Abstract
Single actin filaments stabilized with fluorescent phalloidin exhibit ATP-dependent movement on myosin filaments fixed to a surface. At pH 7.4 and 24 degrees C, the rates of movement average 3-4 micron/s with skeletal muscle myosin and 1-2 micron/s with Dictyostelium myosin. These rates are very similar to those measured in our previous myosin movement assays. The rates of movement are relatively independent of the type of actin used. The filament velocity shows a broad pH optimum between 7.0 and 9.0, and the concentration of ATP required for half-maximal velocity is 50 microM. Evidence was obtained to suggest that movement of actin over myosin requires at most the number of heads in a single thick filament. This system provides a practical, quantitative myosin-movement assay with purified proteins.
Full text
PDF

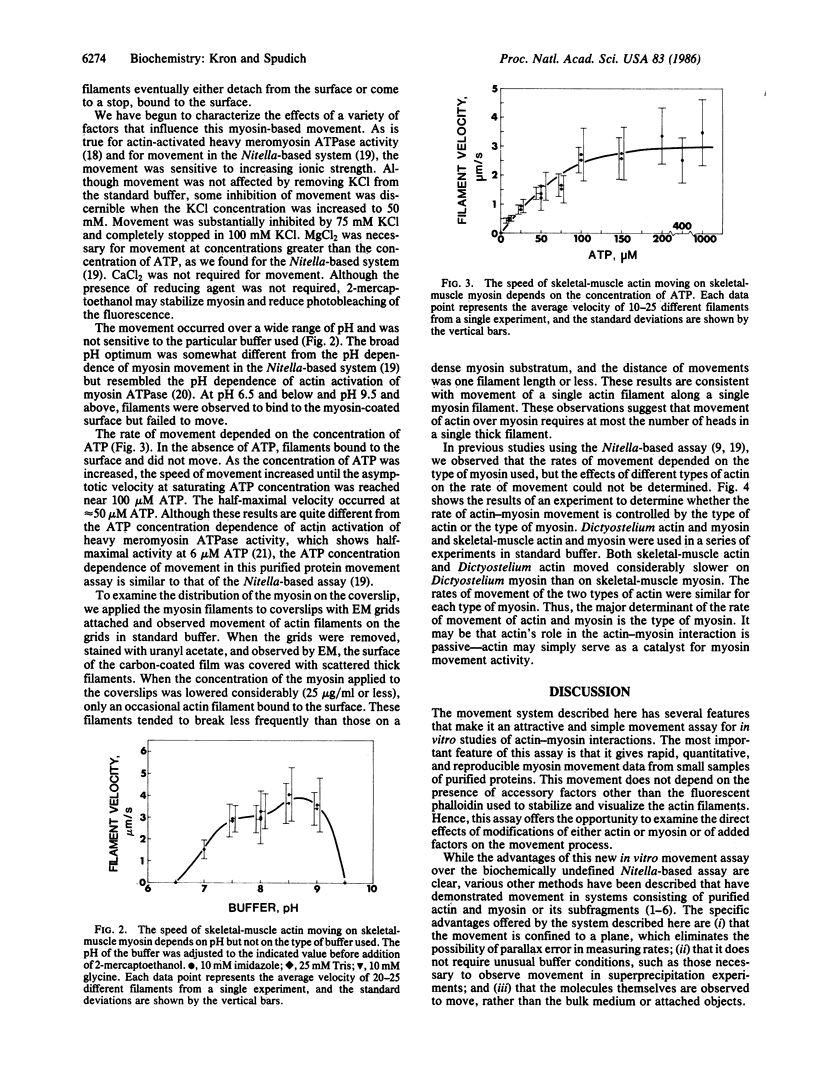
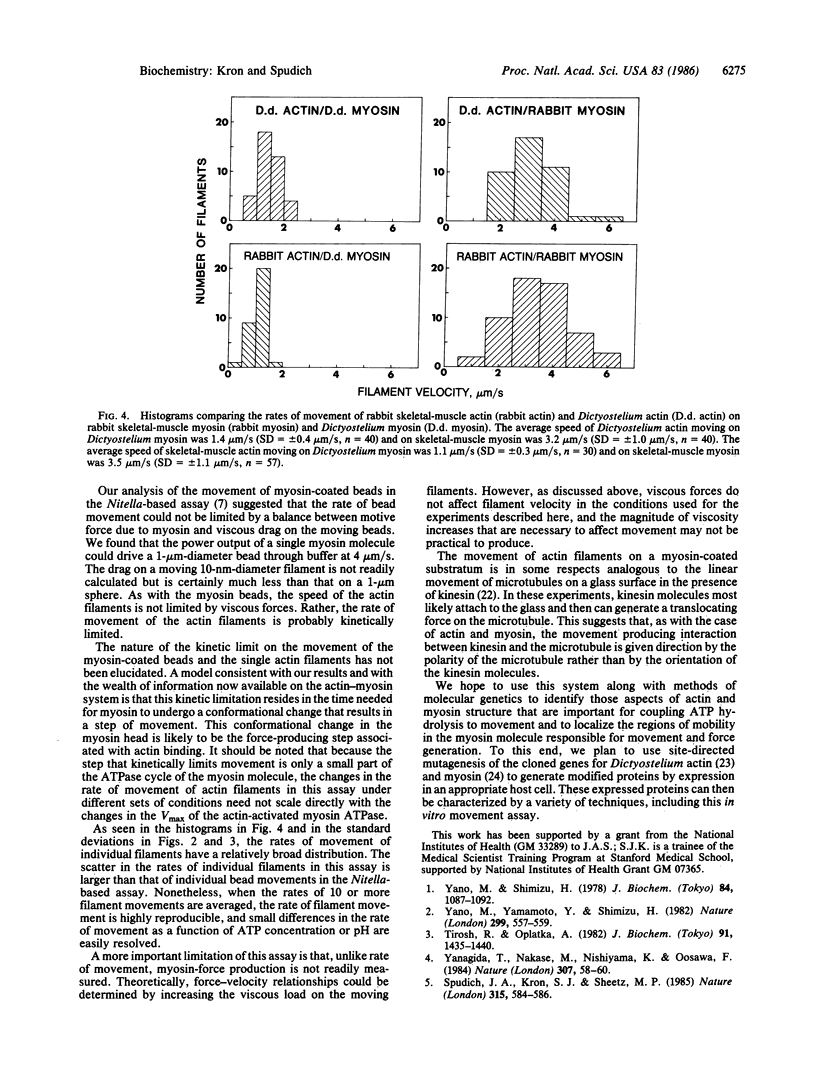

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg H. C., Block S. M. A miniature flow cell designed for rapid exchange of media under high-power microscope objectives. J Gen Microbiol. 1984 Nov;130(11):2915–2920. doi: 10.1099/00221287-130-11-2915. [DOI] [PubMed] [Google Scholar]
- DeLozanne A., Lewis M., Spudich J. A., Leinwand L. A. Cloning and characterization of a nonmuscle myosin heavy chain cDNA. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6807–6810. doi: 10.1073/pnas.82.20.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi-Fujime S. Unidirectional sliding of myosin filaments along the bundle of F-actin filaments spontaneously formed during superprecipitation. J Cell Biol. 1985 Dec;101(6):2335–2344. doi: 10.1083/jcb.101.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIELLEY W. W., HARRINGTON W. F. A model for the myosin molecule. Biochim Biophys Acta. 1960 Jul 15;41:401–421. doi: 10.1016/0006-3002(60)90037-8. [DOI] [PubMed] [Google Scholar]
