Abstract
Background
Cyp11a1, a cytochrome P450 enzyme, is the first and rate-limiting enzyme in the steroidogenic pathway, converting cholesterol to pregnenolone.Cyp11a1 expression is increases in activated T cells.
Objectives
To determine the role of Cyp11a1 activation in the development of peanut allergy and T helper cell functional differentiation.
Methods
A Cyp11a1 inhibitor, aminoglutethimide (AMG), was administered to peanut-sensitized and -challenged mice. Clinical symptoms, intestinal inflammation, and Cyp11a1 levels were assessed. The effects of Cyp11a1 inhibition on Th1, Th2, and Th17 differentiation were determined. Cyp11a1 gene silencing was performed using Cyp11a1-targeted short hairpin RNA.
Results
Peanut sensitization and challenge resulted in diarrhea, inflammation and increased levels of Cyp11a1, IL-13, and IL-17A mRNA in the small intestine. Inhibition of Cyp11a1 with AMG prevented allergic diarrhea and inflammation. Levels of pregnenolone in serum were reduced in parallel. AMG-treatment decreased IL13 and IL17A mRNA expression in the small intestine without impacting Cyp11a1 mRNA or protein levels. In vitro, the inhibitor decreased levels of IL13 and IL17A mRNA and protein in differentiated Th2 and Th17 CD4 T cells, respectively, without affecting GATA3, RORγt or Th1 cells and IFNG and T-bet expression. shRNA-mediated silencing of Cyp11a1 in polarized Th2 CD4 T cells significantly decreased levels of pregnenolone, and IL13 mRNA and protein.
Conclusion
Cyp11a1 plays an important role in the development of peanut allergy, regulating peanut allergic responses through effects on steroidogenesis, an essential pathway in Th2 differentiation. Cyp11a1 thus serves as a novel target in the regulation and treatment of peanut allergy.
Keywords: Cyp11a1, peanut allergy, Th2, Th17, CD4 T cells
Introduction
Steroid hormones, including glucocorticoids (GCs), play an important role in the regulation of the immune system. The inhibitory role of GCs on immune cells is well characterized (1, 2). GCs reduce inflammation through inhibition of NF-κB and by inducing the expression of anti-inflammatory proteins including annexin 1 and MAPK phosphatase 1 (3). GCs and other synthetic derivatives have been used to treat a variety of diseases, including inflammatory diseases of the intestine and asthma (4, 5). Although the anti-inflammatory activity of GCs is well described, accumulating evidence suggests that GCs can also enhance immune cell activation, inducing gene transcription and promoting the pathogenesis of allergic diseases (6, 7). Steroid hormones are mainly produced in the adrenal glands, but other tissues also produce GCs through the induction of steroidogenic enzymes (3, 8). The intestinal mucosa contains steroidogenic enzymes such as cytochrome P450, family 11, subfamily A, polypeptide 1 (Cyp11a1) and synthesizes potent GCs which exhibit both an inhibitory and a co-stimulatory role on intestinal T cell activation (6).
Cyp11a1 is a key regulator of steroid biogenesis as the first and rate-limiting enzyme in the steroidogenic pathway, converting cholesterol to pregnenolone (9). Cyp11a1 is expressed primarily in the cortex of the adrenal gland, but testis, ovary, placenta, thymus, and intestine also express Cyp11a1 (6, 9). Depletion of Cyp11a1 in mice or rabbits results in steroid deficiency, female external genitalia, and death (10-12). In humans, mutations in the Cyp11a1 gene result in a steroid hormone deficiency, causing a rare and potentially fatal form of lipoid congenital adrenal hyperplasia (13, 14). Patients with a heterozygous or homozygous mutation of Cyp11a1 exhibit adrenal insufficiency and sex reversal (15, 16).
We investigated the role of the Cyp11a1 enzyme in an experimental model of peanut-induced intestinal allergy and identified, for the first time, the essential role of this enzyme in the full development of intestinal allergic responses. Moreover, inhibition of the enzymatic activity of Cyp11a1 attenuated Th2 and Th17 differentiation and cytokine production.
Materials and Methods
Mice
Five- to 6-week-old female Balb/c wild-type (WT) mice were purchased from the Harlan Laboratory (Indianapolis, IN). All studies were conducted under a protocol approved by the Institutional Animal Care and Use Committee of National Jewish Health.
Preparation of peanut protein
Crude peanut extract (PE) was prepared as described in the Online Supplement.
Sensitization and intragastric challenge
The experimental protocol for sensitization and challenge to peanut was previously described (17). Control animals were sham sensitized but challenged with peanut.
Cyp11a1 inhibitor treatment in vivo and in vitro
Aminoglutethimide (AMG) was obtained from Sigma (St. Louis, MO). PE sensitized and challenged mice received different doses (0-20 mg/kg) of the inhibitor by gavage, based on doses previously reported (18) and are described in the Online Supplement.
Assessment of peanut intestinal sensitivity reactions
Clinical symptoms were evaluated as previously reported (19) and are described in the Online Supplement.
Histology
Jejunum was fixed in 10% formalin and processed into paraffin blocks. The number of mucus-containing cells from one side of the villus and the total number of villus cells on the same side were counted.
Mucosal mast cells were identified by chloroacetate esterase staining (17). Numbers of mucosal cells expressing Cyp11a1 were identified by immunohistochemical (IHC) staining using anti-human Cyp11a1 antibody (Abcam, Cambridge, MA). At least 4 random fields per slide were examined and analyzed in a blinded manner. Quantification of stained mast cells or Cyp11a1-positive cells per square millimeter of lamina propria was performed with an Olympus microscope linked to the National Institutes of Health (NIH) Image Analysis Program (NIH, Bethesda, MD).
Cytokines levels in cell culture
Levels of IL-4, IL-13, IL-17A, and IFN-γ in cell culture supernatants were measured by ELISA (eBioscience, San Diego, CA) as described by the manufacturer.
Measurement of peanut-specific antibody
Serum peanut-specific IgE, IgG1, and IgG2a levels were measured by ELISA, as described previously (19, 21).
Histamine levels in plasma
Levels of histamine in plasma were measured as described in the Online Supplement.
Pregnenolone levels in serum and cell culture supernatants
Pregnenolone levels in serum and cell culture supernatants were measured by ELISA (ALPCO Diagnostics, Salem, NH), as described by the manufacturer.
T-cell differentiation in vitro
Differentiation of Th1, Th2, or Th17 cells was performed as previously described (22, 23) and is described in the Online Supplement.
Western blot analysis
Cell lysates were prepared from cultured CD4 T cells as previously described (7) and in the Online Supplement.
Quantitative real-time PCR
Real-time PCR (RT-PCR) was performed as previously described (24) and in the Online Supplement.
Expression constructs
The Cyp11a1 short hairpin RNA (shRNA) sequences were generated using the Dharmacon siDESIGN center (Thermo Scientific) web site (http://www.dharmacon.com/designcenter/designcenterpage.aspx) and are described in the Online Supplement.
Retrovirus production and transduction
Retrovirus production (26) and retroviral transduction of Th2 cells (27) were performed as previously described and are detailed in the Online Supplement.
