Abstract
Background
Allogeneic stem cell transplantation is the most effective treatment option for many hematological malignancies, but graft-versus-host-disease (GvHD) remains a major cause of treatment failure. Besides well established risk factors for the outcome of transplantation, recent single-center studies have identified a birth order effect in HLA-identical sibling stem cell transplantation with lower incidence of acute and chronic extensive graft-versus-host disease (aGvHD/cGvHD) and improved overall survival when a donor is younger than the recipient. One hypothesized mechanism is microchimerism due to fetomaternal and transmaternal sibling cell trafficking during pregnancy as the donor is exposed to recipient antigens in utero.
Design and Methods
The aim of this study was to validate single-center data in a multicenter cohort provided by the Center for International Blood and Marrow Transplantation (CIBMTR). All adult and pediatric patients (n=11,365) with a diagnosis of a hematologic malignancy receiving an allogeneic stem cell transplantation from an HLA-identical sibling donor from 1990 to 2007 were included.
Results
When donors were younger than recipients, there was a significantly lower aGvHD II-IV° and cGvHD rate for children as well as lower cGvHD rate for adolescents. However, the hypothesized overall positive effect of lower relapse and better survival when donors are younger than recipients was not observed in this large multicenter study.
Conclusions
Our data suggest that if otherwise equally matched, a graft from a sibling younger than the patient may be superior to an older one for child and adolescent stem cell transplant patients.
Keywords: allogeneic hematopoietic cell transplantation, birth order, HLA-identical sibling donors
Introduction
Allogeneic stem cell transplantation (SCT) is a curative treatment option for many hematological malignancies. However, relapse and graft-versus-host-disease (GvHD) remain the most important causes of treatment failure.1 Since HLA-disparity between donor and recipient is the most critical factor that governs incidence and severity of GvHD, donor search focuses on HLA-identical siblings first.2 Besides well established risk factors for the outcome of transplantation, the impact of birth order in HLA-identical sibling transplantation has been described in recent retrospective analyses.3,4
The mechanism for birth order effects may include microchimerism by fetomaternal cell trafficking during pregnancy which leads to the exposure to non-self antigens in mother and child.5-7 This early perinatal exposure and resultant microchimerism in the mother or siblings may result in B and T cell sensitization and the induction of T regulatory cells, which could affect the activation of donor lymphocytes following later antigen (re-)exposure after stem cell transplantation.8,9 In the context of pregnancy and stem cell transplantation immunology, an increased GvHD risk after transplantation from parous female donors compared to nulliparous donors has been observed in HLA-identical transplantation.10-14 In the haploidentical setting, maternal grafts are superior in terms of disease-free survival, relapse incidence, and mortality, perhaps because of prior exposure of the maternal donor and her children.15,16
To further evaluate the impact of patients' and donors' birth order on outcome of sibling transplantation, we performed a retrospective analysis using the database of the Center for International Blood and Marrow Transplantation (CIBMTR).
Materials and Methods
The aim of this study was to validate single-center data in a multicenter cohort provided by the CIBMTR. This multicenter analysis included patients receiving an HLA-identical sibling transplantation reported to the CIBMTR database between 1990 and 2007. Only HLA-identical sibling transplants were included as birth order effects would not be expected with unrelated donors.
Adult and pediatric patients with the diagnosis of acute leukemia, myelodysplastic syndrome, or chronic myeloid leukemia undergoing first allogeneic transplant were included. Patients with non-malignant disorders were excluded because of our interest in examining any effects on relapse. Cord blood recipients, patients less than 2 years of age as there were only a few patients in this cohort, and pairs in which the age difference was reported as greater than 15 years or as less than one year apart were excluded to improve the homogeneity of the cohort. Disease stage was categorized according to CIBMTR conventions; i. e. early (acute leukemia in first complete remission, refractory anemia, refractory anemia with ringed sideroblasts, or chronic myeloid leukemia in first chronic phase) or late (relapsed or refractory disease). All other disease types and stages were classified as intermediate. Patients were assigned to either the recipient older than donor group (R>D) or donor older than patient group (D>R).
