Abstract
Aim
We hypothesized that microcirculatory dysfunction, similar to that seen in sepsis, occurs in post-cardiac arrest patients and that better microcirculatory flow will be associated with improved outcome. We also assessed the association between microcirculatory dysfunction and inflammatory markers in the post-cardiac arrest state.
Methods
We prospectively evaluated the sublingual microcirculation in post-cardiac arrest patients, severe sepsis/septic shock patients, and healthy control patients using Sidestream Darkfield Microscopy. Microcirculatory flow was assessed using the Microcirculation flow index (MFI) at 6 and 24 hours in the cardiac arrest patients, and within 6 hours of Emergency Department admission in the sepsis and control patients.
Results
We evaluated 30 post-cardiac arrest patients, 16 severe sepsis/septic shock patients, and 9 healthy control patients. Sublingual microcirculatory blood flow was significantly impaired in post-cardiac arrest patients at 6 hours (MFI 2.6 [IQR: 2 - 2.9]) and 24 hours (2.7 [IQR: 2.3 - 2.9]) compared to controls (3.0 [IQR: 2.9 - 3.0]; p < 0.01 and 0.02, respectively). After adjustment for initial APACHE II score, post-cardiac arrest patients had significantly lower MFI at 6-hours compared to sepsis patients (p < 0.03). In the post-cardiac arrest group, patients with good neurologic outcome had better microcirculatory blood flow as compared to patients with poor neurologic outcome (2.9 [IQR: 2.4 – 3.0] vs. 2.6 [IQR: 1.9 – 2.8]; p < 0.03). There was a trend toward higher median MFI at 24 hours in survivors vs. non-survivors (2.8 [IQR: 2.4 – 3.0] vs. 2.6 [IQR: 2.1-2.8] respectively; p < 0.09). We found a negative correlation between MFI-6 and vascular endothelial growth factor (VEGF) (r= −0.49, P= 0.038). However, after Bonferroni adjustment for multiple comparisons, this correlation was statistically non-significant.
Conclusion
Microcirculatory dysfunction occurs early in post-cardiac arrest patients. Better microcirculatory function at 24 hours may be associated with good neurologic outcome.
1. Introduction
Every year 300,000 patients in the United States suffer from out of hospital cardiac arrest.1 Despite improvements in resuscitation efforts over the last fifty years, the in-hospital mortality after restoration of systemic circulation remains high.2 Ischemia/reperfusion injury after return of spontaneous circulation (ROSC) is one of the key pathologic processes contributing to the post-cardiac arrest syndrome3,4. The post-cardiac arrest syndrome is characterized by systemic inflammation and has been referred to a “sepsis-like” syndrome.5,6 Septic shock is accompanied by both macrocirculatory and microcirculatory dysfunction.7,8 Thus, microcirculatory dysfunction may be present in the post-cardiac arrest state as well.
The microcirculation consists of arterioles, capillaries and post-capillary venules (less than 100 micrometers) and is responsible for the fine tuning of tissue perfusion.9 Sublingual microcirculation can be observed directly using Sidestream Dark Field microscopy (SDF).10 SDF records video images of microcirculation over a 1 mm field of view with stroboscopic green (530 nm) illumination. Red blood cells appear dark against a bright background due to light reflected by surrounding tissue surfaces. During cardiac arrest and resuscitation, animal studies using SDF have shown decreased capillary density with increased blood flow heterogeneity in sublingual microcirculation.11 In humans, sublingual microcirculation has been studied in a number of disease states including sepsis7,12, cardiogenic shock 13, cardiac surgery 14 and during cardiopulmonary resuscitation.15 Recent small studies have examined sublingual microvascular flow in post-cardiac arrest patients but have yet to be validated.16,17
We hypothesized that defects in sublingual microcirculation, similar to those seen in sepsis, would be present in the post-cardiac arrest state. We further hypothesized that better microcirculatory dysfunction would be associated with better outcomes in these patients. In order to test these hypotheses, we conducted a prospective observational study to assess the sublingual microcirculation in post-cardiac arrest patients compared to healthy controls and patients with sepsis. We also assessed the association between microcirculatory dysfunction and inflammatory markers in the post-cardiac arrest state.
