 82.3 (5961) | 90.0 (1379) | 87.4 (1547) | 71.5 (1350) | 87.9 (754) | 78.0 (931) |
| Antiplatelets, % (n) | 22.1 (1599) | 16.9 (259) | 17.2 (304) | 27.0 (510) | 18.7 (160) | 30.7 (366) |
| ASA, % (n) | 19.8 (1436) | 14.2 (218) | 16.3 (289) | 24.4 (460) | 16.9 (145) | 27.1 (324) |
| Clopidogrel, % (n) | 4.1 (293) | 3.5 (54) | 2.4 (43) | 4.6 (87) | 4.4 (38) | 6.0 (71) |
| Prasugrel, % (n) | 0.3 (23) | 0.1 (1) | 0.3 (6) | 0.5 (9) | 0.7 (6) | 0.1 (1) |
| Ticagrelor, % (n) | 0.1 (5) | 0.0 (0) | 0.1 (1) | 0.1 (2) | 0.0 (0) | 0.2 (2) |
| Vitamin K antagonists, % (n) | 78.0 (5649) | 86.0 (1318) | 79.1 (1400) | 71.4 (1348) | 80.0 (686) | 75.1 (897) |
| Warfarin, % (n) | 34.1 (2470) | 16.1 (246) | 2.8 (50) | 62.0 (1171) | 12.7 (109) | 74.9 (894) |
| Phenprocoumon, % (n) | 18.4 (1330) | 1.0 (16) | 74.1 (1313) | 0.0 (0) | 0.0 (0) | 0.1 (1) |
| Fluindione, % (n) | 13.1 (948) | 61.8 (947) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.1 (1) |
| Acenocoumarol, % (n) | 12.5 (907) | 7.2 (110) | 2.0 (35) | 9.6 (181) | 67.3 (577) | 0.3 (4) |
| New oral anticoagulants, % (n) | 6.1 (442) | 6.0 (92) | 11.6 (205) | 0.3 (5) | 11.2 (96) | 3.7 (44) |
| Dabigatran, % (n) | 4.0 (291) | 5.0 (76) | 5.5 (97) | 0.2 (3) | 8.9 (76) | 3.3 (39) |
| Rivaroxaban, % (n) | 1.9 (140) | 1.0 (16) | 5.8 (102) | 0.0 (0) | 2.3 (20) | 0.2 (2) |
| Apixaban, % (n) | 0.1 (8) | 0.0 (0) | 0.2 (4) | 0.1 (1) | 0.1 (1) | 0.2 (2) |
| Antiplatelets as mono-therapy, % (n) | 11.2 (808) | 5.9 (91) | 7.6 (135) | 18.1 (342) | 6.4 (55) | 15.5 (185) |
| Vitamin K antagonists as mono-therapy, % (n) | 66.3 (4799) | 74.0 (1133) | 68.1 (1206) | 62.4 (1178) | 66.4 (570) | 59.6 (712) |
| New oral anticoagulants as mono-therapy or in combination, % (n) | 6.1 (442) | 6.0 (92) | 11.6 (205) | 0.3 (5) | 11.2 (96) | 3.7 (44) |
| No antithrombotic therapy, % (n) | 6.5 (474) | 4.1 (62) | 5.0 (89) | 10.4 (196) | 5.7 (49) | 6.5 (78) |
| Combination therapy of antiplatelet agents and oral anticoagulation, % (n) | 10.9 (791) | 11.0 (168) | 9.5 (169) | 8.9 (168) | 12.2 (105) | 15.2 (181) |
