Abstract
We have previously shown that mice challenged with a lethal dose of PR8-OVAI are protected by injection of 4 to 8 × 106 in vitro - generated Tc1 or Tc17 CD8+ effectors. Viral load, lung damage and loss of lung function are all reduced following transfer. Weight loss is reduced and survival increased. We sought here to define the mechanism of this protection. CD8+ effectors exhibit multiple effector activities, perforin-, FasL- and TRAIL- mediated cytotoxicity, secretion of multiple cytokines (IL-2, IL-4, IL-5, IL-9, IL-10, IL-17, IL-21, IL-22, IFN-γ and TNF) and chemokines (CCL3, CCL4, CCL5, CXCL9 and CXCL10). Transfer of CD8+ effectors into recipients, prior to infection, elicits enhanced recruitment of host neutrophils, NK cells, macrophages, and B cells. All of these events have the potential to protect against viral infections. Removal of any one, however, of these potential mechanisms was without effect on protection. Even the simultaneous removal of host T cells, host B cells and host neutrophils combined with the elimination of perforin mediated lytic mechanisms in the donor cells failed to reduce their ability to protect. We conclude that CD8+ effector T cells can protect against the lethal effects of viral infection by means of a large number of redundant mechanisms.
Introduction
Previous studies of the immune response to influenza infection in mice have implicated a variety of different cell types and mechanisms that collectively bring about viral clearance and provide protection. B cells can make neutralizing antibody to the coat proteins of the virus but this occurs too late in the primary response to prevent the lethal effects of the virus and other mechanisms are needed. Studies of heterosubtypic immunity in which mice are challenged with a subtype carrying different coat proteins from the priming strain have shown that CD4+ and CD8+ T cells, non-neutralizing IgA antibodies, NKT cells and γδ T cells can all contribute to heterosubtypic protection in the absence of neutralizing antibodies (1). Our own studies have focused on the role of CD8 T cells and have investigated the multiple ways in which they can protect.
CD8+ T cells are cytolytic and this is often thought of as their primary or even only role. The basic paradigms of elementary immunology tell us that B cells make antibody, T cells mediate cellular immunity; CD4+ T cells help B cells, CD8+ T cells kill infected cells (2, 3). CD8+ T cells enjoyed a period when they were also suppressor cells and have only recently regained that reputation (4), along with CD4+ T cells. Although recognized as generalizations in need of substantial elaboration and qualification, these paradigms still restrict our thinking much more than they should.
The first modification of this picture came when it was shown that cytotoxic CD8+ T cells could also make cytokines (5, 6), and that some CD4+T cells can be also be cytotoxic (7, 8). More recently it has been shown that CD8+ T cells can also make chemokines (9, 10) and that the interaction of CD8+ T cells with epithelial cells induced TNF secretion by the CD8+ T cells (11) and induces chemokine secretion by the epithelial cells (12) These properties lead to a whole further round of secondary effector functions, triggered originally by the CD8+ effector T cells.
As with CD4+ T cells, CD8+ T cells can differentiate along divergent lines to give rise to subsets of cells with different combinations of effector functions and indeed there are few functions of CD4+ T cells that cannot be carried out by CD8+ T cells and vice versa It is even conceivable that Tc17 effectors, which lack cytolytic function, could contribute via the secretion of IL-21 to the B cell response although this has not so far been demonstrated to our knowledge. The breadth of diversity of CD8+ T cell function has been recently illustrated by the demonstration of the very large number of products that can be produced and by the different combinations expressed by different CD8+ T cells (13).
In the model we use, polarized populations of in vitro generated CD8+ effectors from T cell receptor transgenic OT-1 mice, specific for the SIINFEKL peptide of ovalbumin are injected into naïve recipients. Next we infect the recipient mice with a genetically modified strain of influenza, bearing the SIINFEKL peptide inserted in the neuraminidase stalk. We determine the subsequent effectiveness of the injected cells in protecting the mouse from what would otherwise be a lethal challenge with the same strain of virus, using a variety of assays.
The Tc1, Tc2 and Tc17 CD8+ T cell subsets, that parallel the CD4+ Th1, Th2 and Th17, have been mainly characterized by determining the phenotype of polarized subsets generated in vitro under artificial conditions (14–17), but adoptive transfer of all three subsets of effectors have been show to protect against lethal influenza challenge.
To analyze the mechanism of protection mediated by CD8+ T cells we have used polarized populations of in vitro generated CD8+ effectors to dissect the role for each the subsets plays in protection. We show here, that CD8+ T cells can contribute to and shape the immune response via a rather large number of different effector mechanisms and that, in the response to influenza, the ones highlighted in the traditional paradigm may be the least important of their accomplishments.
Cells of the Tc1 subset of CD8+ T cells can indeed kill virally infected cells by a perforin-mediated mechanism but Tc17 cells generated in vitro initially lack lytic activity and Tc17 prepared from perforin deficient OT-1 are as effective at providing protection as those prepared from WT mice (16). Tc17 effectors, however, can kill targets in an in vivo CTL assay by a Fas ligand mediated mechanism after injection into a recipient mouse. This does not appear essential, however, for protection against lethal influenza challenge as Fas deficient lpr mice are protected by Tc17 from perforin deficient donors.
We have previously shown that Tc2 effector cells are also cytolytic (15), but are less protective against viral challenge than are Tc1. Tc2 elicit an enhanced eosinophil influx and bring about a greater impairment of lung functions (17). In the studies presented here, Tc17 are more effective in recruiting host CD4+ and CD8+ T cells, NK cells, eosinophils and macrophages than Tc1.
Polarized subsets of effector CD8+T cells (Tc1, Tc2 and Tc17) can collectively make a wide range of cytokines including IL-2, IL-4, IL-5, IL-9, IL-10, IL-17, IL-21, IL-22, IFN-γ and TNF and chemokines, including CCL3, CCL4, CCL5, CXCL9 and CXCL10 (10, 15). We show here that these mediators recruit eosinophils, NK cells, macrophages, CD4+ and CD8+ T cells and B cells to the lung. We showed also that the CD8+ effectors could bring about B cell growth and differentiation, activation of innate immunity and tissue repair all of which can be assumed to contribute to protection.
In spite of, or more likely because of, this enormous range of functions we were unable to show that any one of the effector mechanisms tested was essential for protection and we conclude that CD8+ T cells act by multiple redundant protective mechanisms. We do show, however, that the transfer of primed CD8+ T cells rapidly induces a wide range of innate cytokines and chemokines and we suggest that it this effect that may be most critical for protection in the early stage of the response.
Materials and Methods
Mice
C57BL/6 (B6), BALB/c (BALB), B6.Thy-1.1, B6.CD45.1, B6.OT-1, B6.OT-1.Thy-1.1, B6.OT-1.CD45.1, B6.OT-1.perforin−/− (pfn), B6.lpr, B6 TCR βδ−/−, B6.RAG-2−/− and clone 4 (BALB.HA) mice were bred at the Trudeau Institute and at University of Massachusetts Medical School, Worcester and were used at 5 to 8 weeks of age for generation of effectors and at 8 to 12 week of age for recipients. B6.OT-1.TRAIL−/− mice were kindly supplied by T.S. Griffiths, University of Iowa, Iowa City, B6.lpr were purchased from Jackson Laboratories. All animal procedures were approved by the Institute Animal Care and Use Committee (IACUC) at the Trudeau Institute and University of Massachusetts Medical School, Worcester.
Influenza virus, infections
Influenza A/Puerto Rico/8/34 (PR8) (H1N1), and PR8-OVAI (kindly provided by Dr. Richard Webby, St. Jude Children’s Res. Hosp., Memphis, TN) and were grown in the allantoic cavity of embryonated hen eggs from virus stocks. Lightly anesthetized mice were infected with influenza by intranasal inoculation of 50 µl virus in PBS. For the stock of PR8 employed 2 × 104 EID50 = 4LD50 and 970 PFU = approximately 2LD50 for PR8-OVAI.
Viral titers
Mice injected with CD8+ T cells and influenza infected were euthanized at various times post infection by cervical dislocation. The lungs were removed, teased into single cell suspensions in a fixed volume of 5ml and then 1ml aliquots frozen and stored at –70 °C. The lysates were thawed and the influenza titer determined using the Madine Darby Canine Kidney Cell plaque assay (MDCK) as detailed previously (15). Results are expressed as plaque forming units (PFU) per lung. In some experiments, viral titer was determined by RT-PCR, see below.
Generation of Tc1 and Tc17 CD8+ effector cells in vitro
Tc1 and Tc17 effectors were generated from B6.OT-1, B6.OT-1.Thy-1.1, B6.OT-1.45.1, B6.OT-1.perforin−/−(pfn), B6.OT-1.TRAIL−/− or Clone 4 mice as previously described (15, 16). T cell-depleted antigen presenting cells (B cell blasts) were prepared by negative selection on MACS columns using FITC-labeled anti-Thy1.2 mAb (53-2.1, eBiosciences) and anti-FITC-MACS beads (Miltenyi Biotech). The B cells were stimulated with LPS (25 µg/ml) and DXS (25 µg/ml) for 3 days and were used as antigen presenting cells. They were loaded with SIINFEKL peptide (10 µg/ml) at 37°C for 30 min and treated with mitomycin C (50 µg/ml) at 37°C for 30 min, and washed 3 times before use. CD8+ T cells from spleens of OT-1 TCR-transgenic mice TcR-transgenic mice were enriched by CD8 MACS beads (Miltenyi Biotech) and incubated with SIINFEKL peptide-pulsed B cell blasts (T:B = 1:3) for 4 days. For Tc17 cultures, IL-1β (10 ng/ml, Peprotech), IL-6 (20 ng/ml, Peprotech), porcine TGF-β (3 ng/ml, R&D systems), IL-21 (80 ng/ml, Peprotech), IL-23 (50 ng/ml, R&D systems), anti-IL-4 mAb (11B11, 10 µg/ml) and anti-IFN-γ mAb (XMG1.2, 10 µg/ml) were added. For Tc1 cultures, IL-2 (4.7 ng/ml), IL-12 (9.2 U/ml, kindly provided by Stanley Wolf, Genetics Institute, Cambridge, MA), anti-IL-4 mAb (11B11, 10 µg/ml) were added. The quality of the effector cell preparations was confirmed by phenotype analysis.
