Abstract
Trait rumination, a tendency to focus on depressive symptoms and negative information, is associated with longer and more severe episodes of depression. This study examined whether trait rumination was also associated with initial remission from unipolar depression in Cognitive Therapy, which we hypothesized would target this coping style. Eighty one patients completed measures of depressive severity and rumination before and after 16–20 sessions of procedurally determined Cognitive Therapy. Pre-treatment rumination and severity were generally associated with later initial remission and lower odds of achieving remission. Limited evidence also suggested that for the most severe patients, rumination was associated with earlier initial remission and greater odds of achieving initial remission. Cognitive Therapy was associated with significant reductions in both rumination and severity. Results suggest that 1) pre-treatment assessment of rumination and severity could help to plan treatment course and 2) Cognitive Therapy is associated with changes in cognitive coping styles.
Keywords: Cognitive Therapy, Rumination, Response Styles Theory, Depression
INTRODUCTION
This study examined relationships of initial remission and self-reported rumination in Cognitive Therapy for unipolar depression. Cognitive Therapy (CT; Beck, 1979) is an empirically supported intervention that is effective for 40–60% of patients with unipolar depression (Hollon, Thase, & Markowitz, 2002). Knowing which patients are likely to benefit from CT could increase response rate and decrease costs by targeted referrals. Moreover, CT is usually “prescribed” in doses of 14–16 weeks. Potentially, some patients need fewer sessions and others need more to achieve remission. Having strong predictors of how long it will take for patients to remit could help in treatment and disposition planning. This study examined the extent to which rumination, a tendency to experience repetitive, intrusive, negative cognitions (Alloy, Abramson, Metalsky, & Hartlage, 1988; Ingram, 1984; Martin & Tesser, 1989, 1996; Nolen-Hoeksema, Morrow, & Fredrickson, 1993; Philippot & Rime, 1998; Wells & Matthews, 1994) is associated with poorer and more delayed initial remission from depression in CT. In particular, we considered the “ruminative response style”, defined as a tendency to reflect elaborately on the causes and consequences of one’s symptoms of depression (Nolen-Hoeksema et al., 1993).
CT involves, among other things, learning compensatory skills that assist in dealing with negative cognitions (Barber & DeRubeis, 1989; Sheppard & Teasdale, 2004; Teasdale et al., 2001). In CT, patients learn to first identify the occurrence of automatic negative cognitions as well as negative core beliefs and then learn to replace negative associations and interpretations with more adaptive or positive interpretations. CT also involves learning explicit problem-solving skills while simultaneously learning to test dysfunctional thoughts and beliefs that impede problem-solving, for example, “There is nothing I can do to solve this.” Researchers have argued and demonstrated that CT works in the short-run by increasing the use of these compensatory skills to cope with negative cognitions rather than by changing the underlying maladaptive cognitive schema or eliminating distorted cognitions (Sheppard & Teasdale, 2004; Teasdale et al., 2001).
As such, a prepotent tendency to negatively elaborate on negative information (i.e., ruminate) could impact remission in CT. Dysphoric ruminators have notoriously impaired problem-solving skills, pessimistic attributions (e.g., “I never succeed’), and distorted interpretations of hypothetical life events (e.g., “I’m a loser if I stay home Saturday nights;”(Lyubomirsky, Caldwell, & Nolen-Hoeksema, 1998). Rumination is associated with stronger responses to mood provocation (Lyubomirsky & Nolen-Hoeksema, 1993), and is predictive of stronger memory for negative emotional stimuli (Lyubomirsky et al., 1998) as well as more sustained physiological and brain reactivity to such stimuli (Siegle, Steinhauer, Carter, Ramel, & Thase, 2003; Siegle, Steinhauer, Thase, Stenger, & Carter, 2002). In addition, depressive symptomatology is also more severe in individuals who ruminate (Just & Alloy, 1997; Morrow & Nolen-Hoeksema, 1990; Nolen-Hoeksema et al., 1993). Therefore depressed individuals who ruminate engage in an elaborative negative cognitive processing style (Teasdale, 1988) that CT was designed to target.
There are many ways in which CT could influence change in rumination. It provides patients with an explanation (i.e., cognitive model) for the cause of their depressed mood, which is one of the primary reasons patients report engaging in rumination (Papageorgiou & Wells, 2003). CT also increases awareness of and a means to challenge negative cognitions located in the ruminative stream rather than passively replaying them (cf. Nolen-Hoeksema & Wisco, In Press). Furthermore, CT also improves problem-solving thereby enabling patients to make progress toward important goals which if unattained can cause rumination (Lavallee & Campbell, 1995; Martin & Tesser, 1996).
Thus, a capitalization model is plausible. In this framework the individuals with the greatest deficits in the treated mechanisms (i.e., repetitive processing of negative cognitions and poor-problem solving skills), would necessitate the highest doses (i.e., longest time-course) of treatment. Evidence supports this conception, as possessing high levels of dysfunctional attitudes (i.e., negative beliefs about the self) in the absence of a negative life event (indicating entrenched negative core beliefs) is predictive of slower remission in CT (Simons, Gordon, Monroe, & Thase, 1995). Similarly, higher initial depressive severity is also associated with poorer and more delayed remission in CT (Coffman, Martell, Dimidjian, Gallop, & Hollon, 2007; Dimidjian et al., 2006). Rumination has specifically been associated with more severe and longer episodes of depression indicating rumination may also be associated with poorer and delayed remission (Nolen-Hoeksema et al., 1993). We have previously used a computational model to suggest that rumination may specifically make it difficult for depressed individuals to learn positive interpretations of otherwise negative information – a key component of CT (Siegle & Ingram, 1997).
Yet, rumination could also be essential to remission in CT. This “compensation” model is supported by the idea that CT addresses the areas of greatest disturbance. The compensation model specifies that if you do not have this form of cognitive deficit you might not respond to CT. Research examining other types of cognitive processing supports this model. Possessing high levels of dysfunctional attitudes in combination with experiencing a negative life event prior to treatment is predictive of faster remission in CT, when compared to low levels of dysfunctional attitudes and the presence of a negative life event (Simons et al., 1995). This finding occurs potentially because CT directly targets relationships between dysfunctional attitudes and provoking life events. The occurrence of a stressful life event in the absence of dysfunctional attitudes leaves CT with little to target. Under the compensation model, individuals who ruminate would thus be more likely to benefit, or might remit more quickly in CT when compared to individuals who do not possess this cognitive processing style. Initial evidence from neuroimaging supports this notion; increased activity in brain structures associated with rumination is predictive of better remission (Siegle, Carter, & Thase, 2006).
In order to clarify the two proposed models, consider the following analogy between CT and antibiotics. Antibiotics (CT) treat bacterial infections (rumination) and will not cure infections caused by viruses (no rumination). Thus, you must have a bacterial infection (rumination) if the antibiotics (CT) are going to work – hence the “compensation” model. However, severe bacterial infections (highly repetitive processing of negative cognitions) require higher doses of antibiotics (CT) to be cured – consistent with a “capitalization” model.
