Abstract
Aberrant expression of c‐Ski oncoprotein in some tumor cells has been shown to be associated with cancer development. However, the role of c‐Ski in cancer‐associated fibroblasts (CAFs) of tumor microenvironment has not been characterized. In the current study, we found that c‐Ski is highly expressed in CAFs derived from breast carcinoma microenvironment and this CAF‐associated c‐Ski expression is associated with invasion and metastasis of human breast tumors. We showed that c‐Ski overexpression in immortalized breast normal fibroblasts (NFs) induces conversion to breast CAFs by repressing p53 and thereby upregulating SDF‐1 in NFs. SDF‐1 treatment or p53 knockdown in NFs had similar effects on the activation of NFs as c‐Ski overexpression. The c‐Ski‐activated CAFs show increased proliferation, migration, invasion and contraction compared with NFs. Furthermore, c‐Ski‐activated CAFs facilitated the migration and invasion of MDA‐MB‐231 breast cancer cells. Our data suggest that c‐Ski is an important regulator in the activation of CAFs and may serve as a potential therapeutic target to block breast cancer progression.
Keywords: CAFs, c-Ski, P53, SDF-1, Tumor microenvironment
Highlights
We demonstrate that c‐Ski can promote trans‐differentiation of NFs to CAFs.
c‐Ski‐activated CAFs can accelerate the invasion of breast tumor cells.
c‐Ski‐induced activation of CAFs may be associated with p53 and SDF‐1.
c‐Ski represses p53 in fibroblasts is closely related to the activation of CAFs.
1. Introduction
Cancer‐associated fibroblasts (CAFs), also called myofibroblasts, are a major component of the tumor microenvironment and contribute to growth, invasion, angiogenesis and metastasis of cancer (Bhowmick et al., 2004; Kalluri and Zeisberg, 2006; Lu et al., 2009). Previous studies have shown that CAFs highly express α‐smooth muscle actin (α‐SMA), fibroblast activation protein (FAP) and platelet‐derived growth factor receptor (PDGFR) β compared with normal fibroblasts (NFs), which have been established as a hallmark of CAFs (Liu et al., 2012; Östman and Augsten, 2009). It is widely accepted that CAFs affect cancer cells mainly through paracrine signaling and mechanical stress.
The c‐Ski oncogene was first identified as homolog of the v‐Ski gene isolated from the avian Sloan–Kettering retroviruses, and its expression induced oncogenic transformation of chicken and quail embryo fibroblasts (Colmenares et al., 1991; Li et al., 1986). c‐Ski is a transcriptional regulator inhibiting the transcription of its target genes through directly binding to co‐repressors or transcription factors (Nomura et al., 1999; Wu et al., 2002). It has been shown that c‐Ski can promote cancer progression and is highly expressed in some of solid cancers including leukemia, melanoma, colorectal cancer, gastric cancer and pancreatic cancer (Boone et al., 2009; Bravou et al., 2009; Heider et al., 2007; Ritter et al., 2006; Takahata et al., 2009). Interestingly, previous studies have also indicated that c‐Ski can act as a tumor suppressor in some cases (Marcelain et al., 2012; Nomura et al., 2004; Shinagawa et al., 2001).
Normal fibroblasts can be activated and become myofibroblasts in the wound healing process, which involve cytokines and chemokines secretion, extracellular matrix (ECM) remodeling, inflammation, angiogenesis and cell migration (Grinnell, 1994). Tumors have been recognized as wounds that do not heal, and the activation of CAFs in the tumor microenvironment is similar to that of myofibroblasts in granulation tissues except CAFs escaping apoptosis (Franco et al., 2010). Recent studies have found that c‐Ski can promote the proliferation of myofibroblasts in wound healing (Li et al., 2012, 2011). Our previous study using cDNA microarrays found that c‐Ski was highly expressed in CAFs isolated from breast tumor tissue (Peng et al., 2013). However, the role of c‐Ski in the activation of CAFs remains unknown.
In this study, we demonstrate that c‐Ski can promote conversion of NFs into CAFs, and can promote the invasion of breast tumor cells via cross‐talk between CAFs and cancer cells. Our data show that c‐Ski is highly expressed in the fibroblasts of invasive breast cancer compared with the fibroblasts of normal tissue. Overexpression of c‐Ski in NFs leads to enhanced invasion and up‐regulation of the hallmarks of CAFs, α‐SMA and FAP. Furthermore, the molecular actions of c‐Ski in the activation of CAFs from NFs are characterized. This effect of c‐Ski in CAFs is mediated by p53‐regulated SDF‐1 in CAFs. Our study sheds light on the role of c‐Ski as a critical regulator in CAF function and as a potential therapeutic target for breast cancer.
2. Materials and methods
2.1. Tissue samples
Human breast tumor tissues were obtained from patients with breast tumors resected at the first affiliated hospital of Chongqing Medical University. None of the patients had previously undergone radiotherapy or chemotherapy treatment. Both tumor tissues and its corresponding normal breast tissues (at least 5 cm far away from the tumor) were harvested within 30 min after resection and were kept in DMEM with 10% FBS and penicillin‐streptomycin on ice for immediate transportation to the laboratory. Hematoxylin and eosin (H&E)‐stained frozen sections of each tissue sample were prepared to confirm its benignity or malignancy and to obtain information about its histological subtype and histopathological grade.