Phenotype of Tc effectors
Tc effectors were prepared as described above. For intracellular cytokine staining, single Tc effector cell suspensions were cultured for 4h with 10 ng/ml PMA, 500 ng/ml ionomycin and 10 mg/ml Brefeldin A. Cells were harvested and incubated with antibody to cell surface markers. Cells were then fixed with 4% formalin and incubated with antibodies to intracellular cytokines: IFN-γ-Pacific Blue and IL-17-PE in 0.1% saponin buffer. Cells were harvested and incubated with antibody to cell surface markers and then fixed with Fixation/Permeabilization solution (BD Biosciences) for 10 min. Cells were then washed with 1× Perm/Wash buffer (BD Biosciences) and then permeabilized with 1× Perm/Wash buffer with 0.05% Triton ×100 for 10 min. Then cells were incubated with antibodies to cytokines in 1× Perm/Wash buffer with 0.05% Triton X100, IL-17-Pacific Blue and IFN-γ-FITC (XMG1.2, BD Biosciences). Cells were analyzed on the CyAN LX9 laser flow cytometer (DAKO), the BD FACS Canto or on the BD LSRll. The staining profiles were analyzed using FlowJo.
In vivo: cytotoxicity
In vivo cytotoxicity was assayed as follows: Tc1 and Tc17 effector populations were prepared from OT-1 mice (CD45.2+Thy1.2+), and 4 × 106 cells of Tc17 or Tc1 were injected into naïve B6.Thy1.1 recipients (CD45.2+ Thy1.1+). One day after Tc effector injection, recipient mice were injected with 2.5 × 106 of SIINFEKL-pulsed spleen cells stained with 1.25 µM of CFSE (CD45.1+ CFSEhi) and 2.5 × 106 of non-pulsed spleen cells stained with 156nM of CFSE (CD45.1+ CFSElo). 24 hours later spleen cells were harvested and the ratio of surviving CD45.1+ CFSEhi to CD45.1+ CFSElo cells was determined by flow cytometry.
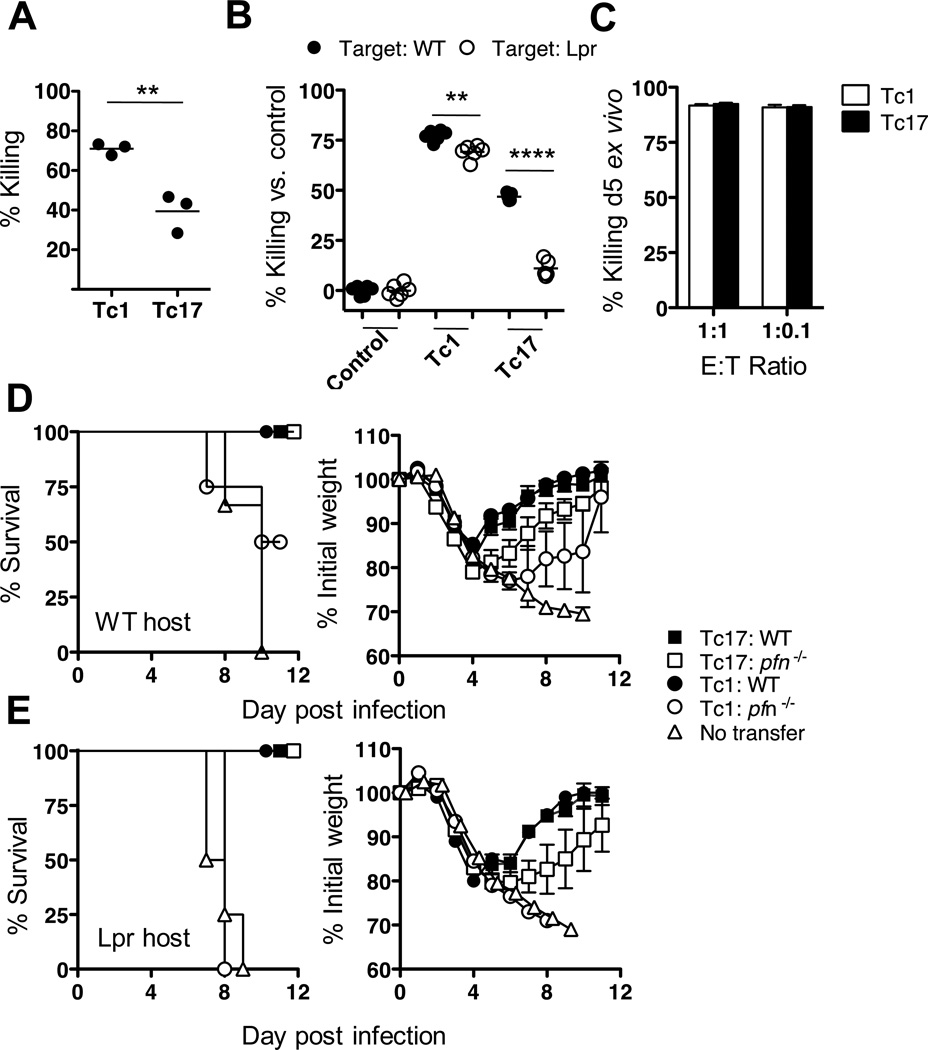
For Figure 1B, Tc1 and Tc17 effectors were prepared from WT OT-1 (CD45.1+ CD45.2+) and 4 × 106 cells of Tc17 or Tc1 were injected into naïve B6.CD45.1 recipients (CD45.1+). One day after Tc effector injection, recipient mice were injected with 1.25 × 106 of SIINFEKL-pulsed wild-type cells (CD45.2+ CFSEhi) and 1.25 × 106 of non-pulsed wild type cells (CD45.2+ CFSElo) or 1.25 × 106 of SIINFEKL-pulsed Fas mutant (lpr) target cells (CD45.2+ CFSEhi) and 1.25 × 106 of non-pulsed lpr cells (CD45.2+ CFSElo). Twenty-four hours after transferring target cells, mice were sacrificed and spleen cells were isolated. Percent killing was calculated by comparing the ratio of surviving CFSEhi targets to CFSElo targets in spleen.
FIGURE 1.
Changes in the cytotoxic activity of adoptively transferred CD8 Effector T cells. 4 × 106 in vitro generated Tc1 or Tc17 OT-1 effectors were injected into groups of 5 naïve B6 recipients. Twenty-four hours later CFSE high concentration labeled, SIINFEKL pulsed target cells and CFSE low concentration labeled control cells were injected. Twenty-four hours later mice were sacrificed and the ratio of high to low CFSE labeled cells was determined by flow cytometry to measure the level of in vivo killing by the OT-1 effectors. Panel A) Tc17 and Tc1 at 24 h; In another assay (panel B) target cells were made from WT or Fas deficient lpr mice to determine whether the killing was FasL dependent. In panel C, the ex-vivo killing assay was performed with Tc17 or Tc1 purified from mice previously injected with Tc1 or Tc17 effectors then infected with PR8-OVAI. Five days post-infection effectors were isolated and injected into naïve B6 mice. One day later, CFSE labeled target cells were then transferred to 5 dpi effector transferred mice and killing was measured (as labeled in A). In panels D and E, 8 × 106 in vitro generated Tc17 or Tc1 effectors from perforin−/− or WT mice were injected into WT (panel D) or lpr (panel E) mice, challenged with 2LD50 PR8-OVAI and weight changes and survival followed for 11 days. Similar results were seen two or more experiments for each panel.
For the ex vivo CTL assay, Tc1 and Tc17 effectors were prepared from OT-1 (CD45.2+) and 4 × 106 cells of Tc17 or Tc1 were injected into naïve B6.CD90.1 recipients. Two days after Tc effector transfer, recipient mice were infected with 0.2LD50 of PR8-OVAI (1:2000 diluted). On day 5 of infection transferred cells were purified from the lung by cell sorting, and 2 × 105 cells of purified Tc17 or Tc1 were injected into naïve B6.CD45.2 mice. One day after transfer, recipient mice were injected with 2 × 105 (1:1) or 2 × 104 (1:0.1) of SIINFEKL-pulsed cells (CFSEhi) and non-pulsed cells (CFSElo). Twenty-four hours after CFSE stained cell transfer, the ratio of surviving CFSEhi cells to CFSElo cells was determined by flow cytometry.
Adoptive transfers, lethal infection, weight changes and survival
C57BL/6 mice were injected intravenously with 4, 8 or 16 × 106 of OT-1 Tc17, or OT-1 Tc1 effector cells on day –1 and challenged on day 0 with an intranasal lethal dose of 1– 2 LD50 influenza PR8-OVAI virus or 1–3 LD50 influenza PR8 virus. In other experiments polyclonal CD8 effectors specific for PR8 antigens were isolated from mice 7 days after sublethal viral challenge and used as the donor cells. Mice were weighed every second day and weight expressed as % of initial. A cohort of mice were followed up to day 12 – 28 post challenge to determine % survival. In some experiments (Figure 8) HA specific Clone 4 TcR transgenic mice were used as donors and BALB/c as recipients.
