Abstract
Scope
Hops contain the phytoestrogen, 8-prenylnaringenin, and the cytoprotective compound, xanthohumol (XH). XH induces the detoxification enzyme, NAD(P)H-quinone oxidoreductase (NQO1) in vitro; however, the tissue distribution of XH and 8-prenylnaringenin and their tissue specific activity have not been analyzed.
Methods and results
A standardized hop extract (p.o.) and XH (s.c.) were administered to Sprague-Dawley rats over four days. LC-MS-MS analysis of plasma, liver and mammary gland revealed that XH accumulated in liver and mammary glands. Compared with the low level in the original extract, 8-prenylnaringenin was enriched in the tissues. Hops and XH induced NQO1 in the liver, while only hops reduced NQO1 activity in the mammary gland. Mechanistic studies revealed that hops modulated NQO1 through three mechanisms. In liver cells, 1) XH modified Keap1 leading to Nrf2 translocation and antioxidant response element (ARE) activation; 2) hop-mediated ARE induction was partially mediated through phosphorylation of Nrf2 by PKC; 3) in breast cells, 8-prenylnaringenin reduced NQO1 likely through binding to ERα, recruiting Nrf2, and downregulating ARE-regulated genes.
Conclusions
XH and 8-prenylnaringenin in dietary hops are bioavailable to the target tissues. While hops and XH might be cytoprotective in the liver, 8-prenylnaringenin seems responsible for hop-mediated NQO1 reduction in the mammary gland.
Keywords: Antioxidant response element, detoxification enzymes, hops, 8-prenylnaringenin, xanthohumol
INTRODUCTION
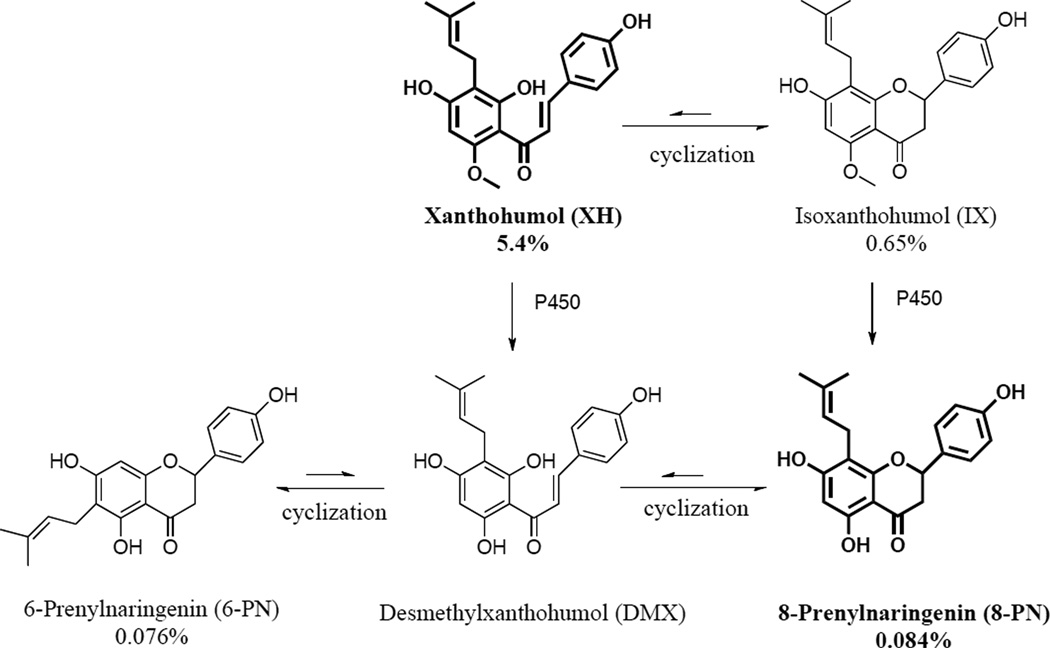
The strobili of Humulus lupulus L. (Cannabaceae, hops) are an essential flavoring agent in beer production and have a long tradition as a dietary supplement for mood and sleep disturbances [1]. More than 1000 constituents have been identified in hop extracts [1]. One such compound of recent pharmacological interest is 8-prenylnaringenin (8-PN) (Figure 1), which has been shown to have potent estrogenic properties in vitro and in vivo [2, 3]. Therefore, hop extracts have gained interest as botanical alternatives to hormone therapy (HT) for the relief of menopausal symptoms [4–6], especially since the release of the Women’s Health Initiative (WHI) has raised significant concerns about traditional HT [7]. The concentration of 8-PN in most hop extracts is relatively low [4]; however, other major prenylated phenols in hops can undergo in vivo conversion to 8-PN (Figure 1) [8, 9]. In addition to the estrogenic activity of hops, chemopreventive properties have been ascribed to hop extracts and XH (Figure 1) [10, 11]. XH has been reported to have multiple biological activities, including anti-inflammatory properties [12, 13] and enhanced detoxification of quinones, such as menadione, through upregulation of NAD(P)H-quinone oxidoreductase 1 (NQO1) [14]. Hence, XH rich hop extracts have been suggested as cancer preventive agents [15].
Figure 1.
Phytoconstituents of hops relevant for the present study.
While the induction of NQO1 by hop extract and purified XH have been shown in liver cells [14], their in vivo activities and influence on estrogen sensitive tissues have so far not been reported. As hop extracts are used as alternative remedies for postmenopausal symptoms, it is essential to evaluate the cytoprotective potential of hops in estrogen sensitive tissues. Therefore, the aim of the present study was to analyze the tissue specific activity of a standardized hop extract on detoxification pathways. In particular, hop-mediated induction of detoxification enzymes in the liver and mammary gland were analyzed, the level of the major prenylated phenols in both tissues was determined, and potential mechanisms of action were studied.
MATERIALS AND METHODS
Chemicals
All chemicals were purchased from Fisher (Hanover Park, IL) or Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. All media for cell culture were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was acquired from Atlanta Biological (Norcross, GA). 4’-bromoflavone (BF) was purchased from Toronto Research Chemicals, (North York, Ontario, Canada).
Plant material and pure phytoconstituents
The hop extract used in this study was prepared by Hopsteiner (Mainburg, Germany/New York) from spent hops using the following procedure. Pelletized strobili of Humulus lupulus cv. Nugget were bulk extracted with food-grade ethanol. The fluid extract was dispersed in diatomaceous earth, dried, and bulk extracted with supercritical CO2 extraction to yield two materials: the bitter acid extract (not used in this study) and the spent hop extract dispersed on the diatomaceous earth (Xantho Extract HHE02) used here. The spent hop extract was free of bitter acids. In preparation of the present experiments, the diatomaceous earth was removed by solubilization in methanol, filtration, and evaporation to dryness in vacuo. Quantitative LC-MS-MS analysis using authentic reference compounds as calibrants revealed that the spent hop extract (Xantho Extract HHE02, bitter acid and diatomaceous earth free) contained 5.4% XH, 0.084% 8-PN, 0.076% 6-PN, and 0.65% IX (w/w % of the spent hops extract). Based on qualitative chromatographic analysis, the general chemical composition of the extract was similar to that of a previously studied spent hops extract from which 20 prenylphenols have been isolated and structurally characterized following a bioassay-guided protocol targeted at the estrogenic active principle [16, 17]. In addition to these bioactive constituents, spent hops has been described as carbohydrate rich and it is likely that a large percentage of the extract contains various carbohydrates [18].
Racemic 8-PN was synthesized as described previously [19]. Purity analysis of 8-PN and XH was performed by quantitative 1H NMR at 400 MHz using the 100% method [20]. Pure 8-PN contained no less than 95.0 % 8-PN. Pure XH (96.5 %) was directly crystallized from XanthoPure extract (Hopsteiner) using a solvent mixture of hexane, EtOH, MeOH, and water (HEMWat +1; 5:5:6:4).
