Abstract
Background and Purpose
Phosphorylation of eNOS, an important post-translational modulator of its enzymatic activity, is reduced in diabetes. We hypothesized that modulation of eNOS phosphorylation could overcome diabetic vascular dysfunction and improves the outcome to stroke.
Methods
We used the db/db mouse model of type 2 diabetes. We mated db/db mice with eNOS knockin mice that carry single-amino acid mutations at the S1176 phosphorylation site; the phosphomimetic SD mutation shows increased eNOS enzymatic activity, while the unphosphorylatable SA mutation shows decreased eNOS activity. We characterized the vascular anatomy, baseline physiologic parameters and vascular reactivity. We used the middle cerebral artery occlusion model of stroke and measured infarct volume and neurological deficits.
Results
db/db mice showed diminished eNOS phosphorylation at S1176. eNOS SD and SA mutations do not change the vascular anatomy at the Circle of Willis, brain capillary density, heart rate, or arterial blood gases of db/db mice. The eNOS SD mutation, but not the SA mutation, lowers blood pressure and improves vascular reactivity to acetylcholine in db/db mice. The eNOS SD mutation reduces stroke size and neurologic deficit following middle cerebral artery occlusion.
Conclusion
Diminished eNOS phosphorylation is a mechanism of vascular dysfunction in db/db mice. We show here that modulation of the eNOS S1176 phosphorylation site in db/db mice is associated with improved vascular reactivity and improved outcome to stroke following middle cerebral artery occlusion.
Keywords: nitric oxide, endothelial dysfunction, diabetes mellitus
Introduction
Cardiovascular disease, including stroke, is the major cause of morbidity and mortality in diabetes1. The precise mechanisms of endothelial dysfunction in diabetes are not known. Here, we test the hypothesis that deficient eNOS phosphorylation is an important cause of diabetic vascular dysfunction and that its modulation can change the outcome to a disease model in vivo.
eNOS phosphorylation at serine 1177 (human sequence numbering) or its equivalents in other species, has been studied2-5. This site is phosphorylated by Akt kinase2, 3, AMP kinase6, and protein kinase A7, resulting in a conformational change, enhancing electron flux through the reductase domain, and increasing NO production8. eNOS-derived NO plays known protective roles in cerebral ischemia, including maintenance of cerebral blood flow9. Models of cerebral ischemia show worse outcome with larger infarcts in eNOS knockout mice10, 11. To study the effects of eNOS phosphorylation, we created eNOS knock-in mice that carry single amino acid mutations at S1176, the murine site corresponding to human S117712. The SD mutation (serine replaced by aspartate) results in the generation of a phosphomimetic form of eNOS with increased enzymatic activity and NO generation. The SA mutation (serine replaced by alanine) results in the generation of an unphosphorylatable form of eNOS. These mutations are located in the endogenous eNOS gene. For a mouse model of type 2 diabetes, we used the db/db mouse, which carries a point mutation in the leptin receptor gene13, 14. These animals show a phenotype similar to type 2 diabetes, with hyperglycemia and insulin resistance, as well as other metabolic abnormalities15, 16. We bred eNOS SD and SA mutant mice to db/db mice to test whether modulation of the eNOS S1176 phosphorylation site could overcome diabetic vascular dysfunction, and whether this affects stroke size in vivo.
Materials and Methods
Animals
Wild type (WT) and db/db mice on C57BL/6 background were obtained from Jackson laboratory. eNOS SA and SD mice were backcrossed to C57BL/6 genetic background by accelerated marker-assisted congenic breeding for 6 generations, equivalent to 10 generations of conventional breeding17. SA and SD mice were mated with db/db mice as described in Supplementary Methods. Male adult mice aged 8-17 weeks were used for all experiments (WT 12.0±3.5 weeks; db/db 12.8±1.7 weeks; SD-db/db 13.5±3.4 weeks; SA-db/db 13.3±2.7 weeks). All procedures were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee.
Biochemical and physiologic characterization
Western blotting, cGMP assay, biopterin measurements, glucose and insulin tolerance tests, microvascular density, cerebrovascular anatomy and cerebral blood flow (CBF) measurements were performed as described in Supplementary Methods. Serum lipoproteins were analyzed by HPLC18. Blood pressure was monitored in the carotid artery by blood pressure transducer (ADInstruments)18. Vessel reactivity studies were performed in a pressurized myograph system (Danish Myotechnologies)18.
