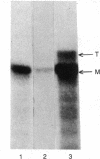
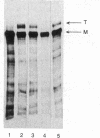
Abstract
The first step in the replication of influenza virion RNAs is the synthesis of full-length transcripts of these RNAs. The synthesis of these transcripts, or template RNAs, requires: unprimed initiation rather than the capped RNA-primed initiation used during viral mRNA synthesis, and antitermination at the polyadenylylation site used during mRNA synthesis. To determine the mechanism of template RNA synthesis, we prepared nuclear extracts from infected cells that were active in the synthesis of both template RNAs and viral mRNAs. By providing the dinucleotide ApG as primer, we circumvented the inefficient unprimed initiation catalyzed by these extracts and, as a consequence, were able to focus on the antitermination step. Antitermination, and hence template RNA synthesis, occurred when ApG but not a capped RNA was used as primer, indicating that the presence of a 5' capped end blocked antitermination at the 3' end of the transcript. Ultracentrifugation of the nuclear extract yielded a pellet fraction that contained viral nucleocapsids active in viral mRNA synthesis but not template RNA synthesis and a supernatant fraction that contained the antitermination factor. When the supernatant, which had essentially no activity by itself, was added to the pellet in the presence of ApG, template RNA synthesis was restored. Depletion experiments in which this supernatant was incubated with protein A-Sepharose containing antibodies to individual viral proteins demonstrated that the viral nucleocapsid protein was required for antitermination. The implications of these results for the control of viral RNA replication are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett T., Wolstenholme A. J., Mahy B. W. Transcription and replication of influenza virus RNA. Virology. 1979 Oct 15;98(1):211–225. doi: 10.1016/0042-6822(79)90539-7. [DOI] [PubMed] [Google Scholar]
- Beaton A. R., Krug R. M. Synthesis of the templates for influenza virion RNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4682–4686. doi: 10.1073/pnas.81.15.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Leppert M., Kolakofsky D. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell. 1981 Mar;23(3):837–845. doi: 10.1016/0092-8674(81)90448-7. [DOI] [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Both the 7-methyl and the 2'-O-methyl groups in the cap of mRNA strongly influence its ability to act as primer for influenza virus RNA transcription. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3952–3956. doi: 10.1073/pnas.77.7.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4886–4890. doi: 10.1073/pnas.75.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam J., Ulmanen I., Krug R. M. Molecular model of a eucaryotic transcription complex: functions and movements of influenza P proteins during capped RNA-primed transcription. Cell. 1983 Sep;34(2):609–618. doi: 10.1016/0092-8674(83)90393-8. [DOI] [PubMed] [Google Scholar]
- Brown L. E., Hinshaw V. S., Webster R. G. Antigenic variation in the influenza A virus nonstructural protein, NS1. Virology. 1983 Oct 15;130(1):134–143. doi: 10.1016/0042-6822(83)90123-x. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Content J., Duesberg P. H. Structure of the ribonucleoprotein of influenza virus. J Virol. 1972 Oct;10(4):795–800. doi: 10.1128/jvi.10.4.795-800.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan D., Krystal M., Nakada S., Arnheiter H., Lyles D. S., Palese P. Expression of influenza virus NS2 nonstructural protein in bacteria and localization of NS2 in infected eucaryotic cells. J Virol. 1985 Jun;54(3):833–843. doi: 10.1128/jvi.54.3.833-843.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J., Lomniczi B., Bellamy A. R., Skehel J. J. Transcription of the influenza virus genome. Virology. 1977 Dec;83(2):337–355. doi: 10.1016/0042-6822(77)90179-9. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Skehel J. J., McCauley J. Characterization of influenza virus RNA complete transcripts. Virology. 1982 Jan 30;116(2):517–522. doi: 10.1016/0042-6822(82)90144-1. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Chen Y. T., Krug R. M. Nuclear-cytoplasmic transport and VAI RNA-independent translation of influenza viral messenger RNAs in late adenovirus-infected cells. Cell. 1984 Jun;37(2):483–490. doi: 10.1016/0092-8674(84)90378-7. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Cytoplasmic and nucleoplasmic viral RNPs in influenza virus-infected MDCK cells. Virology. 1972 Oct;50(1):103–113. doi: 10.1016/0042-6822(72)90350-9. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Priming of influenza viral RNA transcription by capped heterologous RNAs. Curr Top Microbiol Immunol. 1981;93:125–149. doi: 10.1007/978-3-642-68123-3_6. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Ueda M., Palese P. Temperature-sensitive mutants of influenza WSN virus defective in virus-specific RNA synthesis. J Virol. 1975 Oct;16(4):790–796. doi: 10.1128/jvi.16.4.790-796.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D., Fellner P., Newton C. Influenza virus genome consists of eight distinct RNA species. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3045–3049. doi: 10.1073/pnas.73.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. T., Davis N. L., Wertz G. W. Cell-free synthesis and assembly of vesicular stomatitis virus nucleocapsids. J Virol. 1983 Jan;45(1):155–164. doi: 10.1128/jvi.45.1.155-164.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. T., Davis N. L., Wertz G. W. N protein alone satisfies the requirement for protein synthesis during RNA replication of vesicular stomatitis virus. J Virol. 1984 Feb;49(2):303–309. doi: 10.1128/jvi.49.2.303-309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R. W., Moyer S. A. Initiation and replication of vesicular stomatitis virus genome RNA in a cell-free system. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3198–3202. doi: 10.1073/pnas.80.11.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn C. R., Mahy B. W. Capped mRNAs may stimulate the influenza virion polymerase by allosteric modulation. Virus Res. 1984 Jan;1(1):1–13. doi: 10.1016/0168-1702(84)90030-3. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Ulmanen I., Krug R. M. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981 Mar;23(3):847–858. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Influenza virion transcriptase: synthesis in vitro of large, polyadenylic acid-containing complementary RNA. J Virol. 1977 Jan;21(1):24–34. doi: 10.1128/jvi.21.1.24-34.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S., Schubert M., Lazzarini R. A. Polyadenylation sites for influenza virus mRNA. J Virol. 1981 Apr;38(1):157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmanen I., Broni B. A., Krug R. M. Role of two of the influenza virus core P proteins in recognizing cap 1 structures (m7GpppNm) on RNAs and in initiating viral RNA transcription. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7355–7359. doi: 10.1073/pnas.78.12.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmanen I., Broni B., Krug R. M. Influenza virus temperature-sensitive cap (m7GpppNm)-dependent endonuclease. J Virol. 1983 Jan;45(1):27–35. doi: 10.1128/jvi.45.1.27-35.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. F., Desselberger U., Palese P., Ferguson B., Shatzman A. R., Rosenberg M. Efficient expression of influenza virus NS1 nonstructural proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6105–6109. doi: 10.1073/pnas.80.19.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyke K. L., Bean W. J., Jr, Webster R. G. Monoclonal antibodies to the influenza A virus nucleoprotein affecting RNA transcription. J Virol. 1981 Jul;39(1):313–317. doi: 10.1128/jvi.39.1.313-317.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]