Cell sorting and analysis of gene expression
Seventy-two hours after transduction, the cells were collected and labeled with anti-mouse CD4 FITC (eBiosciences). CFP+CD4+ cells were sorted using a Synergy cell sorter (iCyt). Sorted cells were stimulated with 2 μg/ml anti-CD3/CD28 for quantitative RT-PCR and ELISA. Quantitative RT-PCR and ELISA were performed as described above.
Estradiol levels in plasma
Levels of estradiol in plasma were measured by ELISA (Oxford Biomedical Research, Oxford, MI), as described by the manufacturer. The concentration of estradiol was calculated from a standard curve provided by the manufacturer.
Cell viability and growth
Cell viability was determined using the trypan blue dye exclusion assay. Cell growth was determined by counting the number of viable cells.
Statistical analysis
ANOVA was used to determine the levels of difference between all groups. Comparisons for all pairs utilized the Tukey-Kramer highest significance difference test. P values for significance were set at 0.05. All results were expressed as the means±SEM.
Results
Expression of Cyp11a1 in the small intestine of peanut sensitized and challenged mice
We first examined the expression of Cyp11a1 mRNA and protein in the jejunum of WT Balb/c mice. Following PE sensitization and challenge (Fig. 1A), Cyp11a1 mRNA expression was increased 3-fold in the jejunal homogenates (Fig. 1B). Immunohistochemical staining of jejunal tissue with an antibody specific for Cyp11a1 protein was mainly localized to the lamina propria of the small intestine (Fig. 1C). There were few Cyp11a1-positive cells in the mucosa of the small intestine of sham sensitized mice whereas numbers of Cyp11a1-positive cells were significantly increased in the PE sensitized and challenged mice (Fig. 1D). Thus, Cyp11a1 expression was induced following sensitization and challenge.
Figure 1.
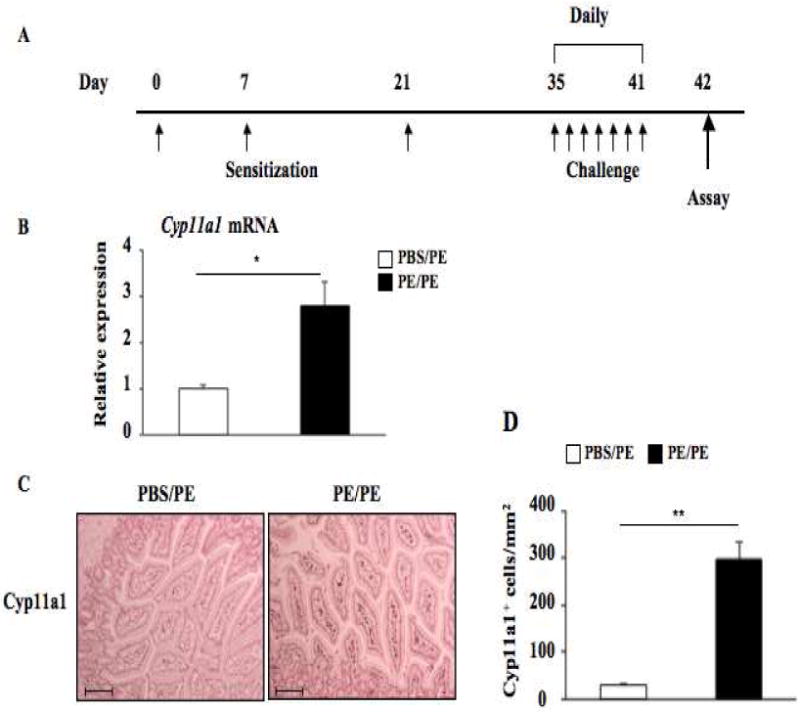
Cyp11a1 is expressed in mouse jejunum. (A) Protocol for induction of peanut allergy. (B) Cyp11a1 mRNA expression detected by quantitative RT-PCR in peanut sensitized and challenged vs. sham sensitized and peanut challenged mice. (C) Representative immunohistochemical staining for Cyp11a1 (magnification ×200. Bar=100 μM). (D) Quantitation of mucosal Cyp11a1-expressing cells. Results were from 3 independent experiments; each experiment included 4 mice per group (n=12). *P<0.05, **P<0.01. PBS/PE, sham sensitized and peanut challenged; PE/PE, peanut sensitized and challenged.
Inhibition of Cyp11a1 attenuates PE-induced allergic responses in vivo
To determine whether Cyp11a1 plays a role in the development of peanut allergy, we investigated the effects of inhibition of Cyp11a1 enzymatic activation on induction of peanut allergy using an inhibitor, AMG. AMG is known to block the enzymatic activity of Cyp11a1, thus preventing conversion of cholesterol to pregnenolone (28). To establish that AMG inhibitory activity was limited to the enzymatic activity, we measured pregnenolone levels in serum following PE sensitization and challenge. As shown in Figure 2A, levels of pregnenolone were significantly increased in the serum of peanut sensitized and challenged mice (4.69±0.92 ng/ml) compared to sham sensitized but PE challenged mice (1.99±0.11 ng/ml). Levels of pregnenolone were significantly decreased (2.98±0.60 ng/ml) in peanut sensitized and challenged mice following treatment with AMG (20 mg/kg). While PE sensitization and challenge increased Cyp11a1 mRNA and numbers of Cyp11a1-positive cells in the small intestine, treatment with AMG (20 mg/kg) did not affect these results (Figs. 2B, 2C). These data confirmed that Cyp11a1 enzymatic activity, in parallel to mRNA and protein expression, was induced by peanut sensitization and challenge and that AMG specifically targeted the enzymatic activity but not protein expression per se.
Figure 2.
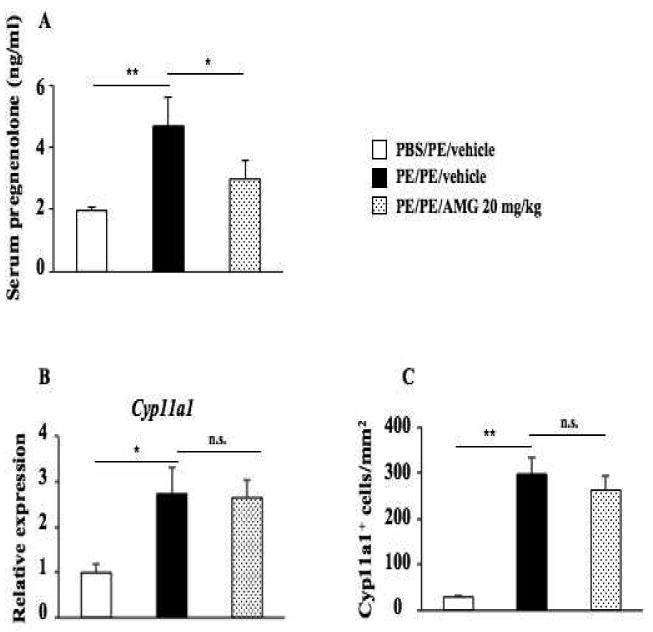
Inhibition of Cyp11a1 enzymatic activity does not impact levels of Cyp11a1 protein and mRNA expression in the mouse jejunum. (A) Pregnenolone levels were assessed in serum of mice. (B) Cyp11a1 mRNA expression in jejunum of mice treated with AMG or vehicle. (C) Quantitation of mucosal Cyp11a1-expressing cells. Results were from 3 independent experiments; each experiment included 4 mice per group (n=12). *P<0.05, **P<0.01, n.s. not significant. PBS/PE, sham sensitized and peanut challenged; PE/PE, peanut sensitized and challenged; PE/PE/AMG 20 mg/kg, peanut sensitized and challenged and treated with AMG at dose of 20 mg/kg.