2. Methods
Study Design and patient selection
This was a prospective, observational single center study in a tertiary care hospital with 50,000 emergency department visits per year. We enrolled patients who presented to the emergency department (ED) after out-of-hospital cardiac arrest or patients who suffered in-hospital cardiac arrest. Patients with severe sepsis or septic shock as well as healthy controls were enrolled as a comparator. Eligible subjects were identified prospectively in the ED or intensive care units (ICU).
Post-cardiac arrest group: Inclusion criteria: (a) Cardiac arrest defined specifically as the absence of detectable circulatory flow (no blood pressure or pulse) with return of spontaneous circulation. Exclusion criteria: (a) Trauma-related arrest (b) Suspected or confirmed septic shock prior to arrest or as suspected etiology of arrest.
Sepsis group: Inclusion criteria: (a) Severe sepsis or septic shock. Severe sepsis was defined as sepsis in association with one or more organ failures (respiratory, cardiovascular, renal, coagulation, hepatic, central nervous system). Septic shock was defined as severe sepsis with hypotension (SBP < 90 or MAP <65) unresponsive to adequate fluid resuscitation.18 Exclusion criteria: (a) suspected myocardial ischemia (b) significant traumatic injury (e.g. fracture or multi-trauma) (c) the existence of a baseline inflammatory syndrome such as arthritis or inflammatory bowel disease
Control group: Inclusion criteria: (a) Presenting to the emergency department with minor complaints without the need for admission
The following exclusion criteria applied to all groups: (a) pregnancy (b) any anticipated inability to tolerate or cooperate with study procedures. All groups consisted of adults (≥ 18 years) and either the patients or their legally authorized surrogate provided written informed consent. The study protocol was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center.
Study Protocol
All consented post-cardiac arrest patients had a microcirculation flow index (MFI) measured at time 6 and 24 hours after return of spontaneous circulation (ROSC). We also measured inflammatory markers for cardiac-arrest patients at 24 hours (E-selectin, V-cam, I-cam, IL_6, IL_8, IL_10, TNFα, and VEGF). The practice at our hospital is to use therapeutic hypothermia (arctic sun) for 24 hours, with a target core body temperature of 33 degrees Celsius. In patients who had induced hypothermia, the MFI measurements (both at 6 and 24 hours) were measured while patient is hypothermic. All consented patients with sepsis and controls had an MFI measured within 6 hours of presentation to the emergency department
Data collection
In all post-cardiac arrest patients, we collected data regarding first rhythm, time to CPR, bystander CPR, time to ROSC, need for defibrillation and drugs administered during CPR. We calculated an Acute Physiology and Chronic Health Evaluation II (APACHE II) score19 and collected demographic data and co-morbidities for all patients. Lab data, including complete blood count, electrolytes, creatinine, bilirubin and lactate, were recorded if ordered by the clinical team. Other parameters, including temperature, hemodynamics, use of vasopressors, mechanical ventilation, renal replacement therapy, 24-hour fluid balance, ICU length of stay and ICU mortality were collected. Neurologic outcome was assessed at hospital discharge using the cerebral performance category (CPC) scale and good neurological outcome was defined as a CPC of 1-2.20
Image Acquisition and scoring
Patients underwent SDF imaging at the bedside (MicroScan provided by Microvision Medical, Amsterdam, Netherlands). SDF is a technique that allows direct visualization of submucosal capillary beds, which have the same embryonal and anatomic origin as splanchnic capillaries. The videomicroscope emits light in the green spectrum that is scattered by background tissue and absorbed by red blood cells. This results in red blood cells that appear dark against a light background, thus outlining arterioles, capillaries, and venules that contain red blood cells. These videos were saved on a laptop without identifying information and uploaded to a server in secure fashion using a customized software package (StudyMaker, Newton, MA).
We obtained multiple video sequences from each patient from multiple locations (5-10 sampling points). Image analysis was performed by an investigator blinded to the clinical data and not involved in image acquisition. Prior to analysis, video sequences were screened and edited to remove portions with severe image artifact. Screening criteria for individual video clips was based on a qualitative scoring metric, the Microscan Image Quality Score, with six categories: Illumination (Brightness/Contrast), Duration, Focus, Content, Stability, and Pressure. If the flow in the medium/large vessels stops and starts (or changes speed or direction) during the course of the video, pressure artifact is deemed present. Video clips with pressure artifacts were rejected from analysis. Each category was given a score of 0 (good), 1 (acceptable), 10 (unacceptable). A Microscan Image Quality Score equal to the sum of scores in all categories was assigned to each video clip. Video clips were sorted by quality score, and the three clips with the best quality were selected for analysis. We then took the average to yield the final MFI for a given timepoint. This resulted in a mean of 2.2 [SD 1.5] videos per patient to address the heterogeneity described. Clips with unacceptable quality scores (≥ 10) were excluded from analysis.