Outcome was analysed in terms of overall survival (OS), relapse rate and mortality, disease free survival (DFS), treatment related mortality (TRM), and acute and chronic graft-versus-host disease (aGvHD/cGvHD).
Statistical analyses were performed with SAS comparing differences between the groups using Chi-square tests, t-tests or non-parametric testing as appropriate. Probabilities of survival and DFS were calculated using the Kaplan-Meier estimate; the log-rank test was used for univariate comparisons. Risk factors for outcomes were evaluated in multivariate analyses, using Cox proportional hazard models. All models included the main effect of interest (R>D vs. D>R), as well as other covariates with statistical significance level of less than p=0.05. Potential covariates included patient and donor sex match, CMV status, race, pre-transplant performance status, disease and stage, time from diagnosis to transplant, conditioning intensity, graft type, GvHD prophylaxis, anti-thymocyte globulin (ATG), and transplant year. Patient and donor age were tested in separate models because of their correlation with birth order.
Results
In this retrospective analysis, a total of 11,365 patients transplanted from HLA-identical siblings (5870 R>D and 5495 D>R) were included. Patient characteristics are shown in Table 1. The median age of patients at SCT was 35 years (range 2-75y.) in the R>D group compared to 31 years (range 2-72y.; p< 0,0001) for the D>R group. Acute myeloid leukemia was the most common SCT indication, 38% in the R>D group and 38% in D>R group. Stem cell source was mainly bone marrow (72% and 73%). Most patients had early stage disease at time of transplantation (80% in R>D group and 81% in D>R group, p= 0.14). Conditioning regimen was myeloablative in more than 90% in each group. In both groups most patients received a calcineurin inhibitor-based GvHD prophylaxis regimen. Few had ATG exposure (4% in R>D and 3% in D>R) and these patients were excluded. Since exposure to CMV increases with age, the D>R group was more likely to be donor positive/patient negative (14% vs. 8%) and less likely to be donor negative/patient positive (12% vs. 20%) than the R>D group.
Table 1.
Patient and donor characteristics.
| Recipient older than donor (R>D) | Donor older than patient (D>R) | P-value | |
|---|---|---|---|
| Variable | N (%) | N (%) | |
| Number of patients | 5870 | 5495 | |
| Age at transplant, median (range), years | 35 (2-75) | 31 (2-72) | < 0.0001 |
| Age at transplant | < 0.0001 | ||
| Children (0 – 9 y) | 319 (5) | 542 (10) | |
| Adolescents (10 – 19 y) | 814 (14) | 911 (17) | |
| Adults (20 y and older) | 4737 (81) | 4042 (74) | |
| Male sex | 3296 (56) | 3248 (59) | 0.001 |
| Karnofsky prior to transplant > 90 | 4546 (77) | 4237 (77) | 0.67 |
| Disease at transplant | < 0.0001 | ||
| AML | 2234 (38) | 2091 (38) | |
| ALL | 1244 (21) | 1399 (25) | |
| CML | 1898 (32) | 1608 (29) | |
| MDS | 494 (8) | 397 (7) | |
| Stem cell source | 0.27 | ||
| Bone marrow | 4244 (72) | 4024 (73) | |
| Peripheral blood stem cells (PBSC) | 1626 (28) | 1471 (27) | |
| Conditioning regimen | |||
| Myeloablative | 5440 (93) | 5152 (94) | 0.02 |
| Reduced intensity/nonmyeloablative | 430 (7) | 343 (6) | |
| GVHD prophylaxis | 0.67 | ||
| Calcineurin inhibitor + MTX ± other | 4420 (75) | 4167 (76) | |
| Calcineurin inhibitor ± other (No MTX) | 1125 (19) | 1043 (19) | |
| T-cell depletion | 325 (6) | 285 (5) | |
| Donor/patient sex match | 0.21 | ||
| Female/Male | 1490 (25) | 1452 (26) | |
| All other | 4380 (75) | 4043 (74) | |
| Donor age, median (range), years | 30 (<1-69) | 37 (3-79) | < 0.0001 |
| Year of transplant | 0.16 | ||
| 1990-1994 | 2504 (43) | 2426 (44) | |
| 1995-1999 | 1756 (30) | 1639 (30) | |
| 2000-2004 | 1086 (19) | 933 (17) | |
| 2005-2009 | 524 (9) | 497 (9) | |
| Follow-up of survivors, median (range) mos | 80 (1-233) | 83 (0.6-228) | 0.49 |
After finding that patient age interacted with birth order, patient age was divided into 3 groups to reduce model complexity: children (older than 2 years but less than 10 years; 8%), adolescents (10 or older but less than 20 years; 15%), and adults (≥ 20 years; 77%) (table 2). Children who are 2 years or younger were excluded because in this subgroup no recipients older than donors could be identified.