- Kersey Y. M., Hepler P. K., Palevitz B. A., Wessells N. K. Polarity of actin filaments in Characean algae. Proc Natl Acad Sci U S A. 1976 Jan;73(1):165–167. doi: 10.1073/pnas.73.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M., Firtel R. A. Differential expression and 5' end mapping of actin genes in Dictyostelium. Cell. 1981 Jun;24(3):799–807. doi: 10.1016/0092-8674(81)90105-7. [DOI] [PubMed] [Google Scholar]
- Mockrin S. C., Spudich J. A. Calcium control of actin-activated myosin adenosine triphosphatase from Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2321–2325. doi: 10.1073/pnas.73.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Purification of muscle actin. Methods Cell Biol. 1982;24:271–289. doi: 10.1016/s0091-679x(08)60661-5. [DOI] [PubMed] [Google Scholar]
- Rizzino A. A., Barouch W. W., Eisenberg E., Moos C. Actin-heavy meromyosin biding. Determination of binding stoichiometry from adenosine triphosphatase kinetic measurements. Biochemistry. 1970 Jun 9;9(12):2402–2408. doi: 10.1021/bi00814a003. [DOI] [PubMed] [Google Scholar]
- Sellers J. R., Spudich J. A., Sheetz M. P. Light chain phosphorylation regulates the movement of smooth muscle myosin on actin filaments. J Cell Biol. 1985 Nov;101(5 Pt 1):1897–1902. doi: 10.1083/jcb.101.5.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Chasan R., Spudich J. A. ATP-dependent movement of myosin in vitro: characterization of a quantitative assay. J Cell Biol. 1984 Nov;99(5):1867–1871. doi: 10.1083/jcb.99.5.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Spudich J. A. Movement of myosin-coated fluorescent beads on actin cables in vitro. Nature. 1983 May 5;303(5912):31–35. doi: 10.1038/303031a0. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Kron S. J., Sheetz M. P. Movement of myosin-coated beads on oriented filaments reconstituted from purified actin. Nature. 1985 Jun 13;315(6020):584–586. doi: 10.1038/315584a0. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stone D. B., Prevost S. C. Characterization of modified myosin at low ionic strength. Enzymatic and spin-label studies. Biochemistry. 1973 Oct 9;12(21):4206–4211. doi: 10.1021/bi00745a026. [DOI] [PubMed] [Google Scholar]