The primary outcome measure (determined a priori) was average microvascular flow index (MFI), a measure of flow velocity, which was determined using a semiquantitative technique employed in previous studies.21 Video images were partitioned into 4 quadrants and each quadrant visually rated based on the predominate flow (0 = absent flow, 1 = intermittent flow, 2 = continuous but slowed flow, 3 = continuous brisk flow). Each image was assigned an average score over all quadrants and all videos for a given patient were averaged to obtain an overall flow velocity score.
Statistical analysis
Simple descriptive statistics were used to describe the study population. Goodness of fit testing was used to analyze the normality of the data prior to statistical modeling. Data for continuous variables are presented as means with standard deviations or medians with inter-quartile ranges (IQR) depending on the distribution of the variable. Categorical data are presented as frequencies with percentages. For our primary outcome, we used a Wilcoxon rank sum test to compare the MFI between groups and subsequently used general linear modeling to adjust for clinically significant variables. For outcome comparison p-value, less than 0.05 was considered statistically significant. Bonferroni adjustment was done for the analysis of multiple vascular biomarkers, and a p value less than 0.0125 was considered significant for that portion of the analysis. All analyses were performed in SAS v9.2 (SAS Institute Inc., Cary, NC, USA).
3. Results
Characteristics of the Study Subjects
We enrolled 30 post-cardiac arrest patients (17 in-hospital cardiac arrest and 13 out-of-hospital cardiac arrest), 16 sepsis patients (9 severe sepsis and 7 septic shock) and 9 controls. Baseline characteristics of all patients are shown in Table 1. Fourteen of the 30 cardiac-arrest patients were treated with therapeutic hypothermia while 16 patients were not due to the presence of contraindications. Microcirculation flow index at 6 hours (MFI-6) was measured in 22 patients and Microcirculation flow index at 24 hours (MFI-24) in 23 patients; both MFI-6 and MFI-24 were obtained in 15 patients. Hemodynamic indices were measured and their median values are shown in Table 1. Fifteen patients had good neurological outcome (CPC 1-2) at hospital discharge after cardiac arrest and overall mortality was 43% in this group.
Table 1. Baseline characteristics and outcomes.
| Demographics | Controls (n = 9) | Sepsis (n = 16) | Cardiac Arrest (n = 30) |
|---|---|---|---|
| Gender (% female) | 22 | 44 | 30 |
| Age (years) | 48 (34 - 68) | 64 (50 - 75) | 66 (54 - 79) |
| Race (% white) | 100 | 88 | 90 |
| Vital signs | |||
| Temperature (F) | 97.8 (97.2 - 98.1) | 100.1 (97.6 - 101.3) |
96.7 (94.9 - 97.9) |
| Heart rate (beat per minute) | 82 (72 - 86) | 93 (80 - 105) | 101 (82 - 121) |
