Abstract
The pharyngeal arch arteries (PAAs) are transient embryonic blood vessels that make indispensable contributions to the carotid arteries and great vessels of the heart, including the aorta and pulmonary artery1, 2. During embryogenesis, the PAAs appear in a craniocaudal sequence to connect pre-existing segments of the primitive circulation after de novo vasculogenic assembly from angioblast precursors3, 4. Despite the unique spatiotemporal characteristics of PAA development, the embryonic origins of PAA angioblasts and the genetic factors regulating their emergence remain unknown. Here, we identify the embryonic source of PAA endothelium as nkx2.5+ progenitors in lateral plate mesoderm long considered to adopt cell fates within the heart exclusively5, 6. Further, we report that PAA endothelial differentiation relies on Nkx2.5, a canonical cardiac transcription factor not previously implicated in blood vessel formation. Together, these studies reveal the heart field origin of PAA endothelium and attribute a novel vasculogenic function to the cardiac transcription factor nkx2.5 during great vessel precursor development.
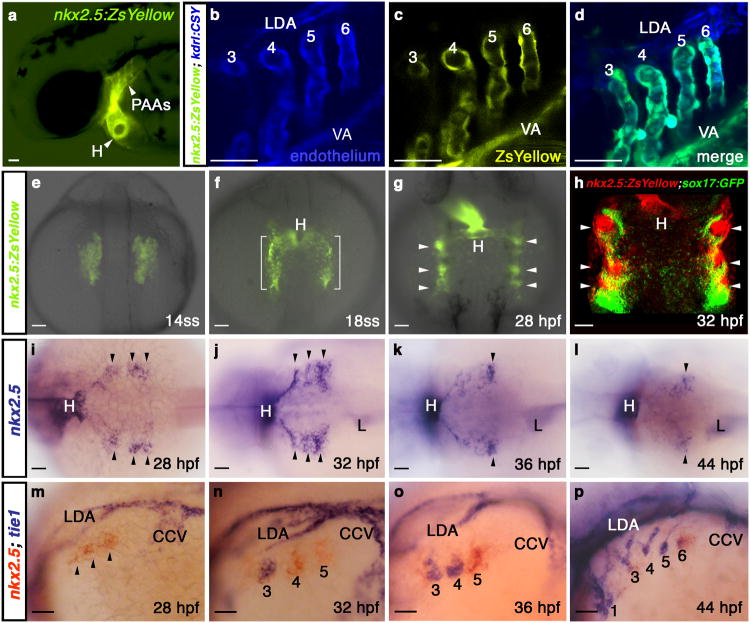
During the course of analyzing zebrafish embryos expressing a yellow fluorescent protein from nkx2.5 cis-regulatory sequences [Tg(nkx2.5:ZsYellow)]7, we observed fluorescence in known nkx2.5+ organs including the heart and liver (Fig. 1a and Supplementary Fig. 2a). Unexpectedly, we also observed ZsYellow fluorescence in pharyngeal structures revealed through co-localization studies to be endothelial cells comprising PAAs 3-6 and the adjoining ventral aorta (VA; Fig. 1a-d). Among the embryonic vasculature, only the PAAs and VA expressed ZsYellow consistent with their unique developmental origin3, 4. To pursue this observation further, we followed the dynamics of ZsYellow fluorescence during developmental stages leading up to PAA establishment. During mid-somitogenesis, we observed ZsYellow in bilateral populations of anterior lateral plate mesoderm (ALPM) previously identified as ventricular myocardial precursors in the zebrafish heart forming region6, 8 (Fig. 1e; Supplementary Fig. 2b, c; Supplementary Fig. 2g-j). As expected, ventricular precursors migrated medially and contributed to the heart. Interestingly, fractions of the ZsYellow+ field remained lateral and condensed by 28 hours post-fertilization (hpf) into pharyngeal clusters (Fig. 1f, g) that we verified were non-endodermal (Fig. 1h; Supplementary Fig. 2d-f).
Figure 1. nkx2.5 is expressed in presumptive PAA endothelial progenitors.
a, Tg(nkx2.5:ZsYellow) embryo at 60 hpf exhibiting fluorescence in the heart and PAAs. b-d, Tg(nkx2.5:ZsYellow); Tg(kdrl:CSY) embryo at 60 hpf with overlapping yellow and blue fluorescence in the endothelium of PAAs 3-6 and the VA. The LDA exhibits blue fluorescence exclusively. e-g, ZsYellow fluorescence in Tg(nkx2.5:ZsYellow) embryos at 14ss (e), 18ss (f), and 28 hpf (g). Brackets and arrowheads highlight non-cardiogenic nkx2.5+ cells in the pharynx. h, Tg(nkx2.5:ZsYellow); Tg(sox17:GFP) embryo at 32 hpf with nkx2.5+ pharyngeal cells (red) and sox17+ pharyngeal endoderm (green); i-l, in situ hybridization time-course of nkx2.5 expression in the pharynx. Arrowheads mark nkx2.5+ pharyngeal clusters. m-p, Double in situ hybridization time-course of nkx2.5 (red) and tie1 (blue) expression. PAA1 (labelled in p) forms earlier in development and never expresses nkx2.5. tie1+ clusters are numbered according to the mature PAA they derive. a-d, m-p, lateral views, anterior left; e-h, dorsal views, anterior up; i-l, dorsal views, anterior left; a-p, n>20 embryos per group. Abbr: VA, ventral aorta; LDA, lateral dorsal aorta; ss, somite-stage; hpf, hours post-fertilization; H, heart; L, liver; CCV, common cardinal vein.
