Abstract
The extent to which changing day lengths synchronize the seasonal molt was assessed in nine cohorts of male and female Siberian hamsters (Phodopus sungorus) born into a simulated natural photoperiod (SNP) beginning 4 weeks before and ending 12 weeks after the summer solstice. Hamsters in early cohorts displayed rapid somatic and gonadal growth and early puberty, whereas those in later cohorts delayed puberty until the next spring. Despite the varying birth dates and puberty strategies, the seasonal pattern of change in pelage is much better predicted by calendar date than by age in both sexes. Males born over the course of 16 weeks first made the transition to the winter pelage during a 5 week interval beginning on October 25; the autumn molt, however, was not significantly synchronized by either age or calendar date. The autumn molt of females on the other hand began 2 weeks later, and was significantly synchronized to calendar date with no detectable age effects. In both sexes, the autumn molt lagged gonadal and somatic seasonal changes by many weeks. Date of birth did not affect the timing of the spring molt, which was significantly synchronized by calendar date in both sexes. Incrementally changing photoperiods exert a strong organizing effect on the seasonal molt by providing hamsters with timing cues that are absent in laboratory analyses that employ static day lengths and abrupt transitions from summer to winter day lengths, thereby extending and validating conclusions derived from previous analyses.
Keywords: natural day length, molt, fur
Introduction
The annual cycle in day length (DL) coordinates seasonal rhythms in mammalian physiology and behavior (Paul et al., 2008; Prendergast et al., 2002). Knowledge of the salient photoperiodic cues that govern and consequently define the calendar date of transitions of between summer and winter phenotypes remains elusive. The vast majority of photoperiodism research employs a protocol of large shifts in DL (many hours on a single day) to initiate and maintain photoperiodic responses. While logistically convenient, these studies implicitly embrace a critical day length model of photoperiodism in which DLs above and below species-specific thresholds promote the summer and winter phenotypes, respectively (Elliott, ’76; Hoffmann, ’82). This approach neglects the potentially meaningful predictive information available in the natural pattern of change in DL over several months (Gorman and Zucker, ’98). Studies of hamsters maintained in photoperiods that simulate incremental changes that occur in nature (simulated natural photoperiods; SNPs) suggest that changes in DL may be as important as absolute DL for synchronization of seasonal traits (Butler et al., 2007a,b; Gorman and Zucker, ’95; Gorman and Zucker, '98). In this study, we examine the regulation of seasonal molting in SNPs in Siberian hamsters (Phodopus sungorus) born over a 16-week interval during mid- to late-summer.
The life history of many small temperate zone rodents depends on the date of birth (Sadleir, 1969). Male Siberian hamsters born in spring and early-summer DLs grow rapidly and attain puberty by 5–7 weeks of age, whereas cohorts born 4–6 weeks after the summer solstice or later manifest slower somatic development and delay puberty until the following spring; females adopt the delayed puberty strategy earlier in the year than do males (Butler et al., 2007a,b). Somatic responses to photoperiod, including pelage growth, color, and density, also vary seasonally, and adapt Siberian hamsters to changing environmental conditions (Paul et al., 2007). As DLs decrease, the grey-brown agouti pelage of spring and early summer gives way to a denser white coat that provides greater insulation (Heldmaier and Steinlechner, '81; Kauffman et al., 2003, Kuhlmann et al., 2003, Paul et al., 2007). These changes in pelage are particularly important, for Siberian hamsters remain active and forego hibernation in the harsh winter conditions of their home range in eastern Kazakhstan and southwest Siberia (Ross, ’98; Weiner, '87). Snowfall is minimal and hamsters contend with air temperatures as low as −45°C (Weiner and Gorecki, '82; Weiner, '87).
Different traits are not all equally sensitive to static DL changes. The abrupt transition from 16 h light/day (16L) to 14L induces testicular regression to the fall-winter reproductive phenotype, but not a molt to the winter pelage (Duncan et al., '85; Freeman et al., 2004). This suggests that the transition to the autumn pelage requires shorter DLs than other seasonal traits. Whether this relation extends to hamsters in incrementally changing photoperiods is unknown. If it does, then pelage changes should lag body mass decreases and gonadal regression. In maintaining Siberian hamsters in an SNP that corresponds to the latitude of origin of our founding stock we sought to identify the calendar dates and DLs at which hamsters molt and the extent to which the molt is synchronized across birth dates. We also assessed whether differences in responsiveness of several traits to changes in static DLs are preserved in a more information-rich (SNP) photoperiodic environment.