| Systolic blood pressure (mm Hg) | 126 (119 -161) | 99 (83 - 120) | 111 (84 - 140) |
| Diastolic blood pressure (mm Hg) | 80 (73 - 89) | 58.5 (46.5 - 74) | 66 (44 - 83) |
| Respiratory rate (minutes−1) | 16 (16 - 18) | 19 (17 - 25) | 20 (16 - 22) |
| Oxygen saturation (%) | 99 (97 - 100) | 97.5 (96 - 100) | 100 (97 - 100) |
| FiO2 (%) | 21 (21 - 21) | 24 (21 - 30) | 100 (100 - 100) |
| Laboratory values | |||
| Hematocrit (%) | 42 (20.4 - 42.9) | 32.4 (27.9 - 37.3) | 34.2 (30.6 - 39.8) |
| White blood count (103/μL) | 9.2 (5.0 - 10.0) | 11.9 (7.9 - 15.9) | 12.1 (8.6 - 15.6) |
| Platelet count (103/μL) | 269 (167 - 310) | 312 (181 - 429) | 222 (177 - 284) |
| INR | 2 (1.0 - 2.3) | 1.4 (1.2 - 2.4) | 1.4 (1.2 - 1.8) |
| Bicarbonate (mEq/L) | 25 (22 - 29) | 22 (18 - 27) | 18.5 (16 - 21) |
| Creatinine (mg/dL) | 1 (0.8 - 1.3) | 2.2 (1.4 - 3.5) | 1.5 (1.0 - 2.3) |
| Glucose (mg/dL) | 104 (78 - 277) | 128 (101 - 159) | 200 (148 - 243) |
| Lactate (mmol/L) | 2.1 (2.1 - 2.1) | 2.3 (1.5 - 3.4) | 5.1 (3.0 - 10.1) |
| APACHE score | 2 (0 - 7) | 19 (14 - 22) | 26 (20 - 33) |
| Vasopressor support (%) | 0 | 38 | 57 |
| Outcome | |||
| Mortality (%) | 0 | 6 | 43 |
| ICU length of stay (days) | 0 | 1 (0 - 3) | 3 (3 - 8) |
| Days on mechanical ventilation (days) |
0 | 0 | 2.5 (1 - 6) |
| Hospital length of stay (days) | 0 | 5 (4 - 9) | 7 (3 - 17) |
(All continuous variables are expressed as median with inter quartile range), INR international normalized ratio, APACHE II acute physiology and chronic health evaluation II)
Results
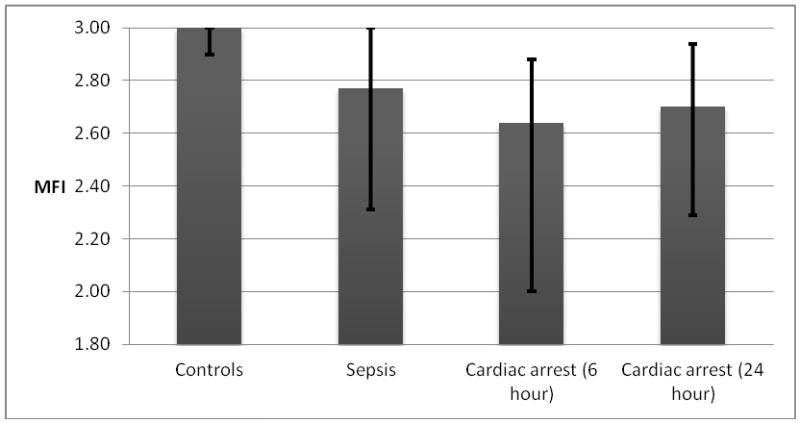
Microcirculatory flow was significantly impaired in post-cardiac arrest patients at 6 and 24 hours compared to controls (MFI: 2.6 [IQR: 2 - 2.9]) and (2.7 [IQR: 2.3 - 2.9]) vs. (3.0 [IQR: 2.9 - 3.0]; p < 0.01 and 0.02 respectively, Figure). There was no difference in median MFI between septic patients (2.8 [IQR: 2.3 - 3.0]) and post-arrest patients in univariate analysis. After adjustment for initial APACHE II score, however, post-cardiac arrest patients had significantly lower MFI at 6 hours compared to sepsis patients (p = 0.03). In the post-cardiac arrest group, microcirculatory flow was significantly higher at 24 hours in patients with good neurological outcome as compared to patients with bad neurological outcome (2.9 [IQR: 2.4 – 3.0] vs. 2.6 [IQR: 1.9 – 2.8]; p = 0.03). There was a trend toward lower median MFI at 24 hours in survivors vs. non-survivors (2.8 [IQR: 2.4 - 3.0] vs. 2.6 [IQR: 2.1-2.8] respectively; p < 0.09). Median MFI-6 and MFI-24 in survivors and non-survivors, patients with good and bad neurologic outcome, cooled and non-cooled patients, patients who received heparin or did not receive heparin and patients on vasopressors or not on vasopressors are shown in Table 2.
Figure 1.