Other existing predictive data is more ambiguous. For example, increased brain function associated with linguistic processing is positively associated with CT outcome (Bruder et al., 1997). This linguistic function could be seen as either associated with rumination, or as a necessity for linguistic reinterpretation of automatic sustained pre-linguistic negative feelings in response to emotional stimuli. In previous studies, ruminative response styles were not reliably associated with differential reduction of depressive symptoms after treatment (Arnow, Spangler, Klein, & Burns, 2004; Bagby et al., 1999; Rector, Segal, & Crawford, 1998; Schmaling, Dimidjian, Katon, & Sullivan, 2002); yet, rumination could have affected the rate of remission rather than its magnitude, which was not tested, and many of these studies did not investigate CT. This study examined whether ruminative individuals take longer to achieve initial remission from depression in CT than non-ruminators.
We hypothesized that among depressed people who engaged in rumination, an increased tendency to ruminate would be associated with a longer time to achieve initial remission. We allowed for the possibility that initial depressive severity could moderate these effects. Hypotheses were tested by examining associations of rumination, severity, and their interaction on remission status and time to initial remission in CT in a relatively large group of depressed outpatients. In addition, to understand whether aspects of remission could be due to change in rumination, the extent to which rumination changed in CT was also examined.
METHOD
Participants
The sample consisted of 120 patients who self-enrolled in an ongoing trial of CT for recurrent depression at the University of Pittsburgh Medical Center from 2001–2007. Thirty-two patients were lost to attrition.1 Seven patients were also excluded from analyses because they were missing data on initial pre-treatment depression severity. The final sample consisted of 81 patients (29 male, M(SD) age = 44.8(11.7), Age Range: 19.7 – 68.4, 91% Caucasian, 6% African American). All participants met diagnostic criteria for unipolar major depressive disorder, recurrent based on a Structured Clinical Interview for DSM-IV diagnosis (SCID, (First, Spitzer, Gibbon, & Williams, 1996), and had no history of psychosis. Participants did not report having abused substances within two weeks of the study, or having met criteria for current substance dependence within the previous six months. On average the sample reported that their first onset of depression occurred at 23.8 years of age (SD = 11.2), and reported a median of 4 major depressive episodes. Patients reported a median duration of 30 weeks for the current episode.
Procedure
After signing informed consent, and completing a diagnostic evaluation using the Structured Clinical Interview for DSM Diagnosis (SCID, First et al., 1996), participants received a full medical evaluation and underwent a drug washout period such that all participants were free of psychotropic medications for at least 1 week before study entry (longer if patients continue to experienced discontinuation symptoms). Before therapy and at the end of treatment, participants completed the Response Style Questionnaire (Nolen-Hoeksema et al., 1993). In addition, weekly Beck Depression Inventory (Beck et al., 1961) scores and weekly Hamilton Rating Scale for Depression (HRSD, Hamilton, 1960) scores were recorded to assess severity of depression over time. CT consisted of 16–20 individual sessions conducted using Beck’s (1979) treatment manual and Greenberger and Padesky’s (1995) patient workbook. A maximum of 14 weeks was permitted so that missed appointments could be rescheduled. Sessions 1–8 occurred twice weekly with 8 weekly sessions thereafter. However if a patient had not experienced at least a 40% reduction in HRSD scores by session 9, twice weekly sessions continued through the 8th week of treatment. Thus, patients who were responding quickly typically received 16 sessions of therapy, whereas those whose response was less rapid received 20 sessions. Therapists received weekly group supervision and individual consultation as needed by a senior CT therapist. All CT sessions were videotaped and tapes were selected at random for review in supervisory groups. The Cognitive Therapy Scale (Young & Beck, 1980) was completed by study supervisors and used to provide feedback to therapists. Patients also attended two psychoeducational sessions, one at intake and one between weeks 7 and 8 in order to provide factual information about relapse/recurrence in MDD, to review the study’s treatment “road map”, to verify continued consent, and to collect self-report questionnaires.
Measures
Beck Depression Inventory (BDI)
The BDI (Beck et al., 1961) is a widely-used 21-item measure of depressive and dysphoric symptoms. Respondents are asked to endorse items varying in severity from 0 to 3 in a number of life areas. For example, “I do not feel sad” scored 0; and “I am so sad or unhappy that I can’t stand it” scored 3. The highest rating for each item was summed across all 21 items to create a continuous measure of depressive symptoms. Based the BDI manual (Beck & Steer, 1987), the BDI was used to classify individuals into three severity categories, Minimal-mild (BDI ≤ 18), Moderate (BDI 19–29), and Severe (BDI ≥ 30). Based on this classification the sample was classified as 21% Mildly Depressed, 38% Moderately Depressed, and 41% Severely Depressed.
Time to initial remission
Time to initial remission was defined, conventionally, as the time to a BDI score < 10 met for 2 weeks in a row (Fava, 2006; Frank, Prien, Jarrett, Keller, & et al., 1991). In the calculation of time to initial remission a missing BDI rating was counted as not having met either criterion and patients who did not remit were given a score of 21.
Hamilton Rating Scale for Depression (HRSD)
The HRSD (Hamilton, 1960) is a 17-item scale in which some items are defined in terms of a series of categories of increasing intensity, while others are defined by a number of equal-valued terms. The variables are measured either on five-point or three-point scales. The measure was completed by a clinical evaluator. As the HRSD is less focused on cognitive aspects of depression than the BDI, the HRSD was used as a secondary measure of depression, primarily for sensitivity analyses regarding robustness of results obtained for the BDI.
Response Styles Questionnaire (RSQ)
The RSQ (Nolen-Hoeksema et al., 1993) is a 71-item scale that was designed to measure the way that an individual typically responds to feelings of depression or sad mood, for example, “analyze recent events to try to understand why you are so depressed.” Patients are asked to rate each item on a 4-point scale ranging from 0 (almost never) to 3 (almost always). The RSQ includes several subscales; the 22-item rumination subscale was used in the main analyses. While other researchers have chosen to administer only the 22-item rumination subscale (e.g., Kasch, Klein, & Lara, 2001), the entire 71-item RSQ was administered in the current study to persevere the validity and reliability of the original scale by not changing the context of the rumination questions. Previous studies have reported acceptable convergent and predictive validity for the measure (Butler & Nolen-Hoeksema, 1994; Nolen-Hoeksema & Morrow, 1991) The reliability of the measure has been shown to vary ranging from .36 to .80 due to both elapsed time between measurement and changes in mood (Bagby, Rector, Bacchiochi, & McBride, 2004). In addition, we calculated the 5-item brooding subscale and the 5-item reflection/pondering subscales to be using in sensitivity analyses. These two subscales attempt to create measures of rumination and reflection that are unconfounded with depressive content (Treynor, Gonzalez, & Nolen-Hoeksema, 2003).