Administration of the inhibitor to sensitized mice resulted in a dose-dependent inhibitory effect on intestinal allergy induction; 20 mg/kg of the inhibitor fully prevented development of diarrhea and significantly diminished clinical symptom scores in PE sensitized and challenged mice (Figs. 3A, 3B). Lower doses of the inhibitor (10 mg/kg) partially inhibited diarrhea and symptom scores, whereas 5 mg/kg of the inhibitor had no observed inhibitory effects on diarrhea occurrence or clinical symptoms.
Figure 3.
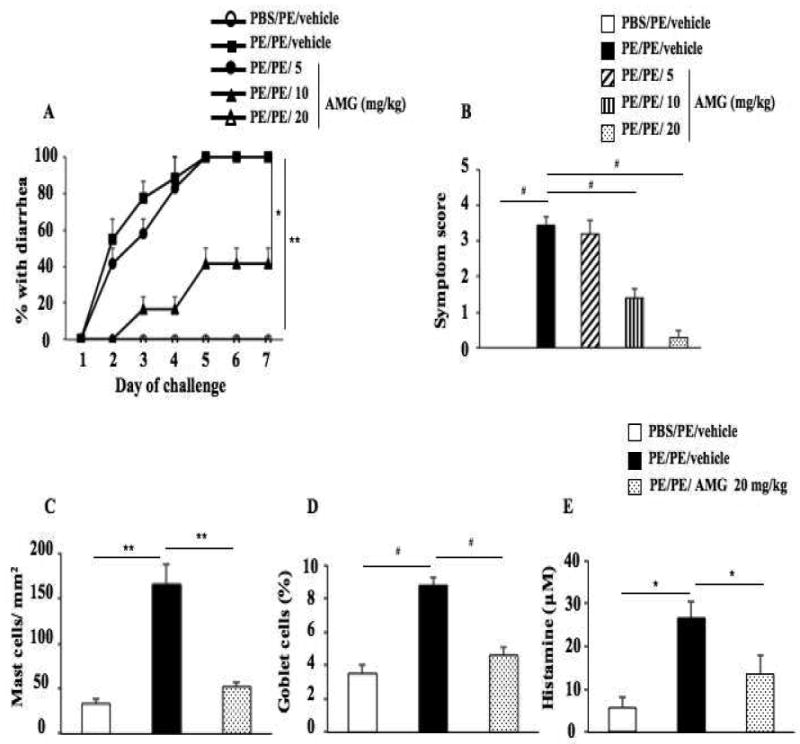
Inhibition of Cyp11a1 enzymatic activity in vivo reduces intestinal responses. (A) Kinetics of development of diarrhea after treatment with AMG (Cyp11a1 inhibitor) vs. vehicle. (B) Scores based on the severity of clinical signs were assessed 30 minutes after oral challenge. (C-D) Quantitation of mucosal mast cell and goblet cell numbers in jejunum. (E) Plasma histamine levels were assessed within 30 minutes of the last oral challenge. Results were from 3 independent experiments; each experiment included 4 mice per group. *P<0.05, **P<0.01, #P<0.001.
Mast cells are involved in allergic responses (17, 29) and we demonstrated increased numbers of mast cells and mucus-producing goblet cells in the small intestine of PE sensitized and challenged mice (Figs. 3C, 3D and Figs. E1, E2 in the Online Supplement). Mice treated with the Cyp11a1 inhibitor at a dose of 20 mg/kg demonstrated markedly reduced numbers of mast cells as well as mucus-producing goblet cells in the mucosa of the small intestine. To detect mast cell degranulation, we measured plasma levels of histamine within 30 minutes of the last antigen challenge. Challenge of sensitized mice resulted in detection of increased levels of histamine in plasma; following treatment with AMG (20 mg/kg), significantly lower levels of plasma histamine were detected (Fig. 3E).
As the inhibitor was administered after sensitization, levels of peanut-specific IgE, IgG1, and IgG2a were unaffected by AMG administration (Fig. E3 in the Online Supplement). To examine whether AMG treatment has effect on estrogen secretion, we measured estradiol levels in plasma following PE sensitization and challenge. Levels of estradiol did not significantly increase in the plasma of peanut sensitized and challenged mice compared to sham sensitized but PE challenged mice (Fig. E4 in the Online Supplement). Levels of estradiol were unaffected by AMG (20 mg/kg) administration (Fig. E4 in the Online Supplement). Together, these results demonstrated that AMG was a potent inhibitor of the enzymatic activity of Cyp11a1 in vivo without affecting mRNA expression or protein levels of the enzyme. These data identified Cyp11a1 to play an essential role in the triggering of allergic diarrhea and symptoms, intestinal inflammation, and goblet cell metaplasia.
Inhibition of Cyp11a1 enzymatic activity suppresses Th2 and Th17 cytokine production without impacting the expression of lineage-specific transcription factors in vivo
Th2 and Th17 cells have been implicated in the development of allergic disorders, including asthma and food allergy (24, 30-33). PE sensitization and challenge increased IL4, IL13, and IL17A but not IFNG mRNA expression in the small intestine (Fig. 4A). In parallel, expression of the lineage-specific transcription factors GATA3 and RORγt mRNA were significantly increased in sensitized and challenged mice while levels of T-bet mRNA were not altered (Fig. 4B). After treatment with AMG (20 mg/kg), IL4, IL13, and IL17A mRNA expression were reduced to baseline levels, but expression levels of T-bet, GATA3, or RORγt mRNA were not affected (Fig. 4B), indicating that the effects on cytokine transcription were mediated downstream of these transcription factors. Given that transcription factor expression was still increased in AMG-treated animals, drug toxicity as an explanation of the effects on cytokine expression was eliminated.
Figure 4.
