Abstract
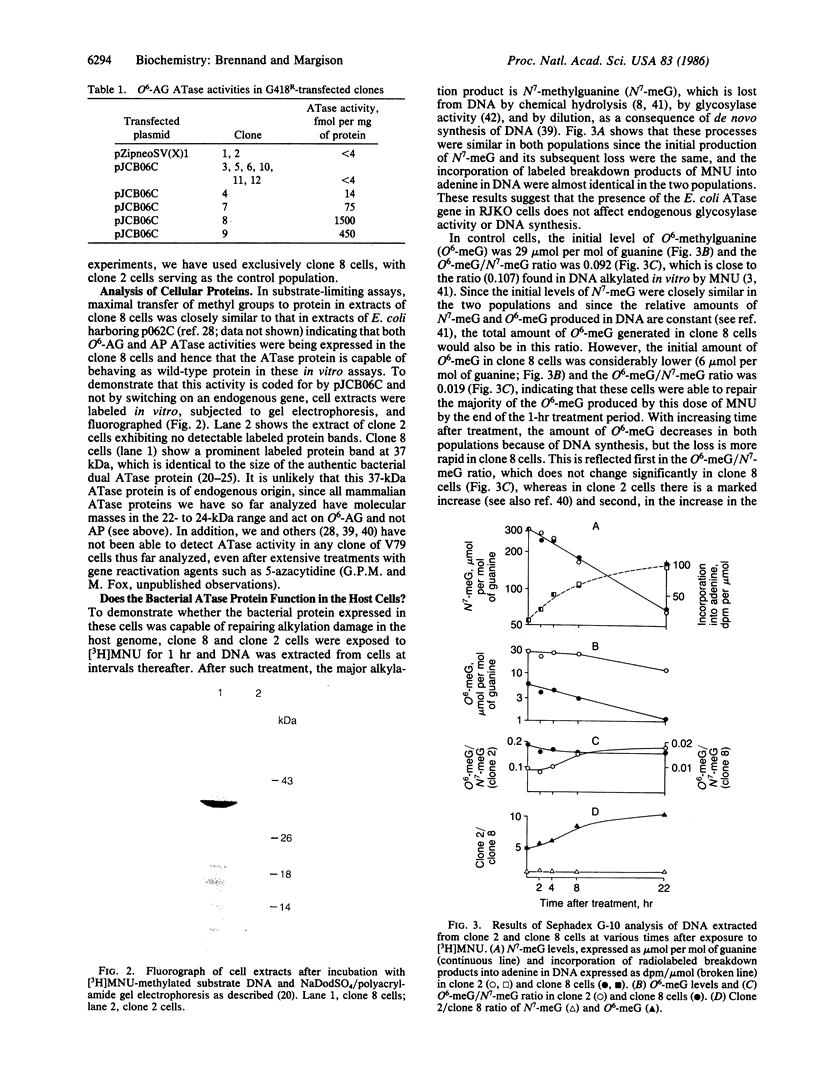
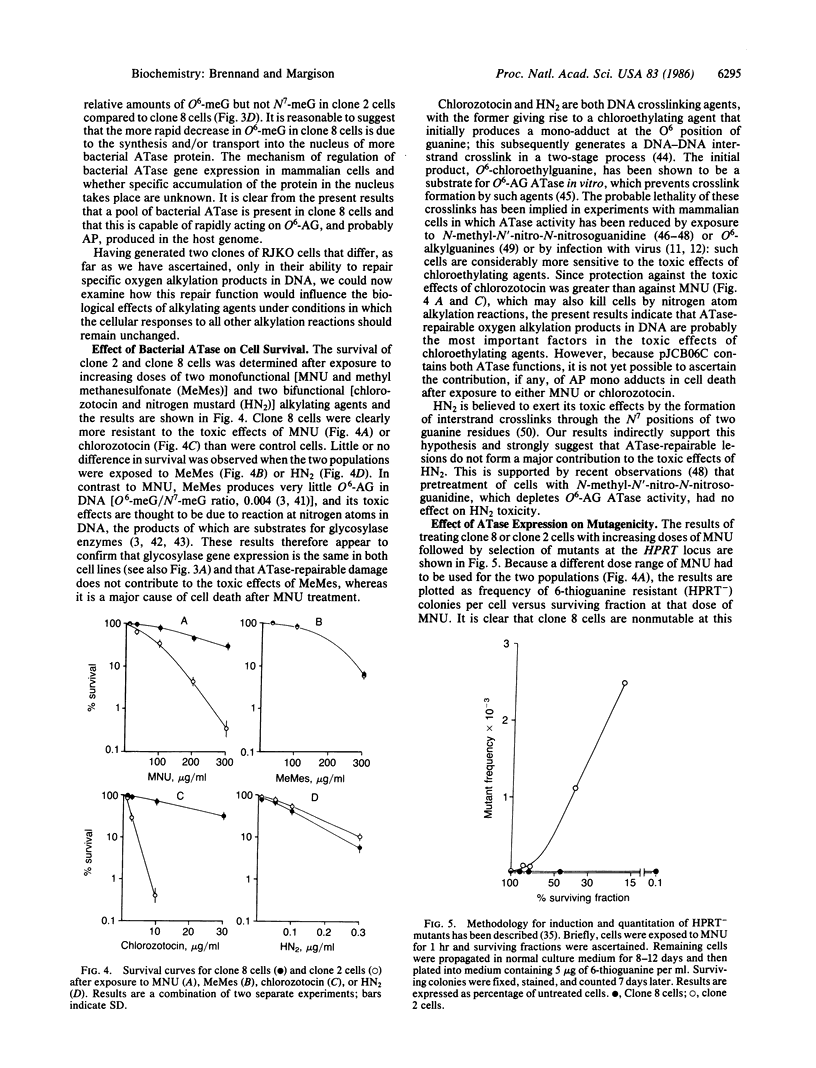
The toxic, mutagenic, and carcinogenic effects of alkylating agents have been attributed to their ability to damage DNA. Reaction at the O6 position of guanine results in miscoding during DNA replication, has been shown to be mutagenic in both bacteriophage and bacteria, and may be responsible for malignant transformation. In common with many other prokaryotes and eukaryotes the Escherichia coli B strain contains a protein that repairs O6-alkylation damage in DNA by transferring the alkyl group to one of its own cysteine residues. We have recently cloned the E. coli O6-alkylguanine alkyltransferase gene and shown it to encode a 37-kDa protein containing an additional activity that removes alkyl groups from alkylphosphotriesters in DNA. To examine the biological effects of this gene in mammalian cells, we have now inserted the coding sequence into a retrovirus-based selectable expression vector and transfected it into Chinese hamster V79 cells that lack endogenous alkyltransferase activity. A clone expressing high levels of the bacterial protein was selected and shown to produce a 37-kDa alkyltransferase protein and to rapidly repair O6-methylguanine produced in the host genome following exposure to N-methyl-N-nitrosourea. In comparison with a control population, this clone is considerably more resistant to the toxic and mutagenic effects of alkylating agents that react extensively with oxygen atoms in DNA. The usefulness of these clones in examining the role of DNA alkylation and other biological effects of alkylating agents is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott P. J., Saffhill R. DNA synthesis with methylated poly(dC-dG) templates. Evidence for a competitive nature to miscoding by O(6)-methylguanine. Biochim Biophys Acta. 1979 Mar 28;562(1):51–61. doi: 10.1016/0005-2787(79)90125-4. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Montesano R. Relevance of nitrosamines to human cancer. Carcinogenesis. 1984 Nov;5(11):1381–1393. doi: 10.1093/carcin/5.11.1381. [DOI] [PubMed] [Google Scholar]
- Beranek D. T., Heflich R. H., Kodell R. L., Morris S. M., Casciano D. A. Correlation between specific DNA-methylation products and mutation induction at the HGPRT locus in Chinese hamster ovary cells. Mutat Res. 1983 Jun-Jul;110(1):171–180. doi: 10.1016/0027-5107(83)90026-x. [DOI] [PubMed] [Google Scholar]
- Brennand J., Margison G. P. Expression of the E. coli O6-methylguanine-methylphosphotriester methyltransferase gene in mammalian cells. Carcinogenesis. 1986 Jan;7(1):185–188. doi: 10.1093/carcin/7.1.185. [DOI] [PubMed] [Google Scholar]