Analyses
Analyses were conducted on all patients with complete data required for the specified analysis. Given the operationalization of time to initial remission, no patients were missing data on this dependant variable. However, 15 patients were missing BDI post-intervention ratings and 23 patients were missing post-rumination ratings, therefore analyses using these measures are reported without these subjects’ data. Analyses were also conducted on just the subset of patients who remitted in CT, since those who did not remit could have been depressed for reasons not addressed by CT (i.e., mechanisms specifically less well related to rumination, such as medication induced depressions). Effect Sizes (Cohen’s d) for within-group differences were calculated as: (M1 − M2)/(sqrt((SD12 + SD22)/2)) (Dunlap, Cortina, Vaslow, & Burke, 1996).
RESULTS
Treatment Response and Initial Severity
After 16–20 sessions of CT, 40 of 81 (49%) patients were considered to have achieved initial remission. Remission varied with initial depression severity status χ2 = 8.80, df = 2, p = 0.012; fewer severely depressed patients achieved remission after 20 sessions of CT (30.3%) compared to 70.6% of the mildly depressed patients, and 58.1% of the moderately depressed patients. On average, patients experienced a significant decrease in the severity of depressive symptoms from pretreatment, M(SD) = 26.5(9.8), to post-treatment, M(SD) = 10.2(10.1), paired t(65) = 12.96, p < .0001, d = 1.65. As expected, patients who remitted experienced a significant decrease in depressive symptoms from pretreatment, M(SD) = 23.6(7.8), to post-treatment, M(SD) = 4.1(5.2), paired t(36) = 13.93, p < .0001, d = 2.94. Non-remitters also experienced a significant decrease in symptom severity from pretreatment, M(SD) = 30.0(10.8) to post-treatment, M(SD) = 17.4(9.9), paired t(28) = 6.20, p < .0001, d = 1.22; 31.7% of the non-remitters experienced at least a 50% drop in symptom severity (i.e., responders who did not remit).
Regression Analyses Predicting Time to Initial Remission
On average, participants had a moderate level of initial severity M(SD)BDI = 27.1(9.6) and a moderate level of rumination, M(SD)RSQ = 33.1(9.3). Initial rumination was significantly correlated with initial severity r(81) = .47, p < .0001 and time to initial remission r(81) = .41, p < .0002. Initial severity was also correlated with time to initial remission r(81) = .35, p = .001.
Hierarchical regression analysis was used to examine the main effects of initial rumination and initial severity and their interaction on time to initial remission in CT. Prior to analyses, predictor variables were standardized to have M(SD) = 0(1). Thus, one standard deviation increase in the predictor was equivalent to a 1 session increase in time to initial remission. Both initial severity and initial rumination were entered into step 1 of a hierarchical regression followed by a severity x rumination interaction, entered into step 2. The severity x rumination interaction was significant, ΔR2 = .05, t(1) = −2.37, p = .021, suggesting that though time to initial remission increased with both initial depression severity, b = 1.28, SE = 0.63, p = .047, sr2 = .040, and initial rumination, b = 1.78, SE = 0.63, p = .006, sr2 = .076, having both high levels of rumination and initial severity lead to decreased time to initial remission, b = −1.19, SE = 0.50, p = .021, sr2 = .054, intercept = 17.40, SE = .60, p < .0001.
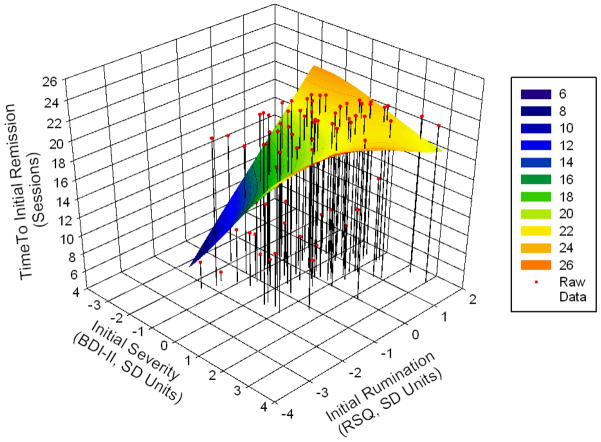
This interaction is shown in Figure 1, which depicts time to initial remission (vertical axis) as a function of initial severity and initial rumination. The presence of the saddle curve indicates that there is a significant initial severity x rumination interaction (in the absence of the interaction a rectangular plane within the three dimensional space would have provided the best fit). The shaded regions on the saddle curve indicate time to initial remission; quicker initial remission times are shaded in dark blue and green and slower initial remission times are shaded in shades of yellow and orange. As can be seen from Figure 1, patients approximately between a ZBDI score = −1.5 (BDI = 12.7, mildly depressed) and ZBDI score = 0 (BDI = 27.1, moderately depressed) on initial severity took longer to remit in treatment the more they reported ruminating. In addition, patients with initial severity ZBDI scores > .50 (BDI > 32, severely depressed) took longer to remit as the more they engaged in rumination up until approximately ZRSQ = .20 on initial rumination (RSQ = 34.9), reflecting main effects of initial severity and initial rumination. The observable negative slope of the saddle curve occurs because the parameter estimate for the initial severity x rumination is negative. As can be seen from the lower right corner of Figure 1, this negative slope indicates that patients with initial severity scores greater than approximately ZBDI = .50 (BDI > 32, severely depressed) were predicted to remit in fewer sessions if they engaged in high levels of rumination (ZRSQ > 1).
Figure 1.
Time to initial remission as a function of initial severity, initial rumination, and their interaction
The interaction was probed using regions of significance tests which indicate over what range of the moderator (severity or rumination) the effect of the focal predictor (severity or rumination) was significantly positive, nonsignificant, or significantly negative (Bauer & Curran, 2005), using an online calculator (Preacher, Curran, & Bauer, 2006). Using initial severity as the focal moderator, results indicated that higher levels of rumination were associated with a longer time to initial remission for individuals with initial BDI < 31 (b = 1.30, SE = .67, p = 0.055, intercept = 17.91, SE = .66, p < .0001). For example, patients whose initial BDI score was 22.3, experienced a 2.4 session increase in time to initial remission for every 1 SD increase in initial rumination (b = 2.37, SE = .68, p = .0008, intercept = 16.75, SE = .67, p < .0001). Patients’ whose initial BDI scores fell between 31 and the highest possible BDI scores experienced longer time to initial remission regardless of rumination (e.g., BDI = 36.7, b = 0.58, SE = .82, p = .477, intercept = 18.68, SE = .89, p < .0001, CI95% 16.9 – 20.44). The non-significant slope and significant intercept indicates that on average severely depressed patients took between 17 – 20 sessions to achieve initial remission. In Figure 1 this represents the orange plateau on the lower right. To determine if rumination was a protective factor for the severely depressed, as indicated in Figure 1, we probed the interaction further. Post hoc probing examining patients with the highest observed initial level of severity (BDI = 55.9) did not demonstrate a significant decrease in time to initial remission with increased rumination (b = −1.80, SE = 1.65, p = .278, intercept = 21.25, SE = 2.01, p < .0001).