Microvascular Flow Index (MFI) in controls, sepsis and cardiac arrest patients. (all values are represented in median with interquartile range)
Table 2. MFI at 6 and 24 hours by subgroup of cardiac arrest patients.
| Groups | MFI at 6 hours | P value | MFI at 24 hours | p value | ||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
| Survived | 2.6 (2.1-2.9) | 2.7 (1.7-2.9) | 0.89 | 2.8 (2.4-3.0) | 2.6 (2.1-2.8) | 0.09 |
| Good neurological outcome |
2.5 (2.0-2.9) | 2.7 (1.7-2.9) | 1.0 | 2.9 (2.4-3.0) | 2.6 (1.9-2.8) | 0.03 |
| On vasopressor | 2.75 (2.0-2.87) | 2.5 (1.91-2.9) | 0.35 | 2.75 (2.29-2.83) | 2.62 (2.37-3.0) | 0.7 |
| On heparin | 2.64 (2.2-2.92) | 2.61(1.8-2.88) | 0.71 | 2.73(2.4-3.0) | 2.67(2.3-2.83) | 0.46 |
| Received therapeutic hypothermia |
2.6 (1.9-2.8) | 2.7 (2.1-2.9) | 0.48 | 2.7 (2.4-2.8) | 2.8 (2.3-3.0) | 0.75 |
All values in median with Inter-quartile range
We evaluated the relationship between the amount of epinephrine used during resuscitation and MFI at 6 hours and found a trend towards a negative correlation (r= −0.37, p = 0.09). However, this trend weakened further and was not statistically significant when controlling for downtime (r= −0.27, p = 0.25). We found a negative correlation between MFI-6 and vascular endothelial growth factor (VEGF) (r= −.49, P= 0.038). However, after Bonferroni adjustment for multiple comparisons this correlation was statistically non-significant. We did not observe any correlation between MFI at 6 or 24 hours with E-selectin, V-cam, I-cam, IL_6, IL_8, IL_10 or TNFα. We did not detect a statistically significant correlation between MFI-6 or MFI-24 and temperature; mean arterial pressure, lactate level, APACHE II score, downtime or initial rhythm. There was no statistically significant difference in lactate clearance, mortality, length of ICU stay, or time on mechanical ventilation between the patients whose MFI increased and patients whose MFI decreased from 6 to 24 hours.
4. Discussion
Our study confirms that sublingual microvascular flow is impaired in post-cardiac arrest patients. This impairment was similar to the microcirculatory impairment in septic patients and was independent of the mean arterial blood pressure. We found that MFI at 24 hours was higher in patients with good neurologic outcome compared to those with poor neurologic outcome.
Investigation into the role of the microcirculation after cardiac arrest has been limited to date. In a recent pilot study, Donadello et al reported that sublingual microvascular flow is impaired within 12 hours post-cardiac arrest and may be affected by body temperature.16 Van Genderen et al reported sublingual microvascular impairment in 25 post-cardiac arrest patients, all of whom were treated with therapeutic hypothermia.17 In this study, Van Genderen et al found decreased microvascular flow at baseline and during therapeutic hypothermia within the first 24 hours, which might be due to vasoconstriction and decreased metabolism. However, one can not exclude the early inflammatory and microvascular changes as a possible cause of their findings. We observed similar early impairment of sublingual microvascular flow within the first 6 hours after ROSC. Meanwhile, we compared MFI between patients who were treated with therapeutic hypothermia and those who were not and we did not observe a statistically significant difference between those groups.