Table 2. Multivariate analysis.
| RR (95% CI) for R>D* | P-value | |
|---|---|---|
| Overall survival | ||
| Children (2-<10) | 0.85 (0.68-1.06) | 0.15 |
| Adolescents (10-<20) | 1.04 (0.90-1.21) | 0.58 |
| Adults (20+) | 1.06 (0.98-1.14) | 0.14 |
| Leukemia-free survival | ||
| Children (2-<10) | 0.83 (0.67-1.04) | 0.10 |
| Adolescents (10-<20) | 1.03 (0.90-1.20) | 0.65 |
| Adults (20+) | 1.03 (0.96-1.10) | 0.48 |
| Non-relapse mortality | ||
| Children (2-<10) | 0.90 (0.61-1.31) | 0.58 |
| Adolescents (10-<20) | 1.11 (0.90-1.37) | 0.34 |
| Adults (20+) | 1.03 (0.95-1.12) | 0.48 |
| Relapse | ||
| Children (2-<10) | 0.85 (0.65-1.11) | 0.23 |
| Adolescents (10-<20) | 1.02 (0.83-1.24) | 0.87 |
| Adults (20+) | 1.04 (0.92-1.17) | 0.58 |
| Acute GVHD II-IV | ||
| Children (2-<10) | 0.68 (0.54-0.87) | 0.002 |
| Adolescents (10-<20) | 0.94 (0.81-1.11) | 0.50 |
| Adults (20+) | 0.97 (0.89-1.05) | 0.38 |
| Acute GVHD III-IV | ||
| Children (2-<10) | 0.89 (0.60-1.32) | 0.56 |
| Adolescents (10-<20) | 0.86 (0.66-1.12) | 0.25 |
| Adults (20+) | 0.96 (0.85-1.08) | 0.46 |
| Chronic GVHD | ||
| Children (2-<10) | 0.51 (0.34-0.76) | 0.001 |
| Adolescents (10-<20) | 0.68 (0.56-0.83) | 0.0001 |
| Adults (20+) | 0.99 (0.91-1.08) | 0.83 |
D>R RR 1.0
See text for listing of significant clinical co-variates. Stratified for significant non-proportional covariates