When rumination was examined as the focal moderator, increasing initial severity was associated with longer time to initial remission for those who ruminated less (ZRSQ < .02; RSQ < 33.3, b = 1.26, SE = .63, p = 0.050, intercept = 17.44, SE = .60, p < .0001). For example, patients with ZRSQ = −.50, RSQ = 28.5, experienced a 1.9 session increase in time to initial remission for every 1 SD increase in initial severity (b = 1.88, SE = .70, p = .009, intercept = 16.51, SE = .68, p < .0001). Patients’ whose rumination scores fell between .02 SD and 6.5 SD above the mean, experienced longer time to initial remission regardless of initial severity (e.g., ZRSQ = 1, b = 0.09, SE = .78, p = .91, intercept = 19.18, SE = .87, p < .0001, CI95% 17.45 – 20.91). Higher levels of initial severity were predicted to be associated with shorter time to initial remission for individuals who ruminated more, (ZRSQ > 6.5, RSQ = 93.55, b = −6.47, SE = 1.97, p = .053, intercept = 28.96, SE = 3.30, p = .053), though in the current sample no patient scored within this range.
In summary, visual and statistical inspection of the interaction yielded similar conclusions. In patients who are mildly-moderately depressed both initial severity and rumination are associated with longer times to initial remission. Visual inspection indicated that for patients who are most severely depressed, those who ruminate more may recover at faster rates than those who ruminate less, however the levels of initial severity and rumination required to reach statistical significance are outside of the bounds of the current measures – indicating that the plausibility of this finding is tentative.
Regression Analyses Predicting Time to Initial Remission in Remitted Patients
Patients who achieved remission had mean initial severity M(SD)BDI = 24.(8.7) and mean initial rumination M(SD)RSQ = 30.9(9.6). Initial rumination was significantly correlated with initial severity r(40) = .64, p < .0001 and time to initial remission r(40) = .49, p = .001. Initial severity was not significantly correlated with time to initial remission r(40) = .26, p = .103.
As for the full sample, standardized initial depression severity and initial rumination were, together, associated with remission F(2,37) = 6.09, p = .005, R2 = .25, which was driven by an independent effect of initial rumination status b = 3.02, SE = 1.02, p = .005, sr2 = .18 but not initial severity b = −.50, SE = 1.02, p = .623, sr2 = .005; intercept = 12.60, SE = .77 p < .0001. Rumination and initial severity did not interact in this restricted sample, ΔR2 = .00, t(1) = 0.45, p = .653. Thus, among just remitters, patients who were high on rumination at the start of treatment took longer to remit regardless of their initial severity.
Rumination Predicting Treatment Outcome
A hierarchical logistic regression was used to examine the main effects of initial rumination and initial severity and their interaction on treatment outcome (i.e., remission vs. non-remission) in CT. Both initial severity and initial rumination were entered on the first step, followed by a severity x rumination interaction, entered on the second step. The severity x rumination interaction was significant, ΔR2 = .04, difference in the −2 Log Likelihoods between the main effects model and the model containing the interaction was 4.53 evaluated on χ2 distribution, df = 1, p = .033; suggesting that though the odds of initial remission decreased with both initial depression severity b = −.28, SE = 0.11, χ2 = 5.98, p = .015, odds (eb) = 0.758 and initial rumination b = −.19, SE = 0.10, p = .032, odds (eb) = 0.823, having high levels of both rumination and initial severity leads to increased odds of initial remission, b = 0.006, SE = 0.003, p = .043, odds (eb) = 1.006, intercept = 8.32, SE = 3.23, 2 = 6.63, p = .010, (Likelihood Ratio χ2 = 14.49, df = 3, p = .002, Gamma = .36, c = .679). These findings are consistent with the time to initial remission analyses; increasing levels of both rumination and initial severity are associated with decreased odds of initial remission; however being high on both rumination and initial severity increase the odds that a person will remit, and remit more quickly in CT.
Changes in Rumination
Paired t-tests comparing pretreatment rumination and post-treatment rumination scores for all available patients indicated that there was a significant decrease in rumination after 16–20 sessions of CT, pretreatment rumination M(SD) = 33.6(9.2), post-treatment rumination M(SD) = 26.2(10.1), t(57) = 5.74, p < .0001, d = .76. To determine whether change in rumination held after controlling for change in depression we conducted a linear mixed model using restricted/residual maximum likelihood estimation with a compound symmetry covariance structure after evaluating other structures based on the AIC and BIC criterion. Pairwise comparisons were conducted on least-squared means. In the analysis, rumination was the repeated factor (time) and change in depressive symptoms (i.e., Pretreatment BDI – Post-treatment BDI) was entered as a covariate. Simultaneous fixed effects indicated that after controlling for change in depression severity t(54) = −0.62, p = .537, change in rumination remained significant, pretreatment M(SE) = 33.6(1.30), post-treatment M(SE) = 26.5(1.30), t(55) = 5.40, p < .0001, d = .73.
Changes in rumination were calculated separately for patients who initially remitted, partial responders who did not remit, and those who remained symptomatic. Patients who achieved initial remission after 16–20 sessions of CT experienced a significant decrease in rumination, pretreatment rumination M(SD) = 31.9(9.5), post-treatment rumination M(SD) = 24.8(10.6), t(31) = 4.03, p = .0003, d = .71. After controlling for change in depression severity t(29) = 0.89, p = .382, change in rumination remained significant, pretreatment M(SE) = 31.5(1.83), post-treatment M(SE) = 24.8(1.83), t(30) = 3.79, p < .001, d = .66. Similarly, patients who were partial responders at the end of treatment (i.e., those who experienced at least a 50% drop in symptom severity, but did not remit) also experienced a significant decrease in rumination, pretreatment rumination M(SD) = 36.3(9.5), post-treatment rumination, M(SD) = 26.4(9.5), t(12) = 3.43, p = 0.005, d = 1.04. After controlling for change in depression severity t(11) = 0.22, p = .832, change in rumination remained significant pretreatment M (SE) = 36.3(2.71), post-treatment M(SE) = 26.4(2.71), t(12) = 11.79, p = .005, d = 1.01. However, non-responders did not experience a significant decrease in rumination, pretreatment rumination M(SD) = 35.9(7.6), post-treatment rumination, M(SD) = 30.9(8.3), t(11) = 1.88, p = 0.087, d = .63. After controlling for change in depression severity t(10) = −2.53, p = .030 change in rumination was not significant, pretreatment M (SE) = 35.9(2.03), post-treatment M(SE) = 30.9(2.03), t(11) = 1.88, p = .087, d = .71. In addition, there were no significant differences in the magnitude of the change in rumination among those who achieved initial remission, partial responders, and non-responders F(2,54) = .81, p > .1 – results were identical after controlling for change in depression severity F(3,52) = .88, p > .1.