Middle cerebral artery (MCA) occlusion (MCAO) model of stroke
For MCAO, a 7-0 nylon filament covered by silicon (Doccol Corp) was inserted into the internal carotid artery and advanced to the origin of the MCA for one hour, as described in Supplementary Methods.
Determination of infarct size
2 mm thick coronal brain sections were stained after 23 hours reperfusion with 2% 2, 3, 5-triphenyltetrazolium chloride (TTC)18. Total and infarcted areas were measured on the side ipsilateral to ischemia, and total area was measured on the contralateral side. Areas were integrated over the five coronal sections to obtain volumes. The percentage of the infarct volume to brain size was calculated as infarct volume divided by total volume of cerebral hemispheres without the cerebellum. To correct for edema, indirect infarct volume was calculated as volume of contralateral hemisphere minus ipsilateral non-infarcted volume.
Neurologic scoring
Mice were examined for neurologic deficits 24 hours after MCA occlusion as described19 with modifications. We excluded from original 5-point scale one point (leaning to the contralateral side), due to inability of overweight db/db, SD-db/db and SA-db/db mice to lean. Normal motor function was scored as 0, flexion of the contralateral torso and forearm on lifting the animal by the tail as 1, circling to the contralateral side as 2, and no spontaneous motor activity as 3. Measurements of infarct volume and neurological scoring were done by a blinded operator.
Statistics
All results are expressed as mean±SD. Statistical analysis was performed using Mann-Whitney test when two groups were compared, or Kruskal-Wallis test with Dunns post hoc test when more than two groups were compared. Statistical analysis for neurological deficit was performed using Kruskal-Wallis 1-way analysis of variance on ranks. Differences of P<0.05 were considered significant.
Results
eNOS phosphorylation is impaired in db/db mice
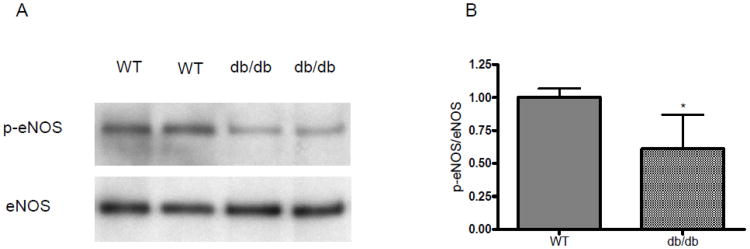
We performed Western blotting to quantitate eNOS phosphorylation in the carotid artery. As seen in Figure 1, db/db mice showed normal levels of total eNOS, while eNOS phosphorylation at S1176 was diminished.
Figure 1. eNOS phosphorylation in the carotid artery of WT and db/db mice.
A. Representative Western blot probed with antibody to p-eNOS and total eNOS. B. Densitometry (n=8 each group). *P<0.05, WT vs. db/db.
SA-db/db and SD-db/db mice
We used homologous recombination to knockin the specific SD and SA mutations into the endogenous mouse eNOS gene (Supplementary Figure IA). These mice were bred with db/db mice to obtain animals homozygous for the eNOS mutations and db/db alleles. Expression of eNOS and nNOS are the same in SD-db/db and SA-db/db mice as in WT and db/db mice (Supplementary Figure IB, C, D). eNOS mutations did not change the levels of total or phosphorylated Akt or AMP kinase (Supplementary Figure IB, E, F). The body weights of db/db (49.2±5.6g), SD-db/db (49.3±7.4g) and SA-db/db (45.9±6.7g) mice were not significantly different from each other, and were higher than WT mice (25.0±3.9g).
Cerebrovascular anatomy
We injected a latex-carbon black solution to outline the Circle of Willis and its major branches. The Circle of Willis was complete in all animals, and no redundant middle cerebral arteries were observed. We measured the diameters of the posterior cerebral arteries (PCA) and posterior communicating (Pcomm) arteries. No significant differences were observed between WT (PCA: 138.1±27.0 μm, Pcomm: 52.9±19.8 μm, n=4), db/db (PCA: 161.4±26.6 μm, Pcomm: 45.9±11.0 μm, n=5), SA-db/db (PCA: 150.6±26.2 μm, Pcomm: 66.7±34.1 μm, n=4) and SD-db/db (PCA: 169.6±25.2 μm, Pcomm: 64.4±37.4 μm, n=6) mice (Supplementary Figure IIA and B). We measured microvascular density by lectin staining in the cerebral cortex (WT, 4.06 ±1.97%, db/db, 4.62±2.09%, SD-db/db 4.44±1.45%, SA-db/db 4.04±0.62%) and striatum (WT, 3.74 ±0.42%, db/db, 4.29±1.18%, SD-db/db 4.05±0.70%, SA-db/db 3.85±0.51%, n=3 per group). No significant differences were seen between any of the groups (Supplementary Figure IIC).