2.2. Plasmid constructs and siRNA
The plasmid containing human telomerase reverse transcriptase (pBABE‐hygro‐hTERT) was purchased from Addgene (Cambridge, MA). The plasmid containing c‐Ski (pcDNA3.0‐Ski) was kindly provided by Dr. Yuan‐Guo Zhou (Third Military Medical University, Chongqing, China). pBABE‐puro‐c‐Ski was constructed by cloning c‐Ski fragment into pBABE‐puro vector using PCR‐based approach. pCMV‐p53 was constructed by cloning human p53 cDNA into pCMV‐HA (Clontech, Palo Alto, CA, USA). For the c‐Ski shRNA plasmid, synthetic DNA inserts for expressing of the specific c‐Ski shRNA were cloned into pLVX‐shRNA1 (Clontech). The p53 siRNA was transiently transferred into NFs. The target sequence for c‐Ski shRNAs is GTACTCGGCCCAGATCGAA. The target sequences for p53 siRNA are 5′‐AAGAAATTTGCGTTTGGAGTA‐3′ (A), and 5′‐AAGGAAGACTCCAGTGGTAAT‐3′ (B). Control siRNA sequence, which do not match any known human cDNAs, is 5′‐AAGGTGTCAGAAACTGACGAT‐3′.
2.3. Fibroblasts isolation/culture and immortalization
Fibroblasts were isolated as described previously (Zhao et al., 2012). Briefly, Tissues were washed with PBS containing 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma, St. Louis, MO, USA), then minced into small pieces and digested for 8 h at 37 °C in DMEM containing 10% FBS and 0.5 mg/ml collagenase (Sigma). After centrifugation and washing with PBS, cell pellets were re‐suspended in DMEM with 10% FBS and transferred into 100‐mm tissue‐culture dishes. CAFs and NFs were routinely maintained in DMEM containing 10% FBS at 37 °C in humidified atmosphere containing 5% CO2.
Fibroblasts were immortalized by hTERT. The retroviral vector pBABE‐hygro‐hTERT or pBABE‐hygro was transfected into the PT67 cell line using Lipofectamine (Invitrogen, Carlsbad, CA, USA). Retroviruses were harvested at 48 h after transfection and were used to infect fibroblasts in the presence of 4 μg/mL polybrene (Sigma). Expression of α‐SMA, FAP and FN was confirmed by immunofluorescence (Figure S1).
2.4. MTT and flow cytometric analysis
Cell growth was determined by 3‐[4,5‐dimethylthiazol‐2yl]‐2,5‐diphenyl tetrazoliumbromide (MTT) assay. The cells were plated at 5 × 103 cells in 200 μl of DMED per well in 96‐well plates. After incubation for the specified time at 37 °C, MTT (5 mg/ml in PBS) was added to each well and incubated for 4 h. After treating with 0.1 ml of isopropanol with 0.04 N of HCl and generally shaken, the absorbance was recorded on an ultraviolet spectrophotometric reader at a wave‐length of 492 nm. The experiments were repeated five times.
The cell cycle S‐phase of fibroblasts was analyzed by flow cytometry. Briefly, cells were cultured in DMEM with 10% FBS (85% confluent), trypsinized, washed twice with cold PBS, fixed by 75% ethanol, incubated with RNAase and stained by propidium iodide. Tritiated thymidine uptake of CAFs, NFs were determined using a Cytomics FC device (Beckman Coulter) as previously described (Liu et al., 2010). The experiments were done in triplicate.
2.5. RNA isolation and quantitative real‐time PCR (qRT‐PCR)
Total RNA was isolated from CAFs and NFs using Trizol (Invitrogen) according to the manufacturer's protocol. RNA quantity was determined by agarose gel electrophoresis and by spectrophotometry. Quantitative real‐time PCR was performed using SYBR Premix Ex Taq™ (Takara Bio, Japan) according to the instruction of the manufacturer. The used primers were listed in the supplemental table (Table S1). All experiments were performed at least three times.
2.6. Immunohistochemistry (IHC) staining and immunofluorescence
Tumor and normal tissues were fixed with 10% buffered formalin. Paraffin‐embedded specimens were then sectioned at 4 μm thickness and stained with H&E staining according to standard histopathological techniques. After treating with 3% hydrogen peroxide in methanol for 10 min to exhaust endogenous peroxidase activity, the sections were incubated with primary antibody [rabbit anti‐c‐Ski polyclonal antibody (Santa cruz, CA) (1/50)] for overnight at 4 °C and 1 h at room temperature. Then, the sections were sequentially incubated with polymer helper solution (ZSBiO, Beijing, China) for 20 min, polyperoxidase‐anti‐mouse/rabbit IgG (ZSBiO) for 30 min at 37 °C, and stained with diaminobenizidine (DAB).