| Mean heart rate (bpm) at enrolment mean (25–75% quartiles)b | 79.1 (67.0–88.0) | 74.5 (64.0–83.0) | 80.3 (69.0–90.0) | 80.8 (68.0–90.0) | 78.3 (68.0–88.0) | 81.4 (67.0–93.0) |
| Sinus rhythm, % (n) | 31.4 (2254) | 36.3 (546) | 25.1 (442) | 38.0 (710) | 34.2 (293) | 22.3 (263) |
| Patients with adequate heart rate control (HR 60–100), % (n) | 78.6 (5530) | 79.4 (1186) | 81.4 (1401) | 78.7 (1452) | 79.5 (673) | 72.5 (818) |
| Patients with acceptable heart rate control (HR 50–59 or 101–110), % (n) | 14.3 (1005) | 14.9 (223) | 12.2 (210) | 13.8 (255) | 15.5 (131) | 16.5 (186) |
| Patients without adequate heart rate control (HR<50 or >110), % (n) | 7.1 (499) | 5.6 (84) | 6.4 (110) | 7.5 (138) | 5.1 (43) | 11.0 (124) |
| Rhythm control therapy, % (n) | 59.8 (4332) | 72.3 (1107) | 54.6 (966) | 66.0 (1246) | 50.2 (431) | 48.7 (582) |
| Amiodarone, % (n) | 24.1 (1746) | 40 (613) | 14.1 (250) | 29.8 (562) | 21.5 (184) | 11.5 (137) |
| Dronedarone, % (n) | 4.0 (291) | 2.7 (41) | 7.5 (132) | 2.1 (40) | 6.3 (54) | 2.0 (24) |
| Flecainide, % (n) | 10.6 (764) | 17.5 (268) | 6.2 (110) | 12.0 (226) | 12.0 (103) | 4.8 (57) |
| Propafenone, % (n) | 2.9 (211) | 2.0 (30) | 1.3 (23) | 7.3 (138) | 1.9 (16) | 0.3 (4) |
| d,I-Sotalol, % (n) | 5.5 (396) | 8.5 (130) | 4.7 (83) | 4.6 (86) | 1.8 (15) | 6.9 (82) |
| Quinidine, % (n) | 0.2 (13) | 0.5 (8) | 0.1 (1) | 0.2 (4) | 0.0 (0) | 0.0 (0) |
| Catheter ablation done in the past 12 months, % (n) | 5.0 (358) | 4.7 (71) | 5.8 (102) | 4.4 (83) | 3.7 (32) | 5.9 (70) |
| Electrical cardioversion done in the past 12 months, % (n) | 18.1 (1306) | 14.4 (216) | 19.1 (337) | 21.0 (394) | 14.5 (124) | 19.7 (235) |
| Pharmacological cardioversion done in the past 12 months, % (n) | 19.5 (1403) | 26.1 (391) | 12.8 (226) | 27.3 (512) | 17.7 (152) | 10.2 (122) |
HR, heart rate.
aIncludes Austria and Switzerland.
bVentricular rate during AF.
Figure 3.
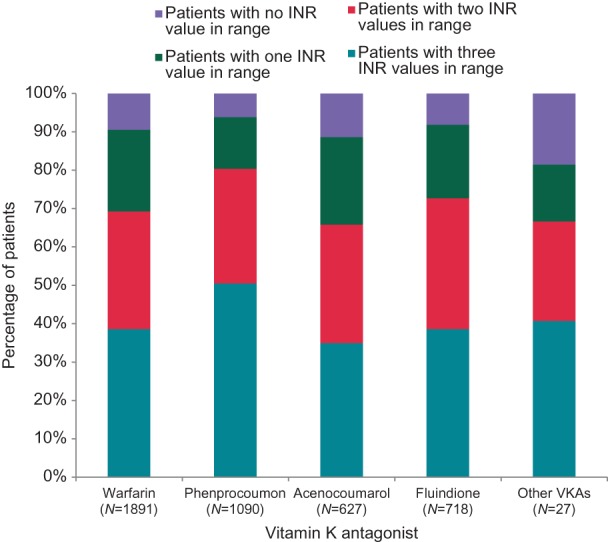
Therapeutic effect of vitamin K antagonist therapy, expressed as the number of the last three INR values prior to enrolment that were within the therapeutic range, split by the different vitamin K antagonists used. INR, international normalized ratio.