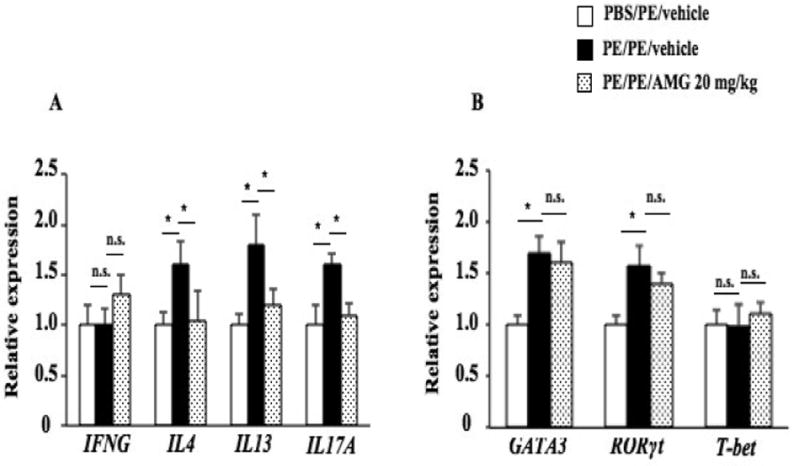
Effects of Cyp11a1 inhibition on cytokine and lineage-specific transcription factor expression in the mouse jejunum. (A) IFNG, IL4, IL13, and IL17A mRNA expression in jejunum of mice treated with AMG or vehicle. (B) Th1, Th2, and Th17 transcription factors T-bet, GATA3, and RORγt expression in jejunum of mice treated with AMG or vehicle. Results were from 3 independent experiments (n=12). *P<0.05, **P<0.01, n.s. not significant.
Inhibition of Cyp11a1 enzymatic activity suppresses Th2 and Th17 cell differentiation in vitro without affecting lineage-specific transcription factor or Cyp11a1 expression
Naive Th cells differentiate into Th1, Th2, and Th17 cells under the control of specific polarizing cytokines and master transcription factors (34). We investigated the effect of Cyp11a1 inhibition on Th cell differentiation in vitro. Isolated CD4+CD45RB+ T cells from spleens of naive TCR transgenic mice (OT II mice) were cultured under Th1, Th2, and Th17 polarizing conditions in the presence or absence of the inhibitor AMG for 6 days and then stimulated with anti-CD3/anti-CD28.
Addition of AMG to cultured CD4 T cells under Th1, Th2, and Th17 polarizing conditions had significant and distinct effects. In polarized cells, Cyp11a1 mRNA expression was approximately 300-fold higher in Th2 cells compared to Th1 cells and 10-fold higher in Th17 cells compared to Th1 cells (Fig. 5A). The addition of AMG (400 μM) to the cell cultures did not suppress expression levels of Cyp11a1 mRNA or protein in the polarized Th1, Th2, or Th17 cells (Figs. 5B, 5C). As shown in Figure 5D, levels of pregnenolone were highest in the culture supernates from polarized Th2 cells, with lower levels released from Th17 cells, followed by release from Th1 cells. Addition of AMG (400 μM) during the polarization of Th cells in vitro significantly decreased levels of pregnenolone in the culture supernates from Th2 cells but not in Th1 cells. Levels in cultures of polarized Th17 cells were also reduced by AMG, but the decreases did not reach statistical significance. These results confirmed the findings that the inhibitory activity of AMG appeared restricted to the enzymatic activity of Cyp11a1 without affecting gene transcription or translation. Further, the data demonstrated the highest levels of Cyp11a1 expression and enzymatic activity in Th2 cells with little to no expression or activity in Th1 cells.
Figure 5.
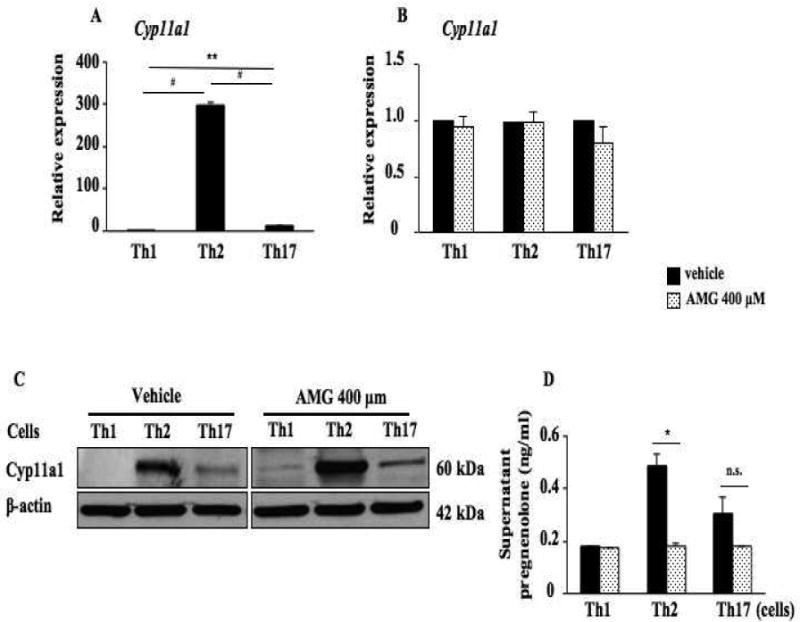
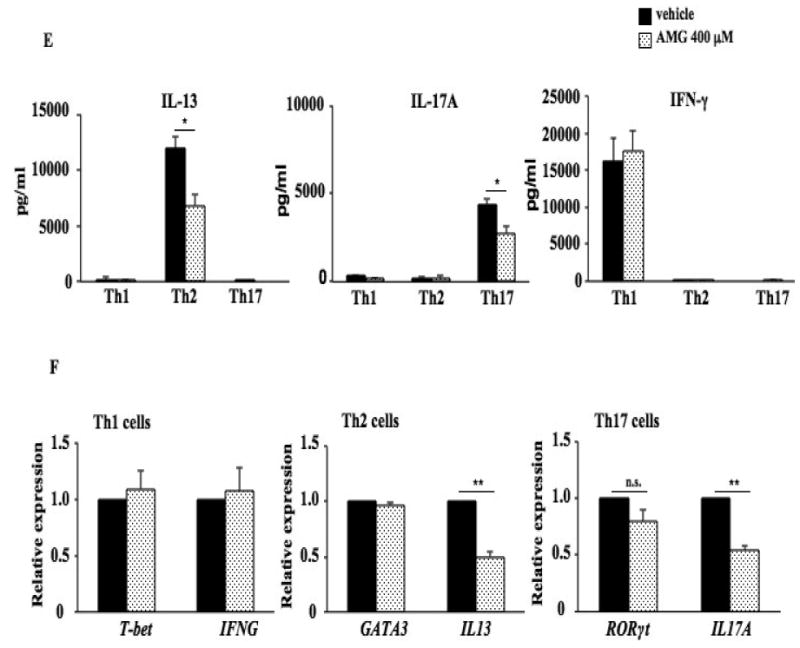
Inhibition of Cyp11a1 enzymatic activity suppresses the differentiation of naive CD4 T cells into Th2 and Th17 cells without affecting lineage-specific transcription factor and Cyp11a1 expression. (A). Relative Cyp11a1 expression in naive CD4 T cells differentiated in vitro into Th1, Th2, and Th17 cells from spleen of naive TCR-transgenic mice (OT II mice) determined by RT-PCR. (B). Cyp11a1 mRNA expression in polarized CD4 T cells in the presence of AMG or vehicle. (C). Western blot analysis of Cyp11a1 protein in polarized Th1, Th2, or Th17 cells treated with AMG or vehicle. (D). Pregnenolone levels were assessed in supernatants of cultured CD4 T cells under Th1, Th2, and Th17 polarizing conditions. (E) Cytokine levels in supernatants of cultured CD4 T cells treated with inhibitor or vehicle under Th1, Th2, and Th17 polarizing conditions. (F) Th1, Th2, and Th17 cytokine and lineage-specific transcription factor mRNA expression in polarized Th1, Th2, or Th17 cells treated with the inhibitor or vehicle. The data shown are from 3 independent experiments (n=12). *P<0.05, **P<0.01, #P<0.001, n.s. not significant.