The pathologic scoring of tumor cells and stromal fibroblasts were performed independently by 3 of pathologists as described previously (Murata et al., 2011). c‐Ski staining intensities (I) were scored as: 0, 1+, 2+, 3+. The percentage of the stained area (A) was scored as: 1 (0%–25%), 2 (26%–50%), 3 (51%–75%), and 4 (76%–100%). The sum of the intensity and percentage scores (I + A) was used as the final IHC score, which defined as follows: 1, negative; 2–3, weak; 4–5, moderate; and 6–7, strong.
For immunofluorescence, CAFs and NFs were grown on pre‐prepared coverslips for 24 h and then fixed within 4% paraformaldehyde for 20 min at room temperature. After washing with PBS for three times, cells were treated by 0.1% triton‐100 for 15 min at room temperature and incubated with 5% goat serum solution for 30 min at 37 °C. Cells were separately stained with antibodies specifically against c‐Ski (1:70; Santa cruz), α‐SMA (rabbit polyclonal antibody, 1:150; Abcam, Cambridge, MA, USA), FAP (rabbit polyclonal antibody, 1:150; Abcam) and fibronectin (rabbit polyclonal antibody FN, 1:150; Abcam) for overnight at 4 °C. After washing with PBS, cells were then incubated with a FITC‐labeled goat anti‐rabbit secondary antibody (1:200, Sigma) for 1 h at 37 °C in a humidified incubator. Sections were mounted in aqueous medium containing DAPI as a nuclear counterstain (Vector LABS). A negative control was used to ensure the specificity of fluorescent immunostaining by replacing the primary antibody with a normal rabbit IgG.
2.7. Western blot analysis
Western blot analysis was performed as described previously (Liu et al., 2010). Briefly, cell lysates were harvested in cell lysis buffer (Boster, China), dissolved in 9% SDS‐PAGE buffer, and subjected to western blotting. The antibodies used were against c‐Ski (1:500, Santa cruz), α‐SMA (1:1000, Abcam), FAP (1:1000, Abcam), p53 (1:500, Boster) and β‐Actin (1:500, Boster), which were incubated for overnight at 4 °C. The appropriate horseradish peroxidase (HRP)‐conjugated secondary antibodies were subsequently applied and immunodetection was conducted by using the enhanced chemiluminescence system (Amersham Pharmacia Biotech).
2.8. Preparation of conditioned medium
CAFs and NFs were cultured for 48 h to obtain approximate 90% confluence. The growth medium was changed to serum‐free or 0.5% FBS DMEM, and cells were continued further for 30–36 h. For neutralization experiments, a neutralizing antibody against human SDF‐1 (500 ng/ml, Abcam) was preincubated at 37 °C with supernatant for 1 h before performing migration assays. The medium was collected and centrifuged at 1200× g for 15 min, and the supernatant was collected as conditioned medium (CM) for further study.
2.9. Wound healing and invasion assay
Cells were seeded in 6‐well plates. After the medium was changed to serum‐free DMEM, the confluent cell monolayers were wounded by manually drawing a gap with a plastic pipette tip. The ability of cells to migrate into the cleared section was monitored by phase contrast microscopy at the specific time point, and the Image J software was employed to measure the wounded area at those time points. The percentage of the non‐healed scratched area (S) for each replicate at specific time point was calculated as follows: % of the non‐healed scratched area = [S (designed time)/S (starting time)]*100%. All the experiments were done in triplicate.
Cell invasion assay was measured via the transwell chamber invasion assay as described previously (Liu et al., 2009). Briefly, 2 × 104 CAFs or NFs cells in 200 μl growth medium were seeded in the wells of 8 μm‐pore Boyden chambers (Millipore, Darmstadt, Germany) coated with ECM (1:7.5) (Sigma). Cells were allowed to invade toward CM in the lower chamber. After 12 h of incubation, cells adhered to the upper surface of the filter were removed using a cotton applicator. Stained with hematoxylin in methanol, the invaded cells on the opposite side of the filter were counted. The data represent three experiments, each in triplicate (mean ± standard error).
2.10. Fibroblast contraction assay
Fibroblast contraction assay was performed according to the published method (Yang et al., 2012). Fibroblasts were trypsinized and mixed with 1.5 mg/ml rat tail collagen I (Becton Dickinson, Franklin Lakes, USA) diluted in DMEM. The mixture of collagen and cells (500 μl) was dispensed into single wells of a 24‐well plate and incubated at 37 °C for 30 min to allow collagen polymerization, followed by the addition of DMEM containing 10% FBS (500 μl). The collagen gels were then freed from attachment to the wells using a pipette tip. The collagen gels containing cells were incubated at 37 °C, and diameters of the collagen gels were measured. Alternatively, gels were kept attached to the culture dish bottom for 2 d and then released and the diameters were measured at 30 min and 60 min after the release.