Animal treatment
Female Sprague–Dawley rats were received at 7 weeks of age from Harlan (Indianapolis, IN). All rats consumed Harlan/Teklad purified diet (Indianapolis, IN). After 1 week, animals were separated into four groups according to their weight (n=5/group): 1) Control diet plus s.c. vehicle control injection (sesame oil); 2) control diet plus s.c. injection of pure XH (100 mg/kg BW per day); 3) experimental diet containing BF (150 mg/kg BW per day) plus s.c. vehicle control; and 4) experimental diet containing powdered hop extract (7.5 g/kg BW per day) plus s.c. vehicle control injection. The exposure dose was calculated to reach an in vivo concentration that was expected to be pharmacologically active, in hope of laying the groundwork for future clinical studies. One treatment group received subcutaneously injected XH, to analyze whether XH would induce NQO1 in vivo without considering its bioavailability. The animals were treated for 4 days. All animals and their food were weighed daily. No difference in food intake was observed between treatment groups. On day 4, animals were sacrificed by CO2 asphyxiation, and blood and tissues were collected, snap frozen, and stored at − 80 °C for later analysis. The animal protocol complied with the Guide for the Care and Use of Laboratory Animals and all procedures were approved by UIC’s Institutional Animal Care and Use Committee (Protocol No. 08–101).
LC-MS-MS quantitation of XH in rat plasma and tissues
a) Sample preparation
Plasma samples were treated with ice-cold methanol (1:6) containing eriodictyol as internal standard to precipitate proteins. After centrifugation at 10,000 g for 10 min, an aliquot of the supernatant was removed and analyzed in duplicate using LC-MS-MS. Calibration curves were prepared by spiking blank plasma with known concentrations of XH (1–1000 ng/ml).
Tissue samples (200–700 mg) were homogenized in 3 volumes of deionized water and eriodictyol (30 µL, 2.5 µM) was added to 300 µL of the homogenate as internal standard. Each homogenate was extracted with ethyl acetate saturated with water (3 mL). The organic layer was removed, evaporated to dryness, and reconstituted with 50 % aqueous methanol (150 µL). After centrifugation, a 10 µL aliquot of supernatant was analyzed using LC-MS-MS.
b) LC-MS-MS analysis
Reversed phase HPLC separation of XH was carried out using a ZORBAX SB 2.1 × 100 mm C18 column (3.5 µm particle size) with a Shimadzu (Columbia, MD) LC-10ADvp solvent delivery system. Separation was achieved using a 10 min linear gradient from 35–90% acetonitrile in 0.1% formic acid at a flow rate of 0.3 ml/min. The eluent was introduced into an API4000 triple quadruple mass spectrometer (Applied Biosystems, Framingham, CT) operated in negative electrospray ionization mode. The data were acquired using selected reaction monitoring (SRM), and the SRM transitions of m/z 353–119 and m/z 287–151 were used for XH and eriodictyol (internal standard), respectively. The limit of detection and limit of quantitation for XH were 1.13 nM and 2.82 nM, respectively.
Qualitative profiling of prenylated flavonoids, XH, and their metabolites by LC-MS-MS
For qualitative analysis, tissue samples (200–300 mg) were homogenized in 70% methanol/water at a concentration of 0.1 g/ml. After centrifugation at 15,000 g for 15 min, the supernatant was filtered and directly injected onto the HPLC column. Metabolites were separated using an Atlantis T3 (Waters, Milford, MA) 2.1 × 100 mm (5 µm particle size) C18 column and a linear gradient from 30–70% acetonitrile in 0.1% formic acid at a flow rate of 0.25 ml/min. The column was maintained at 35°C. Negative ion electrospray with SRM was utilized to monitor ion transitions as follows: m/z 339–219 for 8-PN/6-PN, m/z 353–119 for XH/IX, m/z 529–353 for monoglucuronided XH/IX metabolites, and m/z 515–339 for monoglucuronic acid conjugates of 8-PN/6-PN.
Analysis of NQO1 and GST activity in vivo
Tissue homogenates were prepared as described by Cuendet et al. [21]. NQO1 activity was measured in 50 µL of a suitable dilution of the tissue supernatant (5 µg liver protein, 30 µg mammary gland protein) as described previously [14]. Glutathione S-transferase (GST) activity was determined using the Cayman GST assay kit.
Cell Culture Conditions
The MCF-7 (BUS) cell line was kindly provided by L. Lehmann (University of Würzburg, Germany) and cultured as described previously [22]. The HepG2 cell line obtained from the American Type Culture Collection (ATCC) and HepG2-ARE-luc cells kindly provided by A. N. Kong (Rutgers University, NJ) were cultured as previously described [14]. Cell line authentication was performed by short tandem repeat (STR) profiling using the StemElite ID System by Promega.
Nrf-2 Translocation Study
a) Western blot analysis
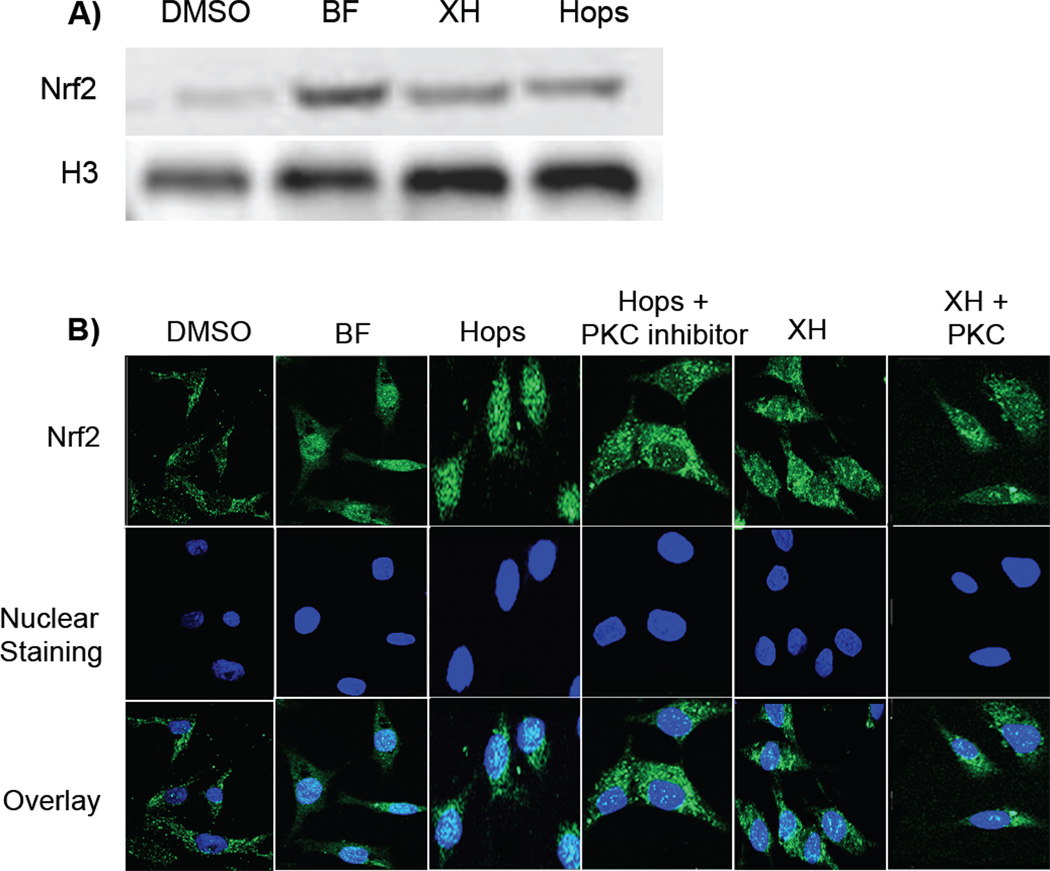
HepG2 cells were treated with test samples for 6 h. Nuclear fractions were prepared by using the nuclear extraction kit from Active Motif (Carlsbad, CA, USA). The Western blot analysis of nuclear lysates (30 µg protein/well) for Nrf2 and histone 3 (loading control) was carried out as previously described [10].
b) Confocal microscopy
HepG2 cells (5 × 104 cells/mL) were plated on each of 8 wells on a sterile NuncTM chambered cover glass. The following day, the cells were treated with test samples for 6 h. The cells were fixed with 3% paraformaldehyde solution, blocked with 10% goat antiserum, and incubated with rabbit polyclonal anti-Nrf2 antibody overnight. Cells were incubated with fluorescein conjugated anti-rabbit IgG secondary antibody for 1 h, and 4’'6-diamidino-2-phenylindole (DAPI) was added to the cells to detect nuclear staining. Imaging was performed with a Zeiss LSM 510 laser-scanning confocal microscope. The fluorescence signal from Nrf2 was monitored with a 488 nm laser and a 530 nm band pass filter. The DAPI nuclear staining signal was monitored with a 345 nm laser and 420 nm band pass filter. Images were analyzed using the analysis tool provided in the Zeiss biophysical software package and ImageJ. Images presented depict a representative picture of three independent experiments.
c) Antioxidant response element (ARE)-Luciferase Activity Assay
Hep-G2-ARE-C8 cells were stimulated with test samples with and without the PKC inhibitor, Ro-320432 (1 µM) (Calbiochem, Gibbstown, NJ). The PKC inhibitor was added 1 h prior to sample treatment and luciferase activity was determined according to the protocol provided by the manufacturer (Promega, Madison, WI) and as described previously [14].