In the culture supernatants of polarized Th2 cell cultures, dose-dependent inhibitory effects of AMG on IL-13 secretion were observed. Levels of IL-13 were significantly decreased in the culture supernatants of polarized Th2 cell cultures in the presence of AMG at 400 μM and 600 μM (Fig. 5E and Fig. E5 in the Online Supplement); levels of IL-13 were decreased in the presence of AMG at 200 μM but the decreases did not reach statistical significance; levels of IL-17A were significantly decreased by AMG at 400 μM and 600 μM in polarized Th17 cell cultures, but levels of IFN-γ were not affected by AMG at any concentration tested in polarized Th1 cell cultures. In parallel, the inhibitor decreased levels of IL13 and IL17A mRNA in polarized Th2 and Th17 cells, respectively (Fig. 5F) but no significant effects of the inhibitor were detected on IFNG mRNA expression in Th1 cells. Consistent with results from the in vivo studies, the inhibitor (400 μM) did not have any effect on lineage-specific transcription factor mRNA expression, T-bet, GATA3, or RORγt mRNA in polarized Th1, Th2, and Th17 cells, respectively (Fig. 5F). In addition, addition of the inhibitor at 400 μM or lower during the polarization of Th cells in vitro had no impact on Th cell viability; in the presence of 600 μM of the inhibitor, cell viability was marginally affected (Fig. E6 in the Online Supplement).
shRNA-mediated silencing of Cyp11a1 reduces Th2 cytokine expression
To confirm the importance of Cyp11a1 in Th cell differentiation, we used shRNA-mediated silencing of Cyp11a1 in polarized Th2 cells. Polarized Th2 T cells were transduced with retroviruses co-expressing cyan fluorescent protein (CFP) with control (luc) or Cyp11a1 shRNA. Seventy-two hours after transduction, CFP+CD4+ cells were sorted and stimulated with 2 μg/ml anti-CD3/anti-CD28 for 6 and 24 hours. To confirm the effectiveness of Cyp11a1 gene silencing, we demonstrated reduced levels of Cyp11a1 mRNA in Th2 T cells compared to silencing with the control shRNA (Fig. 6A). As a result, levels of pregnenolone in supernates of cultured Th2 cells transfected with Cyp11a1 shRNA were significantly reduced compared to those transfected with control shRNA (Fig. 6B).
Figure 6.
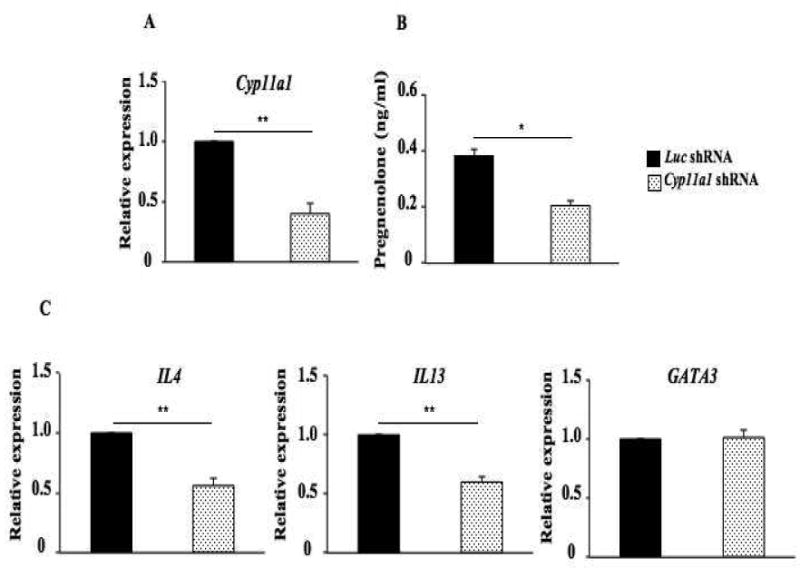
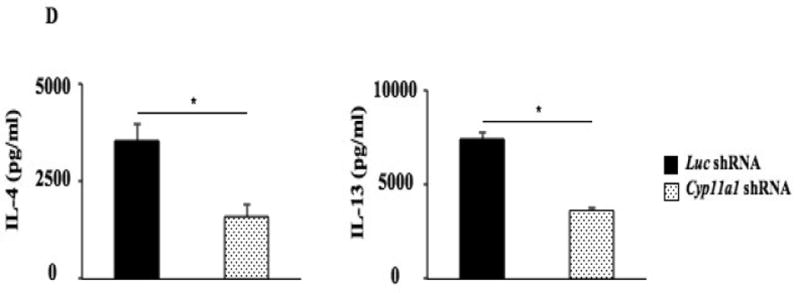
shRNA-mediated silencing of Cyp11a1 regulates levels of IL-13 without affecting levels of GATA3 transcripts in Th2 T cells. (A) Cyp11a1 mRNA expression in shRNA-transduced Th2 cells. (B) Pregnenolone levels were assessed in supernatants of cultured Th2 cells transduced with Cyp11a1 or luc shRNA. (C) Levels of IL4, IL13, and GATA3 mRNA expression in cultured Th2 cells transduced with Cyp11a1 or luc shRNA. (D) Levels of IL-4 and IL-13 in supernatants of cultured Th2 cells transduced with Cyp11a1 or luc shRNA. Results were from 3 independent experiments (n=12). *P<0.05, **P<0.01.
Levels of IL4 and IL13 mRNA were decreased in Th2 T cells transfected with Cyp11a1 shRNA compared to those transfected with control shRNA, without affecting levels of GATA3 mRNA (Fig. 6C). In parallel, levels of IL-4 and IL-13 were reduced in supernatants of Th2 T cell cultures transfected with Cyp11a1 shRNA (Fig 6D). These results demonstrated that silencing of Cyp11a1 in polarized Th2 T cells resulted in decreased levels of IL4 and IL13 mRNA and protein without affecting GATA3 transcription. These results indicated that Cyp11a1 upregulation and activation is downstream of GATA3.