2.11. Statistical analysis
Statistical analysis was done using SPSS standard version 13.0 software. The data were shown as means ± SD for at least three independent determinations. For comparisons between multiple groups, ANOVA followed by the Student–Newman–Keuls multiple comparisons test was used, and for single comparisons between two groups, the Student t test was used. Values of P < 0.05 were considered significant.
3. Results
3.1. c‐Ski is up‐regulated in tumor stromal fibroblasts and correlates with poor prognosis in breast cancer
To determine the biological characteristics of CAFs and NFs in breast cancer, cDNA microarray analysis was performed using the primary cultures of NFs and CAFs isolated from tumors. It was found that c‐Ski was highly expressed in CAFs compared with NFs (Peng et al., 2013; Zhao et al., 2012). To further characterize the expression of c‐Ski in stromal fibroblasts, c‐Ski levels were examined by IHC staining in breast tumor tissue and its paired normal tissue derived from breast cancer patients. As shown in Figure 1A, the stroma was weakly immunostained in normal breast tissues. In cases with DCIS, moderate immunostaining was observed in the stroma. However, in invasive and especially in metastatic tumor tissues, c‐Ski was strongly immunostained in the stromal region.
Figure 1.
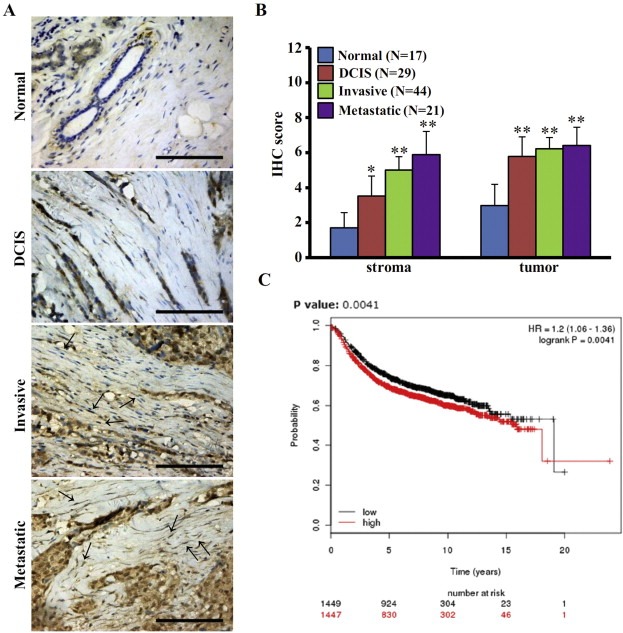
Expression of c‐Ski in breast tumor fibroblasts. (A) Expression of c‐Ski in breast specimens was examined by IHC staining. Less or no staining of c‐Ski was found in normal breast. Moderate levels of c‐Ski were found in the fibroblasts of ducal carcinoma in situ (DCIS). The highest expression of c‐Ski was in the fibroblasts surrounding invasive and metastatic breast cancer (arrows). Scale bars, 200 μm. (B) The expression of c‐Ski in breast tumor cells and stroma were quantitatively displayed by pathology scoring. The data represent means ± s.d. from difference staining slides. (*P < 0.05, **P < 0.01, ANOVA followed by Student–Newman–Keuls test). (C) The data from Kaplan–Meier survival analysis shows the association of poor prognosis with c‐Ski expression.
To evaluate the relationships between stromal c‐Ski immunostaining and clinical features, the cases with positive staining in the stroma and epithelium were scored (Figure 1B). Faint immunostaining was recorded in normal breast stroma, whereas moderate to strong stromal c‐Ski immunostaining was observed in DCIS, invasive and metastatic tumor. In addition, c‐Ski was highly expressed in tumor cells in contrast to the epithelium of normal tissues. These data indicate that c‐Ski oncoprotein is increased in the stromal tissues during breast carcinogenesis, suggesting that it is involved in the development and progression of breast carcinoma.
To further characterize the association of c‐Ski with the prognosis of breast cancer, we performed Kaplan–Meier survival analysis using the follow‐up data on 2977 breast cancer patients in the KM plots database (www.kmplot.com) (Figure 1C). The KM plotter is a database that can be used to assess the effect of gene expression on survival in breast cancer and ovarian cancer patients (Györffy et al., 2010). The log‐rank test displayed that increased c‐Ski expression in breast caner correlates with poor prognosis.
3.2. c‐Ski promotes activation of NFs into CAFs in breast cancer
We then determined whether c‐Ski can regulate the activation of CAFs in human breast cancer. The immortalized NFs and CAFs were used (Figure S1). As shown in Figure 2A, c‐Ski was up‐regulated more than 3 times in CAFs (3.1 ± 0.6) compared with NFs (1.04 ± 0.02), and mainly located in the nucleus (Figure 2B). In consistence with this, the CAFs biomarker α‐SMA was also about 3 times in CAFs than that in NFs. This suggests that c‐Ski may participate in the activation of CAFs. To consolidate the study, c‐Ski was transfected into NFs. Interestingly, two major hallmarks of CAFs, α‐SMA and FAP, were up‐regulated in the NFs overexpressing c‐Ski (NFs/c‐Ski) cells in contrast to its control vector cells (at lest more than 2.0 times, respectively), as detected by western blotting (Figure 2C). Consistent with this, FAP was also found to be up‐regulated in NFs/c‐Ski by immunocytochemistry (Figure 2D). These data suggest that increased c‐Ski expression in fibroblasts leads to trans‐differentiation of NFs to CAFs through enhancing the fibrogenic activity of fibroblasts.