Higher levels of initial severity were associated with greater decreases in rumination over the course of CT, r(58) = −.29, p = .03. Changes in rumination (i.e., Pretreatment RSQ – Post-treatment RSQ) were not associated with changes depression severity (i.e., Pretreatment BDI – Post-treatment BDI) r(56) = .18 or time to initial remission r(58) = −.11, ps > .1. In addition, changes in depression severity were also uncorrelated with time to initial remission r(66) = .16, p > .1. In those patients that remitted, higher levels of initial severity did not significantly correlate with change in rumination r(32) = −.20, p = .24. Changes in rumination were not associated with changes in depression severity r(32) = .05 or time to initial remission r(32)= −.17, ps > .1. In addition, changes in depression severity were also uncorrelated with time to initial remission r(37) = .19, p > .1
Among those who were severely depressed at intake, change in rumination was not significantly correlated with change in depression r(26) = .05,or time to initial remission r(26)= −.09 ps > .1. Similarly, among the severely depressed, there were no significant differences in the magnitude of the change in rumination among those who achieved initial remission, partial responders, and non-responders F(2,23) = .73, p > .1 – results were identical after controlling for change in depression severity F(3,52) = .88, p > .1.
Sensitivity Analyses
Time to initial remission defined by HRSD scores
Sensitivity analyses were conducted using the HRSD (Hamilton, 1960) to define time to initial remission. Time to initial remission was defined, conventionally, as the time until a HRSD score < 7 met for 2 weeks in a row. In this analysis, initial HRSD scores and initial rumination, were together, associated with remission F(2,78) = 12.06, p < .0001, R2 = .24. Significant main effects were detected for initial rumination status β = .23, SE = 0.49, p = .03, sr2 = .05 and for initial HRSD β = .38, SE = 0.13, p = .0004, sr2 = .14, though the HRSD x rumination interaction was not significant. These analyses indicate that patients who were high on rumination or high on initial severity as measured by the HRSD took longer to remit in CT.
Treatment Outcome defined by HRSD scores
Hierarchical logistic regression analyses on outcome status revealed a significant main effect for initial HRSD scores b = −.22, SE = 0.07, χ2 = 9.08, p = .0026, odds (eb) = 0.805 and non significant effect for initial rumination b = −.04, SE = 0.03, p = .120, odds (eb) = 0.957, intercept = 5.99, SE = 1.74, χ2 = 11.83, p = .0006, (Likelihood Ratio χ2 = 16.07, df = 2, p = .0003, Gamma = .51, c = .75), but no significant HRSD x rumination interaction. These results indicate that higher levels of initial severity were associated with significantly lower odds of remission and that initial rumination did not significantly contributed to the decrease in odds of remission.
Brooding & Reflection
All major study analyses were re-analyzed using the brooding and reflection/pondering subscales. The pattern and significance for all time to initial remission analyses were replicated using the brooding subscale – there was a small decrease in magnitude of the calculated effect sizes (see Table 1). However, the pattern and significance for brooding and treatment outcome were not replicated, only initial severity was a significant predictor of treatment outcome. The pattern and significance of all major findings were not replicated using the reflection subscale, which was unrelated to almost all relevant outcomes. However, in both the total sample and those who achieved initial remission a greater increase in reflection after treatment was associated with a greater reduction in depressive symptoms r(56) = −.26, p = .054, r(56) = −.40, p = .026.
Table 1.
Effect Sizes Decomposed by Type of Rumination Scale and Missing Data Strategy Used
| Type of Analysis | Effect Size
|
||||||
|---|---|---|---|---|---|---|---|
|
|
Complete Case Analysis
|
MI (20)
|
|||||
| Type | n | RSQ-Rum | Brooding | Reflection | n | RSQ-Rum | |
| Full Sample | |||||||
| r(Rumination, Initial Severity) | r2 | 81 | .221 * | .238 * | .043 | 119 | .198* |
| r(Rumination, Time to Initial Remission) | r2 | 81 | .168 * | .173 * | .005 | 119 | .104* |
| r(Depression, Time to Initial Remission) | r2 | 81 | .122 * | .122 * | .122 * | 119 | .120* |
|
| |||||||
| Hierarchical Regression: Dependent Variable = Time to Initial Remission | |||||||
| Rumination | sr2 | 81 | .076 * | .076 * | .000 | 119 | .034* |
| Initial Severity | sr2 | 81 | .040 * | .041 * | .127 * | 119 | .062* |
| Initial Severity x Rumination | sr2 | 81 | .054 * | .042 * | .018 | 119 | .028† |
|
| |||||||
| Δ Rumination | d | 58 | .760 * | .611 * | .031 | 119 | .600* |
| r(Δ Rumination, Initial Severity) | r2 | 58 | .084 * | .125 * | .011 | 119 | .088* |
| r(Δ Rumination, Δ Depression) | r2 | 56 | .032 | .059 | .067 * | 119 | .064 |
| r(Δ Rumination, Time to Initial Remission) | r2 | 58 | .026 | .037 | .032 | 119 | .001 |
|
| |||||||
| Remitters | |||||||
| r(Rumination, Initial Severity) | r2 | 40 | .410 * | .298 * | .065 | 70 | .245* |
| r(Rumination, Time to Initial Remission) | r2 | 40 | .240 * | .195 * | .021 | 70 | .134* |
| r(Depression, Time to Initial Remission) | r2 | 40 | .068 | .068 | .068 | 70 | .084* |
|
| |||||||
| Hierarchical Regression: Dependent Variable = Time to Initial Remission | |||||||
| Rumination | sr2 | 40 | .180 * | .127 * | .007 | 70 | .064* |
| Initial Severity | sr2 | 40 | .005 | .001 | .054 | 70 | .019 |
| Initial Severity x Rumination | sr2 | 40 | xx | xx | xx | 70 | xx |
|
| |||||||
| Δ Rumination | d | 32 | .710 * | .584 * | −.202 | 70 | .670* |
| r(Δ Rumination, Initial Severity) | r2 | 32 | .040 | .017 | .048 | 70 | .091 |
| r(Δ Rumination, Δ Depression) | r2 | 32 | .003 | .000 | .161 * | 70 | .054 |
| r(Δ Rumination, Time to Initial Remission) | r2 | 32 | .029 | .122 * | .002 | 70 | .021 |
|
| |||||||
| Partial Responders | |||||||
| Δ Rumination | d | 13 | 1.04 * | .940 * | .193 | 17 | .760* |
|
| |||||||
| Non-Responders | |||||||
| Δ Rumination | d | 12 | .630 | .397 | .563 | 34 | .341* |
|
| |||||||
| Severely Depressed | |||||||
| r(Δ Rumination, Δ Depression) | r2 | 26 | .003 | .041 | .092 | 54 | .045 |
| r(Δ Rumination, Time to Initial Remission) | r2 | 26 | .008 | .036 | .066 | 54 | .006 |
Note:
p = .07,
p < .05.
Δ Rumination = (Pretreatment Rumination) – (Post-treatment Rumination). Δ Depression = Pretreatment BDI) – (Post-treatment BDI). MI = Multiple imputation. Total number of imputations = 20. RSQ-Rum = Response Styles Questionnaire – 22-item rumination subscale.