In vitro NQO1 assay
Induction of NQO1 activity was assessed in the MCF-7 cell line. The cells were seeded in 96-well plates at a density of 9 × 104 cells/mL in 190 µl media. After 24 h incubation, test samples were added to each well and cells were incubated for an additional 48 h. The NQO1 assay and cytotoxicity assay were performed as previously described [14].
mRNA extraction and real-time PCR
MCF-7 cells were lysed in TRIzol reagent (Invitrogen). mRNA was purified as described by White and Kaestner [23]. mRNA was reverse transcribed using qScript cDNA Synthesis Kit (Quanta Biosciences, Gaithersburg, MD). Real-time PCR evaluation of the expression of NQO1 was performed using the TaqMan Gene Expression Assay (Applied Biosystems). The relative expression level of NQO1 mRNA was calculated using the Delta Ct method by comparing it with the relative mRNA levels of actin beta.
Statistical Analysis
Two-tailed Student’s T test (unpaired data) or one-way ANOVA with Dunnett’s post test was performed using GraphPad Prism version 4.0c for Macintosh (GraphPad Software, San Diego, CA). In all cases, a P value < 0.05 was considered to indicate significance. Experimental values are expressed as averages ± SD.
RESULTS
Distribution of XH and 8-PN in plasma, liver, and mammary gland
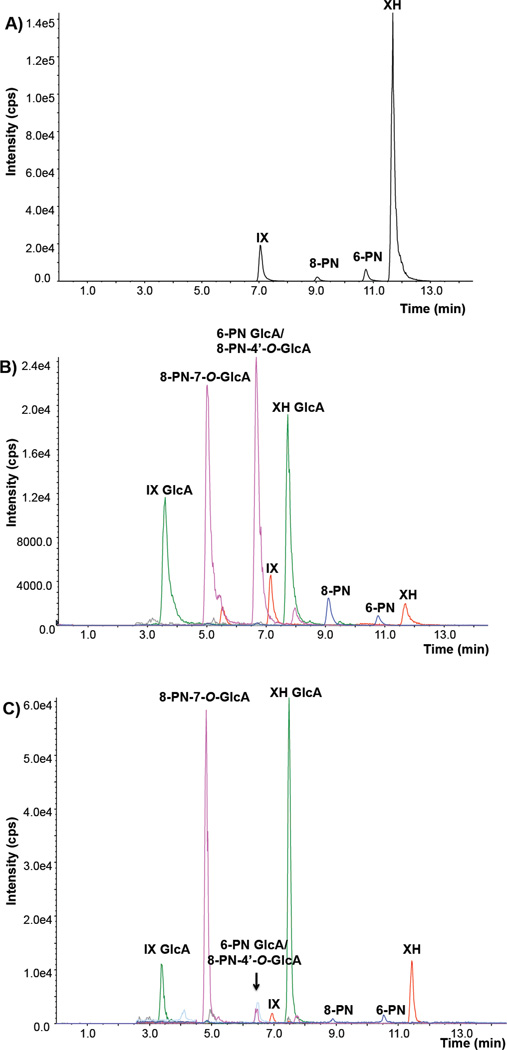
To confirm that XH and 8-PN reached the target tissues after oral administration of a standardized hop extract (Figure 2A), qualitative and quantitative LC-MS-MS analyses of rat plasma, liver, and mammary gland homogenates were carried out. 8-PN in relation to XH was found to be considerably enriched in liver tissue (Figure 2B), in particular considering 8-PN’s low concentration (0.084% 8-PN) and its relative abundance in the administered extract (XH > ~ 60-fold, IX > ~ 8-fold, 6-PN ≈ 8-PN, Figure 2A). Free 8-PN and 8-PN glucuronides were also detected in liver tissue samples of animals receiving pure XH (s.c.) (Supplemental data Figure 1A–C). In general, the prenylated flavonoids were predominantly found in the form of glucuronide conjugates (Figure 2B and 2C). The identities of the glucuronides were determined by comparison with standards obtained from separate incubations using rat liver microsomes and UDPGA (Supplemental data Figure 2A–C). 8-PN-7-O-glucuronide eluted at 5.0 min while 8-PN-4’-O-glucuronide coeluted with the major 6-PN glucuronide at 6.5 min. Based on our previous work with human hepatocytes, 8-PN is primarily glucuronidated at the 7-position, thus the peak eluting at 6.5 min is mostly comprised of 6-PN glucuronide [24]. The position of glucuronidation of 6-PN was not established in the current investigation. One significant difference between the liver and the mammary gland profiles (Figure 2B and 2C) was a notable enrichment of the 8-PN glucuronide compared to the 6-PN glucuronide. This was confirmed in mammary tissue and liver samples from animals receiving pure XH s.c. (Supplemental data: Figure 3A and B and Figure 1A–C).
Figure 2.
Combined negative ion electrospray LC-MS-MS SRM chromatograms of 8-PN, 6-PN, IX, and XH in the hop extract (A), of 8-PN, 6-PN, IX, XH, and their respective glucuronides in rat liver homogenate (B), and in rat mammary gland homogenate (C) from a rat that was treated with hop extract orally. SRM transitions: XH/IX: m/z 353-119; 8-PN/6-PN: m/z 339–219. XH GlcA/IX GlcA: m/z 529–353; 8-PN GlcA/6-PN GlcA: m/z 515–339. Based on our previous work with human hepatocytes 8-PN is primarily glucuronidated at the 7-position, thus the peak eluting at 6.9 min is mostly comprised of 6-PN glucuronide [24].
Regarding Phase I metabolites of XH, no peaks for free XH monohydroxylated metabolites (XH-OH) were detected; however, one monoglucuronide of XH-OH was observed in both plasma and liver tissue samples from rats treated with XH (s.c.) (Supplemental data Figure 4). Since no standards were available, the specific site of glucuronidation of XH-OH was not determined.
Results of the quantitative analysis of the free XH aglycones are summarized in Table 1. Subcutaneous administration of XH led to detectable levels of free XH in plasma and similar concentrations in the liver and mammary gland. In contrast, after oral administration of hop extract, no free XH could be detected in the plasma and the liver had much higher levels of free XH compared to the mammary gland. This likely indicates that XH, when given orally, is absorbed and enriched in the liver during the first pass, although a fraction reaches the mammary gland.
Table 1.
Free XH concentrations determined by LC-MS-MS in rat plasma, liver, and mammary gland.
| Treatment | [XH] | ||
|---|---|---|---|
| Plasma (n = 5) |
Liver (n = 5) |
Mammary gland (n = 5) |
|
| (ng/mL) | (µg/g tissue) | (µg/g tissue) | |
| XH Hops |
14.6 ± 8.2 < 1.0 a |
0.33 ± 0.08 0.70 ± 0.16 |
0.30 ± 0.41 0.05 ± 0.04 |
Values are expressed as mean ± S.D. of two independent experiments.
Below the limit of quantitation.