Metabolic parameters
Glucose tolerance and insulin tolerance testing showed impaired responses in db/db mice as compared with WT mice. The eNOS SA and SD mutations did not significantly affect the glucose tolerance and insulin tolerance curves of the db/db mice (Supplementary Figure III). Cholesterol levels were lower in WT mice compared with all other groups of mice. Triglyceride levels were not different between all groups of mice (Supplementary Table I).
SD mutation reduces stroke size in db/db mice
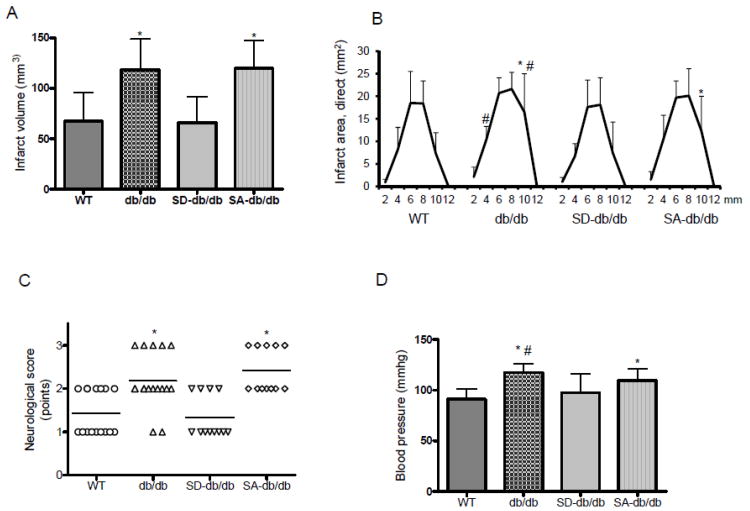
db/db mice show a larger stroke following MCAO than WT mice (Figure 2A, B). The percentage of the infarct volume to brain size of db/db mice was 26.5±5.0%, as compared to 18.0±4.9% in WT mice (n=15, P<0.01). The ratio of ipsilateral to contralateral brain volumes, a reflection of brain edema, was not significantly different between db/db (109.9 ± 10.1%) and WT (114.2 ± 9.0%, n=15) mice. The SD mutation reduced the infarct volume of db/db mice and the percentage of infarct volume to brain (20.2±5.8%, n=12, P<0.05) as compared to db/db mice. The SA mutation did not change the infarct volume or the percentage infarct volume (25.7±4.5%, n=12) of db/db mice. The ipsilateral to contralateral brain volume ratio did not significantly differ between db/db, SD-db/db (115.7±8.2%) and SA-db/db (105.9±13.8%) mice. The coronal infarct areas were smaller in WT mice and SD-db/db mice compared to db/db and SA-db/db mice (Figure 2B). Together, these results show that the eNOS SD phosphomimetic mutation protects brain tissue in db/db mice, decreasing the cerebral infarct volume, with the equivalent brain edema in the mice groups. The reduction of stroke size in SD-db/db mice was associated with functional improvement, as quantitated by neurologic scoring (Figure 2C). We used laser Doppler flowmetry to assess relative CBF values in the core ischemic region. There were no significant differences between mouse groups during MCA occlusion and first 60 minutes of reperfusion (Supplementary Figure IID).
Figure 2. Cerebral infarct volumes, neurological score, and infarct areas.
A. Indirect cerebral infarct volumes (mm3) 23 hours after reperfusion. (n=15, WT and db/db; n=12, SD-db/db and SA-db/db mice. *P<0.01 vs. WT andSD-db/db). B. Infarct areas (mm2) for each coronal section.(*P<0.01 vs. WT and #P<0.01 vs. SD-db/db). C. Neurological scores. (n=15, WT and db/db; n=12, SD-db/db; n=11, SA-db/db mice.*P<0.01 vs. WT and SD-db/db). D. Mean arterial blood pressure (n=15 each group). *P<0.01 vs. WT and #P<0.01 vs. SD-db/db.