Methods
Nine cohorts of Siberian hamsters (Phodopus sungorus) were born at 2 wk intervals beginning 4 wk before and ending 12 wk after the summer solstice in an SNP corresponding to 53°N latitude (annual range of 7.6 –16.9 h of light/day) generated by a latitudinal timer (EC71ST SunTracker; Paragon Electric, Two Rivers, WI). There was no illumination during the dark phase. The last births correspond to the end of the breeding season in the field in mid-September (Weiner, '87). The 53°N latitude is close to the northern extent of this species’ natural range and approximates that at which the founder animals of our colony were trapped (Ross, '98; Wynne-Edwards et al., '99). The midpoint of the SNP light phase was matched to the photophase midpoint of our colony [14 h light/day (14L): lights on 0400 h PST]. For each cohort, colony females were transferred to the SNP 2 wk before they were paired with a colony male. During this time, locomotor activity was monitored by passive infra-red motion detectors to confirm appropriate entrainment of circadian rhythms to the SNP (Butler et al., 2007a). Females were then paired with males 21 d before the target birth date for a cohort. Males remained with females for 1 week. For example, to produce litters born at the summer solstice (cohort 0 = week 0 relative to solstice on June 21), females were transferred to the SNP on May 17, paired with males on May 31, and gave birth in the 4–7 days surrounding the target date. Males of proven fecundity were housed in 14L except during pairing with females. Each dam contributed a single litter to the subject pool.
Litters were weaned at 23–24 d of age, segregated by sex, and housed three per cage with littermates and non-littermates of the same age in clear polypropylene cages (18 × 28 × 12 cm), furnished with Tek-Fresh bedding (Harlan Teklad, Madison, WI). Cages were housed in light-tight boxes that each held 56 cages; hamsters of both sexes were co-housed in each box. Tap water and Lab Diet 5015 Mouse Diet (PMI Nutrition International, Brentwood, MO) were available ad libitum and room temperature was maintained at 22°C. All procedures were approved by the Animal Care and Use Committee of the University of California at Berkeley and conformed to NIH guidelines.
Beginning on the day of weaning and at weekly intervals thereafter, pelage color was assessed using the four-point scale of Duncan and Goldman ('84) with the addition of half steps (summer pelage = 1, winter pelage = 4). Body mass, testis size, and balanopreputial separation (BPS) of males, and body mass and vaginal patency of females were assessed weekly or biweekly as described previously (Butler et al., 2007a,b). These measures identified hamsters unresponsive to seasonal changes in DL (non-responders), as indicated by their failure to arrest reproductive development during exposure to short DLs and other criteria (Butler et al., 2007a,b). The dates of testicular regression and recrudescence were defined by a threshold estimated testis volume of 350 (testis length times width squared in millimeters, measured externally with calipers with hamsters under light isoflurane anesthesia). The dates of vaginal closure and opening were defined as the 1st of five consecutive closed observations and the 1st of two consecutive patent observations, respectively. The date of body mass loss and gain were defined as the point of inflection during the body mass decrease in the autumn and the maximum rate of increase in the spring (Butler et al., 2007a). At the end of the experiment hamsters were euthanized by exposure to CO2, followed by cervical dislocation.
Statistical Analysis
Data from all non-responders were removed from the analyses, based on the criteria described in Butler et al. (2007a,b). A pelage score of 2, previously adopted as marking the transition to the short-day phenotype (Butler et al., 2007a), defined the calendar date of seasonal pelage color change. This value tended to fall on the steepest portions of the curves (Fig. 1). Differences among cohorts in the dates of molt were assessed with 1 way ANOVA followed by Tukey-Kramer post hoc tests to evaluate pairwise differences. Differences between early developing and late developing hamsters were assessed with t-tests. The maximum pelage score was compared between cohorts using the Kruskal-Wallis test. Differences in the calendar dates of seasonal changes of different traits in the same individuals were analyzed with repeated measures ANOVA followed by post-hoc Tukey tests. Results were considered significant when p<0.05. To assess synchronization by date or age, the date of first and last pelage score ≥2 was plotted against date of birth: significant synchronization by date was indicated when the 95% confidence interval of the slope of the regression contained the value 0 and significant synchronization by age when the 95% slope confidence interval included the value 1, as described previously (Butler et al., 2007a).