To rule out a position effect of the transgene, we confirmed that nkx2.5 transcripts also localized to pharyngeal clusters at 28 hpf (Fig. 1i; SupplementaryFig. 2k, l). Over the next 20 hours, however, nkx2.5 transcipts progressively disappeared in a craniocaudal sequence until expression was undetectable specifically in the pharynx at 48 hpf (Fig. 1j-l; Supplementary Fig. 2m, o). By contrast, ZsYellow transcripts persisted longer in Tg(nkx2.5:ZsYellow) embryos, thereby providing an explanation for the robust ZsYellow fluorescence observed in PAAs 3-6 (Supplementary Fig. 2n-p). Intriguingly, a previous report3 described the cranial to caudal appearance of four tie1+ PAA angioblast clusters in pharyngeal mesoderm during a developmental window overlapping with the cranial to caudal disappearance of nkx2.5+ clusters that we observed (Fig. 1j-l). Using double in situ hybridization, we revealed a reciprocal relationship between nkx2.5 and tie1 transcripts in each pharyngeal cluster (Fig. 1m-p). Specifically, nkx2.5 expression precedes that of tie1 (Fig. 1m), yet after a transient period of overlap, nkx2.5 transcripts decline while tie1 expression is maintained throughout PAA morphogenesis3 (Fig. 1n-p). These data suggest that tie1+ PAA angioblasts derive from undifferentiated nkx2.5+ clusters in the pharynx.
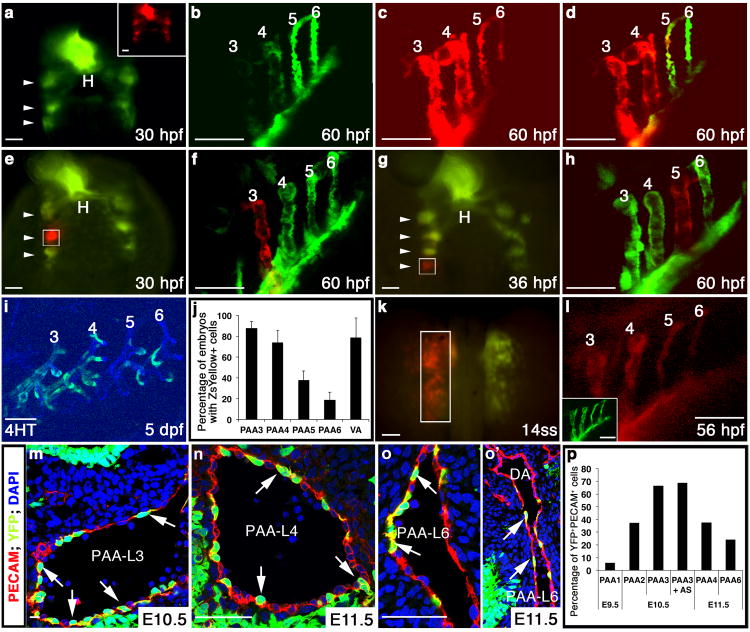
To begin testing this hypothesis, we tracked the derivatives of nkx2.5+ clusters expressing the photoconvertible Kaede protein, which instantly switches from green to red fluorescence following ultraviolet (UV) light exposure9. Pan Kaede photoconversion at 30 hpf resulted in robust red fluorescence in PAAs 3 and 4 with regions of red and green fluorescence or green-only fluorescence in PAAs 5 and 6 (Fig. 2a-d). Based on the red and green fluorescent signal distributions, we conclude that PAAs 3 and 4 derive exclusively from progenitor cells that express nkx2.5 prior to photoconversion at 30 hpf. By contrast, only a small fraction of PAAs 5 and 6 derive from cells expressing nkx2.5 prior to photoconversion with the majority of progenitors initiating nkx2.5 expression thereafter.
Figure 2. nkx2.5+progenitors give rise to PAA endothelium in zebrafish and mouse.