SD mutation normalized blood pressure and vascular reactivity in db/db mice
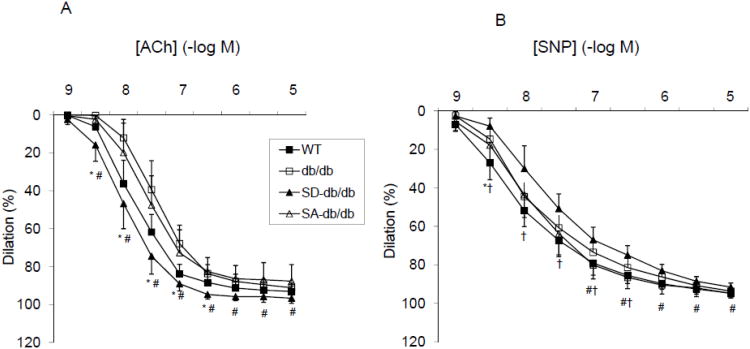
db/db mice are hypertensive compared with WT mice. The blood pressure was significantly lower in SD-db/db mice, but not in SA-db/db mice as compared with db/db mice (Figure 2D). Carotid artery vasodilation in response to ACh was impaired in db/db mice (dose response curve shifted to the right, EC50 43.4±25.3 nM) as compared with WT mice (EC50 13.1±4.9 nM, p<0.01, Figure 3A). The eNOS SD mutation improved the vascular reactivity of db/db mice, shifting the ACh dose response curve back to the left towards the WT curve. The EC50 for the SD-db/db mice was 10.2±5.9 nM (p<0.01 compared with db/db mice and SA-db/db mice). In contrast, the dose response curve of SA-db/db mice was similar to the db/db mice (EC50 30.0±12.3 nM). In response to SNP (Figure 3B), the EC50 for the WT mice (8.0±4.8 nM) was significantly lower than SD-db/db mice (25.8±13.8nM, P<0.05), and not different between SD-db/db mice and db/db mice (18.6±2.8 nM, P=0.51) or SA-db/db mice (15.5±11.6 nM, p=0.24). Together, these results show that the eNOS SD phosphomimetic mutation improves vascular reactivity in db/db mice, normalizing the EC50 for ACh, despite reduced sensitivity of smooth muscle cells of SD-db/db mice to NO.
Figure 3. Effect of eNOS mutations on vascular reactivity.
A. Dose response curves to ACh. WT (n=18); db/db (n=17); SD-db/db (n=6); SA-db/db (n=6). *P<0.05 WT vs. db/db; #P<0.05 SD-db/db vs. db/db and SA-db/db. B. Dose response curves to SNP. WT (n=12); db/db (n=17); SD-db/db (n=6); SA-db/db (n=5). *P<0.05 WT vs. db/db; †P<0.05 WT vs. SD-db/db; #P<0.05 SD-db/db vs. SA-db/db
cGMP production and BH4 levels
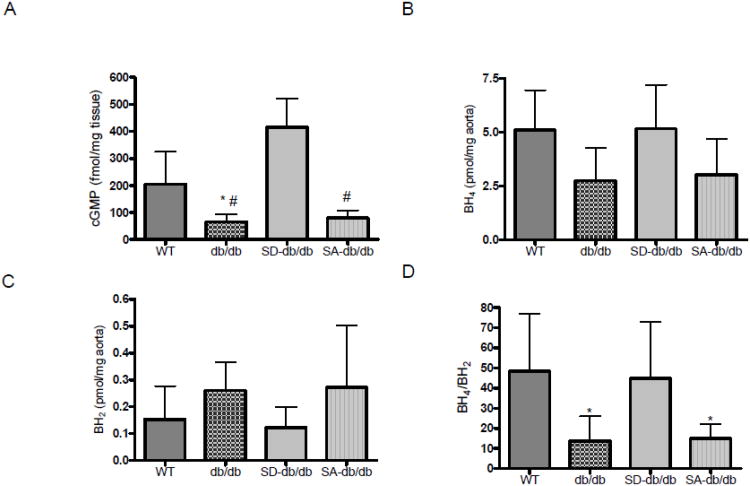
cGMP production, as a reflection of basal NO production, was decreased in aortic rings from db/db mice compared to WT mice (P<0.05). SD-db/db mice showed greater cGMP production compared to db/db, SA-db/db (P<0.01) mice (Figure 4A). BH4 levels were reduced in the aortas of db/db mice as compared to WT mice (Figure 4B), while the oxidative product of BH4, 7,8-BH2, was not different among all studied groups (Figure 4C). The BH4 to 7,8-BH2 ratio was significantly decreased in db/db and SA-db/db mice aortas as compared to WT and SD-db/db mice (Figure 4D).