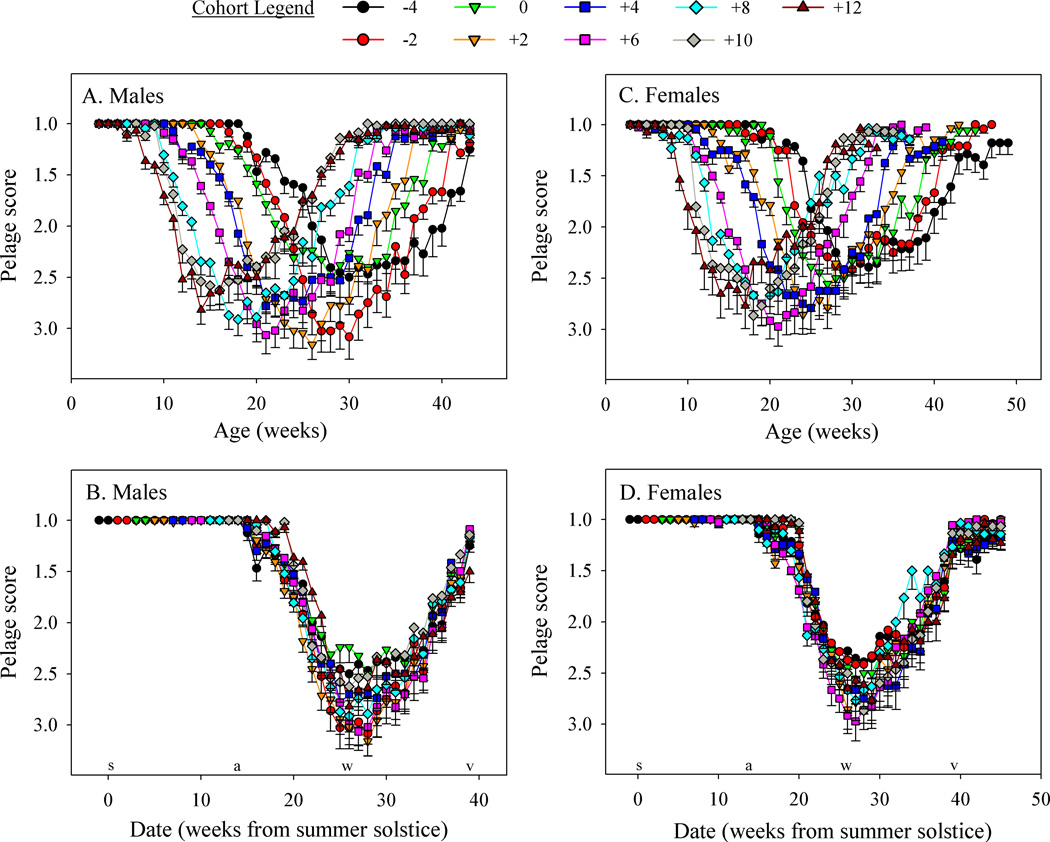
Fig. 1.
Mean pelage score (±SE) as functions of age or date. Although the mean is not entirely meaningful for discrete data, it serves to illustrate that date, rather than age, predicts pelage score. The dates of the summer (s) and winter (w) solstices, and autumnal (a) and vernal (v) equinoxes are shown on the abscissas of B and D. Note that the spring molt proceeds almost entirely between the winter solstice (December 21) and the vernal equinox (March 21). Sample sizes by cohort from −4 are: males: 16, 18, 21, 23, 20, 23, 23, 24, 22; females: 14, 12, 9, 14, 12, 18, 15, 15, 13.
Results
Regardless of birth date, at weaning all hamsters had a pelage score of 1 (spring-summer pelage). The pelage prior to weaning is often a shade paler, but darkens to the typical adult summer pelage soon after weaning. In both sexes, the pattern of change in pelage is much better predicted by calendar date (Fig. 1 panels B and D) than by age (Fig. 1 panels A and C). Across all cohorts, the date of autumn molt was substantially delayed relative to reproductive and body mass measures (Table 1). In contrast, the spring molt occurred at nearly the same calendar date as changes in other seasonal traits. In males, there were significant differences between the timing of testicular recrudescence, body mass gain, and pelage molt, but the three measures were separated by only four weeks. In females, the dates at which the three seasonal traits underwent transitions in the spring did not differ.
Table 1.