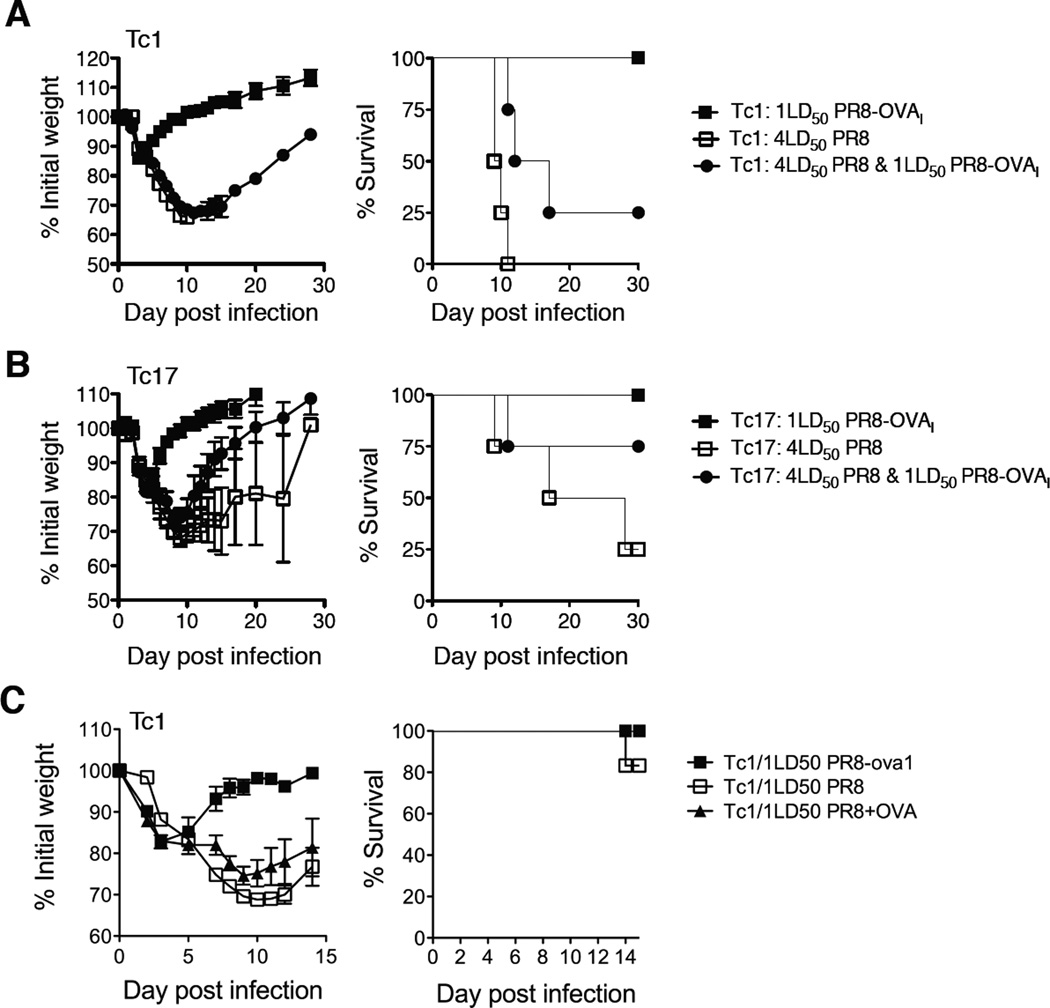
FIGURE 8.
Tc17 donor cells provide bystander protection against viral challenge. 8 × 106 in vitro generated Tc1 (panel A) or Tc17 OT-1 (panel B) effectors were each injected into three groups of mice. One was challenged PR8, a second with PR8-OVAI and the third with both viruses (n=4). In panel C, the third group of mice was injected with Tc1 effectors and 100 µg LPS-free OVA i.n instead of PR8-OVAI (n=5). Weight changes and survival were followed for 15–30 days.
Adoptive transfers, sub-lethal infection, albumin and LDH measurement and lung function
B6.OT-1 effectors were injected into B6.CD45.1 mice. One day later mice were infected i.n. and then sacrificed at various time points after influenza infection (days 2, 4, 6, 8, and 21 post-infection). Lungs were removed following perfusion with 5 ml PBS via the left ventricle of the heart and single cell suspensions prepared by collagenase treatment (5 mg/mL collagenase A and DNase I). Cells were stained with anti CD45.2 to distinguish donor and host cells and surface markers were stained with following antibodies: CD45.2-AlexaFluor647, CD4-PE, CD8-PE-Cy7, CD19-FITC, and AQUA fixable Dead Cell Stain (Invitrogen). Cells were analyzed on the FACS Canto II (BD). Cells were gated on live (AQUA negative) and either CD45.2− (host) or CD45.2+ (donor) and analyzed using FlowJo.
Neutrophils were identified as Gr-1high7/4highCD11b+F4/80−I-Ab-, NK cells were identified as gal-ser/mCD1d positive (PBS-57 tetramer, NIH Tetramer Core). Biotinylated antibodies were counter-stained with Streptavidin-PacificOrange (Invitrogen) and then fixed with 4% formalin. Cells were analyzed on the FACS Canto II (BD).
Measurement of the host response
B6.OT-1.Thy-1.2 effectors were injected into infected B6.Thy-1.1 mice, which were sacrificed at various time points after influenza infection. Bronchoalveolar lavage (BAL) was collected by washing the airways five times with 0.5 ml of PBS. Lungs were removed following perfusion with 5 ml PBS via the left ventricle of the heart and single cell suspensions prepared by collagenase treatment (2.5 mg/ml collagenase D). Cells were stained with anti-thy-1.1 to distinguish donor and host cells and surface markers were stained with following antibodies: CD3-PE-Cy7 (145-2C11, eBiosciences), CD4-FITC (RM4-4, BD Biosciences), CD8-APC-AlexaFluor750 (53-6.7, eBiosciences) and CD19-PE (1D3 BD Biosciences) for B cells. Neutrophils were identified as Gr-1high7/4highCD11b+F4/80−I-Ab-, NK cells were identified as gal-ser/mCD1d positive (PBS-57 tetramer,NIH Tetramer Core). Biotinylated antibodies were counter-stained with Streptavidine-PacificOrange (8 mg/ml, Invitrogen) and then fixed with 4% formalin. Cells were analyzed on the FACS Canto II (BD). Cells were gated on CD3+ and either Thy-1.1 positive (host) or Thy-1.1 negative (donor) and their staining profiles were analyzed using FlowJo.
Depletion of Neutrophils, NK-1.1 cells, Thy-1.1 donor cells
Neutrophils were depleted by intraperitoneal injection on d-2, d0, d2 and d4 of 200µg/mouse the monoclonal anti-Ly6G antibody, 1A8. The isotype controls were injected with rat IgG2a. NK cells were depleted by injection of 200µg/mouse of anti NK-1.1 clone PK136 on days −1, +2 and +5. Thy-1.1 donor cells were depleted by a single i.p. injection of 0.2mg anti-Thy1.1 (clone 19E12) on days 3, 5 or 8
RNA and quantitative PCR
RNA was extracted and purified from CD8+T effector cells, using TRIzol (Invitrogen) and RNeasy kit (Qiagen, La Jolla, CA), sequentially. DNase-treated RNA (2µg) was reverse transcribed with Oligo dT and SuperScript II (Invitrogen). Quantitative PCR was performed using TaqMan Universal PCR Master Mix, following the Applied Biosystems (Foster City, CA) protocol. Probes for GAPDH, FasL and TRAIL were obtained from Applied Biosystems. Quantitative PCR was performed using a PRISM 7700 instrument (Applied Biosystems). Quantitation of viral RNA was performed as previously described (18) utilizing forward (5′-GAGCTGAGGGAGCAATTGAG-3′) and reverse (5′-TCATCACCGCCTAACAGTA-3′) primers that were designed for a viral PA fragment.
Cytokine assay
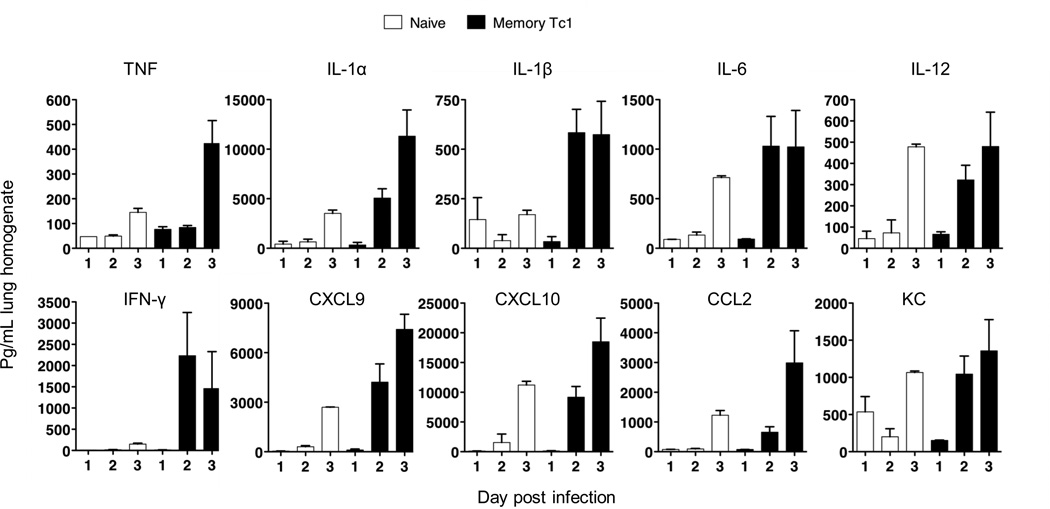
Levels of cytokines and chemokines in lung homogenates (Figure 8) were determined using mouse multiplex luminex kits (Invitrogen) read on a Luminex 100 reader (Luminex Corp.)
Staining Lung Sections
Infected mice were sacrificed and bled by cutting the renal artery. Lungs were perfused in 4% PFA and embedded in paraffin. 5µm paraffin lung sections were cut in a microtome and collected in plus slides. Slides with lung sections (for Suppl. Figure 3) were incubated in a 60°C oven and quickly transferred to xylenes. Lung tissues were progressively hydrated by transferring them to xylenes, alcohol, 96% alcohol, 70% alcohol and finally water. Antigens were unmasked by boiling lung sections in antigen retrieval solution for 30 minutes (Dako). Slides were cooled down for 20 minutes and washed with deionized water. Lung sections were outlined with a pap-pen and blocked for 30 minutes with 5% normal donkey serum and 1:100 of antibodies against Fc receptors 2.4G2 diluted in 0.1% Tween 20/0.1% Triton×100 in PBS. Without washing, primary antibodies (CD3: Santa Cruz Biotechnology, cloneM-20; PCNA, Santa Cruz Biotechnology, clone c-20 and biotinylated B220, BD Pharmingen, RA36B2) were added to the lung sections and incubated overnight at room temperature in a humid chamber. CD3 and PCNA were detected with donkey anti-goat (Jackson ImmunoResearch Laboratories, PA), AlexaFluor-594 (Molecular probes, Eugene, OR) and B220-biotin was detected by adding donkey anti-rat (Jackson ImmunoResearch Laboratories, PA, AlexaFluor-488 (Molecular probes, Eugene OR) and streptavidin, AlexaFluor-488 (molecular probes, Eugene). In other experiments lung sections were stained with anti-pro-surfactant protein C as an indicator of type 2 epithelial cells or cells suspensions were prepared an analyzed by flow cytometry using the same reagents. Tissue sections were mounted with medium for fluorescence with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Pictures were taken with a Carl Zeiss Microscope and representative 200× magnifications are shown.