Figure 2.
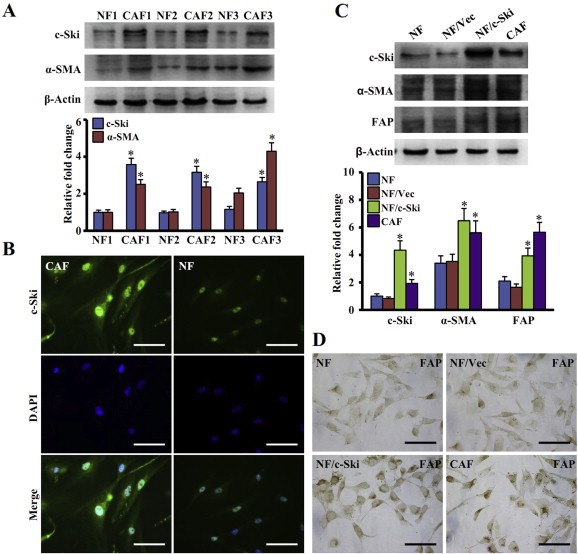
Increase of c‐Ski promotes activation of breast NFs into CAFs. (A) Levels of c‐Ski and α‐SMA were determined by Western blot analysis. Higher levels of c‐Ski and α‐SMA were found in CAFs than in NFs. The data represent means ± s.d. from three different experiments. (*P < 0.01, Student t test). (B) c‐Ski expression in CAFs and NFs detected by immunofluorescence. Green: c‐Ski; Blue: DAPI. Scale bars, 200 μm. (C) Western blot analysis of c‐Ski, α‐SMA and FAP in parental NFs, parental CAFs, NFs with c‐Ski overexpression and corresponding control cells (Upper panel). β‐Actin is loading control. The relative fold change of c‐Ski, α‐SMA and FAP in cells is shown in the lower panel. (*P < 0.01, ANOVA followed by Student–Newman–Keuls test). (D) Levels of FAP in fibroblasts were determined by immunohistochemistry staining. Scale bars, 200 μm.
3.3. c‐Ski‐overexpressing fibroblasts play a stimulatory role in proliferation, migration, invasion and contractile activity
We next asked whether c‐Ski could affect the proliferation, migration and invasion abilities of fibroblasts. The proliferation of parental NFs and NFs/c‐Ski cells were analyzed by MTT assays. Obviously, high levels of c‐Ski were associated with a significant increase of cell numbers versus its control cells (Figure 3A). Consistent with this result, more NFs/c‐Ski cells (38%) were in the S‐phase of cell cycle than parental NFs (26%), as detected by flow cytometry (Figure 3B). Furthermore, transwell assays showed that c‐Ski increased the migration and invasion of NFs. As shown in Figure 3C, there were more than 2.5 times of invasive NFs/c‐Ski cells invaded through the chamber than its control cells (NFs/Vec) or parental NFs. Wound healing assays indicated that the NFs/c‐Ski almost completely healed the wounded area after 48 h, whereas only about half of the scratched area were healed by control cells, NFs/Vec or NFs (Figure 3D). Previous data have shown that CAFs have an enhanced capacity of proliferation, migration and invasion compared with NFs (Micke, 2004). Thus, these results demonstrate that c‐Ski‐overexpressing NFs have similar biological characteristics as CAFs.
Figure 3.
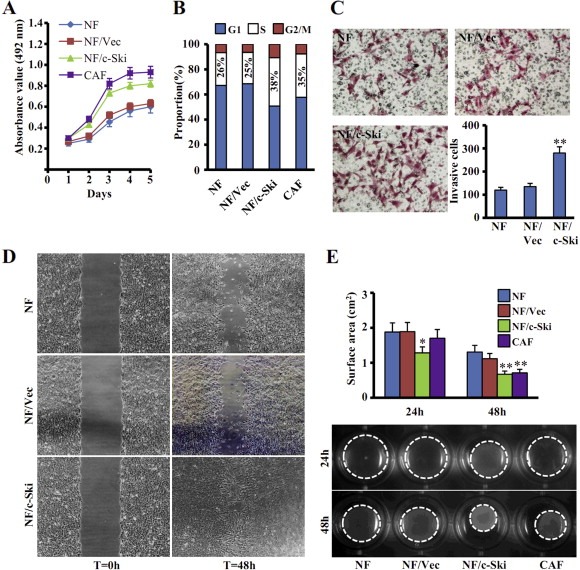
c‐Ski overexpression enhances the proliferation, migration, invasion and contractile activities of fibroblasts. (A, B) Cellular proliferation assays were conducted by using either (A) MTT assay or (B) flow cytometric assay for NFs, NFs stably transfected with c‐Ski (NF/c‐Ski) or control vector (NF/Vec), and parental CAFs. The data represent means ± s.d. from three different experiments. (C, D) Cell invasion or migration were tested by Transwell Chamber (C) or wound healing assay (D) for parental NFs, parental CAFs, NF/c‐Ski and its control cells (NF/Vec). (**P < 0.01, Student t test.) (E) The contractile activity of each fibroblast was checked by contraction assays in collagen gel. Representative images of collagen gels are shown. (*P < 0.05, **P < 0.01, ANOVA followed by Student–Newman–Keuls test.)