Seasonal changes in three traits. Autumn dates given as weeks after summer solstice; spring dates as weeks after winter solstice (mean ± SE). Dates of autumn reproductive seasonal changes (estimated testis volume ≤ 350 in males, or the observation of a closed vagina in females), body mass loss, and pelage score ≥ 2, are shown for all hamsters for which all 3 measures were available. Dates of spring reversals (estimated testis volume > 350, patent vagina, body mass increase, pelage score <2) are for all hamsters with all 3 measures available, regardless of autumn versus spring puberty.
| Autumn | Spring | |||
|---|---|---|---|---|
| Measure | Male (n=94) | Female (n=44) | Male (n=141) | Female (n=115) |
| Reproductive | 12.7 ± 0.2 a | 16.7 ± 0.4 a | 8.2 ± 0.3 a | 9.4 ± 0.4 |
| Body Mass | 15.5 ± 0.3 b | 15.7 ± 0.3 a | 6.0 ± 0.3 b | 9.7 ± 0.4 |
| Pelage | 20.4 ± 0.3 c | 21.6 ± 0.3 b | 9.7 ± 0.3 c | 10.0 ± 0.2 |
Different superscripts denote significant differences within a column as detected by Tukey test when a significant main effect of trait was revealed by repeated measures ANOVA.
Males
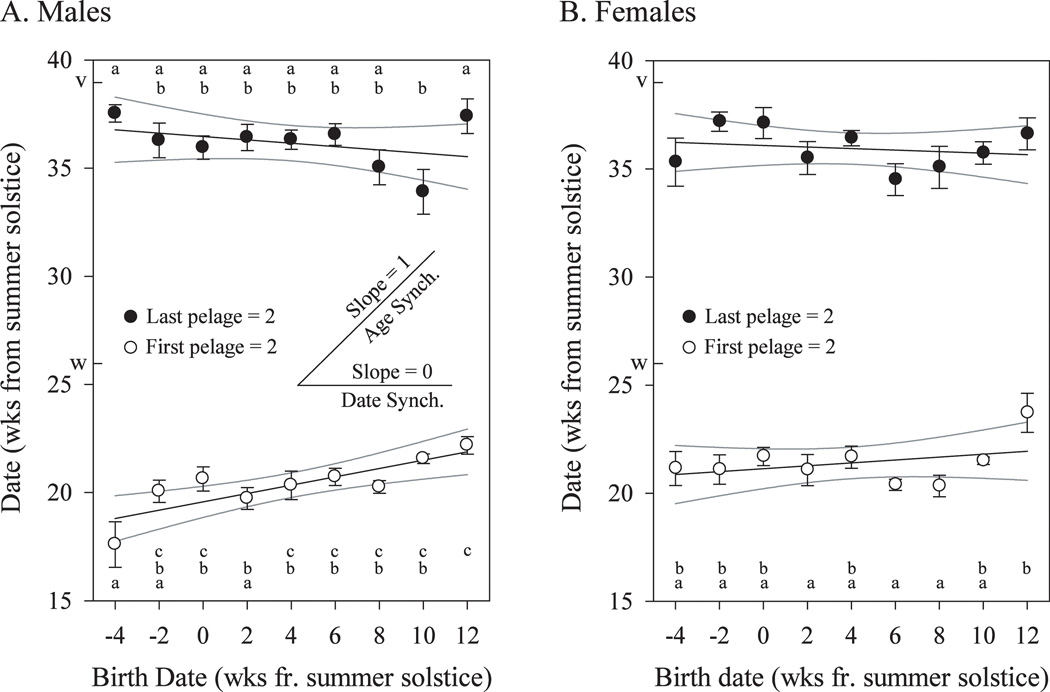
Despite birth dates ranging over 16 weeks, attainment of the first pelage score ≥ 2 was restricted to a 5 week interval, 18–22 weeks after the summer solstice (Oct 25 to Nov 22, Fig. 1B). Within this time window, these dates still differed significantly between cohorts (1 way ANOVA, F8,167 = 5.3, p<0.001), and the timing of the autumn molt was not synchronized with either age or date (Fig. 2A). Cohort −4 underwent a particularly early autumn molt, but exclusion of this cohort did not alter the results (F7,158 = 3.5, p<0.01). The last pelage score ≥ 2 occurred at similar calendar dates in the spring in all cohorts (34–38 wks after the summer solstice; Feb 14 to Mar 14). Although there was a significant effect of cohort on timing of the spring molt (1 way ANOVA, F8,171 = 2.4, p=0.02), this was due to early molt in cohort +10; over-all, the spring molt remained significantly synchronized with calendar date (Fig. 2A). Mean maximum pelage scores among the cohorts varied between 2.7 and 3.3; 94% of males achieved a score ≥2 and 65% a score of ≥3. The maximum pelage score analysis revealed a significant effect of cohort (Kruskal-Wallis test, H=15.9, df=8, p<0.05), but there was no apparent systematic effect; in order of increasing rank, the cohort order was 0, −4, +10, +12, +4, +8, −2, +6, and +2.