Adequate rate control targets
Of 7034 patients in whom information on heart rate was available, 5530 (78.6%) patients were adequately rate controlled at rest (Table 2), and 93% of the patients had resting heart rates of 59–110 bpm. The proportion of patients with adequate rate control was similar between asymptomatic patients (European Heart Rhythm Association, EHRA score=I:9 81% of patients with heart rate 60–100), and highly symptomatic patients (EHRA III: 79% with heart rate 60–100, EHRA IV 75% with heart rate 60–100, Table 3), illustrating the need for additional rhythm control (Figure 4). Patients with severe symptoms (EHRA III–IV) did not show marked differences in the duration of AF since the first diagnosis compared to patients without symptoms (EHRA I, Table 4).
Table 3.
Adequacy of rate control therapy by symptom status
| EHRA Ia | EHRA IIa | EHRA IIIa | EHRA IVa | |
|---|---|---|---|---|
| (N = 534) | (N = 2594) | (N = 2335) | (N = 1516) | |
| Patients with adequate heart rate control (HR 60–100) | 431 (80.7) | 2099 (80.9) | 1834 (78.5) | 1129 (74.5) |
| Patients with acceptable heart rate control (HR 50–59 or 101–110) | 75 (14.0) | 344 (13.3) | 334 (14.3) | 242 (16.0) |
| Patients without adequate heart rate control (HR<50 or >110) | 28 (5.2) | 151 (5.8) | 167 (7.2) | 145 (9.6) |
| Total | 534 (99.9) | 2594 (100.0) | 2335 (100.0) | 1516 (100.1) |
HR, heart rate.
aThe EHRA score was determined as the maximum of the six individual symptoms scores (palpitations, fatigue, dizziness, dyspnea, chest pain, anxiety). Each of these symptoms was scored by the enrolling physician as follows: never, occasional, intermediate, frequent. The EHRA score was then defined as follows: I, maximum score of ‘never’; II, maximum score of ‘occasional’; III, maximum score of ‘intermediate’; IV, maximum score of ‘frequent’.9
Figure 4.
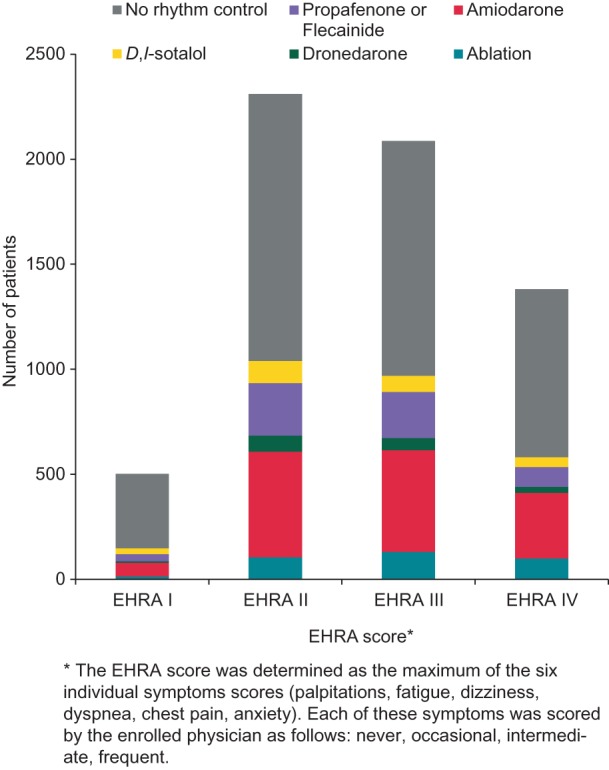
Use of rhythm control therapy options by patient symptoms. Following clinical reasoning and the recommendations in the ESC guidelines, rhythm control therapy was rarely used in asymptomatic patients. The EHRA score is calculated as the maximum of the six symptoms score (palpitations, fatigue, dizziness, dyspnea, chest pain, anxiety) as explained in the legend to Table 3.
Table 4.