Children (older than 2 years but less than 10 years)
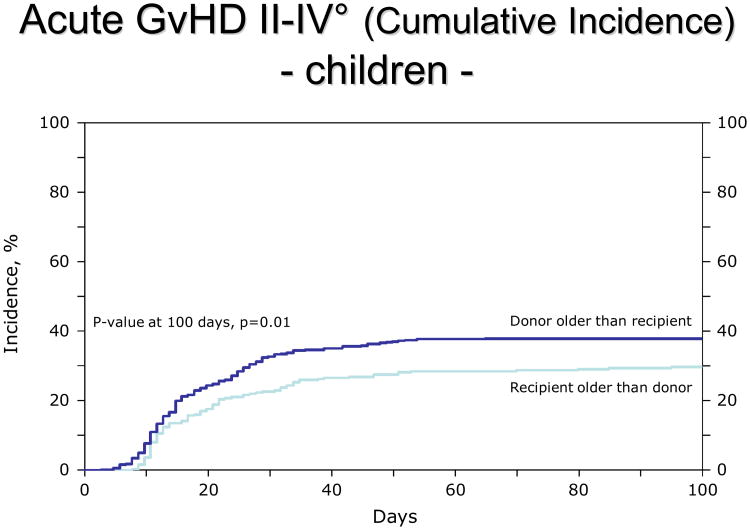
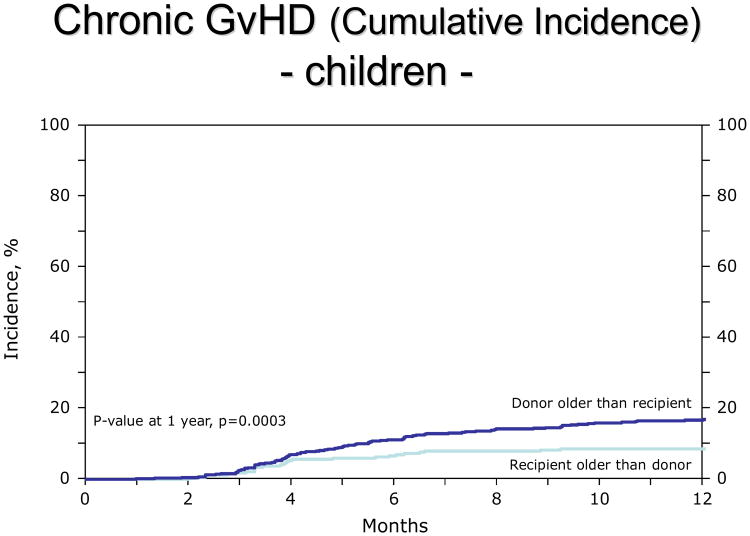
For children, the R>D group had a lower incidence of both acute GvHD (aGvHD) II-IV° (RR 0.68, 95% CI: 0.53-0.86, p=0.0015, adjusted for GvHD prophylaxis; figure 1) and chronic GvHD (cGvHD, RR 0.49, 95% CI: 0.33-0.73, p=0.0005, adjusted for graft type and performance score; figure 2). Other clinical covariates were not statistically associated with GvHD. When the analysis was limited to extensive chronic GVHD (excluding limited presentation), the incidence remained lower in the R>D group (RR 0.51, p=0.02). The distribution of organ involvement in the children was 26% skin, 12% liver, 13% GI in the R>D group. The distribution in the D>R group was 36% skin, 14% liver, 20% GI.
Figure 1.
Lower incidence of aGvHD II-IV° in children (2-9 years old) when the donor is younger than the patient.
Figure 2.
Lower incidence of chronic GvHD in children (2-9 years old) when the donor is younger than the patient.
Adolescents (at least 10 years, but less than 20 years)
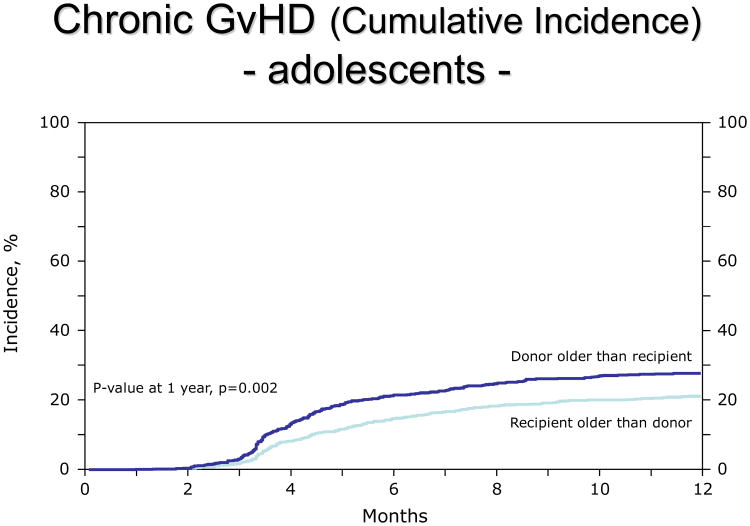
In adolescents, the R>D group had a lower incidence of cGvHD (RR 0.67, 95% CI: 0.56-0.81, p=<0.0001, adjusted for GvHD prophylaxis, graft type, race, sex match, year of transplant; figure 3). Results were similar when the analysis was limited to extensive cGvHD.