Fig. 2.
Synchronization of autumn and spring molts (mean ± SE) for males (A) and females (B). Pairwise differences are indicated (points that share a common letter designate values that do not differ significantly; Tukey-Kramer post-hoc test). Regressions and the 95% confidence interval of the slope (CI) are sensitive measures of synchronization; these are indicated by the lines and the grey curves, respectively. Perfect age synchronization is represented by a regression slope of 1, whereas perfect date synchronization is represented by a slope of 0. Autumn molt in males is not synchronized by either age or date, CI = (0.11, 0.24), but the spring molt in males, CI = (−0.18, 0.02), and both molts in females, CIautumn = (−0.01, 0.14) and CIspring = (−0.13, 0.07), are significantly synchronized by date. Without cohort −4, the CIautumn for males is (0.06, 0.20). The dates of the winter solstice (w) and vernal equinox (v) are shown on the ordinate. Sample sizes by cohort from −4 are: male autumn molt: 10, 17, 19, 22, 18, 22, 23, 23, 22; male spring molt: 15, 18, 19, 24, 19, 18, 25, 22, 20; female autumn molt: 14, 11, 10, 15, 12, 18, 15, 18, 14; female spring molt: 13, 11, 9, 14, 12, 18, 15, 15, 13.
Females
Both autumn and spring molts were significantly synchronized with calendar date (Fig. 2B). Superimposed on this broader pattern, there was a significant effect of cohort on the autumn molt (1 way ANOVA, F8,118 = 3.1, p<0.01), due to delayed molt in cohort +12. There were no significant differences between cohorts in the date of spring molt (1 way ANOVA, F8,111 = 1.5, p=0.18) or maximum pelage score (Kruskal-Wallis test, H=5.4, df=8, p=0.7).
Effect of puberty on molt timing
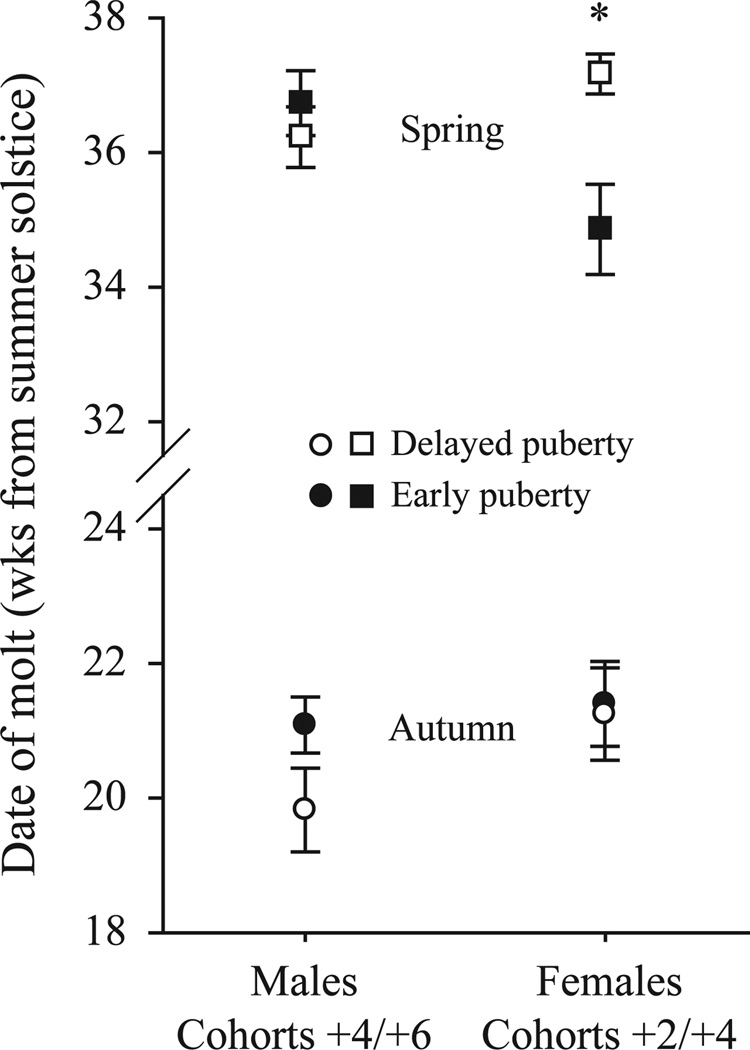
The timing of autumn and spring molts were compared between individuals adopting the two different puberty strategies (early and late) within cohorts +4 and +6 for males and within cohorts +2 and +4 in females. These subsets had comparable representation of both reproductive strategies; this categorization ensured that effects observed were not skewed by uncontrolled age effects. Whether or not hamsters experienced puberty during the autumn did not affect the timing of autumn molt in either sex, but did affect the timing of the spring molt in females. Males that underwent early pubertal development (large gonads and BPS by 5 wks of age; Butler et al., 2007a) molted to the winter pelage later than did hamsters that delayed puberty, but this difference fell short of statistical significance (Fig. 3, lower left quadrant, t = 1.76, df = 38, p = 0.09). The date of spring molt in males did not differ as a function of early versus late puberty (Fig. 3, upper left quadrant, t = 0.75, df = 35, p = 0.5). Early female puberty, defined by vaginal patency by 13 wks of age (Butler et al., 2007b) did not affect timing of the autumn molt (lower right quadrant, t = 0.16, df = 25, p=0.9), but did affect timing of the spring molt (Fig. 3, upper right quadrant, t = 2.98, df = 24, p<0.01).