The duration of atrial fibrillation since its first diagnosis does not differ between patients with or without symptoms
| Duration since initial AF diagnosis | EHRA I (N = 568) | EHRA II (N = 2643) | EHRA III (N = 2377) | EHRA IV (N = 1569) |
|---|---|---|---|---|
| Less than 1 year, % (n) | 30.3 (172) | 25.8 (683) | 27.1 (643) | 28.6 (449) |
| 1–2 years, % (n) | 7.4 (42) | 9.0 (237) | 9.4 (224) | 9.1 (142) |
| 2–3 years, % (n) | 4.8 (27) | 6.4 (169) | 6.3 (149) | 5.4 (84) |
| 3–4 years, % (n) | 4.9 (28) | 5.0 (133) | 4.5 (108) | 4.9 (77) |
| 4–5 years, % (n) | 3.4 (19) | 4.5 (118) | 4.7 (112) | 3.6 (57) |
| More than 5 years, % (n) | 26.9 (153) | 25.0 (661) | 25.2 (598) | 27.8 (436) |
| Unknown, % (n) | 22.4 (127) | 24.3 (642) | 22.8 (543) | 20.7 (324) |
| Duration since initial AF diagnosis | EHRA I (N = 441)a | EHRA II (N = 2001)a | EHRA III (N = 1834)a | EHRA IV (N = 1245)a |
| Duration, mean (years) | 4.6 | 4.6 | 4.5 | 4.9 |
| Duration, lower quartile (years) | 0.5 | 0.6 | 0.5 | 0.4 |
| Duration, median (years) | 2.2 | 2.3 | 2.3 | 2.4 |
| Duration, upper quartile (years) | 7.3 | 6.8 | 6.7 | 7.2 |
aReduced by number of unknown cases.
Rhythm control therapy
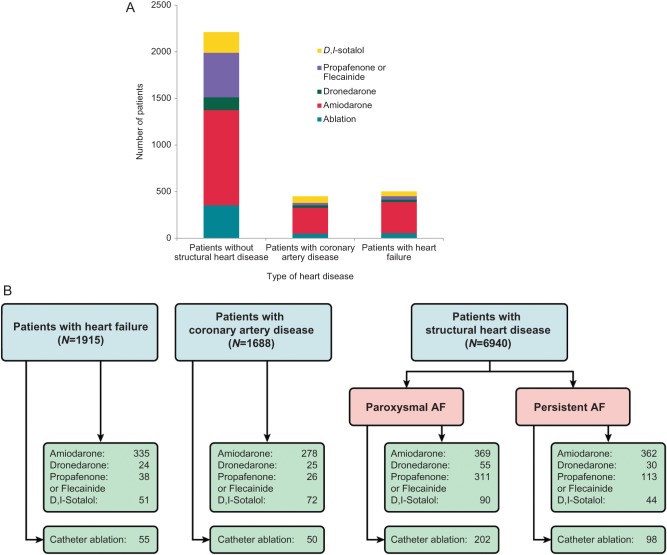
About half of the patients enrolled into PREFER in AF received rhythm control therapy. Electrical cardioversion was performed in 18.1% of patients, pharmacological cardioversion in 19.5% of patients. The following antiarrhythmic drugs were used: amiodarone (24.1%), flecainide or propafenone (13.5%), sotalol (5.5%), dronedarone (4.0%). Cather ablation was performed in 358 patients in the 12 months prior to enrolment (5.0%, Table 2, Figure 5). Rhythm control therapy was more often used in highly symptomatic patients (Figure 4) but more than half of the symptomatic patients did not receive rhythm control at all (Figure 4). Catheter ablation was often used in patients with paroxysmal AF, and sodium channel blockers were mainly used in patients without structural heart disease (Figure 5).
Figure 5.
Type of rhythm control therapy by type of heart disease. (A) Stacked column graph depicting the use of the different antiarrhythmic drugs and catheter ablation in patients with different types of heart disease (coronary artery disease, heart failure, no structural heart disease). (B) Illustration of the use of rhythm control therapies in patients with different types of heart disease in a flow chart illustrating the recommendations of the ESC 2010 guidelines for AF. All numbers reflect the actual patient number.