Figure 3.
Lower incidence of chronic GvHD in adolescents (10-19 years old) when the donor is younger than the patient.
Adults (20 years and older)
Birth order was not predictive of aGvHD or cGvHD in adults.
Because of the concern that either patient or donor age17 rather than birth order accounted for these findings, separate models were created to test whether patient or donor age per se were associated with aGvHD or cGvHD in the child or adolescent groups. Neither patient nor donor age was found to predict GvHD in multivariate models, nor was the age difference between patient and donor predictive of GvHD. Finally, no significant differences between the R>D and the D>R group were found for overall survival, leukemia-free survival, non-relapse mortality or relapse in any age group.
Further, several pre-specified subset analyses were conducted based on prior published literature. In patients with an early stage myeloid disease receiving a T-cell replete peripheral blood stem cell graft (PBSC), no association of birth order and OS, DFS or NRM in the myeloablative or non-myeloablative groups could be observed (data not shown).
Discussion
The aim of this study was to evaluate a possible effect of donor birth order on incidence of graft-versus-host-disease, relapse and overall survival in hematological malignancies in a very large patient cohort. We found a birth order effect on GvHD incidence in HLA-identical sibling SCT that depends on patient age. Our results showed a reduced aGvHD incidence, consistent with previous findings from a Swiss single-center analysis of 311 patients which found less aGvHD irrespective of patient age, although that study also reported an association with OS and relapse incidence.3 In contrast to hematologic malignancies, the impact of birth order on OS could not be reproduced in aplastic anemia patients,18 but a graft-versus-leukemia effect is not needed for a successful allogeneic SCT for this diagnosis.
Feto-maternal and materno-fetal cell trafficking and persistence are believed to result in at least transient microchimerism with either sensitization or tolerance,19-21 and the donor-age dependency of these effects may correspond to microchimerism and immunmodulation disappearing over time. In some individuals, fetomaternal trafficking effects may last until adulthood with HLA-disparate maternal cells persisting in immunocompetent offspring into adult life.22 In multiparous female blood donors, minor histocompatibility-antigen-specific cytotoxic T-cells can be detected in peripheral blood mononuclear cells, presumably due to exposure to the minor histocompatibility antigen during pregnancy, up to 22 years after delivery.10 Maternal cells can cross the placenta to reside in fetal lymph nodes with development of CD4+CD25highFox P3+ Tregs that suppress fetal anti-maternal immunity and persist at least until early adulthood.20,21 Undoubtedly, it would have been very interesting to analyze the influence of the parity of the mother. However, in a large registry study these data are not available and might also be difficult to evaluate as miscarriage, half-siblings, and abortion might have an influence on a potential microchimerism effect but be incompletely recorded in the maternal past medical history.
Further, male DNA can be detected in growth factor mobilized peripheral blood mononuclear cells and CD34-enriched apheresis products from female donors.23 Very recently, Dierselhuis et al. have been able to demonstrate the presence of functional HY-specific cytotoxic T-cells and male microchimerism in female umbilical cord blood.24 This finding can be interpreted as a strong indicative for the existence of a cell flow between siblings via the mother.24
We could not confirm an association between donor birth order and relapse or survival in sibling transplant recipients in our cohort, nor did we find any association of donor birth order with the incidence of GvHD in adult transplant recipients. Differences between the current results and previous studies could be due to center-dependent strategies, such as increased use of peripheral stem cells,25 and the use of ATG3,4 or different GvHD prophylaxis approaches, which may modulate the clinical effects of birth order in HLA-identical sibling-SCT,19-21 although we adjusted for these factors if they were statistically associated with GvHD. The CIBMTR population is more ethnically diverse than previously studied single center populations, which might result in different prognostic genetic markers associated with microchimerism, for example HLA, which could mask any birth order effect. A major strength of this study is the very large sample size of over 10,000 transplant pairs and ability to adjust for other important clinical variables which could potentially confound the post-transplant outcomes of interest.