a-d, Tg(nkx2.5:Kaede) embryo at 30 hpf before (green) and after (red, inset) pan-photoconversion (n=3). Arrowheads highlight Kaede+ pharyngeal clusters. At 60 hpf, embryos were imaged in the green (b) and red (c) channels and these images were merged (d). e-h, Localized Kaede photoconversion of cluster 2 (e, white box, n=2) or 4 (g, white box, n=2) at the indicated developmental stages. Merged red and green images of the PAAs in the same embryos at 60 hpf are shown in (f) and (h). i, ZsYellow reporter fluorescence in PAAs 3-6 at 5 dpf in a Tg(nkx2.5:CreERT2); Tg(kdrl:CSY) embryo treated with 4HT from 10-13 hpf. j, Graph showing the average percentages of embryos with ZsYellow+ reporter fluorescence in each PAA and the VA across four experimental repliates (n=160). Error bars indicate one standard deviation. k, Left ALPM of a Tg(nkx2.5:Kaede) embryo photoconverted (white box) at 14ss and subsequently imaged in the red (l) and green channels (inset), (n=5). m-o′, Nkx2-5IRESCre; ROSAYFP embryos co-stained with PECAM1 (red) and DAPI (blue). Arrows indicate YFP+/PECAM+ lineage traced endothelial cells. o′, Left PAA 6 junction with DA. p, Graph depicting the average percentage of endothelial cells (PECAM1+) co-expressing YFP within each PAA. Cells counted across two embryos: E9.5 (PAA 1, n=244), E10.5 (PAA 2, n=216, PAA 3, n=1357, PAA3 + aortic sac, n=642), and E11.5 (PAAs 4, n=511 and 6, n=649). a,e,g,k, dorsal views, anterior up; b-d,f,h,i,l, lateral views, anterior left. Scale bar = 50μm. Abbr: hpf, hours post-fertilization; dpf, days post-fertilization; H, heart; AS, aortic sac; DA, dorsal aorta; PAA number and left (L) and right (R) designations indicated.
To test if each nkx2.5+ cluster gives rise to a single PAA, we individually traced their cell fates using focused Kaede photoconversion. At 30 hpf, three bilateral pairs of nkx2.5+ clusters were visualized in pharyngeal mesoderm (Fig. 2e). The second cluster gives rise exclusively to PAA3 (Fig. 2e, f). The third cluster, and scattered cells located caudal to the third cluster, become PAA4 and part of PAA5 (Supplementary Fig. 3a-c). Between 30 and 44 hpf, we witnessed the caudal emergence of two new Kaede clusters that when photoconverted individually, resulted in PAAs 5 or 6 being labeled exclusively with red fluorescence (Fig 2g, h). These findings support our hypothesis that individual nkx2.5+ clusters give rise to single PAAs and that the majority of progenitors for the caudal-most PAAs are specified after 30 hpf.
Based on the apparent segregation of ZsYellow+ pharyngeal clusters from myocardial precursors in the ALPM of Tg(nkx2.5:ZsYellow) embryos (Fig. 1e-g), we hypothesized that PAA endothelium derives from nkx2.5+ progenitors located in the classically defined heart forming region, a population long considered to adopt cell fates within the heart exclusively5, 6, 10. We tested this hypothesis by employing two complementary lineage tracing strategies. First, using tamoxifen-inducible Cre/loxP lineage tracing, we transiently induced Cre activity in nkx2.5+ cells for two hours during heart field stages (Supplementary Fig. 3j) and identified their derivatives using endothelial restricted [Tg(kdrl:CSY)]7 or ubiquitous [Tg(ubi:Switch)]11 Cre-responsive “color switching” reporters. Remarkably, we observed scattered reporter labeling of endothelial cells within PAAs 3-6 and the VA (Fig. 2i,j; Supplementary Fig. 3k-n). The majority of animals exhibited reporter fluorescence in PAAs 3 and 4 with lower percentages reporting fluorescence in PAAs 5 and 6 (Fig. 2j; Supplementary Fig. 3o).
In a complementary approach, we photoconverted Kaede protein unilaterally in left-side nkx2.5+ heart field progenitors and observed red fluorescence throughout left-side PAAs 3 and 4 with markedly weaker signal in caudal PAAs 5 and 6 (Fig. 2k,l). As an internal control, PAAs residing contralaterally failed to express red fluorescence (Supplementary Fig. 3d-f). Furthermore, pan-photoconversion of nkx2.5+ heart field cells labelled not only the heart tube, but the three pharyngeal clusters and scattered posterior cells present at 30 hpf (Supplementary Fig. 3g-i). Taken together, these data from Kaede photoconversion and Cre/loxP lineage tracing demonstrate that nkx2.5+ progenitors residing in the zebrafish heart field give rise to PAA endothelium. Further, the decline in labeling efficiency observed in PAAs 5 and 6 supports our previous observation that these caudal vessels derive from progenitor cells that initiate nkx2.5 expression in the ALPM and those that are specified subsequently in pharyngeal mesoderm (Fig. 2a-h).