Figure 4. cGMP and biopterin levels.
A. cGMP levels in aorta: WT (n=8), db/db (n=6), SD-db/db (n=6) and SA-db/db (n=4) mice. *P<0.05, db/db vs. WT; #P<0.01, db/db and SA-db/db vs. SD-db/db. B. BH4 levels. C. BH2 levels. D. BH4/BH2ratio. Measurements were made in aorta of WT (n=7), db/db (n=5), SD-db/db (n=6) and SA-db/db (n=7) mice. *P<0.05, db/db and SA-db/db vs. WT and SD-db/db.
Discussion
Patients with type 2 diabetes display endothelial dysfunction, which is thought to play a key role in the mechanisms of atherogenesis. However, the molecular mechanisms of endothelial dysfunction are not known, nor has it been proven that improving endothelial function in diabetes will reduce cardiovascular events. Here, we use the leptin receptor deficient db/db mouse, which is a commonly used model for type 2 diabetes13, 14. db/db mice develop hyperglycemia and insulin resistance and other metabolic abnormalities, including obesity and hyperlipidemia15, 16. Our results demonstrate that deficient eNOS phosphorylation is a molecular mechanism of vascular dysfunction in db/db mice, and that modulation of eNOS phosphorylation corrects both vascular dysfunction and increased stroke size.
Several lines of evidence suggest that phosphorylation of eNOS at S1176 is essential to the link between metabolism and vascular dysfunction20, 21. First, eNOS phosphorylation is diminished in diabetes, hypercholesterolemia and atherosclerosis. Second, estrogens, statins, and PPARα and PPARγ agonists increase eNOS S1176 phosphorylation. Third, vasculoprotective signaling molecules such as insulin, IGF-1, VEGF, adiponectin and leptin increase S1176 phosphorylation. These agonists act through multiple kinases to converge on eNOS phosphorylation, suggesting that this is a common integration point that underlies endothelial dysfunction from various causes.
To study the effects of phosphorylation, we used single amino acid eNOS mutations at S1176. In the SD mutation, serine is replaced by aspartate, which has a negatively charged carboxyl group. The negative charge and the size of the side chain are similar to the negatively charged phosphate group of phospho-eNOS, hence the designation of the mutation as phosphomimetic. In contrast, the SA mutation replaces serine with alanine, of which the methyl side chain is unphosphorylatable. These mutations have been characterized in vitro, and are known to affect eNOS enzymatic activity, with the SD mutation showing increased NO production at rest and the SA mutation showing decreased NO production2, 3. Both mutations are sensitive to calcium stimulation, and the SA mutation is not a null mutation.
In this study, we used eNOS SD and SA knockin mice in which the endogenous eNOS gene carries these single amino acid mutations. We bred these mice to db/db mice to study vascular function and outcome to stroke. In the MCA occlusion model of stroke, the quantitative endpoint is the infarct volume. A meaningful comparison presupposes that the territories at risk are comparable, and that the effects of the filament occlusion are the same. We sought to address these issues in several ways. First, we assessed the effects of the eNOS mutations on cerebrovascular anatomy relevant to the outcome of the stroke model. We performed carbon black injections to visualize the vessels and to ensure that continuity of the Circle of Willis was not affected. We did not observe redundant MCA or changes in the sizes of the PCA or Pcomm arteries that would affect outcome to filament occlusion. We performed lectin staining to quantitate capillary density and ensure that there were no detectable differences that may affect the stroke volume. Second, we assessed absolute CBF in the animals by hydrogen clearance. Third, we calculated infarct volume in several ways: direct infarct volume, indirect infarct volume, which accounts for ipsilateral edema, and percent infarct volume to brain size, which accounts for differences in brain size. Using all of these measures, the SD mutation reduced the infarct size of db/db mice, while the SA mutation did not. Fourth, we verified by laser Doppler flowmetry that the filament caused the same CBF reductions in the core ischemic zone.
Improvements in CBF in the ischemic penumbra are the most likely mechanism for the eNOS SD mutation to reduce infarct size in db/db mice11. In support of this, we found that the impaired vascular reactivity in db/db mice is improved by the SD mutation but not the SA mutation.