Post-cardiac arrest is often characterized by a systemic inflammatory response that has been described as analogous to septic shock.5 Microvascular flow is known to be dysfunctional in septic shock and we report similar findings in the post-arrest state. The pathphysiology of dysfunctional microvascular flow post-cardiac arrest remains unknown but may be related to the underlying ischemia reperfusion injury and associated systemic inflammation leading to decreased endothelial release of nitric oxide,22,23 leukocyte 24 and platelet 25 activation and marked fibrin activation with impaired fibrinolysis.26
After ROSC, tissue hypoperfusion and ongoing cellular injury may persist.27 Current practice in post-cardiac arrest care primarily targets optimizing systemic hemodynamics, with a focus on monitoring the mean arterial pressure, cardiac output, and/or global tissue oxygenation. Although a critical decrease in macrovascular flow would be expected to influence the microvascular flow,11 our data in conjunction with other post-cardiac arrest studies suggest discordance may exist between these two components of the circulation. For example, we found (similar to Donadello et al and Van Genderen et al) that microvascular impairment post-cardiac arrest is independent of the mean arterial pressure. Adequate microvascular flow would logically play an important role in tissue perfusion, though in our current study we did not find an association with lactic acid levels (i.e., surrogate for tissue perfusion). However, lactate elevation in the post-arrest state may be multi-factorial including ischemia-reperfusion injury from the initial arrest, ongoing tissue hypo-perfusion (from cellular injury, macrocirculatory compromise, or microcirculatory injury), shock liver, and other etiologies. Conversely, induced hypothermia may decrease cellular oxygen utilization and therefore indirectly decrease lactic acidosis, perhaps while even leading to some decreased microcirculatory flow. With these multiple confounders, the lack of association between lactate and microcirculatory flow in our dataset is not surprising and may not necessarily reflect a lack of relationship. The heterogenous patient population and small sample size prevents definitive assessment of the relationship between microcirculatory flow and lactate, hypothermia and microcirculatory flow, and even vasopressors and microcirculation though no clear relations were seen in our data set. Future investigation with larger sample sizes may be able to help better differentiate the relationship between macrovascular flow, microvascular flow, and tissue oxygen utilization. By distinguishing the relationships between these three components of tissue perfusion, an optimal approach to restoration of tissue perfusion could be devised.
Neurologic injury is the leading cause of death after cardiac arrest.28 Sublingual microvascular flow impairment has been associated with increased mortality during cardiopulmonary resuscitation in previous animal studies.29 In post-cardiac arrest patients, Van Genderen et al also reported that impaired MFI was associated with higher mortality and organ failure as determined by Sequential Organ Failure Assessment (SOFA) score. Our study is the first to determine an association between sublingual microvascular flow and neurologic outcome. Specifically, we observed that higher sublingual microvascular flow at 24 hours was associated with better neurologic outcome. Our data in conjunction with the survival data reported by Van Genderen et al suggests that preservation of the microcirculation could be a potential target in improving cardiac arrest outcomes. Further research is needed to test this hypothesis.
Vascular endothelial growth factor (VEGF), as a regulator of angiogenesis and capillary permeability, likely plays a role in microvascular flow.30 Shapiro et al reported that VEGF levels are higher in septic patients compared to controls, VEGF levels were also higher in septic shock than in those with sepsis alone.31 Meanwhile Karlsson et al reported lower VEGF in non-survivors with severe sepsis compared to survivors.32 The significance of VEGF and its correlation with mortality remains controversial in septic shock patients. In a previous study of post-cardiac arrest patients, VEGF levels were lower than controls, but there was no significant difference between survivors and non-survivors.33 In our study, we observed a trend of lower microvascular flow at 6 hours in patients with higher VEGF levels but it was not statistically significant. Further investigations about the role of VEGF in the pathophysiology of the post-cardiac arrest syndrome are needed.
Epinephrine is used routinely during cardiopulmonary resuscitation. Through its α1 action, epinephrine increases large vessel perfusion pressure and flow. Previous studies using an animal model of cardiac arrest demonstrated that epinephrine dramatically decreased cerebral and sublingual microvascular flow.11,34 In our study, we observed a trend toward lower MFI at 6 hours in patients who received higher doses of epinephrine during the initial resuscitation, but this trend essentially disappeared after controlling for down time. Clearly at this point epinephrine remains a key component of cardiopulmonary resuscitation, and the clinical significance of our findings is unclear.
Future studies to assess the potential role of microcirculatory flow monitoring in the post-cardiac arrest population are warranted. Questions to be answered include whether microcirculatory flow can be used to asses the effect of various post-cardiac arrest interventions (specifically vasopressors, inotropes and therapeutic hypothermia), whether targeting microcirculatory goals will improve the overall outcomes of these patients, and finally whether persistence of impaired microcirculatory flow after 24 hours may predict worse neurologic outcome in this population.