PAA establishment appears qualitatively similar across vertebrate species as each PAA forms in a craniocaudal sequence12, 13 through the assembly of nascent angioblasts into discrete vessels3, 4. Although the progenitor source of these angioblasts has not been defined, a previous Cre recombinase-based lineage tracing study noted descendants of Nkx2-5-expressing cells in endocardium as well as putative endothelial cells scattered throughout the first pharyngeal arch14. However, PAAs were not systematically examined, and in the absence of co-labeling studies and high resolution imaging, the molecular identity of the traced cells remains unclear. To conclusively determine if PAA endothelium in the mouse derives from Nkx2-5+ progenitors, we performed Cre/loxP lineage tracing using the previously characterized Nkx2-5IRESCre driver14 with the Z/EG15 or ROSAYFP[16] reporters followed by immunostaining with the endothelial cell marker PECAM1. As anticipated, robust reporter expression was observed in the myocardium and endocardium of the heart (Supplementary Fig. 3p, q)14. Strikingly, reporter fluorescence at E9.5 and E10.5 co-localized with PECAM1 in PAAs 1 and 2, respectively (Fig. 2p; Supplementary Fig. 3r,s). At later stages, reporter fluorescence was also observed in PAAs 3, 4, and 6 (Fig. 2m-p; Supplementary Fig. 3t), the embryonic vessels that generate critical segments of the postnatal carotid arteries, aorta, and pulmonary artery, respectively12, 17. Rare overlap was also observed in the dorsal aorta (DA) endothelium at the sites of PAA attachment (Fig. 2o′). These findings highlight the evolutionary conservation of PAA endothelial cell derivation from an Nkx2-5+ source in mammals.
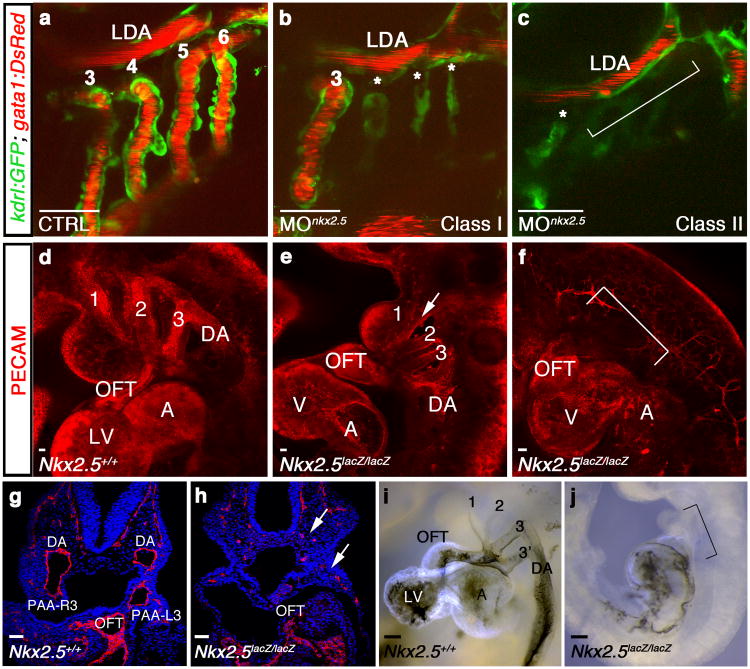
To assess the requirement for nkx2.5 during PAA establishment in zebrafish, we employed a previously validated anti-sense morpholino18 to suppress Nkx2.5 function (Supplementary Fig. 4a,b). While control embryos exhibited strong blood flow through PAAs 3-6 (Fig. 3a), nkx2.5 morphants displayed either a reduction in PAA number (class I; Fig. 3b) or absence of PAAs altogether (class II; Fig. 3c). Importantly, PAA1, which establishes the initial circulatory loop in zebrafish, develops much earlier in an nkx2.5-independent manner (Fig. 1p)13, 19. As such, both morphant classes maintained robust blood flow through the remaining vasculature reducing the likelihood that hemodynamic alterations caused the observed phenotype. Consistent with this idea, PAA vasculogenesis occurs normally in silent heart mutants that completely lack heart function and blood flow20.
Figure 3. Nkx2.5 is required for vertebrate PAA formation.
a-c, Tg(kdrl:GFP); Tg(gata1:DsRed) zebrafish embryos with green endothelial cells and red erythrocytes at 60 hpf. a, Control (CTRL) embryo with patent PAAs 3-6 and LDA (n=60/60). b, Class I nkx2.5 morphant (MOnkx2.5) in which one (shown) or two PAAs support blood flow. Asterisks label malformed PAAs (n=88/116). c, Class II nkx2.5 morphant lacking patent PAAs (bracket, n=22/116). d-f, Whole mount control (d) or Nkx2-5 null (e,f) mouse embryos stained for PECAM1 (red) at E9.5 (n=5 per group). Nkx2-5 null embryos displayed disrupted PAAs 1-3 with residual isolated endothelial cells (arrow, e) or a complete absence of PAAs altogether (bracket, f). g,h, Sections through control and Nkx2-5 null embryos stained with PECAM (red) and DAPI (blue). Arrow shows residual endothelial cells (h). i,j, Ink injections in control and Nkx2-5 null animals at E9.5 (n=5 per group). Bracket in j indicates absence of flow through the mutant OFTs. a-c, lateral views, anterior left; d-f, i,j, lateral views, anterior up; g,h, coronal sections. Scale bar = 50μm. Abbr: LDA, lateral dorsal aorta; DA, dorsal aorta; OFT, outflow tract; LV, left ventricle; A, atrium; V, ventricle; PAA number and left (L) and right (R) designations indicated.