Discussion
CD4 Th cells play a pivotal role in the induction and control of allergic inflammation, including food allergy (35). In a mouse model of food allergy, allergen-specific CD4 T cells were activated in the mesenteric lymph nodes and recruited to the small intestine, resulting in increased levels of Th2 cytokines in the inflamed small intestine (36). We showed previously that increased numbers of CD4 T cells accumulated in the small intestine accompanied by increases in Th2 cytokine (IL4, IL13) mRNA expression in a mouse model of peanut allergy (24). Here, we demonstrated that peanut sensitization and challenge not only resulted in inflammatory and cytokine changes in the small intestine but that mRNA, protein, and enzymatic activity levels of the steroidogenic enzyme Cyp11a1 were also markedly elevated. Administration of an inhibitor of Cyp11a1 enzymatic activity, AMG, prevented development of allergic diarrhea and accumulation of inflammatory cells in the small intestine in a dose-dependent manner. Levels of serum pregnenolone were reduced in parallel. AMG treatment decreased IL13 and IL17 mRNA expression in the small intestine without impacting Cyp11a1 mRNA or protein levels. In vitro, the inhibitor decreased levels of IL13 and IL17 mRNA in polarized Th2 and Th17 T cells, respectively, without affecting levels of GATA3, RORγt, or the polarization of Th1 cells, IFNG, and T-bet expression. The importance of Cyp11a1 was further demonstrated using shRNA-mediated silencing of Cyp11a1 in polarized Th2 T cells which resulted in significantly decreased levels of IL4 and IL13 mRNA and protein. These data indicated that Cyp11a1 played an important role in the development of peanut allergy through its effects on steroidogenesis, a critical pathway in Th2 differentiation.
In humans, allergen-specific Th2 T cells were essential in the development and maintenance of both type I IgE-mediated and non-IgE-mediated food allergic responses. In patients with anaphylactic peanut allergy, increased numbers of peanut-specific IL-5- and IL-4-producing Th2 cells were found in peripheral blood (37). In addition, peanut-specific T cell lines from individuals with peanut anaphylaxis primarily produced Th2 cytokines (IL-4, IL-13) (38). Other food allergies were also characterized by increased levels of Th2 cytokines; in patients with milk-induced gastrointestinal diseases, milk-specific CD4 T cells derived from the duodenal mucosa produced high levels of Th2 cytokines, especially IL-13 (39).
GCs are steroid hormones with important functions in regulating immune responses and inflammation (3). Endogenous GC synthesis is controlled by the hypothalamic-pituitary-adrenal axis (3, 40) and is regulated by the transcriptional control of steroidogenic enzymes of the cytochrome P450 gene family (41). Corticosteroids have been used in treating allergic diseases due to their anti-inflammatory activity (4), but, somewhat paradoxically, increasing evidence indicates that corticosteroids may also enhance disease pathogenesis by activating and enhancing growth of CD4 T cells and inhibiting Th1 cytokine production (42). GCs amplified immune responses in steroid-insensitive CD8+ T cells (7). As well, the corticosteroids themselves may induce Th2 cytokine production while simultaneously suppressing the production of Th1 cytokines (7).
Cyp11a1 is the first and rate-limiting enzyme in the steroid biosynthetic pathway, catalyzing the conversion of cholesterol to pregnenolone. Transcription factors such as steroidogenic factor-1 (SF-1), activator protein-2 (AP-2), and several tissue-specific GATA family proteins enhance the transcription of Cyp11a1 through interactions with AP-1, specificity protein-1 (SP-1) and AP-2 (10). In particular, the GATA protein family plays an important role in the regulation of Cyp11a1 expression (43). GATA binding elements have been identified in the Cyp11a1 promoter and Cyp11a1 expression was decreased in GATA3-deficient mice (44). GATA4 significantly upregulated Cyp11a1 expression in granulosa cells (45). These results identify important events in the transcriptional regulation of Cyp11a1 that directly affect steroid synthesis and release.
In this study, we investigated the role of Cyp11a1 in peanut-induced allergic intestinal responses. We demonstrated that levels of Cyp11a1 protein and mRNA were increased in the jejunum of sensitized and challenged mice. In parallel, enzymatic activity was increased as demonstrated by increased levels of pregnenolone in the serum of sensitized and challenged mice. Next, we determined whether Cyp11a1 enzymatic activity was essential for induction of peanut allergy using an inhibitor, AMG. AMG is a recognized inhibitor of Cyp11a1 enzymatic activity and has been used for the suppression of adrenal function in patients with Cushing's syndrome (46) and as second-line therapy for patients with breast cancer (47, 48) and prostate cancer (49).
Administration of this inhibitor during the oral challenge phase, after sensitization, resulted in significantly lower serum pregnenolone levels and reduced the incidence and severity of diarrhea and intestinal inflammation (mast cell accumulation and goblet cell metaplasia), accompanied by decreases in IL13 and IL17A mRNA in the intestine. The inhibitor did not alter the development of specific antibodies, including peanut-specific IgE, likely because sensitization was completed prior to treatment in the challenge phase. The inhibitor did not affect estradiol secretion following peanut sensitization and challenge nor were serum levels affected by AMG administration. This, in part, may reflect the finding that AMG, per se, does not suppress plasma estrogen levels by inhibiting the adrenal secretion of precursor androgen (50). Although administration of the inhibitor in vivo could not identify specific target cells, these data demonstrated for the first time that Cyp11a1 functioned as a key regulator of the development of peanut-induced allergic responses.
The data showed that inhibition of Cyp11a1 significantly reduced Th2 and Th17 cytokine production in vivo. Interestingly, the inhibitor did not affect expression of the Th1, Th2, and Th17 lineage-specific transcription factors T-bet, GATA3, or RORγt. The results suggested that suppression of Th2 and Th17 cytokine production was not mediated through effects on lineage-specific transcription factor expression but on cytokine transcription. The primary action of Cyp11a1 enzymatic activity in this model thus appeared to manifest downstream of these lineage-specific transcription factors.
To investigate the function of Cyp11a1 in CD4 T cells, we monitored Th1, Th2, and Th17 polarization in vitro in the presence of AMG. The highest levels of Cyp11a1 protein and enzymatic activity were detected in polarized Th2 cells, with significantly lower levels in Th17 cells, and virtually no activity in Th1 cells. Based on AMG dose-dependent inhibitory effects on Th2 and Th17 cytokine secretion as well as cell viability, we chose 400 μM AMG to carry out the in vitro Th cell polarization experiments. The inhibitor decreased IL-13 cytokine production in polarized Th2 cells; however, IFN-γ production was not affected by the inhibitor in polarized Th1 cells. Similar to our in vivo data, the inhibitor did not affect GATA3 mRNA expression in polarized Th2 cells nor levels of T-bet or RORγt in polarized Th1 and Th17 cells, respectively. Thus, inhibition of Cyp11a1 enzymatic activity impaired CD4 Th2 and Th17 cell differentiation, which in turn decreased production of the Th2 cytokine (IL-13) and Th17 cytokine (IL-17A) and these effects were mediated downstream of their respective and essential lineage-specific transcription factors.
To confirm that the results with AMG were specific to inhibition of Cyp11a1, we silenced Cyp11a1 mRNA in cultured Th2 T cells using a shRNA. During Th2 polarization, cells were transduced with retrovirus expressing Cyp11a1-targeted shRNA or control (luc) shRNA and activated under Th2 conditions. Cyp11a1 shRNA decreased the expression of Cyp11a1 mRNA levels by 58%±5.2% and enzymatic activity of Cyp11a1, monitoring pregnenolone levels, was reduced by 47%±4.5%. Levels of Th2 cytokine (IL-4, IL-13) mRNA and protein were decreased upon transduction of Cyp11a1 shRNA. As we observed with Cyp11a1 inhibition in vivo and in vitro with AMG, levels of GATA3 mRNA remained unaffected after silencing of Cyp11a1. These data confirmed in vivo and in vitro AMG inhibition data, indicating that Cyp11a1 critically regulates Th2 cell differentiation and cytokine production.