Results
In our previous studies (15–17), we have seen evidence of the participation of IFN-γ, IL-4 and IL-17 secreting cells in the response to influenza, suggesting that Tc1, Tc2 and Tc17 cells can all play a role in protection. We sought, here, to determine the effect of transfer of CD8+ effectors on the course of the response in the recipient mice. Our first approach was to determine the correlates of protection mediated by the injected CD8+ effector T cells.
Adoptive transfer of CD8+ effectors brings about a reduction in viral load and lung pathology
We have previously shown (15–17) that all three subsets of cells can protect naïve mice from otherwise lethal challenge but our previous studies had measured only weight changes and survival. We show here that injection of Tc1 or Tc17 effectors lowers initial viral load (Suppl. Fig 1A), prevents damage as shown by reduction in leakage of albumin (Suppl. Fig 1B) and LDH (Suppl. Fig 1C) into the lung lavage and alleviates respiratory distress as shown by changes in minute volume (Suppl. Fig 1D) and respiratory rate (Suppl. Fig 1E)
In vivo generated polyclonal CD8+ effectors also provide protection
It is not possible to prepare well-polarized CD8 effectors in vivo but polyclonal CD8 effectors were isolated from mice challenged with 0.5LD PR8 seven days post infection and graded numbers were injected into naïve recipients that were then challenged with 3LD PR8. As few a 4 × 106 effectors reduced weight loss and increased survival establishing that protection was not unique to the use of T cells TcR transgenic (data not shown).
CD8+ effectors lacking perforin-mediated cytotoxic activity are still protective
The prevailing belief is that CD8+ T cells protect against viral infection by killing virally infected cells. We have previously shown that Tc1 and Tc17 effectors are equally protective even though Tc1 are lytic in vitro while Tc17 are not (16). We showed also that protection by Tc1 was diminished in effectors made from perforin−/− mice while protection by Tc17 was not (16). Although Tc17 effectors lacked FasL or TRAIL expression in vitro (Suppl. Fig 2) it remained possible that Tc17 develop in vivo lytic activity dependent on FasL or TRAIL expression on injection back into the animal. To examine this possibility we injected Tc17 effectors from OT-1 mice into uninfected naïve recipients and looked for killing of SIINFEKL labeled targets at 24 hours in an in vivo cytolysis assay.
When Tc17 effector cells are injected into normal mice they do show some killing activity at 24h after injection but less activity that Tc1 (Fig 1A). The Tc17 mediated killing, however, is FasL dependent as Fas negative targets from lpr mice are not killed (Fig 1B). Eventually, however, Tc17 cytolytic activity develops and becomes quantitatively equivalent to that of Tc1 by day 5 (Fig 1C) and many of the injected cells become double producers of IFN-γ and IL-17 (data not shown).
To determine whether FasL-mediated killing plays a role for Tc17 mediated protection we injected Tc1 or Tc17 effectors from WT or perforin deficient mice into WT or lpr recipients to determine whether they could still protect in the absence of both perforin and FasL-mediated killing. WT Tc17, WT Tc1 and perforin deficient Tc17 were fully protective in WT recipients (Fig 1D) but mice that received Tc1 from perforin deficient mice lost more weight (Fig 1D) and 2 out of 4 died. This differential was more marked when the same cells were transferred into lpr recipients where Tc1 cells from perforin deficient mice provided no protection while Tc17 WT or perforin deficient effectors were still protective (Fig 1E). We conclude that while perforin-mediated lysis is important in the protection mediated by Tc1 and Tc2 cells neither perforin nor FasL-mediated killing play a significant role in the protection mediated by Tc17.
In further experiments we attempted to determine whether Tc1 or Tc17 protection is mediated by a TRAIL dependent mechanism using Tc1 and Tc17 effectors prepared from CD8+ T cells from OT-1.TRAIL−/− mice. Tc17 effectors from TRAIL deficient mice were still able to protect (data not shown) but a high proportion of the CD8+ cells in the naïve OT-1.TRAIL−/−mice were CD44 high and it was not possible to make preparations of Tc17 effectors from these mice with anything more than a very low percentage of IL-17 secreting cells. We were thus unable to completely exclude the possibility that Tc17 effectors protect by a TRAIL-mediated lytic mechanism, as suggested by Brincks and his colleagues (19).
We turned, next, to other correlates associated with the protection mediated by the injection of CD8+effectors to determine if any were essential for protection.
Adoptive transfer of CD8 effectors enhances recruitment of host cells
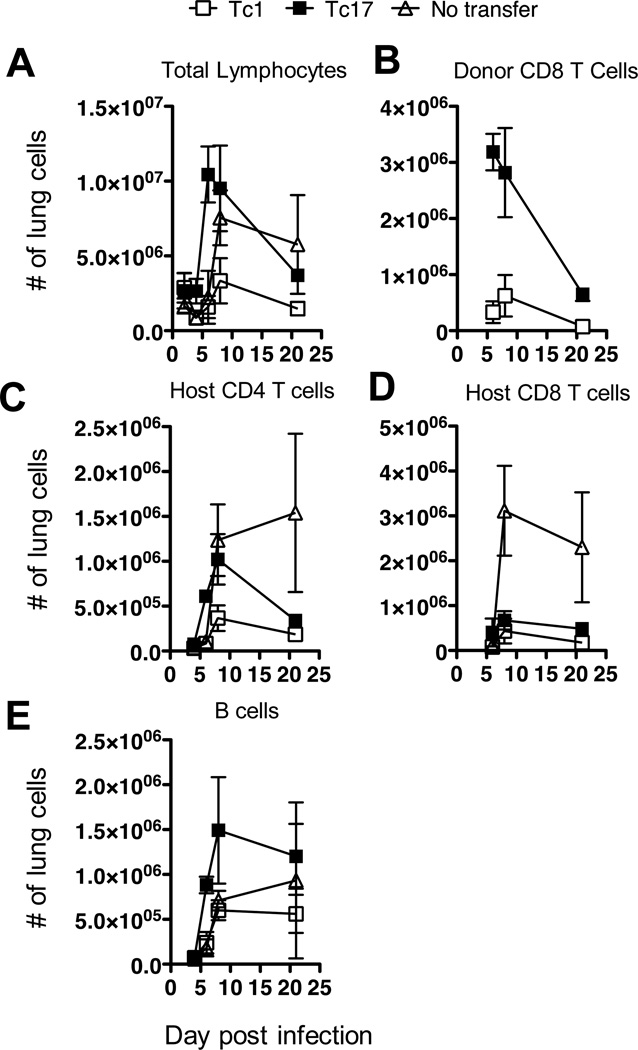
CD4+ and CD8+ T cells, B cells, neutrophils, NK cells and macrophages are all recruited into the lungs of influenza-infected mice (20, 21). The injection of already functional CD8+ effectors from OT-1 mice accelerates the recruitment of most of these cell types after exposure to PR8-OVAI. Differential effects are seen with effectors of different subsets, thus Tc2, for example, recruit greater numbers of eosinophils than Tc1 (17) and Tc17 recruit more B cells (see below). We had also previously shown that the injection of Tc17 effectors led to a greater accumulation of neutrophils than Tc1 following viral challenge (16). In further experiments, we found that following the adoptive transfer of either Tc1 or Tc17 effectors, Tc17 effectors recruited greater numbers of cells to the lung than Tc1 recipient or control mice (Fig 2A) and that Tc17 cells entered the lung more rapidly and in greater numbers than Tc1 cells (Fig 2B). Tc17 effectors were able to recruit host CD4+ (Fig 2C) and CD8+ T cells (Fig 2D) more effectively than Tc1 effectors. However, host CD4+ T cells rapidly declined in the recipients of CD8+ effectors after day 10 while they continued to increase in the untreated controls and the net effect of the transfer was actually to decrease recruitment of host T cells at later time points. There was, however, a striking increase in the number of B cells (Fig 2E) after adoptive transfer of Tc17, which persisted through day 20. We concluded that the cytokines and chemokines released following the transfer of Tc17 and to a lesser extent Tc1 led to an early enhancement of recruitment of many cell types, including neutrophils, NK cells, macrophages and B cells. This correlated with the control of the viral load and was followed by a decline in the numbers of recruited T cells, and to a less extent B cells, in the treated mice while the numbers continued to rise in the untreated controls.
FIGURE 2.
Total cells, B cells, CD4+, host CD8+, donor CD8+ and CD19+ B cells are recruited to the lung after adoptive transfer of CD8 effector T cells and viral challenge. Groups of 5 B6 mice were injected with 8 × 106 in vitro generated Tc1 (open squares) or Tc17 Thy-1.1.OT-1 effectors (filled squares) or left un-injected (open triangles) and challenged one day later with 0.2LD50 PR8-OVAI. Mice were sacrificed at the times indicated and cell suspensions were prepared from the lungs and stained with fluorescently labeled antibody to pro-surfactant C to determine the numbers of donor (Thy-1.1+) and host cells recruited into the lungs. Similar results were seen two experiments.