Fig. 3.
Effect of timing of puberty on the mean (±SE) dates of the autumn and spring molts, defined by first and last pelage scores ≥2, respectively. Data are shown for the subset of cohorts that had comparable representation of both puberty strategies. *t-test, p<0.05. n for early/delayed puberty: male autumn, 23/17; male spring, 22/15; female autumn, 15/12; female spring, 14/12.
Discussion
Seasonal changes in pelage coloration of Siberian hamsters of both sexes were well predicted by calendar date and DL, regardless of the date of birth (Fig. 1). Males and females also tended to undergo molts at similar dates (Fig. 2). Onset of the transition to the autumn molt, defined by the first pelage score ≥2, occurred between the autumnal equinox and winter solstice: weeks 18–22 for males (Oct 25 – Nov 22; DL decreasing from 10:00 to 8:26 h), and weeks 20–24 for females (Nov 8 – Dec 6; DL decreasing from 9:10 to 8:00 h). Molt to the winter pelage lagged other seasonal transitions such as reductions in body mass and reproductive quiescence. The molt to the spring pelage occurred approximately 8–12 weeks after the winter solstice (Feb 14 to Mar 14; DL increasing from 10:01 to 11:57). This corresponds approximately to the timing of somatic and reproductive spring transitions, and occurs in advance of canonically long days (Table 1).
These data indicate that SNPs are sufficient to synchronize pelage molts to similar calendar dates, despite large age differences in the different birth cohorts. Unlike autumn body mass and gonadal changes, molts were not manifested until DLs were short, and indeed below the 12L necessary to induce a winter molt in static photoperiods (Duncan et al., 1985). Inspection of Fig. 1 shows that the first evidence of molt is soon after the autumn equinox (weeks 15 and 16 post-solstice, DL of 11:25 and 10:56 h, respectively, for 7 of 9 cohorts in both males and females). Whether the timing of the autumn molt derives from a true requirement for a short absolute DL, or a rapid change in DL, or a delay between when the molting process is triggered and when it becomes conspicuous, remains to be definitively established. Nevertheless it is clear that use of SNPs provides a clearer snapshot of photoperiodic signals that affect hamsters in the field than is provided in studies that employ static DLs and abrupt transitions from long to shorter DLs.
The lag between autumn changes in body mass and reproductive measures on one hand, and pelage molt on the other, may reflect different neural and hormonal mechanisms. The most important of these is the differential regulation of different seasonal traits by DL. In males in static photoperiods, induction of the winter pelage requires DLs shorter than those sufficient for inducing testicular regression (Duncan et al., '85). Because the influence of DL on the neuroendocrine axis of this species is transduced by the duration of nocturnal melatonin secretion (Carter and Goldman, '83), we infer that longer nightly melatonin durations are required to trigger the autumnal molt than for inhibiting reproduction. Data from SNP-housed Siberian hamsters with lesions of the intergeniculate leaflet (IGLx) support this conjecture. IGLx hamsters have shorter durations of nocturnal activity, and presumably shorter durations of elevated melatonin secretion, than do their intact counterparts in the SNP; whereas all hamsters experienced testicular regression, the IGLx males did not undergo the seasonal pelage molt (Freeman et al., 2004). We observed sequential testicular regression and recrudescence in hamsters without any concomitant molt (n=6 in cohort −4). Not only does pelage molt appear to require shorter DLs than does testicular regression, but under the same conditions, testes appear more responsive to DL cues than does the molt. Whether these individuals had shorter durations of elevated melatonin than their counterparts that molted is unknown. The delay in molt compared with testicular regression in this experiment verifies that the different DL requirements for these traits, identified in static photoperiods, are relevant in more natural light environments.