We wish to point out potential limitations of our study. In addition to vascular effects, eNOS-derived NO is known to inhibit platelet aggregation and adhesion22, and block leukocyte-endothelial interactions23. Although we demonstrate that the SD mutation improves vascular reactivity, we did not examine whether the SD mutation also acts through alterations of hemostasis, inflammation, or other effects. These systemic effects, either in the CNS or other organ systems, may also affect the outcome to cerebral ischemia. Thus, the effects of eNOS phosphorylation may not be exclusively vascular.
eNOS has been reported to affect insulin sensitivity, and high fat-fed eNOS knockout mice display insulin resistance24. We performed glucose tolerance tests, insulin tolerance tests, and measured lipid profiles. The eNOS SD and SA mutations do not significantly affect these metabolic parameters in db/db mice. We measured BH4, which is important to prevent eNOS uncoupling25. The ratio of BH4/BH2 is significantly reduced in db/db mice as compared to WT mice, but it is the same in SD-db/db mice as WT mice. This could occur by upregulation of GTP cyclohydrolase I expression, the rate-limiting step in BH4 production26. It is still possible that there are other metabolic effects of eNOS mutations that could alter the outcome to cerebral ischemia. Alternatively, the eNOS mutations and the db/db mutation may both influence stroke size, but through mechanisms unique to one or the other that do not overlap.
We confirmed the functional significance of the reduced cerebral infarct size by neurologic scoring, using a system tailored for mice19 modified for body habitus. Like the Bederson score developed for use in rats27, it includes forelimb flexion and circling behavior, but it differs because it does not include lateral push which is a less reliable indicator in overweight mice, and it does include absence of spontaneous activity as an indicator of severe neurologic functional deficit.
We previously reported that eNOS knockout mice that carry mutant bovine eNOSS1179 transgenes (bovine numbering corresponding to S1177 in humans and S1176 in mice) could be used to study the effects of those mutations on an eNOS-null background. The current study differs from the previous report in several important ways. First, we are here using knockin mice in which the endogenous eNOS gene is mutated, rather than transgenic mice. Thus, effects due to expression level, transgene copy number, and site of integration are avoided. Second, we are assessing the effects of the eNOS mutations on the phenotype of the db/db mice in the stroke model, not the effects of the mutations by themselves.
Because NO needs to be generated in the proper subcellular location and with precise timing28, targeting phosphorylation of the endogenous eNOS enzyme offers advantages over pharmacologic replacement of NO with nitrate donors or genetic overexpression of endothelial or other NOS isoforms by gene therapy. The appropriate targets for modulation of NO production by eNOS may be the kinases or phosphatases that regulate eNOS phosphorylation. In addition to eNOS phosphorylation, Akt kinase, AMPK, and PKA clearly have other substrates and effects that may impact cell survival.
Conclusion
db/db mice show diminished eNOS phosphorylation, greater stroke size following MCAO, hypertension and impaired vascular reactivity. The phosphomimetic eNOS SD mutation improves NO production, reduces stroke size, corrects hypertension and improves vascular reactivity. These results demonstrate that modulation of the eNOS phosphorylation site in db/db mice has beneficial effects on physiology and outcome to a stroke model.
Supplementary Material
Supplementary Figure I. Generation and characterization of SD-db/db and SA-db/db mice
A. Knock-in construct. Top, genomic DNA with eNOS exons 17 to 26, is shown. The construct includes the mutation indicated with an asterisk (*) in exon 26, a neomycin resistance gene (NEO) flanked by lox P sites (black triangles), and a thymidine kinase gene (TK). Homologous recombination between the genomic DNA and the targeting construct (indicated by crossed lines) replaces the region surrounding exon 26 with the mutated exon, as well as the NEO gene flanked by lox P sites. Treatment with Cre recombinase by mating the chimeric mice with EIIa-Cre mice results in excision of the NEO gene, and one residual lox P site. B. Western blot analysis of brain protein of WT, db/db, SA-db/db and SD-db/db mice (n=4 for each group). Brain tissue was isolated from mice and electrophoresed on SDS-PAGE.C-F, The average expression levels of eNOS/actin (C), nNOS/actin (D), p-Akt/Akt (E) and p-AMPK/AMPK (F).
Supplementary Figure II. Anatomy of cerebrovasculature and CBF
A. Representative images of cerebrovasculature of WT, db/db, SD-db/db and SA-db/db mice after intracardiac carbon black perfusion. Upper panels show representative images of ventral brain surface and lower panels show representative higher magnifications of the posterior Circle of Willis showing the PCA and Pcomm arteries from WT, db/db, SD-db/db and SA-db/db mice.