- Brennand J., McMillan S., Fox M. Effects of metabolic inhibitors on cel lethality and mutation induction in Chinese hamster cells. II. The effect of posttreatment with non-toxic concentrations of thymidine. Chem Biol Interact. 1981 Jul;36(1):89–106. doi: 10.1016/0009-2797(81)90031-4. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H., Scudiero D. A., Meyer S. A., Lubiniecki A. S., Girardi A. J., Galloway S. M., Bynum G. D. Defective repair of alkylated DNA by human tumour and SV40-transformed human cell strains. Nature. 1980 Dec 25;288(5792):724–727. doi: 10.1038/288724a0. [DOI] [PubMed] [Google Scholar]
- Demple B., Sedgwick B., Robins P., Totty N., Waterfield M. D., Lindahl T. Active site and complete sequence of the suicidal methyltransferase that counters alkylation mutagenesis. Proc Natl Acad Sci U S A. 1985 May;82(9):2688–2692. doi: 10.1073/pnas.82.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M. E., Corsico C. D., Pegg A. E. Exposure of HeLa cells to 0(6)-alkylguanines increases sensitivity to the cytotoxic effects of alkylating agents. Biochem Biophys Res Commun. 1985 Oct 15;132(1):178–185. doi: 10.1016/0006-291x(85)91004-6. [DOI] [PubMed] [Google Scholar]
- Doll R., Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981 Jun;66(6):1191–1308. [PubMed] [Google Scholar]
- Durrant L. G., Margison G. P., Boyle J. M. Pretreatment of Chinese hamster v79 cells with MNU increases survival without affecting DNA repair or mutagenicity. Carcinogenesis. 1981;2(1):55–60. doi: 10.1093/carcin/2.1.55. [DOI] [PubMed] [Google Scholar]
- Evensen G., Seeberg E. Adaptation to alkylation resistance involves the induction of a DNA glycosylase. Nature. 1982 Apr 22;296(5859):773–775. doi: 10.1038/296773a0. [DOI] [PubMed] [Google Scholar]
- Fox M., Brennand J. Evidence for the involvement of lesions other than O6-alkylguanine in mammalian cell mutagenesis. Carcinogenesis. 1980 Sep;1(9):795–799. doi: 10.1093/carcin/1.9.795. [DOI] [PubMed] [Google Scholar]
- Fox M., Scott D. The genetic toxicology of nitrogen and sulphur mustard. Mutat Res. 1980 Mar;75(2):131–168. doi: 10.1016/0165-1110(80)90012-3. [DOI] [PubMed] [Google Scholar]
- Gibson N. W., Zlotogorski C., Erickson L. C. Specific DNA repair mechanisms may protect some human tumor cells from DNA interstrand crosslinking by chloroethylnitrosoureas but not from crosslinking by other anti-tumor alkylating agents. Carcinogenesis. 1985 Mar;6(3):445–450. doi: 10.1093/carcin/6.3.445. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Roufa D. J., Beaudet A. L., Caskey C. T. 8-Azaguanine resistance in mammalian cells. I. Hypoxanthine-guanine phosphoribosyltransferase. Genetics. 1972 Oct;72(2):239–252. doi: 10.1093/genetics/72.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heflich R. H., Beranek D. T., Kodell R. L., Morris S. M. Induction of mutations and sister-chromatid exchanges in Chinese hamster ovary cells by ethylating agents. Mutat Res. 1982 Nov;106(1):147–161. doi: 10.1016/0027-5107(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Kohn K. W. Interstrand cross-linking of DNA by 1,3-bis(2-chloroethyl)-1-nitrosourea and other 1-(2-haloethyl)-1-nitrosoureas. Cancer Res. 1977 May;37(5):1450–1454. [PubMed] [Google Scholar]