Protection is accompanied by an accelerated regeneration of type 2 epithelial cells in the lung
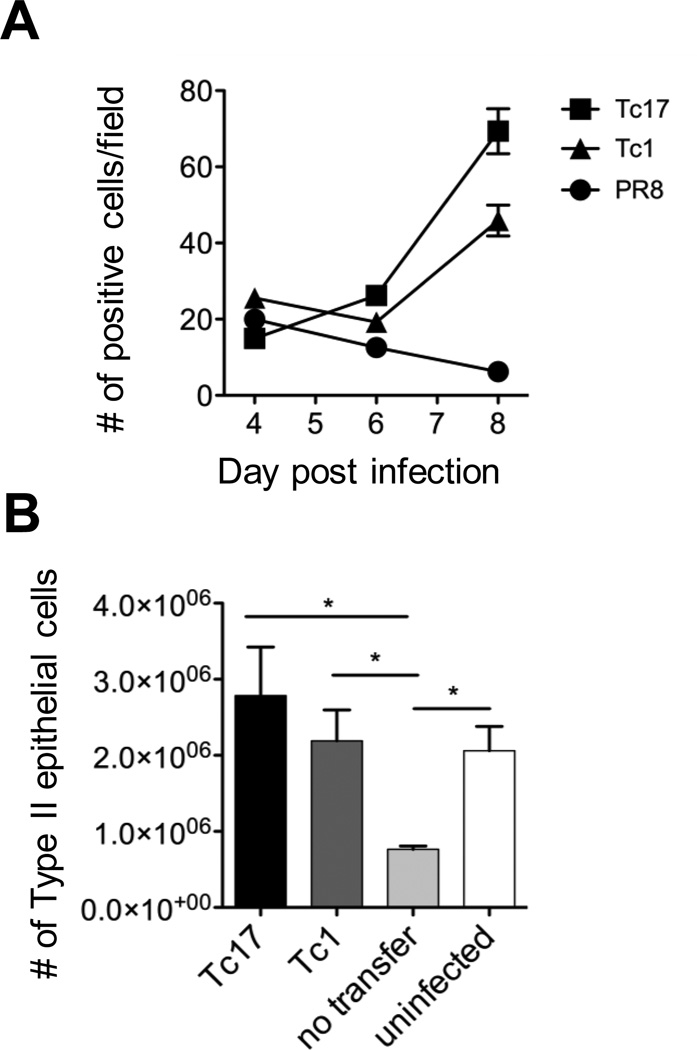
Mice were protected from lethal challenge by the transfer of Tc1 or Tc2 effectors. Mice were sacrificed at days 4, 6 and 8 after challenge and the lungs analyzed by examination of stained lung sections and by flow cytometry of lung cell suspensions. Lung sections were stained with antibody to pro-surfactant protein C (as a marker for type 2 epithelial cells) and the numbers of positive cells counted by field in mice receiving Tc1 or Tc17 effectors or left untreated. Lung cell suspensions were also prepared and stained and analyzed by flow cytometry. It can be seen (Figure 3) that there was a highly significant accelerated increase in the number of stained cells both by histology and by FACS suggesting more rapid recovery of lung epithelium in treated mice but whether this represents the cause or effect could not be deduced. Tc17 effectors were more effective than Tc1.
FIGURE 3.
Accelerated regeneration of type 2 epithelial cells in protected mice. A. Groups of 5 B6 mice were injected with 8 × 106 in vitro generated Tc1 (triangles) or Tc17 Thy-1.1.OT-1 effectors (squares) or left un-injected (circles) and challenged one day later with 0.2LD50 PR8-OVAI. Mice were sacrificed at the times indicated and lung section were prepared and stained with fluorescently labeled antibody to pro-surfactant protein C as a marker for type 2 epithelial cells) to determine the numbers of positive cells per field. For the differences between Tc17 vs. no transfer at days 6 and 8, P < 0.001, for Tc1 vs. no transfer < 0.01, and for Tc17 vs Tc1, < 0.01. B. Cell suspensions from the lungs of similarly treated mice were prepared form mice sacrificed at day 8 and analyzed with the same fluorescent labeled antibody by flow cytometry. Tc17 vs. none, Tc1 vs. none and uninfected vs. none, all P < 0.05.
The above findings suggested a considerable number of candidate mechanisms for protection and we sought to confirm their role by showing a reduction or abrogation of protection in their absence. Our next step was to determine whether any of the identified potential mechanisms were essential for protection.
Protection is still seen when host neutrophils are depleted
Neutrophils are generally thought to exacerbate immunopathology in viral infections but we had seen an early spike in neutrophil numbers in studies of heterosubtypic protection (data not shown) that was accompanied by an early reduction of the viral load. We speculated that an early moderate influx of neutrophils might be beneficial to the outcome of the infection, while the damage done by larger numbers at later times was a correlate of the failure to control infection.
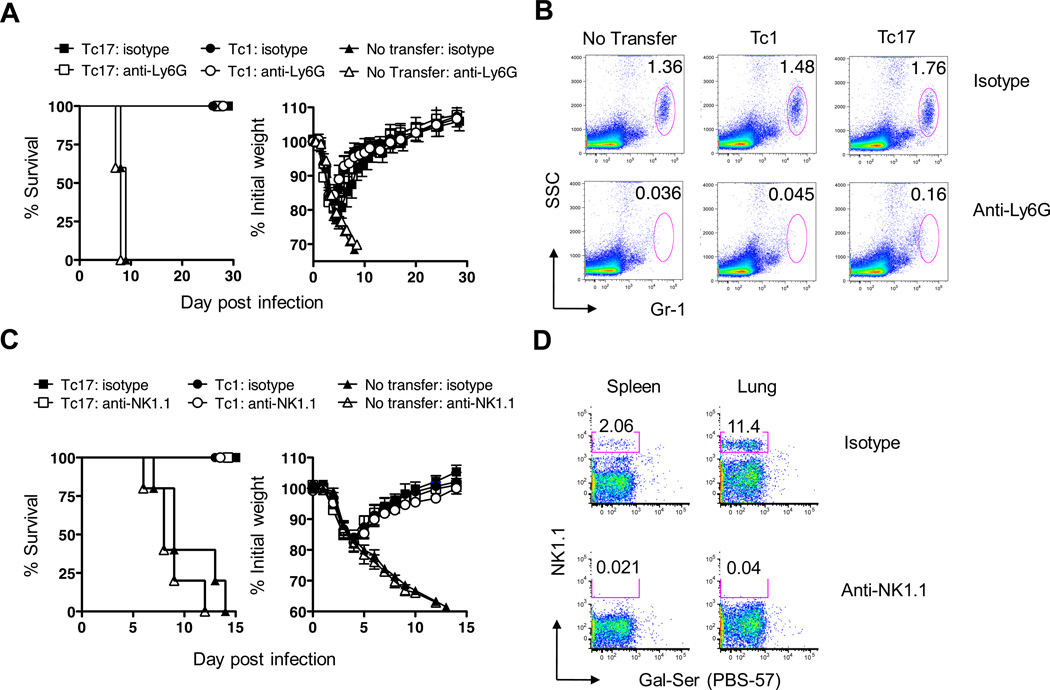
To test the role of neutrophils in infection, two groups of naïve B6 mice were injected with 8 × 106 Tc17 effectors at day zero and infected with 2LD50 PR8-OVAI. One group of 5 mice was injected with 200µg of the monoclonal antibody, 1A8, to deplete the Ly6G-high neutrophils on days minus 1, plus 1 and plus 3 while the second group was injected with an equivalent amount of isotype control immunoglobulin. Two additional groups of mice were run in parallel, receiving Tc1 rather than Tc17. Additional mice from each protocol were sacrificed to check for neutrophil depletion as judged by staining with fluorescently labeled anti GR-1 (RB6) antibody. A fifth group of mice received no CD8+ effectors and no antibody but were challenged with virus. Weight loss and survival were followed for 28 days. No effects were observed on either weight changes or survival (Fig 4A) following effective neutrophil depletion (Fig 4B). We concluded that neutrophil recruitment does not play an essential role in protection.
FIGURE 4.
Depletion of host neutrophils or NK cells does not diminish protection. 8 × 106 in vitro generated Tc1 or Tc17 OT-1 effectors were injected into groups of 5 naïve B6 recipients. Mice were challenged with 2LD50 PR8-OVAI. In panel A mice were depleted of neutrophils or received isotype control immunoglobulin and weight changes and survival were tracked for 30 days. The effectiveness of the neutrophil depletion is shown in panel B. In panel C mice were depleted of NK cells or received isotype control immunoglobulin and weight changes and survival were tracked for 13 days. The effectiveness of the NK cell depletion is shown in D. The details of all of the procedures are as described in the Materials and Methods section. Similar results were seen two experiments.
Protection is still seen when host NK cells are depleted
A similar experiment was carried out to determine whether NK cell depletion, using 200µg NK-1.1 antibody, injected on days −1, +2 and +5, would affect the level of protection. Again, there was no effect on either weight loss or survival (Fig 4C) following effective NK cell depletion (Fig 4D). We concluded that NK cell recruitment does not play an essential role in protection.
Protection is still seen when host T cells are absent
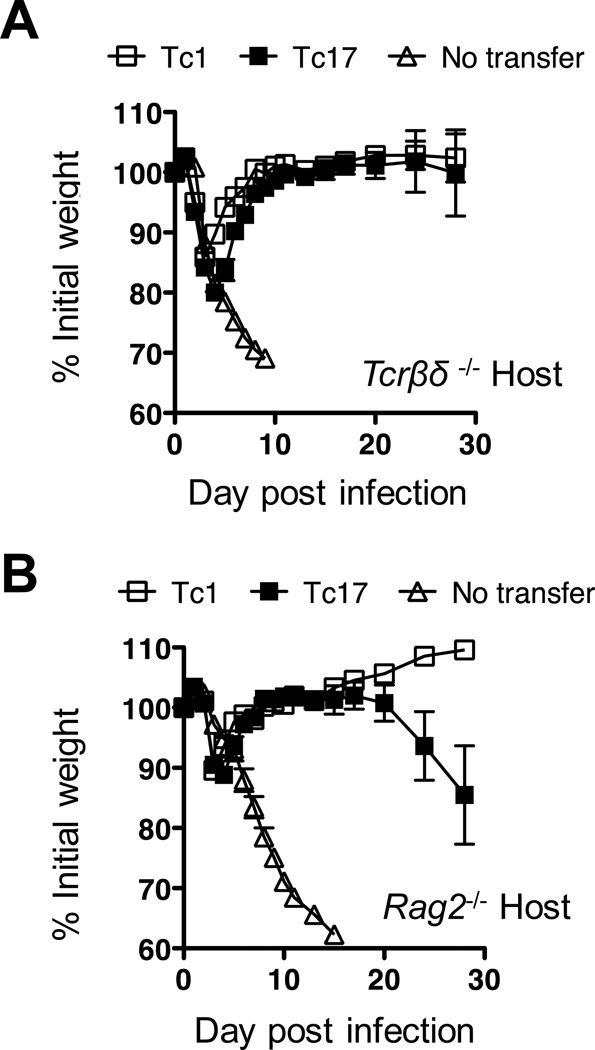
Although adoptive transfer actually decreased the recruitment of host T cells at later time points we considered it possible that they might still play some role in the early clearance of virus. We therefore determined the ability of Tc1 and Tc17 effectors to protect mice lacking both CD4+and CD8+T cells. For this we used TCRβ/TCRδ deficient recipient mice. Un-injected β/δ−/− mice lost weight rapidly and all died by day 10 when challenged with 3LD50 PR8-OVAI (Fig 5A). β/δ −/− mice injected with either 8 × 106 Tc17 or Tc1 effectors at day minus -1 started to regain weight by day 5 and were fully recovered by day 10. Individual mice began to lose weight again around day 30 and eventually died (not shown), possibly following development of viral escape mutants although this was not analyzed. We concluded that neither recruited host CD4+nor CD8+T cells are crucial for early protection.