Missing Data
The data presented use a complete case analysis strategy, to test the stability of our estimates of error we re-analyzed the data using multiple imputation to deal with missing data (Schafer & Graham, 2002). Data was imputed 20 times for 119/120 participants using SAS procedure PROC MI (Yuan, 2000), Markov Chain Monte Carlo (MCMC) method –imputed data sets were analyzed using SAS procedure PROC MIANALYZE. The pattern and significance for almost all time to initial remission analyses were replicated (see Table 1). However, the pattern and significance for rumination and treatment outcome analyses were not replicated – only initial severity was a significant predictor of treatment outcome.
Gender
Gender did not moderate any observed results, and results did not differ by gender. Thus, while rumination may differentially predispose men and women to become depressed, once clinically depressed, men and women may ruminate similarly, and thus, display similar patterns of remission.
DISCUSSION
Our goal was to investigate the influence of rumination on the time course of initial remission in 16–20 sessions of CT. Indeed, patients who were mildly-moderately depressed took longer to remit in treatment the more they endorsed a tendency to engage in a ruminative coping style. Patients who were severely depressed took longer to achieve initial remission (i.e., approximately 17–20 sessions) regardless of the tendency to engage in ruminative coping. There was tentative statistical and descriptive evidence that at extreme thresholds of depressive severity, increasing levels of rumination may be associated with recovery at faster rates. Similarly, patients who are severely depressed and engaged in high levels of ruminative coping had greater odds of remitting in CT. To our knowledge this is the first investigation that has examined and demonstrated the joint role of rumination and initial severity on the time course of remission and treatment outcome in CT. The interaction of rumination and severity on time to initial remission was not observed when just remitters were examined. Among those who remitted in CT, greater levels of rumination were associated with a longer time to achieve initial remission, regardless of initial severity.
These findings predominately support a capitalization model of depression where patients with the greatest deficits – both in terms of initial severity and rumination – need the longest courses (and, possibly the highest doses) of CT to achieve remission. These findings are consistent with existing research which indicates that rumination interacts with high levels of negative cognition (negative inferential styles and dysfunctional attitudes) to predict greater onset, number, and longer duration of major depressive and hopelessness depressive episodes when compared to individuals who do not ruminate (Robinson & Alloy, 2003). It is plausible that more time is needed for ruminators to adopt skills relevant to remission in CT given the context in which learning occurs. Patients are typically asked to recall emotionally triggering events and are then asked to identify, evaluate, and challenge distorted cognitions that occurred during the situation. Individuals who engage in high levels of ruminative coping may be more likely to process these situations in an immersed manner, promoting reliving of the experience and significantly enhancing the associated maladaptive cognitions and intensifying negative affect, making it more difficult to acquire new learning (Ciesla & Roberts, 2007; Kross, Ayduk, & Mischel, 2005). Thus it may take more time for these patients to learn to step back and focus on their experience without reactivating excessive negative affect and cognitive biases that interfere with the skills of CT.
Yet, tentative evidence suggested that patients who were the most severely depressed benefited most quickly and had a greater chance of remitting in CT if they did ruminate. This finding suggests a more complex interpretation for these patients consistent with a compensation model. That is, more severely depressed patients who did not ruminate appeared to obtain less benefit from CT than those who did ruminate, perhaps reflecting a different psychopathophysiologic process not readily addressed in psychotherapy (Thase, Buysse, Frank, Cherry, & et al., 1997; Thase, Dube, Bowler, Howland, & et al., 1996; Thase, Simons, & Reynolds, 1996). A similar explanation was also used by Simons and colleagues (1995) to explain their finding that patients who experienced a negative life event prior to treatment (which may be conceptualized as a proxy for greater depression severity) and endorsed low levels of dysfunctional attitudes (i.e., maladaptive cognitive processing similar to rumination) remitted at slower rates when compared to patients who experienced a negative life event and who endorsed high levels of dysfunctional attitudes. Thus, severely depressed patients who engage in maladaptive cognitive processing (e.g., rumination or dysfunctional attitudes) may have specific affective styles that are addressed in CT and thereby are more likely to achieve remission at faster rates than patients who do not posses these cognitive vulnerabilities.
However, as stated previously, the evidence supporting the compensation model in the severely depressed is tentative. Future research designed to specifically tease apart the validity of the compensation model among severely depressed patients is required to adequately address this question. Until such research is conducted it appears that a capitalization model provides the best fit to the data – higher initial levels of rumination and severity make it harder to achieve initial remission in CT. Our ability to conclude that rumination and initial severity effect the time course of initial remission in CT is bolstered by the results from sensitivity analyses. These results indicated that the brooding but not reflection subscale provided an almost identical pattern of findings as those previously described. Similarly, the pattern of findings were also consistent with a capitalization model when missing data was imputed twenty times rather than using complete case analysis.
At the end of 16–20 sessions of treatment, patients engaged in significantly less ruminative coping, in both those who remitted and those who were partial responders; however, non-responders did not significantly decrease their use of ruminative coping. This reduction in the use of this cognitive processing style may reflect that patients in CT learned to increase executive control in the face of automatic emotional stimuli. Therefore the evidence provides modest support for one of the proposed mechanisms of change in CT. In addition, patients who started out at higher levels of severity experienced the greatest decrease in rumination over the course of treatment. However, consistent with other research findings changes in rumination were not associated with differential reduction of depressive symptoms after therapy (Arnow et al., 2004; Bagby et al., 1999; Rector et al., 1998; Schmaling et al., 2002) and changes in rumination remained significant after controlling for change in depressive symptoms. Changes in rumination were also not associated with depression remission in severely depressed patients. If this were in fact true it would have provided additional support for the compensation model. As such, these findings suggest that while rumination changes over time in CT, it is not necessarily as a function of the magnitude of symptom change. Rather, changes in rumination and symptoms may be dissociable – future research is required to answer this question.
The above pattern of findings in conjunction with findings demonstrating that the observed decrease in a ruminative coping style is not unique to CT has lead researchers to question if rumination is simply a concomitant factor in depression. Specifically, Bagby and colleagues (2004) demonstrate that patients treated with antidepressant medication also experienced a significant decrease in rumination. Similarly, Schmaling and colleagues (2002) reported significant changes in ruminative coping after patients received either 11 weeks of Problem Solving Therapy – Primary Care, or antidepressant medication.
The current findings call this conclusion into question. If rumination were simply a concomitant factor we would not have observed that rumination interacts with initial severity to predict time to initial remission in treatment or treatment outcome. If rumination was just proxy for severity it should not have had additive predictive utility in the complete sample. In addition, evidence suggests that one of the unique benefits of CT is that patients have lower relapse rates than when they are treated with anti-depressant medication when medication is discontinued (Gloaguen, Cottraux, Cucherat, & Blackburn, 1998). This question of whether or not a mechanism of depression was addressed would warrant examining whether or not changes in rumination (proxy for mechanism) is a predictor of relapse.