Influence of hops and XH on NQO1 and GST activity in vivo
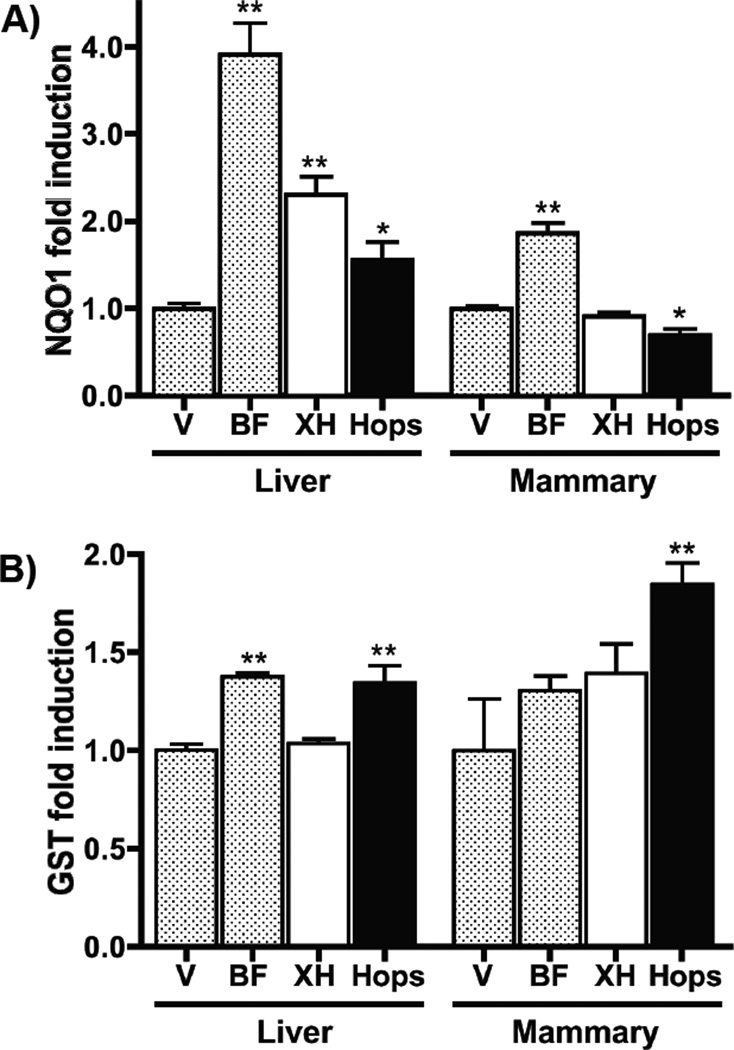
Consistent with a previous report [25], the positive control, BF, significantly induced NQO1 activity in the liver and mammary gland (Figure 3A). XH (s.c.) and the hop extract (p.o.) significantly induced NQO1 activity in the liver, but not in the mammary gland (Figure 3A). In fact, the oral hop extract significantly reduced NQO1 activity in the latter tissue. In the liver and mammary gland, the hop extract, but not XH, produced a significant induction of GST activity (Figure 3B).
Figure 3.
Influence of dietary hop extract and XH on NQO1 (A) and GST (B) activity in liver and mammary gland tissue. Sprague-Dawley rats (n=5) were treated with control diet, or diet containing either the positive control, BF (150 mg/kg BW per day), or the hop extract (7.5 g/kg BW per day), or control diet plus s.c. XH (100 mg/kg BW per day) for 4 days. The groups that were not treated with XH received additionally a vehicle control injection (s.c.). On day 4, the animals were sacrificed, and the liver and mammary gland were analyzed for enzyme activities as mentioned in Material and Methods. Enzyme induction was calculated by comparing the XH and hops group with the negative control group. Treatment groups were significantly different from the vehicle control group when (p<0.05) (*), p < 0.01 (**) using the unpaired, two-tailed t-test (n=5).
Hops and XH induce Nrf2 translocation (Figure 4, Mechanism A)
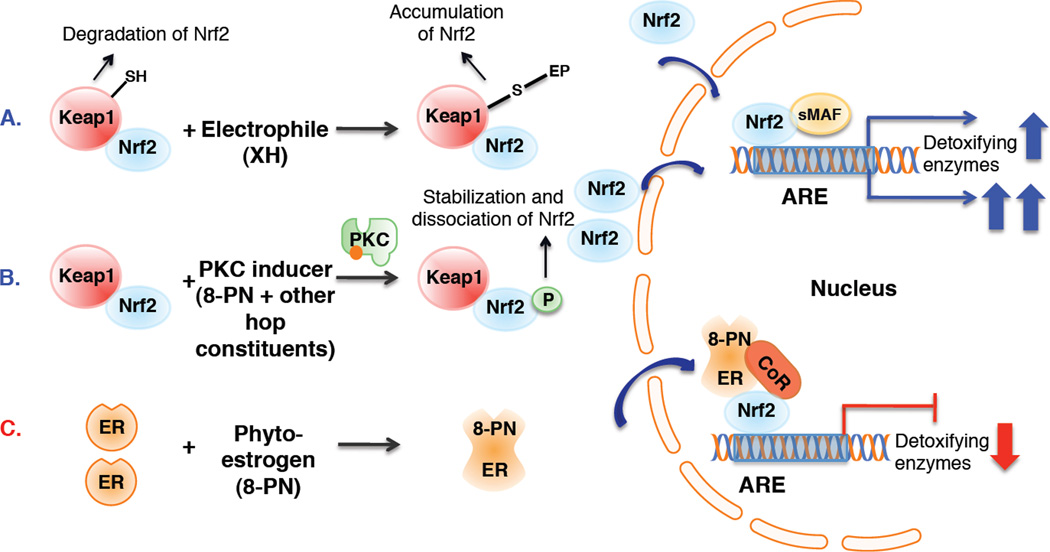
Figure 4.
Postulated mechanisms of ARE mediated upregulation of detoxification enzymes (A and B) by XH or other hop compounds, such as 8-PN, in liver cells and repression of Nrf2-ARE regulated genes (C) by the phytoestrogen 8-PN in ER positive breast cancer cells (MCF-7). A. In unstressed cells, Keap1 forms a complex with Nrf2 and facilitates the degradation of Nrf2 in the cytoplasm [14, 43]. The Michael acceptor, XH, covalently modifies Keap1, causing Nrf2 nuclear accumulation. In the nucleus, Nrf2 binds to the ARE with association of small MAF proteins and increases transcription of ARE regulated detoxifying enzymes [41]. B. Antioxidants have been reported to enhance ARE-regulated detoxification enzymes through phosphorylation of Nrf2 at serine 40 by PKC [27]. The data suggest that hops-mediated ARE induction is partially mediated by PKC and that 8-PN contributes towards this effect. C. The postulated mechanism for 8-PN mediated repression of ARE-regulated genes in MCF-7 cells is based on mechanistic studies by Ansell et al. and Yao et al. [46, 49]. Upon binding of estradiol or 8-PN to ERα, the activated ER might recruit corepressors, such as the histone deacetylase, SIRT, to Nrf2 leading to repression of Nrf2-ARE regulated transcription of detoxifying enzymes. The exact mechanism is still under investigation. Other regulating proteins are omitted in these figures for clarity.
It has previously been demonstrated that XH covalently modifies human Keap1 leading to induction of ARE-mediated detoxifying genes (Figure 4A) [14, 26]. To further elucidate the mechanism of enzyme induction, nuclear translocation of Nrf2 was analyzed in human hepatoma cells (HepG2). The concentrations chosen for hops (20 µg/mL) and XH (8 µM) showed high NQO1 inducing properties, but were not cytotoxic [14]. The known NQO1 inducer BF was chosen as a positive control [25]. Western blot analysis revealed that hops and XH treatment for 6 h lead to an enhanced concentration of free Nrf2 in the nucleus compared with vehicle control treated cells (Figure 5A and Supplemental data Figure 5). These results were confirmed by confocal microscopy (Figure 5B). Cells treated with vehicle exhibited diffuse staining with anti-Nrf2 across the entire cell, whereas cells treated with BF, hops, and XH clearly showed translocation of Nrf2 to the nucleus as visualized with the DAPI nuclear marker. However, hops induced Nrf2 translocation was stronger than XH mediated Nrf2 nuclear translocation.
Figure 5.