Our results might be of particular interest for the pediatric setting since cGvHD is the primary cause of late morbidity and NRM in long-term transplant survivors.26 As it is impossible to perform prospective clinical trials with sufficient power to definitely address the impact of birth order in HLA-identical sibling SCT, our observational data suggest that an HLA-identical sibling donor younger than the pediatric or adolescent patient may be superior to an older one if equally matched for all other established parameters relevant for donor choice. The advantage for the recipient may balance other concerns that usually favour an older sibling, such as larger size, understanding of the procedure, and a better ability to participate in the assent process.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Allos Inc; Amgen Inc; an anonymous donation to the Medical College of Wisconsin; Astellas Pharma US Inc; Be the Match Foundation; Biogen Idec Inc; BioMarin Pharmaceutical Inc; Biovitrum AB; Blood Center of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix GmbH; Children's Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai Inc; Genentech Inc; Genzyme Corporation; Histogenetics Inc; HKS Medical Information Systems; Hospira Inc; Kirin Brewery Co Ltd; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals Inc; Miller Pharmacal Group; Milliman USA Inc; Miltenyi Biotec Inc; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics Inc; Otsuka America Pharmaceutical Inc; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix Inc; StemCyte Inc; StemSoft Software Inc; Sysmex America Inc; Therakos Inc; Vidacare Corporation; ViraCor Laboratories; ViroPharma Inc; and Wellpoint Inc.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Presented in part at the 16th Congress of the European Hematology Association (EHA) in London 9.-12.6.2011
Authorship: Contribution: C.D. proposed the initial study idea, analyzed data and wrote the manuscript. K.W.A., M.H. and S.R.S. collected data and performed statistical analyses. G.A.H., J.J.v.R., D.M., E.W., M. F.-V., M.A., M.B., V.G., S.M., M.S.P. and V.R. contributed to data analysis. A.G., M.E. and S.J.L. supervised and contributed to the development of the study concept, data analysis and writing of the manuscript. All authors critically reviewed the manuscript.
Conflict-of interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357(15):1472–5. doi: 10.1056/NEJMp078166. [DOI] [PubMed] [Google Scholar]
- 2.Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117(11):3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bucher C, Stern M, Buser A, et al. Role of primacy in HLA-identical sibling transplantation. Blood. 2007;110(1):468–469. doi: 10.1182/blood-2007-02-076257. [DOI] [PubMed] [Google Scholar]
- 4.Dobbelstein C, Kamal H, Dammann E, et al. Lower relapse incidence and relapse mortality in patients transplanted from a younger sibling in HLA-identical stem cell transplantation: does microchimerism matter? Blood. 2008;112(11):334. [Google Scholar]
- 5.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93(2):705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchi DW, Fisk NM. Fetomaternal cell trafficking and the stem cell debate: gender matters. JAMA. 2007;297(13):1489–1491. doi: 10.1001/jama.297.13.1489. [DOI] [PubMed] [Google Scholar]
- 7.O'Donoghue K, Chan J, de la Fuente J, et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364(9429):179–182. doi: 10.1016/S0140-6736(04)16631-2. [DOI] [PubMed] [Google Scholar]
- 8.van Rood JJ, Stevens CE, Smits J, Carrier C, Carpenter C, Scaradavou A. Reexposure of cord blood to noninherited maternal HLA antigens improves transplant outcome in hematological malignancies. Proc Natl Acad Sci USA. 2009;106(47):19952–19957. doi: 10.1073/pnas.0910310106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rood JJ, Scaradavou A, Stevens CE. Indirect evidence that maternal microchimerism in cord blood mediates a graft-versus-leukemia effect in cord blood transplantation. Proc Natl Acad Sci USA. 2012;109(7):2509–2514. doi: 10.1073/pnas.1119541109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdijk RM, Kloostermann A, Pool J, et al. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 2004;103(5):1961–1964. doi: 10.1182/blood-2003-05-1625. [DOI] [PubMed] [Google Scholar]