- Lawley P. D., Orr D. J. Specific excision of methylation products from DNA of Escherichia coli treated with N-methyl-N'-nitro-N-nitrosoguanidine. Chem Biol Interact. 1970 Aug;2(2):154–157. doi: 10.1016/0009-2797(70)90047-5. [DOI] [PubMed] [Google Scholar]
- Lemotte P. K., Walker G. C. Induction and autoregulation of ada, a positively acting element regulating the response of Escherichia coli K-12 to methylating agents. J Bacteriol. 1985 Mar;161(3):888–895. doi: 10.1128/jb.161.3.888-895.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Margison G. P., Cooper D. P., Brennand J. Cloning of the E. coli O6-methylguanine and methylphosphotriester methyltransferase gene using a functional DNA repair assay. Nucleic Acids Res. 1985 Mar 25;13(6):1939–1952. doi: 10.1093/nar/13.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margison G. P., Margison J. M., Montesano R. Methylated purines in the deoxyribonucleic acid of various Syrian-golden-hamster tissues after administration of a hepatocarcinogenic dose of dimethylnitrosamine. Biochem J. 1976 Sep 1;157(3):627–634. doi: 10.1042/bj1570627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margison G. P., Pegg A. E. Enzymatic release of 7-methylguanine from methylated DNA by rodent liver extracts. Proc Natl Acad Sci U S A. 1981 Feb;78(2):861–865. doi: 10.1073/pnas.78.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy T. V., Karran P., Lindahl T. Inducible repair of O-alkylated DNA pyrimidines in Escherichia coli. EMBO J. 1984 Mar;3(3):545–550. doi: 10.1002/j.1460-2075.1984.tb01844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Factors governing the expression of a bacterial gene in mammalian cells. Mol Cell Biol. 1981 May;1(5):449–459. doi: 10.1128/mcb.1.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y., Kondo H., Kawabata S., Iwanaga S., Sekiguchi M. Purification and structure of the intact Ada regulatory protein of Escherichia coli K12, O6-methylguanine-DNA methyltransferase. J Biol Chem. 1985 Jun 25;260(12):7281–7288. [PubMed] [Google Scholar]
- Newbold R. F., Warren W., Medcalf A. S., Amos J. Mutagenicity of carcinogenic methylating agents is associated with a specific DNA modification. Nature. 1980 Feb 7;283(5747):596–599. doi: 10.1038/283596a0. [DOI] [PubMed] [Google Scholar]
- Olsson M., Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O6-methylguanine to a protein cysteine residue. J Biol Chem. 1980 Nov 25;255(22):10569–10571. [PubMed] [Google Scholar]
- Pegg A. E. Formation and metabolism of alkylated nucleosides: possible role in carcinogenesis by nitroso compounds and alkylating agents. Adv Cancer Res. 1977;25:195–269. doi: 10.1016/s0065-230x(08)60635-1. [DOI] [PubMed] [Google Scholar]