FIGURE 5.
Tc1 and Tc17 protect TCRβ/TCRδ−/− recipient mice or RAG-2−/− mice from viral challenge. 8 × 106 in vitro generated Tc1 (open squares) or Tc17 (filled squares) OT-1 effectors were injected into groups of 5 naïve recipients. Recipients were either B6.TCRβ/TCRδ−/− (A) or B6.RAG-2−/− mice (B). In both A and B a control group of recipient mice was left un-injected (open triangles). Mice were challenged with approximately 2LD50 of PR8-OVAI for TCRβ/TCRδ−/− and RAG-2−/− mice respectively and the weight changes followed for 28 days. Similar results were seen in an earlier experiment in which the mice were challenged with only 0.2LD50.
Protection is still seen when host T cells and B cell are absent
Finally we examined whether protection could be seen in the absence of both αβ and γδ T cells and B cells. 8 × 106 Tc1 or Tc17 effectors were injected into RAG-2−/− host, which were challenged with 3LD50 PR8-OVAI. Yet again, the injected effectors were able to reverse weight loss and protect the mice for at least 15 days as shown in Fig 5B. As with the β/δ deficient recipient mice, individual mice started to die at later time points (data not shown) but, again, it was clear that none of absent cell types were required to generate the initial protection.
Protection is still seen when multiple cell lineages are absent or depleted and donors are perforin deficient
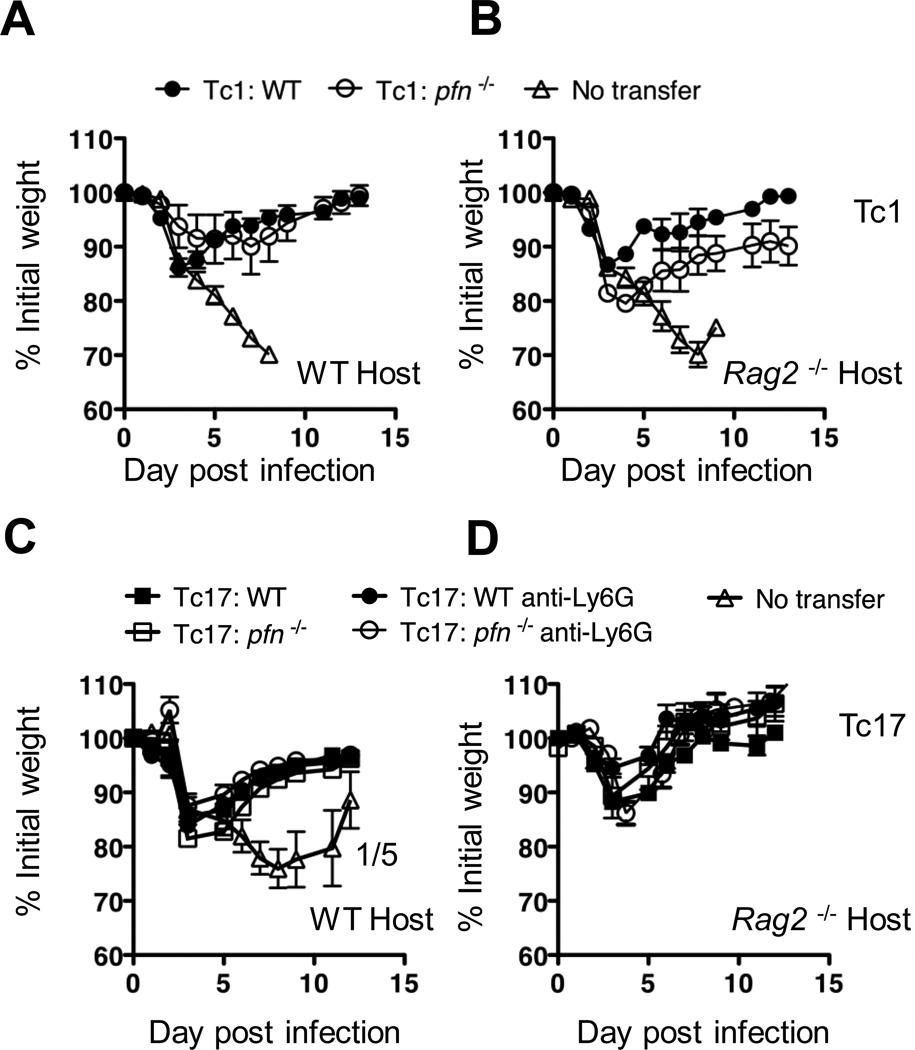
In a further attempt a show clusters of mechanisms that, collectively, were essential for protection we engineered multiple deficiencies into a single protocol. Tc1 (Fig 6A,B) or Tc17 (Fig 6C,D) effectors were prepared from CD8+ T cells from either WT or perforin deficient OT-1 mice and these were injected into WT (Fig 5A,C) or RAG-2−/−(Fig 6B,D) recipients followed by infection with 3LD50 PR8-OVAI. In addition, a second set of recipients were injected with the same sets of Tc17 cells but were also subjected to the regiment previously used to deplete neutrophils (Fig 6C,D). Thus, in the most compromised protocol, the recipient mice lacked four of the postulated protective mechanisms, CD4+ and CD8+ T cells, B cells and neutrophils and, in addition, the donor cells were deprived of perforin-mediated killing. Under these conditions, the Tc17 perforin deficient effectors were still able to protect RAG-2 deficient recipients from lethal challenge, even when neutrophils were depleted (Fig 6C and D). Tc1 effectors from perforin deficient donors, however, were less protective than Tc1 effectors from WT mice in RAG-2−/− hosts, again exposing a difference in the mechanism of protection by Tc17 and Tc1 effectors.
FIGURE 6.
Perforin deficient Tc17 protect neutrophil depleted RAG-2−/− recipients from viral challenge. The design of the experiment was similar in design to that in Figure 4B except that the CD8 effectors were generated with cells from WT or perforin deficient mice and the recipients were left untreated or were injected as in Figure 3 A to deplete neutrophils. All mice were challenged with 2LD50 PR8-OVAI Similar results were seen in two experiments. The upper two panels, indicate the kinetics of weight change for WT mice (panel A) that were injected with Tc1 WT or perforin deficient effectors and for RAG-2−/− mice that were injected with Tc1 WT or perforin deficient effectors (panel B). The lower two panels, for WT mice that were injected with Tc17 WT or perforin deficient effectors (panel C) and for RAG-2−/− mice that were injected with Tc17 wt or perforin deficient effectors (panel D). In addition, in the lower panels, the recipient mice were either neutrophil depleted or left untreated. Similar results were seen in three experiments.
We were somewhat surprised at the robustness of the protective effect, thus revealed, and next attempted to determine which parts of the protective effect were antigen specific and which were antigen non-specific. We carried out experiments in two models: in the first, to determine how long the donor cells needed to be present in the recipient after transfer to retain protection, and, in the second, to determine whether protection against the specific virus provided any bystander protection against a virus lacking the SIINFEKL insert, given at the same time.
Donor cells can be depleted at later times after transfer without loss of protection
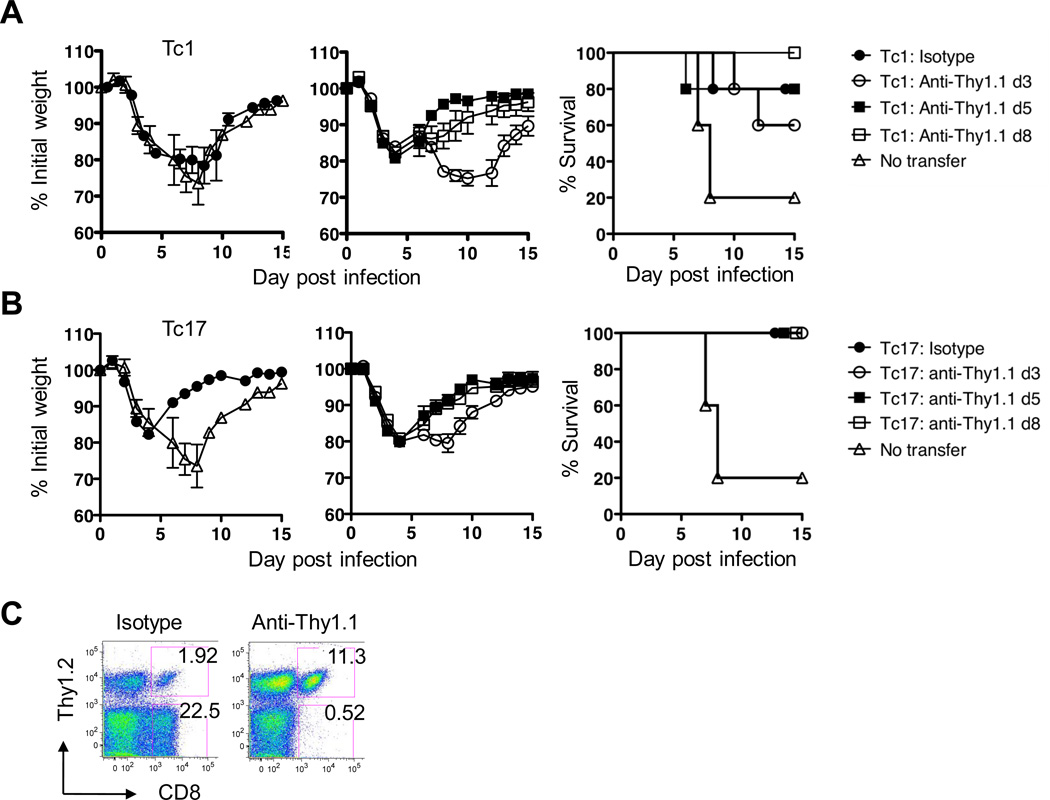
We injected 8 × 106 Tc1 or Tc17 effectors from B6.OT-1.Thy-1.1 mice into WT naïve B6.Thy-1.2 recipients on day zero and then challenged with 1LD50 PR8-OVAI. Mice in one group of five mice were left without further manipulation and were protected as before. Mice in a second group were injected with anti Thy-1.1 to eliminate the donor cells at day 3, mice in a third group were injected at day 5 and mice in a fourth group at day 8. Control, no transfer, mice (the same curve for the same mice is shown in the left panels of both A and B) lose weight, and some died at days 7 and 8, but the remainder began to regain weight as shown in the survival plots (shown in A and B survival panels) Mice injected with Tc1 effector T cells showed little difference in weight change (A, left panel) but most survived. If the Tc1 were depleted at day 3 the recipients lost more weight (A middle panel) and fewer survived (A, right panel). Depletion at days 5 and 8 had little effect.