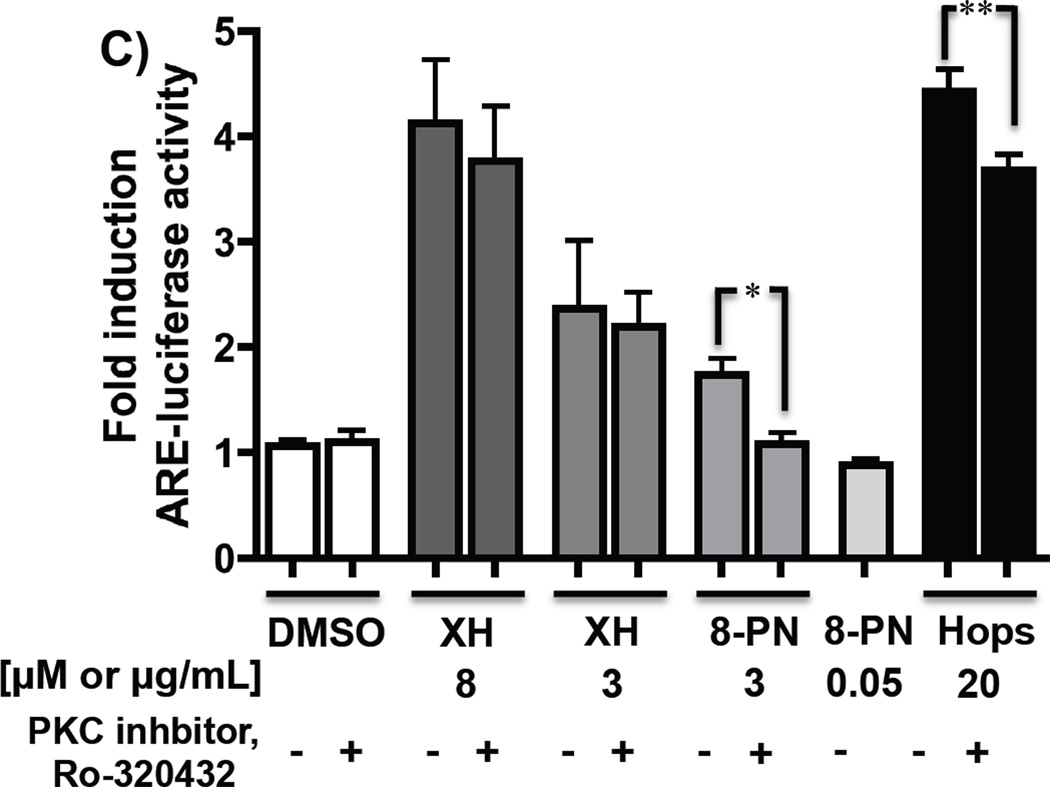
A and B: Hops and XH induce Nrf2 nuclear translocation. HepG2 cells were treated with DMSO (0.2%), positive control BF (1.7 µM), hop extract (20 µg/mL), and XH (8 µM) for 6 hrs and Nrf2 protein was visualized by western blot analysis (A) and confocal microscopy (B). A. Representative Western blot of nuclear lysates (30 µg protein/well) for Nrf2. Anti-Histone 3 (H3) was used as a nuclear marker and loading control. B. Nrf2 signal is indicated as green, DAPI nuclear staining as blue, and overlay shows co-localization of Nrf2 in the nucleus. The images obtained by confocal microscopy and Western blot represent typical representation of three independent experiments. C: Hops-induced ARE induction is partially mediated by PKC. Stably transfected HepG2-ARE-C8 cells were stimulated with vehicle control (DMSO (0.2%)), XH (8 µM), XH (3 µM), 8-PN (3 µM), hop extract (20 µg/mL) with and without the PKC inhibitor, Ro-320432 (1 µM) and 8-PN (50 nM) only treatment. Cells were harvested after 16 hours and the ARE-luciferase induction was determined and normalized to the protein concentration. Results are depicted as fold induction relative to the DMSO control values. Data represent mean values of at least three independent determinations in duplicate ± SD. **: P < 0.01 hops plus PKC inhibitor treatment is significantly different from hops only treatment; *: P < 0.05 8-PN plus PKC inhibitor treatment is significantly different from 8-PN only treatment (unpaired t-test).
Hops induced ARE activity is partially mediated by PKC (Figure 4, Mechanism B)
Alternatively, activation of PKC pathways can lead to direct phosphorylation of Nrf2, causing accumulation of Nrf2 in the nucleus [27] (Figure 4B). To analyze whether Nrf2 phosphorylation through PKC is involved in hops mediated upregulation of ARE-regulated genes, selective inhibition studies with the PKC inhibitor, Ro-320432, were performed. Pretreatment with the PKC inhibitor did not influence XH induced Nrf2 translocation as visualized by confocal microscopy (Figure 5B). However, hops induced Nrf2 translocation was partially inhibited by the PKC inhibitor. The involvement of PKC was further investigated by an ARE-luciferase assay in HepG2 cells stably transfected with an ARE-luciferase construct. Polyphenols, such as epigallocatechin, have been described to induce detoxification enzymes through this mechanism [28]. Therefore, 8-PN (3 µM and 50 nM) was also analyzed for its ARE-inducing properties. As expected, hops and XH (8 µM) induced ARE activity significantly (Figure 5C), while 8-PN moderately induced ARE-luciferase activity at a concentration of 3 µM. Only the ARE-luciferase induction by the hop extract and by 8-PN (3 µM) was significantly inhibited by the PKC inhibitor.
Influence of hops, XH, and 8-PN on NQO1 in breast cancer cells (Figure 4, Mechanism C)
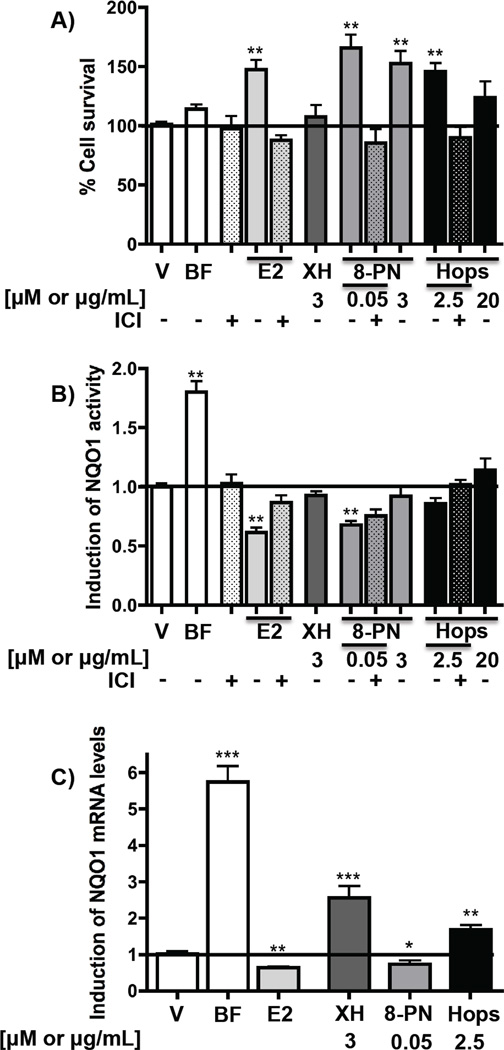
As the hop extract reduced NQO1 activity in the mammary gland in vivo, the influence of the extract, XH, and 8-PN on NQO1 was analyzed in the estrogen receptor (ER) positive breast cancer cell line, MCF-7. Concentrations that showed estrogenic activities as demonstrated by an increase of cell survival were used for estradiol (200 pM), 8-PN (50 nM), and hops (2.5 µg/mL) (Figure 6A). As described previously, estradiol (200 pM) significantly reduced NQO1 mRNA levels and activity (Figure 6B and C) [22]. Although the hop extract and XH increased NQO1 mRNA levels (Figure 6C), both treatments did not significantly modify NQO1 activity at the tested concentration (Figure 6B). 8-PN was similar to estradiol by decreasing NQO1 mRNA levels and NQO1 activity at a concentration at which it exhibited strong proliferative properties (50 nM) (Figure 6A–C). The NQO1 reducing activities of estradiol and 8-PN were inhibited by the antiestrogen, ICI 182,780 (Fulvestrant™), suggesting the involvement of ERα.
Figure 6.
Influence of hop extract, XH, and 8-PN on NQO1 in ER positive breast cancer cells (MCF-7). Cells were treated with DMSO (0.5%), BF (664.2 nM), estradiol (E2, 200 pM), the antiestrogen ICI 182,780 (100 nM) or XH, 8-PN and hop extract at the indicated concentrations for 48 hours. Determinations included % cell survival (A), NQO1 activity (B), and NQO1 mRNA levels (C). Results are depicted as fold induction relative to DMSO control values. Data represent mean of three independent determinations in triplicate ± SD. *: P < 0.05, **: P< 0.01 significantly different from DMSO control values (ANOVA).
DISCUSSION
The present study examines the influence of dietary hops on cytoprotective enzymes and the tissue distribution of its bioactive components, XH and 8-PN. XH was quantified in plasma, liver, and mammary gland homogenates of rats after treatment with a standardized hop extract (p.o., Figure 2A) and XH (s.c.) for 4 days. As previously reported [29], unconjugated XH was not detected in plasma after oral administration of the extract (Table 1), but free XH was detected in liver and mammary tissues (Table 1, Figure 2B and C). This demonstrates that XH has some oral bioavailability in vivo and accumulates in the liver and the mammary gland. Previously, various hydroxylated XH metabolites have been identified by rat and human liver microsomal incubations [8, 30]. Hydroxylation of a prenyl methyl group and epoxidation of the double bond leading to the formation of cyclized products were the major in vitro metabolism pathways [8, 30]. In this study, no peaks for free XH-OH metabolites were detected; however, one monoglucuronide of XH-OH was observed in both plasma and liver tissue samples from rats treated with XH (s.c.) (Supplemental data Figure 4). In general, XH and the prenylated flavonoids were mainly found in the form of glucuronide conjugates (Figure 2B and 2C).