Because enhanced contractile activity is an important pathological feature of CAFs (Orimo and Weinberg, 2006), we examined whether high levels of c‐Ski expression could enhance the cell contractility of NFs and cause mechanical stress similar as that of CAFs. As shown in Figure 3E, fibroblast contraction assays showed that the area of collagen gel with NFs/c‐Ski was smaller than that containing NFs/Vec or NFs, but almost similar with CAFs. Thus, high levels of c‐Ski indeed promote the contractile activity of NFs.
3.4. c‐Ski‐activated NFs promote the migration and invasion of breast cancer cells
Previous studies have suggested that CAFs can promote cancer cell growth and survival through paracrine signaling (Anderberg et al., 2009). We wondered whether c‐Ski‐activated NFs (NF/c‐Ski) can play a similar role. The effect of conditioned medium (CM) collected from NF/c‐Ski cells on the migration of MDA‐MB‐231 breast cancer cells was tested by wound‐healing scratch assays. As shown in Figure 4A, MDA‐MB‐231 cells cultured in the CM from NF/c‐Ski cells (NF/c‐Ski‐CM) or parental CAFs (CAFs‐CM) retained 44% (3.73 mm2/8.48 mm2) or 48% (3.94 mm2/8.21 mm2) of the non‐healed wound scratched area within 48 h respectively, but cells in the CM of control NFs (NFs‐CM) or NFs/Vec (NFs/Vec‐CM) retained 74% (5.36 mm2/7.24 mm2) or 72% (5.67 mm2/7.88 mm2) of the unclosed scratched area respectively. These data indicate that c‐Ski‐activated NFs can promote the mobility of MDA‐MB‐231 cells.
Figure 4.
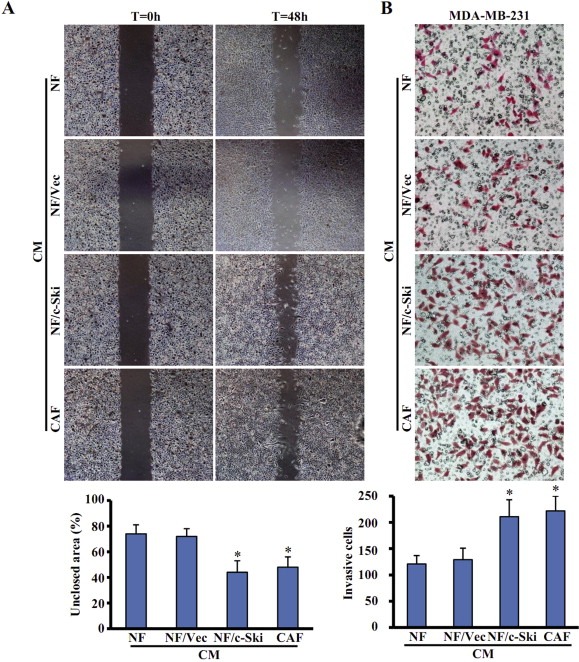
c‐Ski activated NFs promote the migration and invasion of breast cancer MDA‐MB‐231 cells. (A) The migration of breast cancer MDA‐MB‐231 cells was tested by the wound healing assay. Cells were cultured in conditioned media (CM) derived from parental NFs, parental CAFs, c‐Ske‐activated NFs (NF/c‐Ski) and control cells (NF/Vec). The migration ability was determined as the percentage of non‐healed scratched area by using Image J software described in Material. The data represent means ± s.d. from three different experiments. (*P < 0.01, ANOVA followed by Student–Newman–Keuls test). (B) The invasive potential was examined by the chambers coated with ECM Gel. MDA‐MB‐231 cells were seeded in the upper chambers of transwell, and CM derived from labeled fibroblasts was added into the lower chambers of transwell as chemoattractants. (*P < 0.01, ANOVA followed by Student–Newman–Keuls test).
Subsequently, the invasion capacity of MDA‐MB‐231 cells was detected by using CM from NFs/c‐Ski, control NFs and CAFs as chemoattractants. Transwell assays showed that NF/c‐Ski‐CM or CAF‐CM increased MDA‐MB‐231 cells invasion (at least ∼1.75 folds) than control NFs‐CM did (Figure 4B). Taken together, these data suggest that c‐Ski‐activated NFs facilitate the migration and invasion of breast tumor cells.