A similar pattern was seen for the Tc17 half of the experiment (B) except that Tc17 effectors were more protective (B, left panel). Depletion of Tc17 effectors at day 3 resulted in slightly more weight loss while depletion at day 5 and 8 was without effect (B center panel). All Tc17 treated mice survived whenever they were depleted (B right panel).
The efficacy of the donor cell depletion by injection of anti-Thy-1.1 is illustrated in Fig 7C which shows that the percent of Thy-1.2 negative CD8 positive donor cells was reduced from 22.5 to 0.52.
FIGURE 7.
Tc17 donor cells still protect against viral challenge when depleted at 5 or 8 but not at day 3. 8 × 106 in vitro generated Tc1 (A) or Tc17 (B). OT-1 effectors were injected into groups of 5 naïve WT B6 recipients and challenged one day later with approximately 1LD50 PR8-OVAI. Donor T cells were from OT-1.Thy-1.1 mice and were depleted by the injection of anti-Thy-1.1 at day 3, 5 or 8. Weight changes and survival were followed for 15 days. The data displayed in A and B all come from the same experiment but for ease of display the weight change curves are separated into two parts. The weight changes for untreated mice (no transfer) and the isotype control for the CD8 effector treated mice are shown in the left panels of A and B. The effect of depletion at 3, 5 and 8 days are shown in the middle panels. The corresponding survival curves for A and B are shown on the right. Panel C provides a representative example of the efficacy of donor cell depletion using anti-Thy-1.1 injection. Similar results were seen in two experiments.
Note that all of the weight change data presented in A and B are from a single experiment but are separated into different panels so that the weight loss curves may be distinguished. This pattern of results was seen in each of two experiments and suggests that an initial period which requires the continued presence of the donor cells is followed by, at day 5 by a period in which the original injected cells are no longer required. This implies that the CD8 effectors had set in process a train of protective events that no longer required their presence. The requirement for the persistence of Tc1 effectors would appear to be more prolonged for Tc17 mediated protection.
The OT-1 response to the ova-bearing virus (PR8-OVAI) provides bystander protection against the virus lacking the ova- epitope (A/PR8)
Mice were injected with Tc1 (Fig. 8A) or Tc17 (Fig. 8B) effectors generated from OT-1 mice and were challenged with approximately 1LD50 of the “specific” virus, PR8-OVAI, or a lethal dose of the non-specific virus, 4LD50 PR8, or with both.
All the mice receiving Tc1 effectors from ova peptide specific OT-1 mice died when challenged with PR8 alone, while one out of four survived when challenged with both viruses and regained weight (Fig. 8A). All mice challenged with1LD50 PR8-OVAI lost less weight, recovered and all survived. The difference was more marked when mice received Tc17 effectors (Fig. 8B). Three out of four mice challenged with PR8, lost weight rapidly and died. Mice challenged with both viruses also lost weight as rapidly as those challenged with PR8 alone but started to recover at day 10 and three out of four survived, suggesting that the addition of the specific virus led to protection against the non-specific virus. Only one out of four mice survived PR8 alone. A similar pattern of reduced weight loss when both viruses were given was seen in a second experiment in which a lower challenge dose of PR8 was used. In a further experiment we used LPS-free ovalbumin instead of PR8-OVAI and again observed a bystander effect with protection against a lethal challenge with PR8 (Fig 8C). This eliminates the possibility that the apparent bystander effect was really because SIINFEKL specific T cells killed doubly infected cells and the possibility that the two viruses in some way compete with one another. There was, a small reduction in viral load at day 3 in mice given PR8 plus ovalbumin compared with PR8 alone but it was not sustained and the titer had rebounded by day 7 (Suppl. Fig. 4).
We conclude that, together, the effector depletion and the bystander protection experiments support a model in which an antigen specific step leads to a subsequent protective mechanism that is no longer antigen specific.
The transfer of CD8+ rested effectors leads to activation of an early host innate response
We have previously shown that the adoptive transfer of memory CD4+ cells can bring about an induction of a number of innate cytokines and chemokines early in the response to influenza infection (22) and that this was correlated with a 10 fold reduction in viral titer and an increase in survival. CD8+ effectors express many of the same effector mechanisms expressed in CD4+ and it seemed possible that they too might elicit early innate responses.
We found, here, that the adoptive transfer of rested CD8+ effectors from the HA specific TcR transgenic Clone 4 BALB/c mice were also able to induce this same early response (Fig. 9). In this experiment we used effectors that were rested three days before transfer. Such cells adopt a memory phenotype (23) but become reactivated to effectors on transfer to infected mice (24). The rested CD8+ effectors were transferred into naive BALB/c recipients, which were then challenged with 2LD50 PR8. The mice were sacrificed at days 1, 2 or 3 and the supernatant from the lung homogenates assayed for a panel of cytokines and chemokines using Luminex. Mice injected with memory CD4 T cells show enhanced survival (25) following viral challenge and memory CD8 are also effective (data not shown). The factors indicated were induced by day 2 and the level of induction was comparable to that seen with CD4+ cell transfer (22).
FIGURE 9.
CD8 rested effectors responses in the lung acutely enhance a broad panel of cytokine and chemokines. Rested effectors were prepared by four day in vitro stimulation of CD8 cells from Clone 4 mice bearing the T cell receptors for the IYSTVASSL peptide from the HA of PR8. The effectors were then washed and cultured in the absence of cytokines for a further three days. 5 × 106 naïve or CD8 rested effectors were injected into naïve BALB/c mice then challenged with 2LD50 PR8. Mice were sacrificed at on day 1, 2 or 3 and the supernatants of lung homogenates assayed for the cytokines and chemokines indicated by Luminex. Similar results were seen in two experiments.
Discussion
CD8+ effector T cells of multiple subsets are generated in the response of a normal mouse to influenza infection. The dominant subset is the interferon secreting Tc1 like, which reaches numbers 50 fold higher than those of the Tc17 like subset (16). There were approximately 4 × 106 for IFN-γ secreting Tc1 like cells but only 8 × 104 for IL-17 secreting Tc17 like cells. Nonetheless, the numbers of Tc17 cells expands almost 100 fold during the response and we conclude they must also play some significant role in these responses (16).
The goal of this study was to define the roles of these cells by determining how the adoptively transferred CD8+ effector T cells protected recipient mice against otherwise lethal influenza infection in recipient mice. In the model employed we transferred large numbers of CD8+ effector T cells generated, in vitro, from SIINFEKL/Kb specific T cell receptor transgenic mice. A number of investigators, including us (26) have shown that the adoptive transfer of large numbers of naïve transgenic cells is both unnecessary and un-physiological and that the expansion of such cells in response to challenge is inversely proportional to the input number. We argue, however, that the transfer of large numbers of effector cells is needed to mimic the normal response. We have previously shown (15) that following the adoptive transfer of large numbers of effector cells, significant numbers of the donor cells could be detected in the lung and bronchoalveloar lavage by day 1 and that several million donor cells could be found at days 3 and 5. This number is approximately equal to the number of polyclonal CD8+ T cells seen at the peak of the response of a normal mouse to infection with 1LD50 PR8 in the absence of any transferred cells as shown in our previous publications (16, 21). Effectors whether generated at the peak of the response or adoptively transferred produce very high levels of cytokines and these can be expected to play a major role in protection.
All three subsets of CD8 effectors (Tc1, Tc2 and Tc17) were separately able to provide effective protection against a lethal dose of influenza virus when 4 to 8 million cells were transferred (15–17, and this paper). It is clear, in the experiments presented here, that the adoptive transfer of either Tc1 or Tc17 effectors brings about a large number of potentially protective changes in the host. This was also true for Tc2 as shown in earlier studies (15, 17). We showed, for both Tc1 and Tc17 that donor cells enter the lung and secrete an assortment of cytokines and chemokines (10, and this paper). Further, host B cells, neutrophils, NK cells and macrophages and are all recruited in large numbers following adoptive transfer of CD8+ effectors. Tc17 cells are more effective than Tc1 in this regard. Tc17 also recruit higher numbers of host CD4+ and CD8+ T cells, but the numbers recruited are actually less than in the untreated mice. It seems that recruitment stops as soon as the virus is cleared in the treated mice while the influx of T cells continues in the untreated mice and reaches much higher levels. We also found some evidence for accelerated regeneration of type II epithelial cells (Figure 3) and accelerated antibody responses (data not shown) but did not establish whether this was the cause of protection or the consequence of the mice surviving and thus being able to recover. These are all potential candidates for bringing about viral clearance and protection against influenza.
We had initially sought to identify the essential mode of protection of CD8+ effectors by the deletion of some element that would diminish or eliminate the protective effect of the adoptive transfer. Our first thought was that protection would be dependent on the cytolytic activity of the CD8+ effectors and it was therefore surprising that Tc17 effectors that lacked lytic activity when prepared in vitro were as effective as the lytic Tc1 effectors. We found that Tc17 regained some slight activity on introduction into a normal recipient and eventually became as lytic as the re-injected Tc1. We found, however, that the protective activity was still present in Tc17 effectors obtained from perforin deficient mice showing that perforin-mediated lytic activity was not essential for Tc17 effectors. Further experiments showed that Tc17 cells were still protective in the absence of both perforin and FasL mediated lysis. We could find no evidence that protection was TRAIL mediated but preparations of Tc17 effectors that we generated from the TRAIL−/− mice had much lower numbers of IL-17 secreting cells that the other Tc17 preparations from WT or perforin deficient mice.