- Tirosh R., Oplatka A. Active streaming against gravity in glass microcapillaries of solutions containing acto-heavy meromyosin and native tropomyosin. J Biochem. 1982 Apr;91(4):1435–1440. doi: 10.1093/oxfordjournals.jbchem.a133832. [DOI] [PubMed] [Google Scholar]
- Uyemura D. G., Brown S. S., Spudich J. A. Biochemical and structural characterization of actin from Dictyostelium discoideum. J Biol Chem. 1978 Dec 25;253(24):9088–9096. [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Reese T. S., Sheetz M. P. Organelle, bead, and microtubule translocations promoted by soluble factors from the squid giant axon. Cell. 1985 Mar;40(3):559–569. doi: 10.1016/0092-8674(85)90204-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Pardee J. D., Reidler J., Stryer L., Spudich J. A. Mechanism of interaction of Dictyostelium severin with actin filaments. J Cell Biol. 1982 Dec;95(3):711–719. doi: 10.1083/jcb.95.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida T., Nakase M., Nishiyama K., Oosawa F. Direct observation of motion of single F-actin filaments in the presence of myosin. Nature. 1984 Jan 5;307(5946):58–60. doi: 10.1038/307058a0. [DOI] [PubMed] [Google Scholar]
- Yano M., Shimizu H. Studies of the chemo-mechanical conversion in artificially produced streamings. II. An order--disorder phase transition in the chemo-mechanical conversion. J Biochem. 1978 Nov;84(5):1087–1092. doi: 10.1093/oxfordjournals.jbchem.a132222. [DOI] [PubMed] [Google Scholar]
- Yano M., Yamamoto Y., Shimizu H. An actomyosin motor. Nature. 1982 Oct 7;299(5883):557–559. doi: 10.1038/299557a0. [DOI] [PubMed] [Google Scholar]