Discussion
Main findings
This snapshot of AF management in seven European countries in 2012 suggests that treatment patterns have changed in recent years: The guideline-recommended use of oral anticoagulation has increased compared to prior European,10 National,11–13 and international14 registries, reflecting a rapid implementation of the 2010 ESC guidelines.8 Furthermore, most patients were adequately rate controlled. The use of antiarrhythmic drugs and catheter ablation procedures increased compared to prior registries.
Patient characteristics
The PREFER in AF enrolled a comparable number of patients from Western, Central, and Southern European countries and the UK, thereby providing decent information on the current management of AF in Europe. Patient characteristics were comparable to other registries,8,15,16 supporting the assumption that this cohort is representative for the management of AF. More comprehensive information, especially on regional differences in other, smaller European countries, can be expected from the pilot general AF registry of the EORP programme.17
Types of atrial fibrillation and concomitant diseases
The distribution of different types of AF is comparable to those reported in other registries.12,14,16,18 We could replicate that patients with concomitant cardiovascular diseases are more likely to suffer from permanent AF,16 while the proportion of patients with persistent forms of AF is relatively constant (Figure 1). This distribution supports the concept that persistent AF is a transient disease state, and that underlying heart disease and advanced age contribute to the progression to permanent AF in most patients.3,18,19
Appropriate use of oral anticoagulants
Overall, antithrombotic therapy seen in PREFER in AF suggests much better adherence to evidence and recommendations than prior reports of similar registries:10,16 Only 70% of eligible patients received oral anticoagulants during 2005–2008,10,16 while over 85% of clearly eligible patients received oral anticoagulants in PREFER in AF (Figures 2 and 3). This is consistent with smaller recent reports from Germany,12,13 while lower usage of anticoagulants has recently been reported in data sets from Italy20 and by GARFIELD.21 It is conceivable that enrolment by cardiologists contributed to the high use of oral anticoagulants in PREFER in AF.11
The PREFER in AF informs about the uptake of new oral anticoagulants after their approval in Europe in 2012. With an overall rate of about 6%, the number of patients treated with NOACs was rather low, and mainly limited to the use of Dabigatran (4%). However, it should be considered that NOACs were available and reimbursed by the health care systems in 2012 only in Germany and Spain, which is reflected in a higher rate of about 11% in these two countries. Also in the UK, NOACs were on the market in 2012, but due to local reimbursement limitations, the use of NOACs was less than 4%. In France NOACs were launched only in July 2012; at a time where almost all patients of the registry have had their baseline visit, resulting in 6% of patients with NOAC treatment. In Italy the first NOAC (Dabigatran) is available since May 2013, explaining why (almost) no patients were treated at the time of patient enrolment into the registry (Table 2).
Vitamin K antagonists remain the most commonly used anticoagulant (Figure 3, Table 2), and two of three the patients on VKAs were adequately INR controlled (Figure 3). Of note, while the use of adequate anticoagulation has increased compared to prior registries, the rate of inappropriate therapy with oral anticoagulants in patients without stroke risk factors remains high (Figure 2).12,15,16 Hence, there appears to be a need to better communicate that oral anticoagulation is not indicated in these patients.22 Interestingly, new oral anticoagulants were given to younger patients than VKAs, probably reflecting both patient preference and a tendency to use these new medications cautiously at first, despite their proven safety in clinical trials.
Adequate rate control
According to the lenient definition of adequate rate control suggested by RACE-II23 and proposed in the 2010 ESC guidelines,8 the vast majority of patients enrolled in the PREFER in AF were adequately rate controlled (Table 3). It is worth to note that adequacy of rate control therapy hardly differed between asymptomatic and symptomatic patients (Table 3), suggesting that AF-related symptoms reflect a suffering from AF per se. Alternatively, some of these patients may require stricter rate control to better control their symptoms. Further analyses of the relation of heart rate and symptoms may be warranted in this data set.