Depletion of neutrophils or NK cells was also without effect showing that these cell types were not essential for early protection. Protection was also seen in the absence of host CD4+ and CD8+ T cells and even in the absence of all T cells and B cells. In our earlier study (16) we had shown that the absence of perforin mediated cytolytic activity was without effect on the protective activity of Tc17 but led to some loss of protection by Tc1 cells. We had also shown that Tc17 cells from IFN-γ deficient mice were somewhat less effective in preventing reduction in their ability to reduce weight loss and improving survival (16). We did not establish any mechanism for the reduced efficiency of the deficient cells, here, but in an earlier study we showed that IFN-γ was important in recruiting donor cells into lungs (17) and into tumors in a model in which Tc1 effectors from OT-1 mice rejected ova secreting EG7 intradermal tumors (27). Titration of the IFN-γ deficient donor cells showed that higher numbers of donor cells were able to provide full protection.
The elimination of potential protective mechanisms two at a time, three at a time, four at a time or even at set of five at a time had little, if any, effect on the degree of protection by Tc17 but revealed some dependence on perforin, by Tc1. It is important, here, to note that the relative importance of different mechanisms is probably different in the various protocols we employed, thus in the absence of host T cells and B cells perforin mediated killing by the Tc1 donor cells begins to be of some importance while in the presence of host T cells and B cells the removal of perforin has no effect. It was beyond our resources to titrate the number of CD8+ effectors injected but at the dose selected of 8 × 106 Tc17 effector cells per mouse there was no loss of protection against a viral dose that killed four out of five of the unprotected WT recipients. We also recognized the caveat that donor T cells injected into RAG-2−/− mice might expand much more extensively than in WT recipients and the larger numbers of effector cells were perhaps able to make up for other deficiencies and may also rely on a different mix of protective mechanisms from WT effectors in WT hosts, thus invalidating any simple interpretation of the result.
The deletion of dendritic cells using diphtheria toxin and CD11cDTR recipient mice led to no loss of protection but the collateral effects of the treatment were excessive inflammation and precluded the drawing of any firm conclusion (data not shown). The deletion of macrophages using clodronate liposomes lead to the death of all of the mice (data not shown) even those challenged with a sublethal dose of virus and it was therefore not possible to conclude whether removal of the macrophages had any effect on the protection provided by injection of CD8 effectors. We previously showed that Tc17 secrete IL-22 (16, and data not shown) and obtained some evidence that Tc17 effectors accelerated type II epithelial cell proliferation (data not shown) but antibody to IL-22 was without effect on protection (data not shown) which suggested IL-22-mediated repair of lung epithelium was not essential.
We were thus not able to identify any essential single or group of mechanisms necessary for protection. Therefore, it would appear that protection is afforded by multiple pathways many of which are redundant and that there is no single key mechanism that is essential for survival. Our major conclusion is that CD8 carry out a very large number of effector functions providing multiple layers of redundant protection against otherwise very dangerous pathogens.
Some further insight was gained in experiments that showed that there was an antigen specific phase in the protective mechanism that was followed by an antigen independent phase starting around day 4. Depletion of donor cells at later time points did not interfere with protection and the addition of a small amount of the PR8-OVAI virus to PR8 was sufficient for the SIINFEKL specific OT-1 effectors to provide bystander protection to the PR8, suggesting that an initial antigen specific phase was followed by a phase in which protection was mediated by some non specific mechanism. This latter effect was more pronounced with Tc17 effectors than with Tc1.
Because of the extreme redundancy of the mechanisms of protection none of them were essential. The induction of host innate cytokines and chemokines as shown in Figure 9 was striking but we were not able to design an experiment in which only this mechanism is disabled to test how much protection was dependent on this effect. We had previously seen that innate cytokines are strongly induced at 48 hours following heterosubtypic challenge (unpublished) and others have shown that stimulation with TLR ligands can provide some level of protection in a number of models. It is thus possible, that the induction of host innate cytokines and chemokines as shown in Figure 9 is of critical importance and further studies will be required to determine whether it is essential for protection.
Supplementary Material
Acknowledgements
We thank Joyce Reome for excellent technical assistance.
PBS-57-loaded mCD1d tetramers were provided by the NIH Tetramer Core Facility.
This work was supported by National Institutes of Health grant P01AI46530 and by the Trudeau Institute.
Abbreviations used in this article
- FasL
Fas ligand
- HA
hemagglutinin
- i.n.
intranasal
- WT
wildtype.
Footnotes
Disclosures
The authors have no financial conflict of interest
References
- 1.Benton KA, Misplon JA, Lo CY, Brutkiewicz RR, Prasad SA, Epstein SL. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or gamma delta T cells. J Immunol. 2001;166:7437–7445. doi: 10.4049/jimmunol.166.12.7437. [DOI] [PubMed] [Google Scholar]
- 2.Cantor H, Boyse EA. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975;141:1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantor H, Boyse EA. Functional subclasses of T lymphocytes bearing different Ly antigens. II. Cooperation between subclasses of Ly+ cells in the generation of killer activity. J Exp Med. 1975;141:1390–1399. doi: 10.1084/jem.141.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rifa'i M, Shi Z, Zhang SY, Lee YH, Shiku H, Isobe K, Suzuki H. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. Int Immunol. 2008;20:937–947. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]
- 5.Klein JR, Raulet DH, Pasternack MS, Bevan MJ. Cytotoxic T lymphocytes produce immune interferon in response to antigen or mitogen. J Exp Med. 1982;155:1198–1203. doi: 10.1084/jem.155.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris AG, Lin YL, Askonas BA. Immune interferon release when a cloned cytotoxic T-cell line meets its correct influenza-infected target cell. Nature. 1982;295:150–152. doi: 10.1038/295150a0. [DOI] [PubMed] [Google Scholar]
- 7.Lancki DW, Hsieh CS, Fitch FW. Mechanisms of lysis by cytotoxic T lymphocyte clones. Lytic activity and gene expression in cloned antigen-specific CD4+ and CD8+ T lymphocytes. J Immunol. 1991;146:3242–3249. [PubMed] [Google Scholar]
- 8.Brown DM, Kamperschroer C, Dilzer AM, Roberts DM, Swain SL. IL-2 and antigen dose differentially regulate perforin- and FasL-mediated cytolytic activity in antigen specific CD4+ T cells. Cell Immunol. 2009;257:69–79. doi: 10.1016/j.cellimm.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catalfamo M, Karpova T, McNally J, Costes SV, Lockett SJ, Bos E, Peters PJ, Henkart PA. Human CD8+ T cells store RANTES in a unique secretory compartment and release it rapidly after TcR stimulation. Immunity. 2004;20:219–230. doi: 10.1016/s1074-7613(04)00027-5. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Hernandez Mde L, Hamada H, Reome JB, Misra SK, Tighe MP, Dutton RW. Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J Immunol. 2010;184:4215–4227. doi: 10.4049/jimmunol.0902995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Yoon H, Zhao MQ, Liu J, Ramana CV, Enelow RI. Cutting edge: pulmonary immunopathology mediated by antigen-specific expression of TNF-alpha by antiviral CD8+ T cells. J Immunol. 2004;173:721–725. doi: 10.4049/jimmunol.173.2.721. [DOI] [PubMed] [Google Scholar]
- 12.Zhao MQ, Stoler MH, Liu AN, Wei B, Soguero C, Hahn YS, Enelow RI. Alveolar epithelial cell chemokine expression triggered by antigen-specific cytolytic CD8(+) T cell recognition. J Clin Invest. 2000;106:R49–R58. doi: 10.1172/JCI9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity. 2012;36:142–152. doi: 10.1016/j.immuni.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerwenka A, Morgan TM, Harmsen AG, Dutton RW. Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection. J Exp Med. 1999;189:423–434. doi: 10.1084/jem.189.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiley JA, Cerwenka A, Harkema JR, Dutton RW, Harmsen AG. Production of interferon-gamma by influenza hemagglutinin-specific CD8 effector T cells influences the development of pulmonary immunopathology. Am J Pathol. 2001;158:119–130. doi: 10.1016/s0002-9440(10)63950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, Swain SL. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J Immunol. 2008;181:4918–4925. doi: 10.4049/jimmunol.181.7.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell TJ, Brown DM, Hollenbaugh JA, Charbonneau T, Kemp RA, Swain SL, Dutton RW. CD8+ T cells responding to influenza infection reach and persist at higher numbers than CD4+ T cells independently of precursor frequency. Clin Immunol. 2004;113:89–100. doi: 10.1016/j.clim.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Powell TJ, Dwyer DW, Morgan T, Hollenbaugh JA, Dutton RW. The immune system provides a strong response to even a low exposure to virus. Clin Immunol. 2006;119:87–94. doi: 10.1016/j.clim.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Strutt TM, McKinstry KK, Dibble JP, Winchell C, Kuang Y, Curtis JD, Huston G, Dutton RW, Swain SL. Memory CD4+ T cells induce innate responses independently of pathogen. Nat Med. 2010;16:558–564. doi: 10.1038/nm.2142. 551p following 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinstry KK, Golech S, Lee WH, Huston G, Weng NP, Swain SL. Rapid default transition of CD4 T cell effectors to functional memory cells. J Exp Med. 2007;204:2199–2211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strutt TM, McKinstry KK, Kuang Y, Bradley LM, Swain SL. Memory CD4+ T-cellmediated protection depends on secondary effectors that are distinct from and superior to primary effectors. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1205894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinstry KK, Strutt TM, Kuang Y, Brown DM, Sell S, Dutton RW, Swain SL. Memory CD4+ T cells protect against influenza through multiple synergizing mechanisms. J Clin Invest. 2012;122:2847–2856. doi: 10.1172/JCI63689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemp RA, Powell TJ, Dwyer DW, Dutton RW. Cutting edge: regulation of CD8+ T cell effector population size. J Immunol. 2004;173:2923–2927. doi: 10.4049/jimmunol.173.5.2923. [DOI] [PubMed] [Google Scholar]
- 27.Hollenbaugh JA, Dutton RW. IFN-gamma regulates donor CD8 T cell expansion, migration, and leads to apoptosis of cells of a solid tumor. J Immunol. 2006;177:3004–3011. doi: 10.4049/jimmunol.177.5.3004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.