3.5. Loss of c‐Ski expression partly reverses the function of CAFs
To further characterize the role of c‐Ski in regulating CAFs function, we tested whether c‐Ski silence in CAFs affects the expression of α‐SMA and FAP. Knockdown of c‐Ski in CAFs (CAF‐sh/c‐Ski) by siRNA decreased the expression of α‐SMA and FAP, which was comparable to those in NFs (Figure 5A). In contrast, there were no significant differences in the expression levels of α‐SMA and FAP between control shRNA CAFs (CAF‐sh/vec) and parental CAFs (Figure 5A). So, loss of c‐Ski expression in CAFs may inhibit the expression of CAF markers.
Figure 5.
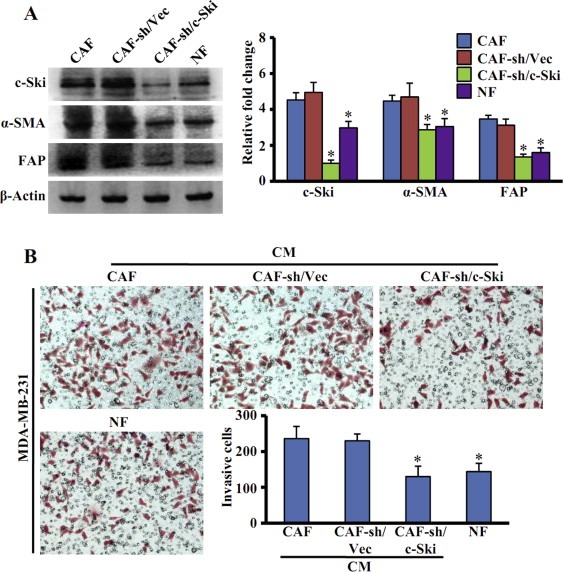
Silence of c‐Ski abrogates CAFs activation and function. (A) Levels of c‐Ski, α‐SMA, FAP in CAFs stably transfected with pLVX‐shRNA1‐Ski (CAF‐sh/c‐Ski) or control vector (CAF‐sh/Vec) were determined by Western blot analysis (Left panel). β‐Actin was used as loading control. The relative fold changes of proteins levels were quantified by density value relative to the loading control (Right panel). The data represent means ± s.d. from three different experiments. (*P < 0.01, ANOVA followed by Student–Newman–Keuls test). (B) The invasion of breast cancer MDA‐MB‐231 cells was analyzed by transwell assays. CM collected from parental CAFs, parental NFs, CAFs‐sh/c‐Ski and its control cells (CAFs‐sh/Vec) were separately added into lower chambers of transwell as chemoattractants. (*P < 0.01, ANOVA followed by Student–Newman–Keuls test.)
In addition, we asked whether c‐Ski knockdown can affect the role of CAFs in breast cancer cell invasion. The invasive capacity of MDA‐MB‐231 cells was measured using CM from CAFs‐sh/c‐Ski, CAFs‐sh/Vec, parental CAFs and NFs. As shown in Figure 5B, MDA‐MB‐231 cells stimulated with CM from CAFs‐sh/c‐Ski displayed lower invasive ability compared with CM from CAFs‐sh/Vec or from parental CAFs. CM from NFs had a similar effect as CM from CAFs‐sh/c‐Ski. Thus, c‐Ski is essential for maintaining the functions of CAFs.
3.6. c‐Ski activates stromal fibroblasts through elevating SDF‐1 expression by repressing p53
CAFs were previously found to promote tumor progression through autocrine and paracrine signaling (Orimo and Weinberg, 2006; Östman and Augsten, 2009). To understand the molecular mechanism of c‐Ski‐induced activation of CAFs, we examined the expression of 9 cytokines which have been reported to be associated with activation and function of CAFs and also with cancer development. These cytokines are: TGF‐β1, HGF, VEGF‐C, VEGF‐A, FGF2, PDGFRα, PDGFRβ, SDF‐1/CXCL12, HB‐EGF (heparin binding‐epidermal growth factor). Interestingly, only SDF‐1 mRNA was remarkably up‐regulated in c‐Ski‐activated NFs and CAFs, whereas the levels of other cytokines kept in less or moderate up‐regulation (Figure 6A). Moreover, the level of SDF‐1 was repressed in c‐Ski silenced CAFs or inhibited by chalcone 4, a specific small‐molecule inhibitor of SDF‐1 (Figure 6B). In contrast, silenced c‐Ski did not significantly alter the expression of other 8 cytokines (Figure S2). Thus, SDF‐1 may be a key cytokine factor regulated by c‐Ski in breast cancer CAFs.
Figure 6.