Different DL requirements between traits may also be mediated by distinct neuroendocrine pathways. In male and female Syrian hamsters (Mesocricetus auratus), lesions of the dorsomedial nucleus of the hypothalamus abolish gonadal responses to short days but do not affect the normal short-day induced decline in prolactin secretion (Lewis et al., 2002; Maywood and Hastings, '95). Photoperiodic control of prolactin secretion is controlled by the pars tuberalis of the pituitary in Syrian hamsters (Stirland et al., 2001). In Siberian hamsters and meadow voles (Microtus pennsylvanicus), decreases in prolactin secretion in short DLs mediate changes in pelage color (Duncan and Goldman, '84; Smale et al., '90).
In males, the delay in the pelage molt compared to seasonal reproductive and somatic changes may reflect residual effects of androgens on the winter pelage (Duncan and Goldman, '84). In Syrian hamsters, a decrease in testosterone concentrations per se does not induce a molt, but elevated testosterone can slow fur re-growth during molts (Paul et al., 2007). We anticipated that increased steroid hormone secretion associated with early puberty might delay autumn molts relative to those of hamsters that delay puberty. An analysis of the cohorts with substantial representation of individuals with early and late puberty onset did not support this conjecture, although there may be a small non-significant modulatory effect of testosterone in males.
As in males, puberty strategy had no effect on the timing of the autumn molt in females. Early puberty did, however, advance the spring molt. There was a significant effect of the timing of puberty on the date of the spring molt in females; a similar difference between overwintering adults and juveniles was observed in the timing of vaginal opening but not on body mass increases (Butler et al., 2007b). The basis for this difference between traits is unknown.
In both male and female Siberian hamsters, autumn and spring pelage molts occur at similar calendar dates, regardless of birth date. SNPs organize annual rhythms in ways not apparent from static DL analyses, but do not synchronize changes among several different traits to a common calendar date. As hamsters attend to incremental changes in DL present in their natural environment, several traits are differentially responsive to these cues. The use of SNPs extends understanding of how DL can control seasonal traits and their transitions in nature and validates some of the conclusions obtained with static DL manipulations.
Acknowledgements
We thank Kevin Turner, Justin Trumbull, Chris Tuthill, and Kim Pelz for technical assistance and animal care. MPB was a Howard Hughes Medical Institute Predoctoral Fellow, a Robert Katz Fellow, and a Wang Family Fellow.
Grant sponsor: NIMH; Grant number MH-61171.
Literature Cited
- Butler MP, Turner KW, Park JH, Butler JP, Trumbull JJ, Dunn SP, Villa P, Zucker I. Simulated natural day lengths synchronize seasonal rhythms of asynchronously born male Siberian hamsters. Am J Physiol. 2007a;293:R402–R412. doi: 10.1152/ajpregu.00146.2007. [DOI] [PubMed] [Google Scholar]
- Butler MP, Trumbull JJ, Turner KW, Zucker I. Timing of puberty and synchronization of seasonal rhythms by simulated natural photoperiods in female Siberian hamsters. Am J Physiol. 2007b;293:R413–R420. doi: 10.1152/ajpregu.00216.2007. [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology. 1983;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Goldman BD. Hormonal regulation of the annual pelage color cycle in the Djungarian hamster, Phodopus sungorus. I. Role of the gonads and pituitary. J Exp Zool. 1984;230:89–95. doi: 10.1002/jez.1402300112. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Goldman BD, Di Pinto MN, Stetson MH. Testicular function and pelage color have different critical daylengths in the Djungarian hamster, Phodopus sungorus sungorus. Endocrinology. 1985;116:424–430. doi: 10.1210/endo-116-1-424. [DOI] [PubMed] [Google Scholar]
- Elliott JA. Circadian rhythms and photoperiodic time measurement in mammals. Fed Proc. 1976;35:2339–2346. [PubMed] [Google Scholar]