Compared to control mouse embryos that displayed well-formed PAAs 1-3 at E9.5 (Fig. 3d), Nkx2-5lacZ/lacZ null animals exhibited either disrupted PAAs with residual mis-patterned endothelial cells (Fig. 3e) or a complete absence of PAAs altogether (Fig. 3f; Supplementary Fig. 4g-i). While ink injections in wild-type embryos revealed patent cardiac outflow tracts (OFTs) with prominent forward flow into the paired PAA3 vessels (Fig. 3i), the OFTs in Nkx2-5lacZ/lacZ mutants ended in a blind sac (Fig. 3j). These data demonstrate a previously unappreciated requirement for Nkx2-5 in PAA establishment that is conserved from zebrafish to mammals.
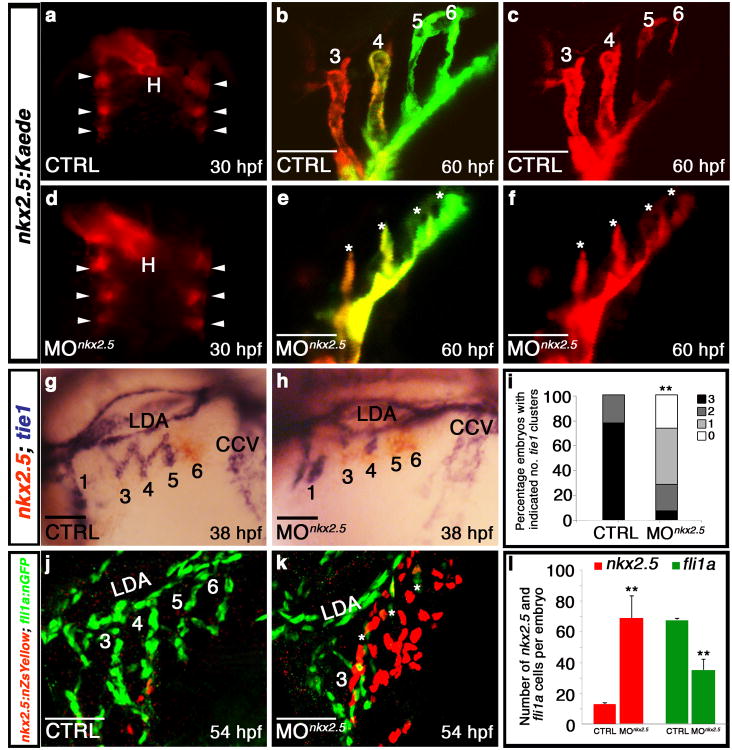
To elucidate the cellular mechanism underlying the PAA defect in Nkx2.5-deficient zebrafish, we evaluated nkx2.5 morphants for PAA progenitor cell specification and differentiation. Using a transgenic strain that highlights nkx2.5+ nuclei, we documented equivalent numbers of nkx2.5+ progenitors in control and morphant heart fields (Supplementary Fig. 4j-l), indicating proper specification. Examination of fluorescence in morpholino-injected Tg(nkx2.5:Kaede) embryos revealed that PAA progenitor cells also clustered properly by 30 hpf (Fig. 4a,d). Photoconversion of kaede expressed within morphant clusters revealed that they were maintained properly in the pharynx, but failed to form organized vessels, suggesting that Nkx2.5 is required specifically for PAA vasculogenesis (Fig. 4b,c,e,f). Next, we evaluated morphants for nkx2.5 and tie1 expression in pharyngeal clusters undergoing endothelial differentiation. In control embryos, we observed differentiated tie1+ clusters that had successfully downregulated nkx2.5 (Fig. 4g,i). In contrast, morphant embryos exhibited persistent expression of nkx2.5 in clusters that failed to appropriately upregulate tie1 (Fig. 4h,i), a phenotype that can be rescued by co-injection of full-length zebrafish nkx2.5 mRNA (Supplementary Fig. 4c-f). Using a double transgenic strain expressing unique fluorescent proteins in the nuclei of either PAA progenitors (red) or endothelial cells (green), we quantified the high degree to which PAA progenitors accumulate at the expense of endothelial cell differentiation in morphant embryos (Fig. 4j-l). Together, these data reveal that Nkx2.5 is dispensable for PAA progenitor specification and maintenence but essential for endothelial differentiation.
Figure 4. Nkx2.5 is required for PAA progenitor cell differentiation.
a-f, Control (n=3) and nkx2.5 morphant (n=5) Tg(nkx2.5:Kaede) embryos were pan-photoconverted at 30 hpf (a,d). Arrowheads show Kaede+ pharyngeal clusters. At 60 hpf, embryos were imaged in red and green channels with red (c,f) and merged (b,e) images shown. Asterisks (e,f) mark abnormal PAAs 3-6 in nkx2.5 morphant embryos. g,h, nkx2.5 (red) and tie1 (blue) transcripts evaluated by double in situ hybridization in control (g) and morphant (h) embryos. i, Quantification of tie1+ clusters in control (n=95) and nkx2.5 morphant (n=42) embryos across three experimental replicates; two-tailed t test **P=0.0001. j,k, Control and nkx2.5 morphant Tg(nkx2.5:nZsYellow); Tg(fli1a:nEGFP) embryos showing nkx2.5+ PAA progenitors (red) and fli1a+ endothelial cells (green) detected by immunohistochemistry. l, Quantification of nkx2.5+ and fli1a+ cells in control (n=4) and nkx2.5 morphant (n=4) embryos; Error bars indicate one standard deviation, two-tailed t test nkx2.5 **P=0.0073, fli1a **P=0.0008 across three independent experiments. Scale bar = 50μm. a,d, dorsal views, anterior up; b,c,e,f,g,h,j,k, lateral views, anterior left; PAA numbers indicated.