5. Limitations
We recognize several limitations in our study. First, our patient numbers were small. Second, some images were excluded from analysis due to poor quality, which might have affected our analysis. In addition, there are a number of metrics that may be used to assess microcirculatory flow. For this initial study, we chose apriori to use flow disturbances represented by MFI as our readout, which is the primary metric reported in a number of initiatives. The focus of the biomarker initiative was within the cardiac arrest population as a novel initiative within cardiac arrest. We did not measure biomarkers with this sepsis population. Moreover, we were not able to calculate the oxygen extraction for comparison to microcirculatory flow indices as measuring ScvO2 or SvO2 were not part of our study protocol. Finally, some studies suggest that sublingual microcirculation may not accurately correlate with other vital microvascular beds such as cerebral microcirculation.35 On the other hand, the correlation between sublingual and intestinal microvascular beds have previously been validated.36
6. Conclusion
Microcirculatory dysfunction occurs early in post-cardiac arrest patients. Better microcirculatory function at 24 hours may be associated with good neurologic outcome.
Acknowledgements
Michael W. Donnino is supported by 1R21AT005119 from the National Institute of Health and 1K02HL107447 from the National Heart Lung and Blood Institute. The authors wish to thank Francesca Montillo for her editorial support in submitting the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. Conflict of interest statement
All authors declare no conflicts of interest
References
- 1.McNally B, Robb R, Mehta M, et al. Out-of-hospital cardiac arrest surveillance --- Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005--December 31, 2010. MMWR Surveill Summ. 2011 Jul 29;60(8):1–19. [PubMed] [Google Scholar]
- 2.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA: the journal of the American Medical Association. 2006 Jan 4;295(1):50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 3.Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA: the journal of the American Medical Association. 2002 Dec 18;288(23):3035–3038. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- 4.Opie LH. Reperfusion injury and its pharmacologic modification. Circulation. 1989 Oct;80(4):1049–1062. doi: 10.1161/01.cir.80.4.1049. [DOI] [PubMed] [Google Scholar]
- 5.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002 Jul 30;106(5):562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 6.Samborska-Sablik A, Sablik Z, Gaszynski W. The role of the immuno-inflammatory response in patients after cardiac arrest. Archives of medical science: AMS. 2011 Aug;7(4):619–626. doi: 10.5114/aoms.2011.24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. American journal of respiratory and critical care medicine. 2002 Jul 1;166(1):98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 8.Trzeciak S, Dellinger RP, Parrillo JE, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Annals of emergency medicine. Jan;20074998(1):88–98. e81–82. doi: 10.1016/j.annemergmed.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Pittman RN. Oxygen transport in the microcirculation and its regulation. Microcirculation. 2013 Feb;20(2):117–137. doi: 10.1111/micc.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groner W, Winkelman JW, Harris AG, et al. Orthogonal polarization spectral imaging: a new method for study of the microcirculation. Nat Med. 1999 Oct;5(10):1209–1212. doi: 10.1038/13529. [DOI] [PubMed] [Google Scholar]
- 11.Fries M, Weil MH, Chang YT, Castillo C, Tang W. Microcirculation during cardiac arrest and resuscitation. Critical care medicine. 2006 Dec;34(12 Suppl):S454–457. doi: 10.1097/01.CCM.0000247717.81480.B2. [DOI] [PubMed] [Google Scholar]
- 12.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Critical care medicine. 2004 Sep;32(9):1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 13.De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J. 2004 Jan;147(1):91–99. doi: 10.1016/j.ahj.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 14.De Backer D, Dubois MJ, Schmartz D, et al. Microcirculatory alterations in cardiac surgery: effects of cardiopulmonary bypass and anesthesia. The Annals of thoracic surgery. 2009 Nov;88(5):1396–1403. doi: 10.1016/j.athoracsur.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Elbers PW, Craenen AJ, Driessen A, et al. Imaging the human microcirculation during cardiopulmonary resuscitation in a hypothermic victim of submersion trauma. Resuscitation. 2010 Jan;81(1):123–125. doi: 10.1016/j.resuscitation.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 16.Donadello K, Favory R, Salgado-Ribeiro D, et al. Sublingual and muscular microcirculatory alterations after cardiac arrest: a pilot study. Resuscitation. 2011 Jun;82(6):690–695. doi: 10.1016/j.resuscitation.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 17.van Genderen ME, Lima A, Akkerhuis M, Bakker J, van Bommel J. Persistent peripheral and microcirculatory perfusion alterations after out-of-hospital cardiac arrest are associated with poor survival. Critical care medicine. 2012 Aug;40(8):2287–2294. doi: 10.1097/CCM.0b013e31825333b2. [DOI] [PubMed] [Google Scholar]