Rhythm control therapy
Long-term rhythm control therapy was mainly used in symptomatic patients (Figures 4 and 5), in line with current and prior recommendations. Rhythm control therapy was more often used in PREFER in AF than in 2004–2006,15,16 and similar to data collected in 2009.12,14 Still, over 50% of highly symptomatic patients (EHRA III–IV) did not receive rhythm control (Figure 4). This may be due to the fact that these patients underwent unsuccessful rhythm control attempts in the past, illustrating the need to improve our ability to successfully deliver rhythm control therapy. Patient preferences or a reluctance to use rhythm control therapy may also contribute to this apparent underuse which invites further study.
Sodium channel blockers were mainly used in patients without structural heart disease (Figure 5), in line with recommendations.4 At first sight, it comes as a slight surprise that amiodarone was the most common antiarrhythmic drug in patients without structural heart disease, where it is recommended only as a second-line therapy.4,8 We can only speculate that this may reflect that amiodarone was used as a second-line drug, e.g. after failure of other antiarrhythmic drugs. Dronedarone was less often used than other antiarrhythmic drugs, possibly reflecting the uncertainty about its appropriate use in 2012 and the need to gain further clinical confidence in the use of this novel antiarrhythmic drug.
Limitations
The PREFER in AF provides a contemporary snapshot of the management of AF in seven European countries, and illustrates the changes in AF management after publication of the ESC guidelines on AF in 2010. Apart from the selection of the countries, all design aspects were decided by the scientific steering committee and executed by an independent CRO. Consecutive enrolment and selection of ‘representative sites’ (outpatients and inpatients, cardiologists and other physicians) were used to provide a real-life data set. Nonetheless, and inherent to other similar registries, we cannot rule out selection bias at the centre or patient level. Additional, comprehensive information, especially on regional differences in other European countries, can be expected from the EORP general AF pilot registry of the ESC17 and other registry initiatives in Europe.
Supplementary Material
Acknowledgements
We would like to thank all centres that participated in this registry, and all patients who gave their consent to participate. Furthermore, we would like to thank Markus Schwertfeger for medical advice and Paul-Egbert Reimitz for statistical advice and programming (both employees of Daiichi Sankyo Europe).
Conflict of interest: R.D.C.: lecture fees and honoraria from Daiichi-Sankyo, Boehringer-Ingelheim, Bayer, Bristol-Myers Squibb, Pfizer, and Lilly; J.-Y.L.H.: consultant/conferences/advisory board for Sanofi-Aventis, BMS/Pfizer, Meda, Boehringer Ingelheim, MSD, Bayer, Servier, and Daiichi-Sankyo; H.D.: Steering Committee member and National Coordinator for Germany RE-LY, APPRAISE-1 and 2, Garfield Registry, and PREFER in AF. Fees, honoraria, and research funding from AstraZeneca, Bayer, Berlin-Chemie, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Daiichi-Sankyo, Lilly, MSD Sharp&Dohme, BMFT, Harvard Med. Res. Inst., and Thrombosis Research Institute; J.L.Z.: speaker honoraria from Sanofi, Servier, and Daiichi-Sankyo; B.A. and J.S.: employees of Daiichi-Sankyo Europe.
Funding
PREFER in AF was sponsored by Daiichi Sankyo Europe GmbH. The members of the steering committee received honoraria for their advice in the planning of the registry.
Supplementary material
A full list of Study sites is given as supplementary material.