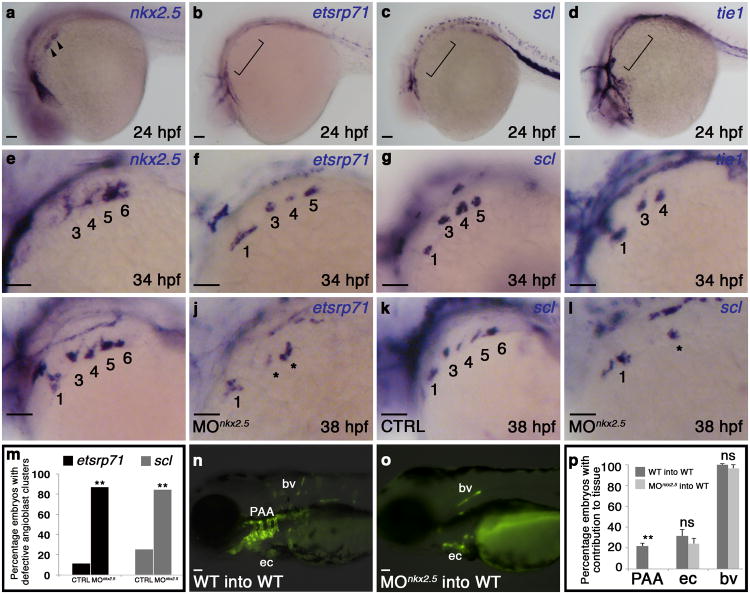
To identify potential downstream mediators of nkx2.5-dependent endothelial cell differentiation, we examined pharyngeal mesoderm for the expression of two transcription factors, etsrp71 and scl, shown previously to be associated with early specification of the angioblast lineage21. At 24 hpf, only nkx2.5 transcripts were visible in pharygneal clusters (Fig. 5a-d). At 34 hpf, however, we observed four nkx2.5+ clusters (Fig. 5e), three etsrp71+, scl+ clusters (Fig. 5f,g), and two tie1+ clusters (Fig. 5h), indicating that etsrp71 and scl transcripts appear subsequent to nkx2.5 but prior to tie1 in each cluster. Further, we learned that knocking down nkx2.5 inhibits the expression of etsrp71 and scl in PAA progenitors (Fig. 5i-m) demonstrating that nkx2.5 function is required for initating the angioblast program.
Figure 5. Cell-autonomous requirement for Nkx2.5 in promoting the PAA progenitor to angioblast transition.
a-h, in situ hybridization analysis of nkx2.5(a,e), etsrp71(b,f), scl(c,g), and tie1(d,h) at 24 (a-d) and 34 (e-h) hpf. i-letsrp(i,j) and scl(k,l) transcripts evaluated by in situ hybridization in control (CTRL; i,k) and nkx2.5 morphant (MOnkx2.5;j,l) embryos, asterisks mark abnormal PAAs 3-6, numbers are quantified in m. (etsrp CTRL n=61, MOnkx2.5 n=53, two-tailed t test **P=0.001 across two independent experiments; scl CTRL n=106, MOnkx2.5 n=116, two-tailed t test **P=0.001 across two independent experiments). n-p, WT (n) and MOnkx2.5(o) donor-derived GFP+ PAA endothelium in unlabelled WT hosts. p, Quantification of host embryos showing GFP+ donor cells from WT (n=208) or MOnkx2.5 (n=86) embryos in the PAAs, endocardium (ec), and body vasculature (bv). Fishers exact test, PAA two tailed **P=0.0001; ec two-tailed P=0.3239 (not significant, ns); bv two-tailed P=0.2710, across four independent experiments. Scale bar = 50μm. a-l, lateral views, anterior left; PAA islands indicated; a-h n>20 embryos per group; m,p, Error bars indicate one standard deviation.
To determine if Nkx2.5 is required cell autonomously for PAA vasculogenesis, we generated chimeric embryos via blastula transplantation. Wild-type or nkx2.5- deficient donor cells carrying an endothelial transgene [Tg(kdrl:GFP)] were transplanted into unlabeled wild-type blastula hosts. Both control and nkx2.5-deficient donor cells contributed equally to the body vasculature and endocardium (Fig. 5n,p). However, nkx2.5-deficient donor cells failed to contribute to PAA endothelium (Fig. 5o,p), demonstrating that Nkx2.5 is required cell autonomously in PAA progenitors for endothelial differentiation. Moreover, because the hemodynamic environments of the host embryos were unaltered, these findings further solidify the conclusion that Nkx2.5 plays a primary role in PAA establishment.