Interestingly, although the concentration of XH in the administered hop extract was ~100 times higher than 8-PN (5.4% XH, 0.084% 8-PN, Figure 2A), 8-PN is more abundant in the liver of hop treated animals (Figure 2B), suggesting either poor absorption of XH and/or in vivo conversion of XH. Liver homogenates from animals that were treated with pure XH, also showed the presence of free 8-PN as well as 8-PN glucuronides suggesting at least in part in vivo conversion of XH into 8-PN (Supplemental data Figure 1A–C). This conversion occurs through a reaction sequence by which XH first non-enzymatically cyclizes into IX, which is then metabolically converted to 8-PN by cytochrome P450 enzymes and/or possibly another still to be identified enzyme(s) (Figure 1) [8, 9]. The relative levels of 8-PN versus XH in the liver tissue of hop treated animals were higher than those in the XH only treated animals (Figure 2B and Supplemental Figure 1A). There are several reasons that can account for this observation. First, after oral administration, XH has been shown to be poorly absorbed [9, 29, 31], which is in contrast to 8-PN that has excellent oral bioavailability [24, 32, 33]. Thus, the relative ratios of 8-PN versus XH present in the original extract (Figure 2A) will be distorted by very different oral bioavailabilities of these compounds. Furthermore, the conversion of XH into 8-PN through the sequence described above might be better after oral administration since the conditions for cyclization might be more favorable in the gut [8]. Previous studies have also demonstrated that IX can be metabolized by human intestinal microflora to form 8-PN [34]. Moreover, in addition to XH, there are other precursors of 8-PN present in the hop extract such as IX and desmethylxanthohumol (DMX) that are converted into 8-PN in vivo (Figure 1). Overall, the hop extract contains many precursors of 8-PN, which results in the increased ratio of 8-PN versus XH observed in the liver homogenates.
Remarkably, the concentration of 8-PN glucuronide in mammary gland was substantially higher than that of 6-PN glucuronide (Figure 2C, Supplemental data Figure 3A and B). In contrast, the concentration of both metabolites was similar in liver samples (Figure 2B, Supplemental data Figure 1A–C). This might be explained by a selective accumulation of the more estrogenic constituent, 8-PN, compared with 6-PN, in estrogen sensitive tissue through sequestration with the ER. For example, enrichment of estrogenic compounds in the nucleus of ER positive cells (Trojan Horse hypothesis) has been reported previously [35].
NQO1 and GST are important detoxification enzymes that protect cells from oxidative damage and represent important targets for chemoprevention [36]. One pathway that is considered to be important in estrogen carcinogenesis of the breast is the chemical pathway involving estrogen metabolism to reactive intermediates in the mammary gland, leading to damage of DNA and proteins [37–39]. NQO1 and GSTs are involved in detoxification of these reactive estrogen quinones, suggesting a role in prevention of estrogen-induced breast carcinogenesis [37]. In the present in vivo study, XH and the hop extract significantly induced NQO1 in liver tissue of rats (Figure 3A). Ferk et al. [40] administered XH (71 µg/kg BW) in the drinking water of rats for 7 days and analyzed the effect on NQO1 and GST activities in the liver. No significant differences in enzyme activities were detected between the control and XH group. However, the administered amount of XH in this study (71 µg/kg BW) was much lower than the XH contained in the hop extract in the present study (405 mg/kg BW per day), which might explain the lack of activity in the XH study. Also, other phytoconstituents in the hop extract might enhance the bioavailability of XH and/or add to the NQO1 inducing properties of the hop extract in the present study.
In contrast to the NQO1 inducing properties observed in liver tissue, XH (s.c.) did not influence NQO1 activity in the mammary gland (Figure 3A). Since the concentrations of XH were similar in the mammary and liver after s.c. XH administration (Table 1), bioavailability can be excluded as the reason for the lack of pharmacodynamic effect. Administration of oral hop extract unexpectedly decreased NQO1 activity in the mammary tissue suggesting that other hop constituents might be responsible for its NQO1 downregulating activity.
Differences in NQO1 modulation patterns by hops and XH in the liver and mammary gland suggest that various mechanisms are involved. Several mechanisms of NQO1 modulation have been reported [41]. Many detoxification enzymes, such as NQO1 and some GST isozymes, contain an ARE sequence in their promotor region [42]. Three general ARE mechanisms have been proposed that lead to modulation of NQO1 activity by bioactive compounds (Figure 4) [14, 22, 28]. Genes with an ARE-promoter are activated by the transcription factor Nrf2, which is sequestered by Keap1 in unstressed cells [14, 43]. The Nrf2 translocation studies in HepG2 cells (Figure 5A and B) demonstrate that XH and the hop extract enhance Nrf2 concentration in the nucleus. These data along with the Keap1 modification studies and ARE induction experiments [14] further substantiate the conclusion that the electrophile XH induces NQO1 through covalently modifying Keap1, leading to accumulation of Nrf2 in the nucleus and to induction of ARE-regulated genes in liver cells (Mechanism A, Figure 4A).
Another mechanism that is involved in the regulation of Nrf2-ARE-mediated gene expression implies phosphorylation of Nrf2 by PKC (Figure 4B) [27, 44]. Phosphorylation of Nrf2 by PKC in response to antioxidants, such as black tea polyphenols, has been shown to support the release, stabilization, and nuclear translocation of Nrf2 [44, 45]. Therefore, 8-PN was also analyzed for ARE-induction. The results suggest that hop induction of detoxification enzymes is partially mediated by PKC (Figure 5C). 8-PN (3 µM) increased ARE-induction moderately, which could be inhibited by the PKC inhibitor. Apart from 8-PN, other polyphenols in the hop extract may contribute to this activity, in particular since concentrations of 8-PN (50 nM) that are contained in 20 µg/mL of the hop extract did not enhance ARE-induction. XH seems to be mainly responsible for hop-mediated induction of ARE-regulated genes. However, 3 µM of XH equal to the XH concentration, which is contained in 20 µg/ml of the hop extract, did not induce ARE-luciferase activity as strongly as the hop extract (Figure 5C), suggesting that other compounds, such as other chalcones, might add to the overall activity of the extract.
In contrast to the upregulation of ARE-mediated genes, a third pathway suggests the repression of cytoprotective enzymes through involvement of the ER and the ARE in estrogen sensitive cells (Figure 4C) [46]. For example, it has been demonstrated that estradiol down-regulates NQO1 in the uterus of mice and in the mammary gland of ACI rats [47, 48]. Wagner et al. showed that also the phytoestrogen, genistein, inhibited the expression of NQO1 mRNA in MCF-7 cells [22]. Due to the observed decrease of NQO1 activity by the hop extract in mammary tissue, the effect of hops, XH, and 8-PN was analyzed in MCF-7 cells. Applying an estrogenic active concentration (2.5 µg/ml) of the hop extract only moderately reduced NQO1 activity (Figure 6B). Interestingly, similar to estradiol, 8-PN (50 nM) significantly decreased NQO1 mRNA and NQO1 activity (Figure 6B and C). Cotreatment of estradiol and 8-PN with the antiestrogen ICI 182,780 prevented reduction of NQO1 activity by estradiol and 8-PN.
The mechanism of repression of Nrf2 regulated genes by estrogenic compounds has not been entirely elucidated. However, recent investigations suggest the involvement of the ER and Nrf2 in the modulation of NQO1 by estradiol. The investigations by Ansell et al. [49] demonstrated that estradiol represses ARE mediated enzymes through recruiting ERα and Nrf2 at the ARE promoter. Chip assay in the breast cancer cell line, T47D, revealed that estrogen recruits ERα and the class III histone deacetylase, SIRT1, to the NQO1 promoter [46]. It is likely that similar to estradiol, 8-PN binds to ERα and leads to repression of Nrf2-ARE regulated detoxification enzymes through recruiting corepressors to the Nrf2 complex (Figure 4C, Mechanism C).