- 18.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Critical care medicine. 2013 Feb;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 19.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical care medicine. 1985 Oct;13(10):818–829. [PubMed] [Google Scholar]
- 20.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975 Mar 1;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 21.Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002 Nov 2;360(9343):1395–1396. doi: 10.1016/s0140-6736(02)11393-6. [DOI] [PubMed] [Google Scholar]
- 22.Lefer AM, Lefer DJ. The role of nitric oxide and cell adhesion molecules on the microcirculation in ischaemia-reperfusion. Cardiovascular research. 1996 Oct;32(4):743–751. [PubMed] [Google Scholar]
- 23.Ma XL, Weyrich AS, Lefer DJ, Lefer AM. Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circulation research. 1993 Feb;72(2):403–412. doi: 10.1161/01.res.72.2.403. [DOI] [PubMed] [Google Scholar]
- 24.Oliver MG, Specian RD, Perry MA, Granger DN. Morphologic assessment of leukocyte-endothelial cell interactions in mesenteric venules subjected to ischemia and reperfusion. Inflammation. 1991 Oct;15(5):331–346. doi: 10.1007/BF00917350. [DOI] [PubMed] [Google Scholar]
- 25.Gando S, Kameue T, Nanzaki S, Igarashi M, Nakanishi Y. Platelet activation with massive formation of thromboxane A2 during and after cardiopulmonary resuscitation. Intensive care medicine. 1997 Jan;23(1):71–76. doi: 10.1007/s001340050293. [DOI] [PubMed] [Google Scholar]
- 26.Gando S, Kameue T, Nanzaki S, Nakanishi Y. Massive fibrin formation with consecutive impairment of fibrinolysis in patients with out-of-hospital cardiac arrest. Thrombosis and haemostasis. 1997 Feb;77(2):278–282. [PubMed] [Google Scholar]
- 27.Trzeciak S, Jones AE, Kilgannon JH, et al. Significance of arterial hypotension after resuscitation from cardiac arrest. Critical care medicine. 2009 Nov;37(11):2895–2903. doi: 10.1097/ccm.0b013e3181b01d8c. quiz 2904. [DOI] [PubMed] [Google Scholar]
- 28.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive care medicine. 2004 Nov;30(11):2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 29.Fries M, Tang W, Chang YT, Wang J, Castillo C, Weil MH. Microvascular blood flow during cardiopulmonary resuscitation is predictive of outcome. Resuscitation. 2006 Nov;71(2):248–253. doi: 10.1016/j.resuscitation.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med (Berl) 1999 Jul;77(7):527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro NI, Yano K, Okada H, et al. A prospective, observational study of soluble FLT-1 and vascular endothelial growth factor in sepsis. Shock. 2008 Apr;29(4):452–457. doi: 10.1097/shk.0b013e31815072c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karlsson S, Pettila V, Tenhunen J, Lund V, Hovilehto S, Ruokonen E. Vascular endothelial growth factor in severe sepsis and septic shock. Anesthesia and analgesia. 2008 Jun;106(6):1820–1826. doi: 10.1213/ane.0b013e31816a643f. [DOI] [PubMed] [Google Scholar]
- 33.Wada T, Jesmin S, Gando S, et al. Angiogenic factors and their soluble receptors predict organ dysfunction and mortality in post-cardiac arrest syndrome. Crit Care. 2012 Sep 29;16(5):R171. doi: 10.1186/cc11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ristagno G, Tang W, Huang L, et al. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Critical care medicine. 2009 Apr;37(4):1408–1415. doi: 10.1097/CCM.0b013e31819cedc9. [DOI] [PubMed] [Google Scholar]
- 35.Wan Z, Ristagno G, Sun S, Li Y, Weil MH, Tang W. Preserved cerebral microcirculation during cardiogenic shock. Critical care medicine. 2009 Aug;37(8):2333–2337. doi: 10.1097/CCM.0b013e3181a3a97b. [DOI] [PubMed] [Google Scholar]
- 36.Verdant CL, De Backer D, Bruhn A, et al. Evaluation of sublingual and gut mucosal microcirculation in sepsis: a quantitative analysis. Critical care medicine. 2009 Nov;37(11):2875–2881. doi: 10.1097/CCM.0b013e3181b029c1. [DOI] [PubMed] [Google Scholar]