Our observations support a model (Supplementary Fig. 1) in which nkx2.5+ PAA progenitors segregate from cardiac precursors in the heart field and condense into clusters concomitant with pharyngeal segmentation22. Clusters 2 and 3 give rise to PAAs 3 and 4, respectively. Further, a small number of nkx2.5-expressing heart field progenitors migrate to arches 5 and 6 where naïve mesodermal cells initiate nkx2.5 expression in the pharynx.
Our findings also highlight a previously unknown and conserved role for Nkx2.5 in blood vessel development. This pro-vasculogenic function of Nkx2.5 was largely unanticipated because previous work demonstrated that misexpression of Nkx2.5 repressed scl+ hemangioblast fates in a region of the ALPM anterior to the heart field23 (Supplementary Fig. 5a, b). However, overexpression of nkx2.5 did not reduce or expand tie1+ PAA endothelial cluster formation in the pharynx (Supplementary Fig. 5c-e), highlighting context dependent roles for Nkx2.5 in angioblast specification. Further, these data demonstrate that PAA progenitor cell differentiation to the endothelial lineage does not require downregulation of nkx2.5 that otherwise occurs naturally (Fig. 1i-p). Futhermore, FGF signaling was shown to promote ALPM hemangioblast fates at the expense of cardiac fates23 (Supplementary Fig 5f,g). Inhibition of FGF signaling did not alter PAA progenitor cluster formation or endothelial differentiation indicating a dispensable role for FGF signaling in early PAA morphogenesis (Supplementary Fig. 5h-l). Together, these findings demonstrate that Nkx2.5 actively represses angioblast differentiation early in the ALPM and is required, but not sufficient, for angioblast differentiation later in the pharynx. Although Nkx2.5 targets have been identified within the myocardium24 and endocardium25, the cis-acting regulatory sequences that are directly activated or repressed by Nkx2.5 during PAA angioblast emergence remain uncharacterized.
Although nkx2.5 expression in PAA progenitors commences by 14 hpf, Nkx2.5 function is not required until approximately 30+ hpf to activate the PAA vasculogenic program (Fig. 1m-p; Fig. 4). This delay suggests that nkx2.5+ PAA progenitors integrate a stage- and/or location-specific external cue to cooperatively promote the angioblast fate. However, following endothelial program initiation, nkx2.5 expression downregulates indicating a specific requirement in promoting the progenitor to angioblast transition. In zebrafish, each PAA wholly derives from nkx2.5+ cells, and Nkx2.5 is essential cell-autonomously for initiating PAA morphogenesis. However, our lineage tracing and knockout studies in the mouse highlight the possibility that more than one progenitor population contributes to PAA endothelium, as suggested for endocardium26, 27. Nonetheless, our work overwhelmingly supports a conserved role for Nkx2-5 in PAA development across vertebrate species. Intriguingly, NKX2.5 mutations in humans can lead to interrupted aortic arch (IAA) type B28, a great vessel malformation involving left PAA4. Although the etiology of this congenital heart defect has been attributed to abnormal regression of left PAA4, IAA type B might also arise from defects in PAA establishment.
Supplementary Material
Acknowledgments
We are grateful to S. Paskaradevan and I. Scott for their training in blastula transplantation. We thank C. Kinney for creating Supplementary Figure 1; P. Obregon and T. Cashman for technical assistance in generating the Tg(nkx2.5:Kaede) and Tg(nkx2.5:nZsYellow) transgenic lines, respectively; W. Goessling for providing Tg(sox17:GFP) fish; T. North, and L. Zon for providing Tg(kdrl:GFP) fish; I. Drummond for providing bonm425 fish; B. Barut and L. Zon for providing bacterial artificial chromosomes (BACs); T. Evans for providing nkx2.5 plasmid for probe generation; G. Wilkinson and S. Sumanas for providing etsrp71 plasmid for probe generation; and the MGH Nephrology Division for access to their confocal microscopy facilities. We thank A. Vasilyev for assistance with confocal microscopy. N.P.L is supported by the Harvard Stem Cell Institute Training Grant (5HL087735). B.G.-A. was funded by an American Heart Association (AHA) Post-Doctoral Fellowship (10POST4170037). K.R.N. is funded by a National Research Service Award (5F32HL110627) from the National Heart, Lung and Blood Institute (NHLBI). R.P.H is supported by grants from the National Health and Medical Research Council of Australia (NHMRC; 573732, 573703), Atlantic Philanthropies (19131) and National Heart Foundation of Australia (G08S3718). R.P.H holds an NHMRC Australia Fellowship (573705). This work was funded by awards from the National Heart Lung and Blood Institute (5R01HL096816), American Heart Association (Grant in Aid no.10GRNT4270021), and Harvard Stem Cell Institute (Seed Grant) awards to C.G.B. and the National Heart Lung and Blood Institute (5R01HL111179), the March of Dimes Foundation (FY12-467), and the Harvard Stem Cell Institute (Seed Grant and Young Investigator Award) to C.E.B.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/ncb.