The current investigation has several limitations. First, rumination was not repeatedly assessed during the course of treatment. Repeated assessment over the course of the study would allow for a true decomposition of the way that rumination and depressed mood change during the course of treatment. Second, given that in the current study dosages of CT were limited to a maximum of 20 sessions, it is not truly possible to test how many additional sessions it would have required for non-remitters to fully respond to treatment. Third, the joint protective effect of being severely depressed and also engaging in rumination did not replicate when clinician rated response to treatment was used as our measure of depression severity. This potentially occurred because the BDI focuses more on cognitive aspects of depression – whereas the HRSD does not have the same focus. Thus, it is plausible that CT helps the patients who ruminate and have clearly cognitive depressions more quickly because these are the aspects of depression it addresses. However, having severity in the absence of a strong cognitive component is not protective. Fourth, the current investigation did not have a comparison group (e.g., medication management) that would allow us to demonstrate clearly that changing elaborative processing of negative information is one of the mechanisms via which CT works. While it is possible that change in rumination occurred simply due to regression to the mean it is not probable. Multiple investigations of large samples have demonstrated that ruminative response styles are stable over 5 – 12 month periods in the absence of mood change with test-retest reliabilities ranging from .62 to .80 (Nolen-Hoeksema, 2000; Nolen-Hoeksema, Parker, & Larson, 1994).
These limitations notwithstanding, our findings suggest that by considering both the level of initial severity and initial tendency to engage in ruminative coping style clinicians can identify who will require more than the traditional 15–16 weeks of CT. Specifically individuals who are have higher levels of severity and who also engage in rumination will require more sessions to remit in treatment. In addition, the findings begin to help clarify which types of severely depressed patients may remit more effectively in CT, i.e., severely depressed patients who also engage in maladaptive cognitive processing are good candidates for CT, other patients may require alternate forms of treatment such as behavioral activation – future investigations are warranted. These findings are consistent with existing research that that has demonstrated that depressive rumination is predictive of maintenance or exacerbation of depressive symptoms (Nolen-Hoeksema & Morrow, 1991; Nolen-Hoeksema et al., 1993). Future studies should focus on examining relapse rates to help understand the role of rumination in remission from depression.
Acknowledgments
We thank the University of Pittsburgh Mood Disorders Treatment and Research Program for help in recruitment and administration of rumination and severity measures. We also thank the reviewers for their thoughtful comments which greatly improved this article.
This research was supported by MH16804, MH58356, MH58397, MH69618, MH064159, MH074807
Footnotes
Patients who did not complete the full protocol were compared in terms of baseline demographic variables, initial rumination, initial severity scores (Beck Depression Inventory; (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) total number of episodes, and duration of current episode. No significant differences were found for gender, initial severity, pre-treatment rumination, total number of episodes, and duration of current episode. Patients who complete the full protocol were significantly older (M = 44.7, SD = 11.8), than did the 32 patients who did not complete the full protocol (M = 36.3, SD = 11.6), t(118) = −3.46, p < .0008.
References
- Alloy LB, Abramson LY, Metalsky GI, Hartlage S. The hopelessness theory of depression: attributional aspects. British Journal of Clinical Psychology. 1988;27(Pt 1):5–21. doi: 10.1111/j.2044-8260.1988.tb00749.x. [DOI] [PubMed] [Google Scholar]
- Arnow BA, Spangler D, Klein DN, Burns DD. Rumination and Distraction Among Chronic Depressives in Treatment: A Structural Equation Analysis. Cognitive Therapy and Research. 2004;28(1):67–83. [Google Scholar]
- Bagby R, Rector NA, Bacchiochi JR, McBride C. The Stability of the Response Styles Questionnaire Rumination Scale in a Sample of Patients With Major Depression. Cognitive Therapy and Research. 2004;28(4):527–538. [Google Scholar]
- Bagby R, Rector NA, Segal ZV, Joffe RT, Levitt AJ, Kennedy SH, et al. Rumination and distraction in major depression: Assessing response to pharmacological treatment. Journal of Affective Disorders. 1999;55(2–3):225–229. doi: 10.1016/s0165-0327(99)00015-4. [DOI] [PubMed] [Google Scholar]
- Barber JP, DeRubeis RJ. On second thought: Where the action is in cognitive therapy for depression. Cognitive Therapy and Research. 1989;13(5):441–457. [Google Scholar]
- Bauer DJ, Curran PJ. Probing interactions in fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behavioral Research. 2005;40(3):373–400. doi: 10.1207/s15327906mbr4003_5. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- Beck AT, Steer RA. Beck Depression Inventory Manual. San Antonio, TX: The Psychological Corporation: Harcourt Brace Javanovich; 1987. [Google Scholar]
- Beck AT, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Mercier MA, Agosti V, Leite P, Donovan S, et al. Outcome of cognitive-behavioral therapy for depression: relation to hemispheric dominance for verbal processing. J Abnorm Psychol. 1997;106(1):138–144. doi: 10.1037//0021-843x.106.1.138. [DOI] [PubMed] [Google Scholar]
- Butler LD, Nolen-Hoeksema S. Gender differences in responses to depressed mood in a college sample. Sex Roles. 1994;30(5–6):331–346. [Google Scholar]
- Ciesla JA, Roberts JE. Rumination, negative cognition, and their interactive effects on depressed mood. Emotion. 2007;7(3):555–565. doi: 10.1037/1528-3542.7.3.555. [DOI] [PubMed] [Google Scholar]
- Coffman SJ, Martell CR, Dimidjian S, Gallop R, Hollon SD. Extreme nonresponse in cognitive therapy: Can behavioral activation succeed where cognitive therapy fails? Journal of Consulting and Clinical Psychology. 2007;75(4):531–541. doi: 10.1037/0022-006X.75.4.531. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, et al. Randomized Trial of Behavioral Activation, Cognitive Therapy, and Antidepressant Medication in the Acute Treatment of Adults With Major Depression. Journal of Consulting and Clinical Psychology. 2006;74(4):658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Dunlap WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychological Methods. 1996;1(2):170–177. [Google Scholar]
- Fava M. Pharmacological approaches to the treatment of residual symptoms. J Psychopharmacol. 2006;20(3_suppl):29–34. doi: 10.1177/1359786806064325. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM IV Axis I Disorders Patient Edition. Vol. 20. New York: Biometrics Research Department New York State Psychiatric Institute; 1996. [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: Remission, recovery, relapse, and recurrence. Archives of General Psychiatry. 1991;48(9):851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Gloaguen V, Cottraux J, Cucherat M, Blackburn IM. A meta-analysis of the effects of cognitive therapy in depressed patients. Journal of Affective Disorders. 1998;49(1):59–72. doi: 10.1016/s0165-0327(97)00199-7. [DOI] [PubMed] [Google Scholar]