- Freeman DA, Dhandapani KM, Goldman BD. The thalamic intergeniculate leaflet modulates photoperiod responsiveness in Siberian hamsters. Brain Res. 2004;1028:31–38. doi: 10.1016/j.brainres.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Zucker I. Seasonal adaptations of Siberian hamsters. II. Pattern of change in day length controls annual testicular and body weight rhythms. Biol Reprod. 1995;53:116–125. doi: 10.1095/biolreprod53.1.116. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Zucker I. Mammalian seasonal rhythms: New perspectives gained from the use of simulated natural photoperiods. In: Touitou Y, editor. Biological Clocks, Mechanisms and Applications. Amsterdam: Elsevier; 1998. pp. 195–204. [Google Scholar]
- Heldmaier G, Steinlechner S. Seasonal control of energy requirements for thermoregulation in the Djungarian hamster (Phodopus sungorus), living in natural photoperiod. J Comp Physiol. 1981;142:429–437. [Google Scholar]
- Hoffmann K. The critical photoperiod in the Djungarian hamster Phodopus sungorus. In: Aschoff J, Daan S, Gross G, editors. Vertebrate Circadian Systems. Berlin: Springer-Verlag; 1982. pp. 297–304. [Google Scholar]
- Kauffman AS, Paul MJ, Butler MP, Zucker I. Huddling, locomotor, and nest-building behaviors of furred and furless Siberian hamsters. Physiol Behav. 2003;79:247–256. doi: 10.1016/s0031-9384(03)00115-x. [DOI] [PubMed] [Google Scholar]
- Kuhlmann MT, Clemen G, Schlatt S. Molting in the Djungarian hamster (Phodopus sungorus Pallas): seasonal or continuous process? J Exp Zool. 2003;295:160–171. doi: 10.1002/jez.a.10211. [DOI] [PubMed] [Google Scholar]
- Lewis D, Freeman DA, Dark J, Wynne-Edwards KE, Zucker I. Photoperiodic control of oestrous cycles in Syrian hamsters: mediation by the mediobasal hypothalamus. J Neuroendocrinol. 2002;14:294–299. doi: 10.1046/j.1365-2826.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Hastings MH. Lesions of the iodomelatonin-binding sites of the mediobasal hypothalamus spare the lactotropic, but block the gonadotropic response of male Syrian hamsters to short photoperiod and to melatonin. Endocrinology. 1995;136:144–153. doi: 10.1210/endo.136.1.7828525. [DOI] [PubMed] [Google Scholar]
- Paul MJ, George NT, Zucker I, Butler MP. Photoperiodic and hormonal influences on fur density and regrowth in two hamster species. Am J Physiol. 2007;293:R2363–R2369. doi: 10.1152/ajpregu.00520.2007. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Zucker I, Schwartz WJ. Tracking the seasons: the internal calendars of vertebrates. Philos Trans R Soc Lond B Biol Sci. 2008;363:341–361. doi: 10.1098/rstb.2007.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Zucker I, Nelson RJ. Mammalian seasonal rhythms: Behavior and neuroendocrine substrates. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego, CA: Academic Press; 2002. pp. 93–156. [Google Scholar]
- Ross PD. Phodopus sungorus. Mammalian Species. 1998;595:1–9. [Google Scholar]
- Sadleir RMFS. The Ecology of Reproduction in Wild and Domestic Mammals. London: Methuen; 1969. [Google Scholar]
- Smale L, Lee TM, Nelson RJ, Zucker I. Prolactin counteracts effects of short day lengths on pelage growth in the meadow vole, Microtus pennsylvanicus. J Exp Zool. 1990;253:186–188. doi: 10.1002/jez.1402530208. [DOI] [PubMed] [Google Scholar]
- Stirland JA, Johnston JD, Cagampang FR, Morgan PJ, Castro MG, White MR, Davis JR, Loudon AS. Photoperiodic regulation of prolactin gene expression in the Syrian hamster by a pars tuberalis-derived factor. J Neuroendocrinol. 2001;13:147–157. doi: 10.1046/j.1365-2826.2001.00611.x. [DOI] [PubMed] [Google Scholar]
- Weiner J, Gorecki A. Small mammals and their habitats in the arid steppe of central eastern Mongolia. Pol Ecol Stud. 1982;8:7–21. [Google Scholar]
- Weiner J. Limits to energy budget and tactics in energy investments during reproduction in the Djungarian hamster (Phodopus sungorus sungorus Pallas 1770) Symp Zool Soc Lond. 1987;57:167–187. [Google Scholar]
- Wynne-Edwards KE, Surov AV, Telitzina AY. Differences in endogenous activity within the genus Phodopus. J Mammal. 1999;80:855–865. [Google Scholar]