Author Contributions N.P.L. designed and performed the zebrafish experiments, analyzed data, and co-wrote the paper; R.S. designed, performed, and analyzed the mouse experiments, and co-wrote the paper; C.G.B. and B.G.A. created the Tg(nkx2.5:CreERT2) and Tg(nkx2.5:nZsYellow) lines; K.R.N., E.O., and L.J. performed and analyzed zebrafish experiments; R.P.H. analyzed data, designed experiments, and co-wrote the paper; C.G.B. and C.E.B. initiated and directed the study, analyzed data, and co-wrote the paper with input from all authors.
Author Information The authors declare no competing financial interests.
References
- 1.Congdon ED. Transformation of the aortic arch system during the development of the human embryo. Contributions to Embryology. 1922;68:49–110. [Google Scholar]
- 2.Moore KL, Persaud TVN. The developing human: clinically oriented embryology. 5th. W.B. Saunders; Philadelphia, PA: 1993. [Google Scholar]
- 3.Anderson MJ, Pham VN, Vogel AM, Weinstein BM, Roman BL. Loss of unc45a precipitates arteriovenous shunting in the aortic arches. Developmental biology. 2008;318:258–267. doi: 10.1016/j.ydbio.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li P, Pashmforoush M, Sucov HM. Mesodermal retinoic acid signaling regulates endothelial cell coalescence in caudal pharyngeal arch artery vasculogenesis. Developmental biology. 2012;361:116–124. doi: 10.1016/j.ydbio.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma Q, Zhou B, Pu WT. Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity. Developmental biology. 2008;323:98–104. doi: 10.1016/j.ydbio.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoenebeck JJ, Keegan BR, Yelon D. Vessel and blood specification override cardiac potential in anterior mesoderm. Developmental cell. 2007;13:254–267. doi: 10.1016/j.devcel.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, et al. Latent TGF-beta binding protein 3 identifies a second heart field in zebrafish. Nature. 2011;474:645–648. doi: 10.1038/nature10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serbedzija GN, Chen JN, Fishman MC. Regulation in the heart field of zebrafish. Development. 1998;125:1095–1101. doi: 10.1242/dev.125.6.1095. [DOI] [PubMed] [Google Scholar]
- 9.Guner-Ataman B, et al. Zebrafish second heart field development relies on progenitor specification in anterior lateral plate mesoderm and nkx2.5 function. Development. 2013;140:1353–1363. doi: 10.1242/dev.088351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hami D, Grimes AC, Tsai HJ, Kirby ML. Zebrafish cardiac development requires a conserved secondary heart field. Development. 2011;138:2389–2398. doi: 10.1242/dev.061473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosimann C, et al. Ubiquitous transgene expression and Cre-based recombination driven by the ubiquitin promoter in zebrafish. Development. 2011;138:169–177. doi: 10.1242/dev.059345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiruma T, Nakajima Y, Nakamura H. Development of pharyngeal arch arteries in early mouse embryo. Journal of anatomy. 2002;201:15–29. doi: 10.1046/j.1469-7580.2002.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Developmental biology. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- 14.Stanley EG, et al. Efficient Cre-mediated deletion in cardiac progenitor cells conferred by a 3′UTR-ires-Cre allele of the homeobox gene Nkx2-5. The International journal of developmental biology. 2002;46:431–439. [PubMed] [Google Scholar]
- 15.Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 16.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC developmental biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufman MH, Bard JBL. The Anatomical Basis of Mouse Development. 1. Academic Press; London: 1999. [Google Scholar]
- 18.Targoff KL, Schell T, Yelon D. Nkx genes regulate heart tube extension and exert differential effects on ventricular and atrial cell number. Developmental biology. 2008;322:314–321. doi: 10.1016/j.ydbio.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westerfield M. The zebrafish book A guide for the laboratory use of zebrafish (Danio rerio) 4th. Univeristy of Oregon Press; Eugene OR: 2000. [Google Scholar]
- 20.Nicoli S, et al. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS biology. 2006;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schilling TF, Kimmel CB. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development. 1994;120:483–494. doi: 10.1242/dev.120.3.483. [DOI] [PubMed] [Google Scholar]
- 23.Simoes FC, Peterkin T, Patient R. Fgf differentially controls cross-antagonism between cardiac and haemangioblast regulators. Development. 2011;138:3235–3245. doi: 10.1242/dev.059634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He A, Kong SW, Ma Q, Pu WT. Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5632–5637. doi: 10.1073/pnas.1016959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferdous A, et al. Nkx2-5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:814–819. doi: 10.1073/pnas.0807583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris IS, Black BL. Development of the endocardium. Pediatric cardiology. 2010;31:391–399. doi: 10.1007/s00246-010-9642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milgrom-Hoffman M, et al. The heart endocardium is derived from vascular endothelial progenitors. Development. 2011;138:4777–4787. doi: 10.1242/dev.061192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McElhinney DB, Geiger E, Blinder J, Benson DW, Goldmuntz E. NKX2.5 mutations in patients with congenital heart disease. Journal of the American College of Cardiology. 2003;42:1650–1655. doi: 10.1016/j.jacc.2003.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.