- Greenberger D, Padesky CA. Mind Over Mood. New York: Guilford; 1995. [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon SD, Thase ME, Markowitz JC. Treatment and prevention of depression. Psychological Science in the Public Interest. 2002;3(2):39–77. doi: 10.1111/1529-1006.00008. [DOI] [PubMed] [Google Scholar]
- Ingram RE. Toward an information processing analysis of depression. Cognitive Therapy and Research. 1984;8:443–478. [Google Scholar]
- Just N, Alloy LB. The response styles theory of depression Tests and an extension of the theory. Journal of Abnormal Psychology. 1997;106:221–229. doi: 10.1037//0021-843x.106.2.221. [DOI] [PubMed] [Google Scholar]
- Kasch KL, Klein DN, Lara ME. A Construct Validation Study of the Response Styles Questionnaire Rumination Scale in Participants With a Recent-Onset Major Depressive Episode. Psychological Assessment. 2001;13(3):375–383. doi: 10.1037//1040-3590.13.3.375. [DOI] [PubMed] [Google Scholar]
- Kross E, Ayduk O, Mischel W. When Asking “Why” Does Not Hurt: Distinguishing Rumination From Reflective Processing of Negative Emotions. Psychological Science. 2005;16(9):709–715. doi: 10.1111/j.1467-9280.2005.01600.x. [DOI] [PubMed] [Google Scholar]
- Lavallee LF, Campbell JD. Impact of personal goals on self-regulation processes elicited by daily negative events. Journal of Personality and Social Psychology. 1995;69(2):341–352. [Google Scholar]
- Lyubomirsky S, Caldwell ND, Nolen-Hoeksema S. Effects of ruminative and distracting responses to depressed mood on retrieval of autobiographical memories. Journal of Personality & Social Psychology. 1998;75(1):166–177. doi: 10.1037//0022-3514.75.1.166. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Nolen-Hoeksema S. Self-perpetuating properties of dysphoric rumination. Journal of Personality & Social Psychology. 1993;65(2):339–349. doi: 10.1037//0022-3514.65.2.339. [DOI] [PubMed] [Google Scholar]
- Martin LL, Tesser A. Toward a motivational and structural theory of ruminative thought. In: Uleman JS, Bargh JA, editors. Unintended thought. New York, NY: Guilford Press; 1989. pp. 306–326. [Google Scholar]
- Martin LL, Tesser A. Some ruminative thoughts. In: Wyer RS Jr, editor. Ruminative thoughts. Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc; 1996. pp. 1–47. [Google Scholar]
- Morrow J, Nolen-Hoeksema S. Effects of responses to depression on the remediation of depressive affect. Journal of Personality & Social Psychology. 1990;58(3):519–527. doi: 10.1037//0022-3514.58.3.519. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109(3):504–511. [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology. 1991;61(1):115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the duration of episodes of depressed mood. Journal of Abnormal Psychology. 1993;102(1):20–28. doi: 10.1037//0021-843x.102.1.20. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Parker LE, Larson J. Ruminative coping with depressed mood following loss. Journal of Personality and Social Psychology. 1994;67(1):92–104. doi: 10.1037//0022-3514.67.1.92. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE. Rethinking Rumination. Perspectives on Psychological Science. doi: 10.1111/j.1745-6924.2008.00088.x. (In Press) [DOI] [PubMed] [Google Scholar]
- Papageorgiou C, Wells A. An Empirical Test of a Clinical Metacognitive Model of Rumination and Depression. Cognitive Therapy and Research. 2003;27(3):261–273. [Google Scholar]
- Philippot P, Rime B. Social and cognitive processing in emotion A heuristic for psychopathology. In: Flack WF, Laird JD, editors. Emotions in Psychopathology. New York: Oxford University Press; 1998. pp. 114–130. [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- Rector NA, Segal ZV, Crawford C. Ruminative and distractive coping responses in major depression: Assessing response to cognitive behavior therapy. Paper presented at the Presentation at the meeting of the Association for the Advancement of Behavior Therapy.1998. [Google Scholar]
- Robinson MS, Alloy LB. Negative Cognitive Styles and Stress-Reactive Rumination Interact to Predict Depression: A Prospective Study. Cognitive Therapy and Research. 2003;27(3):275–292. [Google Scholar]
- Schafer JL, Graham JW. Missing Data: Our View of the State of the Art. Psychological Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- Schmaling KB, Dimidjian S, Katon W, Sullivan M. Response styles among patients with minor depression and dysthymia in primary care. Journal of Abnormal Psychology. 2002;111(2):350–356. doi: 10.1037//0021-843x.111.2.350. [DOI] [PubMed] [Google Scholar]
- Sheppard LC, Teasdale JD. How Does Dysfunctional Thinking Decrease During Recovery From Major Depression? Journal of Abnormal Psychology. 2004;113(1):64–71. doi: 10.1037/0021-843X.113.1.64. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Ingram RE. Modeling individual differences in negative information processing biases. In: Matthews G, editor. Cognitive Science Perspectives on Personality and Emotion. New York NY: Elsevier; 1997. pp. 301–353. [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003;27(3):365–382. [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Simons AD, Gordon JS, Monroe SM, Thase ME. Toward an integration of psychologic, social, and biologic factors in depression: Effects on outcome and course of cognitive therapy. Journal of Consulting and Clinical Psychology. 1995;63(3):369–377. doi: 10.1037//0022-006x.63.3.369. [DOI] [PubMed] [Google Scholar]
- Teasdale JD. Cognitive vulnerability to persistent depression. Cognition & Emotion. 1988;2(3):247–274. [Google Scholar]
- Teasdale JD, Scott J, Moore RG, Hayhurst H, Pope M, Paykel ES. How does cognitive therapy prevent relapse in residual depression? Evidence from a controlled trial. Journal of Consulting and Clinical Psychology. 2001;69(3):347–357. doi: 10.1037//0022-006x.69.3.347. [DOI] [PubMed] [Google Scholar]
- Thase ME, Buysse DJ, Frank E, Cherry CR, et al. Which depressed patients will respond to interpersonal psychotherapy? The role of abnormal EEG sleep profiles. American Journal of Psychiatry. 1997;154(4):502–509. doi: 10.1176/ajp.154.4.502. [DOI] [PubMed] [Google Scholar]
- Thase ME, Dube S, Bowler K, Howland RH, et al. Hypothalamic-pituitary-adrenocortical activity and response to cognitive behavior therapy in unmedicated, hospitalized depressed patients. American Journal of Psychiatry. 1996;153(7):886–891. doi: 10.1176/ajp.153.7.886. [DOI] [PubMed] [Google Scholar]
- Thase ME, Simons AD, Reynolds CF., III Abnormal electroencephalographic sleep profiles in major depression: Association with response to cognitive behavior therapy. Archives of General Psychiatry. 1996;53(2):99–108. doi: 10.1001/archpsyc.1996.01830020013003. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination Reconsidered: A Psychometric Analysis. Cognitive Therapy and Research. 2003;27(3):247–259. [Google Scholar]
- Wells A, Matthews G. Attention and emotion: A clinical perspective. London, England: Erlbaum; 1994. [Google Scholar]
- Young J, Beck AT. Cognitive Therapy Scale: Rating Manual. Philadelphia: Center for Cognitive Therapy; 1980. [Google Scholar]
- Yuan YC. Multiple imputation for missing data: Concepts and new development. Paper presented at the Twenty-Fifth Annual SAS Users Group International Conference (Paper No. 267); Cary, NC: SAS Institute; 2000. [Google Scholar]