- Robins P., Cairns J. Quantitation of the adaptive response to alkylating agents. Nature. 1979 Jul 5;280(5717):74–76. doi: 10.1038/280074a0. [DOI] [PubMed] [Google Scholar]
- Robins P., Harris A. L., Goldsmith I., Lindahl T. Cross-linking of DNA induced by chloroethylnitrosourea is presented by O6-methylguanine-DNA methyltransferase. Nucleic Acids Res. 1983 Nov 25;11(22):7743–7758. doi: 10.1093/nar/11.22.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffhill R., Margison G. P., O'Connor P. J. Mechanisms of carcinogenesis induced by alkylating agents. Biochim Biophys Acta. 1985 Dec 17;823(2):111–145. doi: 10.1016/0304-419x(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Sedgwick B. Molecular cloning of a gene which regulates the adaptive response to alkylating agents in Escherichia coli. Mol Gen Genet. 1983;191(3):466–472. doi: 10.1007/BF00425764. [DOI] [PubMed] [Google Scholar]
- Shiloh Y., Becker Y. Kinetics of O6-methylguanine repair in human normal and ataxia telangiectasia cell lines and correlation of repair capacity with cellular sensitivity to methylating agents. Cancer Res. 1981 Dec;41(12 Pt 1):5114–5120. [PubMed] [Google Scholar]
- Sklar R., Strauss B. Removal of O6-methylguanine from DNA of normal and xeroderma pigmentosum-derived lymphoblastoid lines. Nature. 1981 Jan 29;289(5796):417–420. doi: 10.1038/289417a0. [DOI] [PubMed] [Google Scholar]
- Sukumar S., Notario V., Martin-Zanca D., Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983 Dec 15;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- Teo I., Sedgwick B., Demple B., Li B., Lindahl T. Induction of resistance to alkylating agents in E. coli: the ada+ gene product serves both as a regulatory protein and as an enzyme for repair of mutagenic damage. EMBO J. 1984 Sep;3(9):2151–2157. doi: 10.1002/j.1460-2075.1984.tb02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren W., Crathorn A. R., Shooter K. V. The stability of methylated purines and of methylphosphotriesters in the DNA of V79 cells after treatment with N-methyl-N-nitrosourea. Biochim Biophys Acta. 1979 Jun 20;563(1):82–88. doi: 10.1016/0005-2787(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]
- Zlotogorski C., Erickson L. C. Pretreatment of human colon tumor cells with DNA methylating agents inhibits their ability to repair chloroethyl monoadducts. Carcinogenesis. 1984 Jan;5(1):83–87. doi: 10.1093/carcin/5.1.83. [DOI] [PubMed] [Google Scholar]
- Zlotogorski C., Erickson L. C. Pretreatment of normal human fibroblasts and human colon carcinoma cells with MNNG allows chloroethylnitrosourea to produce DNA interstrand crosslinks not observed in cells treated with chloroethylnitrosourea alone. Carcinogenesis. 1983;4(6):759–763. doi: 10.1093/carcin/4.6.759. [DOI] [PubMed] [Google Scholar]