These studies demonstrate for the first time that activation of the steroidogenic enzyme Cyp11a1 plays a critical role in the development of intestinal allergic responses through its effects on Th2 polarization and IL-13 production. Although studies in experimental animal models are difficult to extrapolate to human conditions, identification of the functional significance of Cyp11a1 represents a novel and potential target for the regulation and treatment of peanut-induced allergy.
Supplementary Material
Clinical Implications.
At present, the only recognized therapy for peanut-sensitive individuals is peanut avoidance. The data identify the important role of the steroidogenic pathway in the triggering of peanut-induced intestinal anaphylaxis and identify potential targets for intervention.
Capsule Summary.
Peanut sensitization and challenge result in the upregulation of Cyp11a1 enzymatic activity which is essential as well as restricted to Th2 differentiation and the full manifestations of intestinal inflammation, cytokine increases, and symptoms.
Acknowledgments
We thank Carissa Dege for culturing Phoenix cells and Diana Nabighian for her assistance in preparation of the manuscript.
Sources of Funding: This work was supported by NIH grants T32 AI-07405 (J.R.), AI-81878 and AI-98417 (J.H.), and HL-36577 and AI-77609 (E.W.G.). M.W. was supported by a fellowship from the Eugene F. and Easton M. Crawford Charitable Lead Unitrust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH.
Abbreviations
- AMG
Aminoglutethimide
- AP-2
Activator protein-2
- CFP
Cyan fluorescent protein
- Cyp11a1
Cytochrome P450, family 11, subfamily A, polypeptide 1
- GC
Glucocorticoid
- IHC
Immunohistochemistry
- Luc
Luciferase
- PAS
Periodic acid-Schiff
- PE
Peanut extract
- RORγt
Retinoic acid-related orphan receptor γt
- RT-PCR
Real-time polymerase chain reaction
- SF-1
Steroidogenic factor-1
- shRNA
Short hairpin RNA
- SP-1
Specificity protein-1
- ΦNX
Phoenix
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 2.De Bosscher K, Haegeman G. Minireview: latest perspectives on antiinflammatory actions of glucocorticoids. Mol Endocrinol. 2009;23:281–91. doi: 10.1210/me.2008-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–62. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29–43. doi: 10.1111/j.1476-5381.2010.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–60. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 6.Cima I, Corazza N, Dick B, Fuhrer A, Herren S, Jakob S, et al. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J Exp Med. 2004;200:1635–46. doi: 10.1084/jem.20031958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohnishi H, Miyahara N, Dakhama A, Takeda K, Mathis S, Haribabu B, et al. Corticosteroids enhance CD8+ T cell-mediated airway hyperresponsiveness and allergic inflammation by upregulating leukotriene B4 receptor 1. J Allergy Clin Immunol. 2008;121:864–71. doi: 10.1016/j.jaci.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 8.Payne AH. Hormonal regulation of cytochrome P450 enzymes, cholesterol side-chain cleavage and 17 alpha-hydroxylase/C17-20 lyase in Leydig cells. Biol Reprod. 1990;42:399–404. doi: 10.1095/biolreprod42.3.399. [DOI] [PubMed] [Google Scholar]
- 9.Pazirandeh A, Xue Y, Rafter I, Sjövall J, Jondal M, Okret S. Paracrine glucocorticoid activity produced by mouse thymic epithelial cells. FASEB J. 1999;13:893–901. doi: 10.1096/fasebj.13.8.893. [DOI] [PubMed] [Google Scholar]
- 10.Shih MC, Chiu YN, Hu MC, Guo IC, Chung BC. Regulation of steroid production: analysis of Cyp11a1 promoter. Mol Cell Endocrinol. 2011;336:80–4. doi: 10.1016/j.mce.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Pang S, Yang X, Wang M, Tissot R, Nino M, Manaligod J, et al. Inherited congenital adrenal hyperplasia in the rabbit: absent cholesterol side-chain cleavage cytochrome P450 gene expression. Endocrinology. 1992;131:181–6. doi: 10.1210/endo.131.1.1611996. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Iwamoto K, Wang M, Artwohl J, Mason JI, Pang S. Inherited congenital adrenal hyperplasia in the rabbit is caused by a deletion in the gene encoding cytochrome P450 cholesterol side-chain cleavage enzyme. Endocrinology. 1993;132:1977–82. doi: 10.1210/endo.132.5.7682938. [DOI] [PubMed] [Google Scholar]
- 13.Kim CJ, Lin L, Huang N, Quigley CA, AvRuskin TW, Achermann JC, et al. Severe combined adrenal and gonadal deficiency caused by novel mutations in the cholesterol side chain cleavage enzyme, P450scc. J Clin Endocrinol Metab. 2008;93:696–702. doi: 10.1210/jc.2007-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Kandari H, Katsumata N, Alexander S, Rasoul MA. Homozygous mutation of P450 side-chain cleavage enzyme gene (CYP11A1) in 46,XY patient with adrenal insufficiency, complete sex reversal, and agenesis of corpus callosum. J Clin Endocrinol Metab. 2006;91:2821–6. doi: 10.1210/jc.2005-2230. [DOI] [PubMed] [Google Scholar]
- 15.Tajima T, Fujieda K, Kouda N, Nakae J, Miller WL. Heterozygous mutation in the cholesterol side chain cleavage enzyme (p450scc) gene in a patient with 46,XY sex reversal and adrenal insufficiency. J Clin Endocrinol Metab. 2001;86:3820–5. doi: 10.1210/jcem.86.8.7748. [DOI] [PubMed] [Google Scholar]
- 16.Parajes S, Kamrath C, Rose IT, Taylor AE, Mooij CF, Dhir V, et al. A novel entity of clinically isolated adrenal insufficiency caused by a partially inactivating mutation of the gene encoding for P450 side chain cleavage enzyme (CYP11A1) J Clin Endocrinol Metab. 2011;96:E1798–806. doi: 10.1210/jc.2011-1277. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Takeda K, Shiraishi Y, Okamoto M, Dakhama A, Joetham A, et al. Peanut-induced intestinal allergy is mediated through a mast cell-IgE-FcepsilonRI-IL-13 pathway. J Allergy Clin Immunol. 2010;126:306–16. doi: 10.1016/j.jaci.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oka H, Emori Y, Hayashi Y, Nomoto K. Breakdown of Th cell immune responses and steroidogenic CYP11A1 expression in CD4+ T cells in a murine model implanted with B16 melanoma. Cell Immunol. 2000;206:7–15. doi: 10.1006/cimm.2000.1715. [DOI] [PubMed] [Google Scholar]
- 19.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106:150–8. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 20.Tomkinson A, Cieslewicz G, Duez C, Larson KA, Lee JJ, Gelfand EW. Temporal association between airway hyperresponsiveness and airway eosinophilia in ovalbumin-sensitized mice. Am J Respir Crit Care Med. 2001;163:721–30. doi: 10.1164/ajrccm.163.3.2005010. [DOI] [PubMed] [Google Scholar]