- 11.Loren AW, Bunin GR, Boudreau C, et al. Impact of donor and recipient sex and parity on outcomes of HLA-identical sibling allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(7):758–69. doi: 10.1016/j.bbmt.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Gratwohl A, Hermans J, Niederwieser D, van Biezen A, van Houwelingen HC, Apperley J Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation EBMT. Female donors influence transplant-related versus-host disease in patients with aplastic anaemia treated by allogeneic marrow transplantation. Hematol J. 2001;2(6):363–70. doi: 10.1038/sj.thj.6200117. [DOI] [PubMed] [Google Scholar]
- 13.Flowers ME, Pepe MS, Longton G, et al. Previous donor pregnancy as a risk factor for acute graft-versus-host disease in patients with aplastic anaemia treated by allogeneic marrow transplantation. Br J Haematol. 1990;74(4):492–496. doi: 10.1111/j.1365-2141.1990.tb06340.x. [DOI] [PubMed] [Google Scholar]
- 14.Hahn T, McCarthy PL, Jr, Zhang MJ, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplantation for adults with leukemia. J Clin Oncol. 2008;26(35):5728–5734. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamaki S, Ichinohe T, Matsuo K, Hamajima N, Hirabayashi N, Dohy H Japan Society of Hematopoietic Cell Transplantation. Superior survival of blood and marrow stem cell recipients given maternal grafts over recipients given paternal grafts. Bone Marrow Transplant. 2001;28(4):375–80. doi: 10.1038/sj.bmt.1703146. [DOI] [PubMed] [Google Scholar]
- 16.Stern M, Ruggeri L, Mancusi A, et al. Survival after T cell-depleted haplidentical stem cell transplantation is improved using the mother as donor. Blood. 2008;112(7):2990–2995. doi: 10.1182/blood-2008-01-135285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kollman C, Howe CWS, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bione marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 18.Gratwohl A, Doehler B, Stern M, Bucher C, Passweg J, Opelz G. Birth order and outcome after HLA-identical sibling donor transplantation. Blood. 2009;114(27):5569–5570. doi: 10.1182/blood-2009-10-249060. [DOI] [PubMed] [Google Scholar]
- 19.van Halteren AG, Jankowska-Gan E, Joosten A, et al. Naturally acquired tolerance and sensitization to minor histocompatibility antigens in healthy family members. Blood. 2009;114(11):2263–2272. doi: 10.1182/blood-2009-01-200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mold JE, Michaelsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;(322):1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burlingham WJ. A lession in tolerance – maternal instruction to fetal cells. N Engl J Med. 2009;360(13):1355–1357. doi: 10.1056/NEJMcibr0810752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maloney S, Smith A, Furst DE, et al. Microchimerism of maternal origin persists into adult life. J Clin Invest. 1999;104(1):41–47. doi: 10.1172/JCI6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams KM, Lambert NC, Heimfeld S, et al. Male DNA in female donor apheresis and CD34-enriched products. Blood. 2003;102(10):3845–3847. doi: 10.1182/blood-2003-05-1570. [DOI] [PubMed] [Google Scholar]
- 24.Dierselhuis MP, Blokland EC, Pool J, Schrama E, Scherjon SA, Goulmy E. Transmaternal cell flow leads to antigen-experienced cord blood. Blood. 2012;120(3):505–510. doi: 10.1182/blood-2012-02-410571. [DOI] [PubMed] [Google Scholar]
- 25.Körbling M, Anderlini P. Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood. 2001;98(10):2900–2908. doi: 10.1182/blood.v98.10.2900. [DOI] [PubMed] [Google Scholar]
- 26.Zecca M, Prete A, Rondelli R, et al. Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood. 2002;100(4):1192–1200. doi: 10.1182/blood-2001-11-0059. [DOI] [PubMed] [Google Scholar]