Regarding the GST activity, hops but not XH induced GST activity in the liver and mammary tissue (Figure 3B) suggesting that other compounds in hops might be responsible for the observed GST induction. The GST enzyme family consists of a variety of GST isozymes that are induced through a variety of different receptors or transcription factors, such as Nrf2, the pregnane X, and the aryl hydrocarbon receptors [50].
Various pharmacodynamic effects have been described for hops and its prenylated phenols. For example, it has been shown that the same hop extract (5 µg/mL) and 8-PN (50 nM) inhibit 4-hydroxylation of estradiol and estrogen induced malignant cell transformation in an ERα negative human breast epithelial cell line (MCF-10A) [10]. In addition, aromatase inhibiting activities have been reported for 8-PN and XH [51]. Various reports describe anti-inflammatory activities, in particular for hop extracts and XH [12, 52, 53]. The phytoestrogen, 8-PN, increases uterine growth in ovariectomized rats. However, the hop extract did not show uterotrophic effects, suggesting that other compounds in hops might counteract the estrogenic activity of 8-PN [19]. These pharmocodynamic effects of hops might provide beneficial health effects in postmenopausal women.
Overall, the present studies demonstrate that after oral administration of a hop extract, XH and 8-PN reach the liver and the mammary gland. Compared to plasma, XH accumulated in these tissues. Considering the low concentration (0.084% 8-PN) and relative abundance of 8-PN in the administrated extract (XH >~60-fold, IX >~8-fold), 8-PN was considerably enriched in the liver and the mammary gland. While hops and XH induced NQO1 in the liver suggesting a cytoprotective effect, 8-PN is likely responsible for hop-mediated reduction of NQO1 activity in the mammary gland.
Since hop extracts show various biological activities, future in vivo studies are warranted to determine whether hop dietary supplements have potential as cytoprotective agents.
Supplementary Material
Acknowledgements
We acknowledge the provision of the hop extract from Hopsteiner (S. S. Steiner, New York, NY, and Steiner Hopfen GmbH, Mainburg, Germany) and the support by A. Schwarz and M. Biendl. We are thankful to J. P. Whitlock, Jr. for providing the Hepa1c1c7 cells, to A. N. Kong, C. Chen (Rutgers University, Piscataway, NJ), and W. E. Fahl (University of Wisconsin, Madison, WI) for supplying the HepG2-ARE-C8 cells, and L. Lehman (University of Würzburg, Würzburg, Germany) for providing the MCF-7 (BUS) cell line. The authors thank D. Lantvit and C. Overk (UIC) for their help in the animal study, M. Main (UIC) for phytochemical assistance, and A. Menassi and G. Appendino (Università del Piemonte Orientale, Novara, Italy) for synthetic 8-PN. Support for this work was through Grant P50 AT00155 from the Office of Dietary Supplements, and the National Center for Complementary and Alternative Medicine, and through grant CA135237 from the National Cancer Institute.
Abbreviations
- ARE
antioxidant response element
- BF
4′-bromoflavone
- DMX
desmethylxanthohumol
- ER
estroge receptor
- 8-PN
8-prenylnaringenin
- GST
Glutathione S-transferase
- HT
hormone therapy
- IX
isoxanthohumol
- Keap1
Kelch-like ECH-associated protein
- NQO1
NAD(P)H-quinone oxidoreductase 1
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- SRM
selected reaction monitoring
- WHI
Women’s Health Initiative
- XH
xanthohumol
- XH-OH
xanthohumol monohydroxylated metabolites
Footnotes
The authors have declared no conflict of interest.
References
- 1.Chadwick LR, Pauli GF, Farnsworth NR. The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties. Phytomedicine. 2006;13:119–131. doi: 10.1016/j.phymed.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowe J, Li XF, Kinsey-Jones J, Heyerick A, et al. The hop phytoestrogenm 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes. J. Endocrinol. 2006;191:399–405. doi: 10.1677/joe.1.06919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overk CR, Yao P, Chadwick LR, Nikolic D, et al. Comparison of the in vitro estrogenic activities of compounds from hops (Humulus lupulus) and red clover (Trifolium pratense) J. Agric. Food Chem. 2005;53:6246–6253. doi: 10.1021/jf050448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolca S, Possemiers S, Maervoet V, Huybrechts I, et al. Microbial and dietary factors associated with the 8-prenylnaringenin producer phenotype: a dietary intervention trial with fifty healthy post-menopausal Caucasian women.Br. J. Nutr. 2007;98:950–959. doi: 10.1017/S0007114507749243. [DOI] [PubMed] [Google Scholar]
- 5.Erkkola R, Vervarcke S, Vansteelandt S, Rompotti P, et al. A randomized, double-blind, placebo-controlled, cross-over pilot study on the use of a standardized hop extract to alleviate menopausal discomforts. Phytomedicine. 2010;17:389–396. doi: 10.1016/j.phymed.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Heyerick A, Vervarcke S, Depypere H, Bracke M, et al. A first prospective, randomized double-blind placebo-controlled study on the use of a standardized hop extract to alleviate menopausal discomforts. Maturitas. 2006;54:164–175. doi: 10.1016/j.maturitas.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Nikolic D, Li Y, Chadwick LR, Pauli GF, et al. Metabolism of xanthohuml and isoxanthohumol, prenylated flavonoids from hops (Humulus lupulus L.), by human liver microsomes. J. Mass Spectrom. 2005;40:289–299. doi: 10.1002/jms.753. [DOI] [PubMed] [Google Scholar]
- 9.Legette L, Ma L, Reed RL, Miranda CL, et al. Pharmacokinetics of xanthohumol and metabolites in rats after oral and intravenous administration. Molecular nutrition & food research. 2012;56:466–474. doi: 10.1002/mnfr.201100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemachandra LP, Madhubhani P, Chandrasena R, Esala P, et al. Hops (Humulus lupulus) inhibits oxidative estrogen metabolism and estrogen-induced malignant transformation in human mammary epithelial cells (MCF-10A) Cancer Prev Res. 2012;5:73–81. doi: 10.1158/1940-6207.CAPR-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plazar J, Filipic M, Groothuis GM. Antigenotoxic effect of Xanthohumol in rat liver slices. Toxicol. In Vitro. 2008;22:318–327. doi: 10.1016/j.tiv.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Dorn C, Heilmann J, Hellerbrand C. Protective effect of xanthohumol on toxin-induced liver inflammation and fibrosis. Int J Clin Exp Pathol. 2012;5:29–36. [PMC free article] [PubMed] [Google Scholar]
- 13.Harikumar KB, Kunnumakkara AB, Ahn KS, Anand P, et al. Modification of the cysteine residues in IkappaBalpha kinase and NF-kappaB (p65) by xanthohumol leads to suppression of NF-kappaB-regulated gene products and potentiation of apoptosis in leukemia cells. Blood. 2009;113:2003–2013. doi: 10.1182/blood-2008-04-151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietz BM, Kang YH, Liu G, Eggler AL, et al. Xanthohumol isolated from Humulus lupulus Inhibits menadione-induced DNA damage through induction of quinone reductase. Chem. Res. Toxicol. 2005;18:1296–1305. doi: 10.1021/tx050058x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magalhaes PJ, Carvalho DO, Cruz JM, Guido LF, et al. Fundamentals and health benefits of xanthohumol, a natural product derived from hops and beer. Nat Prod Commun. 2009;4:591–610. [PubMed] [Google Scholar]