B. The representative diameter of PCA (left panel) and Pcomm (right panel) in WT (n=4), db/db (n= 6), SD-db/db (n=6) and SA-db/db (n=5) mice.
C. Microvascular density measured by lectin staining in cerebral cortex (left panel) and striatum (right panel). n=3 for each group.
D. CBF measured in the core ischemic region by LDF, during 1 hour of MCA occlusion and 60 minutes of reperfusion. There were no significant differences between WT (n=15), db/db (n=15), SD-db/db (n=12) and SA-db/db (n=12) mice.
Supplementary Figure III. Insulin and glucose tolerance tests
A. Insulin tolerance test. Time course of blood glucose levels in WT, db/db, SA-db/db and SDdb/db mice after intraperitoneal injection of insulin (*p<0.05 WT vs. db/db, SD-db/db and SAdb/db mice).
B. Glucose tolerance test. Time course of glucose levels after intraperitoneal injection of glucose (*p<0.05 WT vs. db/db, SD-db/db and SA-db/db mice).
Supplementary Table I: Lipid profile, CBF, blood gases and heart rate
Total cholesterol, LDL-cholesterol, HDL-cholesterol, total triglyceride in serum (WT, n=8; db/db, n=5; SD-db/db, n=4; SA-db/db, n=4, * p<0.05 vs. WT). CBF, blood gases (n=3 for each group), heart rate (n=15 for each group). #P<0.05 db/db vs. SD-db/db mice.
Acknowledgments
We are grateful to Helen Swanson for technical assistance.
Funding Sources: This work was supported by NIH R01 HL57818 and NS33335 to PLH and American Heart Association Scientist Development Grant 0835344N to DNA.
Footnotes
Disclosures: None.
Supplemental Material: Deficient eNOS phosphorylation is a mechanism for diabetic vascular dysfunction contributing to increased stroke size
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease: A statement for healthcare professionals from the american heart association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 2.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 3.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, et al. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem. 2001;276:16587–16591. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- 5.Michell BJ, Chen Z, Tiganis T, Stapleton D, Katsis F, Power DA, et al. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase c and the camp-dependent protein kinase. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 6.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, et al. Amp-activated protein kinase phosphorylation of endothelial no synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 7.Butt E, Bernhardt M, Smolenski A, Kotsonis P, Frohlich LG, Sickmann A, et al. Endothelial nitric-oxide synthase (type iii) is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide-dependent protein kinases. J Biol Chem. 2000;275:5179–5187. doi: 10.1074/jbc.275.7.5179. [DOI] [PubMed] [Google Scholar]
- 8.McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “Calcium-independent” Enos activation by phosphorylation. J Biol Chem. 2000;275:6123–6128. doi: 10.1074/jbc.275.9.6123. [DOI] [PubMed] [Google Scholar]
- 9.Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting enos for stroke protection. Trends Neurosci. 2004;27:283–289. doi: 10.1016/j.tins.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, et al. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-l-arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Lo EH, Hara H, Rogowska J, Trocha M, Pierce AR, Huang PL, et al. Temporal correlation mapping analysis of the hemodynamic penumbra in mutant mice deficient in endothelial nitric oxide synthase gene expression. Stroke. 1996;27:1381–1385. doi: 10.1161/01.str.27.8.1381. [DOI] [PubMed] [Google Scholar]
- 12.Kashiwagi S, Atochin DN, Li Q, Schleicher M, Pong T, Sessa WC, et al. Enos phosphorylation on serine 1176 affects insulin sensitivity and adiposity. Biochem Biophys Res Commun. 2013;431:284–290. doi: 10.1016/j.bbrc.2012.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman DL. Obese and diabetes: Two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 14.Coleman DL. A historical perspective on leptin. Nat Med. 2010;16:1097–1099. doi: 10.1038/nm1010-1097. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, et al. Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 16.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 17.Markel P, Shu P, Ebeling C, Carlson GA, Nagle DL, Smutko JS, et al. Theoretical and empirical issues for marker-assisted breeding of congenic mouse strains. Nat Genet. 1997;17:280–284. doi: 10.1038/ng1197-280. [DOI] [PubMed] [Google Scholar]