References
- 1.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–53. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Lip GY, Van Gelder IC, Bax J, Hylek E, Kaab S, et al. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options—a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace. 2012;14:8–27. doi: 10.1093/europace/eur241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirchhof P, Curtis AB, Skanes AC, Gillis AM, Samuel Wann L, John Camm A. Atrial fibrillation guidelines across the Atlantic: a comparison of the current recommendations of the European Society of Cardiology/European Heart Rhythm Association/European Association Of Cardiothoracic Surgeons, the American College of Cardiology Foundation/American Heart Association/Heart Rhythm Society, and the Canadian Cardiovascular Society. Eur Heart J. 2013 doi: 10.1093/eurheartj/ehs446. [DOI] [PubMed] [Google Scholar]
- 5.Bo S, Valpreda S, Scaglione L, Boscolo D, Piobbici M, Bo M, et al. Implementing hospital guidelines improves warfarin use in non-valvular atrial fibrillation: a before-after study. BMC Public Health. 2007;7:203. doi: 10.1186/1471-2458-7-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimetbaum P, Reynolds MR, Ho KK, Gaziano T, McDonald MJ, McClennen S, et al. Impact of a practice guideline for patients with atrial fibrillation on medical resource utilization and costs. Am J Cardiol. 2003;92:677–81. doi: 10.1016/s0002-9149(03)00821-x. [DOI] [PubMed] [Google Scholar]
- 7.Frykman V, Beerman B, Ryden L, Rosenqvist M. Management of atrial fibrillation: discrepancy between guideline recommendations and actual practice exposes patients to risk for complications. Eur Heart J. 2001;22:1954–9. doi: 10.1053/euhj.2000.2300. [DOI] [PubMed] [Google Scholar]
- 8.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 9.Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener HC, et al. Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork and the European Heart Rhythm Association. Europace. 2007;9:1006–23. doi: 10.1093/europace/eum191. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwlaat R, Olsson SB, Lip GY, Camm AJ, Breithardt G, Capucci A, et al. Guideline-adherent antithrombotic treatment is associated with improved outcomes compared with undertreatment in high-risk patients with atrial fibrillation. The Euro Heart Survey on Atrial Fibrillation. Am Heart J. 2007;153:1006–12. doi: 10.1016/j.ahj.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Kirchhof P, Nabauer M, Gerth A, Limbourg T, Lewalter T, Goette A, et al. Impact of the type of centre on management of AF patients: surprising evidence for differences in antithrombotic therapy decisions. Thromb Haemost. 2011;105:1010–23. doi: 10.1160/TH11-02-0070. [DOI] [PubMed] [Google Scholar]
- 12.Meinertz T, Kirch W, Rosin L, Pittrow D, Willich SN, Kirchhof P. Management of atrial fibrillation by primary care physicians in Germany: baseline results of the ATRIUM registry. Clin Res Cardiol. 2011;100:897–905. doi: 10.1007/s00392-011-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosch RF, Kirch W, Theuer JD, Pittrow D, Kohlhaussen A, Willich SN, et al. Atrial fibrillation management, outcomes and predictors of stable disease in daily practice: prospective non-interventional study. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.03.053. [DOI] [PubMed] [Google Scholar]
- 14.Camm AJ, Breithardt G, Crijns H, Dorian P, Kowey P, Le Heuzey JY, et al. Real-life observations of clinical outcomes with rhythm- and rate-control therapies for atrial fibrillation RECORDAF (Registry on cardiac rhythm disorders assessing the control of atrial fibrillation) J Am Coll Cardiol. 2011;58:493–501. doi: 10.1016/j.jacc.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the euro heart survey on atrial fibrillation. Eur Heart J. 2005;26:2422–34. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 16.Nabauer M, Gerth A, Limbourg T, Schneider S, Oeff M, Kirchhof P, et al. The Registry of the German Competence NETwork on Atrial Fibrillation: Patient characteristics and initial management. Europace. 2009;11:423–34. doi: 10.1093/europace/eun369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lip G. EUR Observational research programme: atrial fibrillation general registry pilot phase. Eur H J. 2013;34:794. [PubMed] [Google Scholar]
- 18.de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–31. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 19.Jahangir A, Lee V, Friedman PA, Trusty JM, Hodge DO, Kopecky SL, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007;115:3050–6. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 20.Zoni-Berisso M, Filippi A, Landolina M, Brignoli O, D'Ambrosio G, Maglia G, et al. Frequency, patient characteristics, treatment strategies, and resource usage of atrial fibrillation (from the Italian Survey of Atrial Fibrillation Management [ISAF] study) Am J Cardiol. 2013;111:705–11. doi: 10.1016/j.amjcard.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, et al. Risk Profiles and Antithrombotic Treatment of Patients Newly Diagnosed with Atrial Fibrillation at Risk of Stroke: Perspectives from the International, Observational, Prospective GARFIELD Registry. PLoS ONE. 2013;8:e63479. doi: 10.1371/journal.pone.0063479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–73. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.