- 16.Chadwick LR, Nikolic D, Burdette JE, Overk CR, et al. Estrogens and congeners from spent hops (Humulus lupulus L.) J. Nat. Prod. 2004;67:2024–2032. doi: 10.1021/np049783i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chadwick LR. Estrogens and congeners from spent hops. University of Illinois at Chicago: 2004. Ph.D. dissertation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer K, Bipp HP. Generation of organic acids and monosaccharides by hydrolytic and oxidative transformation of food processing residues. Bioresource technology. 2005;96:831–842. doi: 10.1016/j.biortech.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Overk CR, Guo J, Chadwick LR, Lantvit DD, et al. In vivo estrogenic comparisons of Trifolium pratense (red clover) Humulus lupulus (hops), and the pure compounds isoxanthohumol and 8-prenylnaringenin. Chem. Biol. Interact. 2008 doi: 10.1016/j.cbi.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauli GF, Jaki BU, Lankin DC. A routine experimental protocol for qHNMR illustrated with Taxol. J. Nat. Prod. 2007;70:589–595. doi: 10.1021/np060535r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuendet M, Guo J, Luo Y, Chen S, et al. Cancer chemopreventive activity and metabolism of isoliquiritigenin, a compound found in licorice. Cancer Prev Res. 2010;3:221–232. doi: 10.1158/1940-6207.CAPR-09-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner J, Jiang L, Lehmann L. Phytoestrogens modulate the expression of 17alpha-estradiol metabolizing enzymes in cultured MCF-7 cells. Adv. Exp. Med. Biol. 2008;617:625–632. doi: 10.1007/978-0-387-69080-3_65. [DOI] [PubMed] [Google Scholar]
- 23.White P, Kaestner KH, Stocker C, editors. Type 2 Diabetes, Methods in Molecular Biology. Springer; 2009. pp. 239–261. [DOI] [PubMed] [Google Scholar]
- 24.Nikolic D, Li Y, Chadwick LR, van Breemen RB. In vitro studies of intestinal permeability and hepatic intestinal metabolism of 8-prenylnaringenin, a potent phytoestrogen from hops (Humulus lupulus L.) Pharm. Res. 2006;23:864–872. doi: 10.1007/s11095-006-9902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song LL, Kosmeder JW, 2nd, Lee SK, Gerhäuser C, et al. Cancer chemopreventive activity mediated by 4'-bromoflavone a potent inducer of phase II detoxification enzymes. Cancer Res. 1999;59:578–585. [PubMed] [Google Scholar]
- 26.Liu G, Eggler AL, Dietz BM, Mesecar AD, et al. A screening method for the discovery of potential cancer chemoprevention agents based on mass spectrometric detection of alkylated Keap1. Anal. Chem. 2005;77:6407–6414. doi: 10.1021/ac050892r. [DOI] [PubMed] [Google Scholar]
- 27.Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 28.Ogborne RM, Rushworth SA, O'Connell MA. Epigallocatechin activates haem oxygenase-1 expression via protein kinase Cdelta and Nrf2. Biochem. Biophys. Res. Commun. 2008;373:584–588. doi: 10.1016/j.bbrc.2008.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avula B, Ganzera M, Warnick JE, Feltenstein MW, et al. High-performance liquid chromatographic determination of xanthohumol in rat plasma, urine, and fecal samples. J. Chromatogr. Sci. 2004;42:378–382. doi: 10.1093/chromsci/42.7.378. [DOI] [PubMed] [Google Scholar]
- 30.Yilmazer M, Stevens JF, Deinzer ML, Buhler DR. In vitro biotransformation of a xanthohumol, a flavonoid from hops (Humulus lupulus) by rat liver microsomes. Drug Metabolism & Disposition. 2001;29:223–231. [PubMed] [Google Scholar]
- 31.Pang Y, Nikolic D, Zhu D, Chadwick LR, et al. Binding of the hop (Humulus lupulus L.) chalcone xanthohumol to cytosolic proteins in Caco-2 intestinal epithelial cells. Mol. Nutr. Food Res. 2007;51:872–879. doi: 10.1002/mnfr.200600252. [DOI] [PubMed] [Google Scholar]
- 32.Yuan Y, Nikolic D, Wright B, Dahl JH, et al. Proceedings of the 59th ASMS Conference on Mass Spectrometry and Allied Topics. Denver, CO: Quantitative analysis of hop prenylflavonoids in human serum us ing UHPLC-MS-MS; p. 1267. [Google Scholar]
- 33.Rad M, Humpel M, Schaefer O, Schoemaker RC, et al. Pharmacokinetics and systemic endocrine effects of the phyto-oestrogen 8-prenylnaringenin after single oral doses to postmenopausal women. Br. J. Clin. Pharmacol. 2006;62:288–296. doi: 10.1111/j.1365-2125.2006.02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Possemiers S, Bolca S, Grootaert C, Heyerick A, et al. The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine. J. Nutr. 2006;136:1862–1867. doi: 10.1093/jn/136.7.1862. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Wijewickrama GT, Peng KW, Dietz BM, et al. Estrogen Receptor {alpha} Enhances the Rate of Oxidative DNA Damage by Targeting an Equine Estrogen Catechol Metabolite to the Nucleus. J. Biol. Chem. 2009;284:8633–8642. doi: 10.1074/jbc.M807860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwak MK, Kensler TW. Targeting NRF2 signaling for cancer chemoprevention. Toxicol. Appl. Pharmacol. 2010;244:66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolton JL, TM P, editors. Chemical Carcinogenesis. Springer; 2011. pp. 74–94. [Google Scholar]
- 38.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. The New England journal of medicine. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 39.Lehmann L, Jiang L, Wagner J. Soy isoflavones decrease the catechol-O-methyltransferase-mediated inactivation of 4-hydroxyestradiol in cultured MCF-7 cells. Carcinogenesis. 2008;29:363–370. doi: 10.1093/carcin/bgm235. [DOI] [PubMed] [Google Scholar]
- 40.Ferk F, Huber WW, Filipic M, Bichler J, et al. Xanthohumol, a prenylated flavonoid contained in beer, prevents the induction of preneoplastic lesions and DNA damage in liver and colon induced by the heterocyclic aromatic amine amino-3-methyl-imidazo[4,5-f]quinoline (IQ) Mutat. Res. 2010 doi: 10.1016/j.mrfmmm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Keum YS, Jeong WS, Kong AN. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat. Res. 2004;555:191–202. doi: 10.1016/j.mrfmmm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 42.Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 43.Eggler AL, Liu G, Pezzuto JM, van Breemen RB, et al. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci USA. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niture SK, Jain AK, Jaiswal AK. Antioxidant-induced modification of INrf2 cysteine 151 and PKC-delta-mediated phosphorylation of Nrf2 serine 40 are both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. J. Cell Sci. 2009;122:4452–4464. doi: 10.1242/jcs.058537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Patel R, Maru G. Polymeric black tea polyphenols induce phase II enzymes via Nrf2 in mouse liver and lungs. Free Radic. Biol. Med. 2008;44:1897–1911. doi: 10.1016/j.freeradbiomed.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Yao Y, Brodie AM, Davidson NE, Kensler TW, et al. Inhibition of estrogen signaling activates the NRF2 pathway in breast cancer. Breast Cancer Res. Treat. 2010;124:585–591. doi: 10.1007/s10549-010-1023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ansell PJ, Espinosa-Nicholas C, Curran EM, Judy BM, et al. In vitro and in vivo regulation of antioxidant response element-dependent gene expression by estrogens. Endocrinology. 2004;145:311–317. doi: 10.1210/en.2003-0817. [DOI] [PubMed] [Google Scholar]
- 48.Singh B, Bhat NK, Bhat HK. Induction of NAD(P)H-quinone oxidoreductase 1 by antioxidants in female ACI rats is associated with decrease in oxidative DNA damage and inhibition of estrogen-induced breast cancer. Carcinogenesis. 2012;33:156–163. doi: 10.1093/carcin/bgr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ansell PJ, Lo SC, Newton LG, Espinosa-Nicholas C, et al. Repression of cancer protective genes by 17beta-estradiol: ligand-dependent interaction between human Nrf2 and estrogen receptor alpha. Mol. Cell. Endocrinol. 2005;243:27–34. doi: 10.1016/j.mce.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Higgins LG, Hayes JD. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab. Rev. 2011;43:92–137. doi: 10.3109/03602532.2011.567391. [DOI] [PubMed] [Google Scholar]
- 51.Monteiro R, Becker H, Azevedo I, Calhau C. Effect of hop (Humulus lupulus L.) flavonoids on aromatase (estrogen synthase) activity. J. Agric. Food Chem. 2006;54:2938–2943. doi: 10.1021/jf053162t. [DOI] [PubMed] [Google Scholar]
- 52.Dorn C, Kraus B, Motyl M, Weiss TS, et al. Xanthohumol, a chalcon derived from hops, inhibits hepatic inflammation and fibrosis. Molecular nutrition & food research. 2010;54(Suppl 2):205–213. doi: 10.1002/mnfr.200900314. [DOI] [PubMed] [Google Scholar]
- 53.Hougee S, Faber J, Sanders A, Berg WB, et al. Selective inhibition of COX-2 by a standardized CO2 extract of Humulus lupulus in vitro and its activity in a mouse model of zymosan-induced arthritis. Planta Med. 2006;72:228–233. doi: 10.1055/s-2005-916212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.