- 18.Atochin DN, Wang A, Liu VW, Critchlow JD, Dantas AP, Looft-Wilson R, et al. The phosphorylation state of enos modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest. 2007;117:1961–1967. doi: 10.1172/JCI29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 20.Huang PL. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab. 2009;20:295–302. doi: 10.1016/j.tem.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedman JE, Sauter R, Battinelli EM, Ault K, Knowles C, Huang PL, et al. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the nosiii gene. Circ Res. 1999;84:1416–1421. doi: 10.1161/01.res.84.12.1416. [DOI] [PubMed] [Google Scholar]
- 23.Lefer DJ, Jones SP, Girod WG, Baines A, Grisham MB, Cockrell AS, et al. Leukocyte-endothelial cell interactions in nitric oxide synthase-deficient mice. Am J Physiol. 1999;276:H1943–1950. doi: 10.1152/ajpheart.1999.276.6.H1943. [DOI] [PubMed] [Google Scholar]
- 24.Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104:342–345. doi: 10.1161/01.cir.104.3.342. [DOI] [PubMed] [Google Scholar]
- 25.Katusic ZS, d'Uscio LV, Nath KA. Vascular protection by tetrahydrobiopterin: Progress and therapeutic prospects. Trends Pharmacol Sci. 2009;30:48–54. doi: 10.1016/j.tips.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Sun X, Sharma S, Aggarwal S, Ravi K, Fineman JR, et al. Gtp cyclohydrolase i expression is regulated by nitric oxide: Role of cyclic amp. Am J Physiol Lung Cell Mol Physiol. 2009;297:L309–317. doi: 10.1152/ajplung.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 28.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure I. Generation and characterization of SD-db/db and SA-db/db mice
A. Knock-in construct. Top, genomic DNA with eNOS exons 17 to 26, is shown. The construct includes the mutation indicated with an asterisk (*) in exon 26, a neomycin resistance gene (NEO) flanked by lox P sites (black triangles), and a thymidine kinase gene (TK). Homologous recombination between the genomic DNA and the targeting construct (indicated by crossed lines) replaces the region surrounding exon 26 with the mutated exon, as well as the NEO gene flanked by lox P sites. Treatment with Cre recombinase by mating the chimeric mice with EIIa-Cre mice results in excision of the NEO gene, and one residual lox P site. B. Western blot analysis of brain protein of WT, db/db, SA-db/db and SD-db/db mice (n=4 for each group). Brain tissue was isolated from mice and electrophoresed on SDS-PAGE.C-F, The average expression levels of eNOS/actin (C), nNOS/actin (D), p-Akt/Akt (E) and p-AMPK/AMPK (F).
Supplementary Figure II. Anatomy of cerebrovasculature and CBF
A. Representative images of cerebrovasculature of WT, db/db, SD-db/db and SA-db/db mice after intracardiac carbon black perfusion. Upper panels show representative images of ventral brain surface and lower panels show representative higher magnifications of the posterior Circle of Willis showing the PCA and Pcomm arteries from WT, db/db, SD-db/db and SA-db/db mice.
B. The representative diameter of PCA (left panel) and Pcomm (right panel) in WT (n=4), db/db (n= 6), SD-db/db (n=6) and SA-db/db (n=5) mice.
C. Microvascular density measured by lectin staining in cerebral cortex (left panel) and striatum (right panel). n=3 for each group.
D. CBF measured in the core ischemic region by LDF, during 1 hour of MCA occlusion and 60 minutes of reperfusion. There were no significant differences between WT (n=15), db/db (n=15), SD-db/db (n=12) and SA-db/db (n=12) mice.
Supplementary Figure III. Insulin and glucose tolerance tests
A. Insulin tolerance test. Time course of blood glucose levels in WT, db/db, SA-db/db and SDdb/db mice after intraperitoneal injection of insulin (*p<0.05 WT vs. db/db, SD-db/db and SAdb/db mice).
B. Glucose tolerance test. Time course of glucose levels after intraperitoneal injection of glucose (*p<0.05 WT vs. db/db, SD-db/db and SA-db/db mice).
Supplementary Table I: Lipid profile, CBF, blood gases and heart rate
Total cholesterol, LDL-cholesterol, HDL-cholesterol, total triglyceride in serum (WT, n=8; db/db, n=5; SD-db/db, n=4; SA-db/db, n=4, * p<0.05 vs. WT). CBF, blood gases (n=3 for each group), heart rate (n=15 for each group). #P<0.05 db/db vs. SD-db/db mice.