- 21.Pons L, Ponnappan U, Hall RA, Simpson P, Cockrell G, West CM, et al. Soy immunotherapy for peanut-allergic mice: modulation of the peanut-allergic response. J Allergy Clin Immunol. 2004;114:915–21. doi: 10.1016/j.jaci.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 22.Komine O, Hayashi K, Natsume W, Watanabe T, Seki Y, Seki N, et al. The Runx1 transcription factor inhibits the differentiation of naive CD4+ T cells into the Th2 lineage by repressing GATA3 expression. J Exp Med. 2003;198:51–61. doi: 10.1084/jem.20021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashino S, Wakita D, Shiohama Y, Iwakura Y, Chamoto K, Ohkuri T, et al. A T(h)17-polarized cell population that has infiltrated the lung requires cells that convert to IFN-{gamma} production in order to induce airway hyperresponsiveness. Intl Immunol. 2010;22:503–13. doi: 10.1093/intimm/dxq034. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Okamoto M, Domenico J, Han J, Ashino S, Shin YS, et al. Inhibition of Pim1 kinase prevents peanut allergy by enhancing Runx3 expression and suppressing T(H)2 and T(H)17 T-cell differentiation. J Allergy Clin Immunol. 2012;130:932–44. doi: 10.1016/j.jaci.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musselman CA, Ramírez J, Sims JK, Mansfield RE, Oliver SS, Denu JM, et al. Bivalent recognition of nucleosomes by the tandem PHD fingers of the CHD4 ATPase is required for CHD4-mediated repression. Proc Natl Acad Sci USA. 2012;109:787–92. doi: 10.1073/pnas.1113655109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maier H, Ostraat R, Parenti S, Fitzsimmons D, Abraham LJ, Garvie CW, et al. Requirements for selective recruitment of Ets proteins and activation of mb-1/Ig-alpha gene transcription by Pax-5 (BSAP) Nucleic Acids Res. 2003;31:5483–9. doi: 10.1093/nar/gkg785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pham D, Vincentz JW, Firulli AB, Kaplan MH. Twist1 regulates IFNγ expression in Th1 cells by interfering with Runx3 function. J Immunol. 2012;189:832–40. doi: 10.4049/jimmunol.1200854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dexter RN, Fishman LM, Ney RL, Liddle GW. Inhibition of adrenal corticosteroid synthesis by aminoglutethimide: studies of the mechanism of action. J Clin Endocrinol Metab. 1967;27:473–80. doi: 10.1210/jcem-27-4-473. [DOI] [PubMed] [Google Scholar]
- 29.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–77. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 31.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–98. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 32.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nature Immunol. 2010;11:928–35. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nature Med. 2012;18:705–15. doi: 10.1038/nm.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knight AK, Blázquez AB, Zhang S, Mayer L, Sampson HA, Berin MC. CD4 T cells activated in the mesenteric lymph node mediate gastrointestinal food allergy in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1234–43. doi: 10.1152/ajpgi.00323.2007. [DOI] [PubMed] [Google Scholar]
- 37.Prussin C, Lee J, Foster B. Eosinophilic gastrointestinal disease and peanut allergy are alternatively associated with IL-5+ and IL-5− TH2 responses. J Allergy Cli Immunol. 2009;124:1326–32. doi: 10.1016/j.jaci.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeLong JH, Simpson KH, Wambre E, James EA, Robinson D, Kwok WW. Ara h 1-reactive T cells in individuals with peanut allergy. J Allergy Clin Immunol. 2011;127:1211–8. doi: 10.1016/j.jaci.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beyer K, Castro R, Birnbaum A, Benkov K, Pittman N, Sampson HA. Human milk-specific mucosal lymphocytes of the gastrointestinal tract display a TH2 cytokine profile. J Allergy Clin Immunol. 2002;109:707–13. doi: 10.1067/mai.2002.122503. [DOI] [PubMed] [Google Scholar]
- 40.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids-new mechanisms for old drugs. N Engl J Med. 2005;353:1711–23. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 41.Mueller M, Cima I, Noti M, Fuhrer A, Jakob S, Dubuquoy L, et al. The nuclear receptor LRH-1 critically regulates extra-adrenal glucocorticoid synthesis in the intestine. J Exp Med. 2006;203:2057–62. doi: 10.1084/jem.20060357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cima I, Fuhrer A, Brunner T. Antagonistic and synergistic effects of glucocorticoids and IL-7 on CD4+ T cell activation. Immunol Lett. 2006;106:99–102. doi: 10.1016/j.imlet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 43.LaVoie HA, King SR. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp Biol Med. 2009;234:880–907. doi: 10.3181/0903-MR-97. [DOI] [PubMed] [Google Scholar]
- 44.Wei G, Abraham BJ, Yagi R, Jothi R, Cui K, Sharma S, et al. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity. 2011;35:299–311. doi: 10.1016/j.immuni.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sher N, Yivgi-Ohana N, Orly J. Transcriptional regulation of the cholesterol side chain cleavage cytochrome P450 gene (CYP11A1) revisited: binding of GATA, cyclic adenosine 3′,5′-monophosphate response element-binding protein and activating protein (AP)-1 proteins to a distal novel cluster of cis-regulatory elements potentiates AP-2 and steroidogenic factor-1-dependent gene expression in the rodent placenta and ovary. Mol Endocrinol. 2007;21:948–62. doi: 10.1210/me.2006-0226. [DOI] [PubMed] [Google Scholar]
- 46.Gross BA, Mindea SA, Pick AJ, Chandler JP, Batjer HH. Medical management of Cushing disease. Neurosurg Focus. 2007;23:E10. doi: 10.3171/foc.2007.23.3.12. [DOI] [PubMed] [Google Scholar]
- 47.Stuart-Harris R, Dowsett M, D'Souza A, Donaldson A, Harris AL, Jeffcoate SL, et al. Endocrine effects of low dose aminoglutethimide as an aromatase inhibitor in the treatment of breast cancer. Clin Endocrinol (Oxf) 1985;22:219–26. doi: 10.1111/j.1365-2265.1985.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 48.Harris AL, Powles TJ, Smith IE. Aminoglutethimide in the treatment of advanced postmenopausal breast cancer. Cancer Res. 1982;42:3405s–8s. [PubMed] [Google Scholar]
- 49.Kruit WH, Stoter G, Klijn JG. Effect of combination therapy with aminoglutethimide and hydrocortisone on prostate-specific antigen response in metastatic prostate cancer refractory to standard endocrine therapy. Anticancer Drugs. 2004;15:843–7. doi: 10.1097/00001813-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Cocconi G. First generation aromatase inhibitors--aminoglutethimide and testololactone. Breast Cancer Res Treat. 1994;30:57–80. doi: 10.1007/BF00682741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