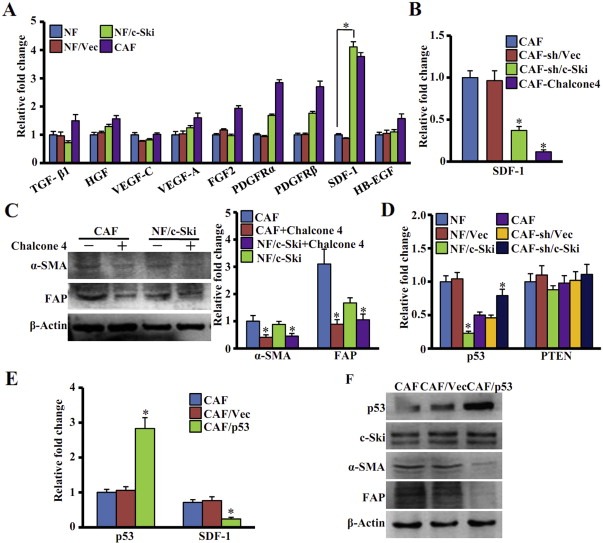
c‐Ski activates stromal fibroblasts through upregulating SDF‐1 and repressing p53. (A) Levels of 9 cytokines in parental NFs, parental CAFs, NFs stably transfected with c‐Ski (NF/c‐Ski) or control vector (NF/Vec) were analyzed using qRT‐PCR. The data represent means ± s.d. from three experiments. (*P < 0.01, Student t test). (B) SDF‐1 expression was determined by qRT‐PCR in CAFs, CAFs treated with Chalcone 4, CAFs with silenced c‐Ski (CAF‐sh/c‐SKi) and control cells (CAF‐sh/Vec). The relative fold change was compared with CAFs. (*P < 0.01, ANOVA followed by Student–Newman–Keuls test). (C) Western blot analysis was used to test the levels of α‐SMA, FAP in cells treated with or without Chalcone 4 (5 μg/mL) for 48 h β‐Actin was used as a loading control. The relative fold change was compared with CAFs. (*P < 0.01, Student t test). (D) Levels of P53 and PTEN in cells were checked by qRT‐PCR. The relative fold change of P53 and PTEN in each cell was compared with NFs. (*P < 0.01, ANOVA followed by Student–Newman–Keuls test). (E) qRT‐PCR was used to analyze the P53 and SDF‐1 expression in parental CAFs, CAFs transfected with pCMV‐HA‐p53 (CAF/p53) and control vector cells (CAF/Vec). The relative fold change of P53 and SDF‐1 in each cell was compared with parental CAFs. (*P < 0.01, ANOVA followed by Student–Newman–Keuls test). (F) Western blot analysis was used to determine the expression of p53, c‐Ski, α‐SMA and FAP in parental CAFs, CAFs transfected with pCMV‐HA‐p53 (CAF/p53) and control vector cells (CAF/Vec). β‐Actin was used as an internal control.
SDF‐1 has been reported to initiate and maintain the transformation of NFs into tumor‐promoting CAFs through autocrine signaling loops in breast cancer stroma during tumor progression (Kojima et al., 2010; Moskovits et al., 2006; Orimo et al., 2005; Shimoda et al., 2010). To further ascertain the effect of SDF‐1 on stimulating CAFs, we tested whether SDF‐1 inhibition affects levels of CAFs marker. As shown in Figure 6C, Chalcone 4 reduced the expression of α‐SMA and FAP in CAFs and NFs/c‐Ski.
Some tumor suppressors, such as p53 and PTEN, were repressed in the breast cancer stroma (Addadi et al., 2010; Trimboli et al., 2009). c‐Ski, known as a transcriptional co‐repressor, was reported to directly bind to other co‐repressors and inhibited the transcription of target genes (Kim et al., 2012; Nomura et al., 1999; Takahata et al., 2009). To determine whether c‐Ski could up‐regulate SDF‐1 through repressing p53 and PTEN in CAFs, levels of p53 and PTEN were assessed in NFs and CAFs. As shown in Figure 6D, p53 was down‐regulated in c‐Ski‐activated NFs (vs parental NFs) and up‐regulated in c‐Ski‐silenced CAFs (vs parental CAFs). Interestingly, c‐Ski had a moderate effect on the expression of PTEN in fibroblasts.
Because p53 was reported to repress SDF‐1 expression in some of cancers (Addadi et al., 2010; Moskovits et al., 2006), we reasoned that c‐Ski might elevate SDF‐1/CXCL12 levels through repressing p53 in CAFs. Thus, p53 was transfected into CAFs. It was found that restoration of p53 expression in CAFs repressed SDF‐1 (Figure 6E), α‐SMA and FAP (Figure 6F), without affecting the expression of the other examined cytokines (Figure S3). These data indicate that p53 mediates the effect of c‐Ski on SDF‐1 in CAFs.
3.7. c‐Ski regulates the invasion of MDA‐MB‐231 cells through SDF‐1 and p53
The above data demonstrate that c‐Ski activates NFs through SDF‐1 and p53. To ascertain that c‐Ski/p53/SDF‐1 signaling directly contributes to breast tumor cell invasion, we performed transwell invasion assays. As shown in Figure 7A (upper left) and 7B, more MDA‐MB‐231 cells invaded towards SDF‐1 protein‐supplemented CM from NFs than towards control CM from NFs. Similarly, CM from CAFs neutralized with an anti‐SDF‐1 antibody (Anti‐SDF‐1) led to decreased invasion of MDA‐MB‐231 cells (Figure 7A upper right panel, and 7B). In addition, MDA‐MB‐231 cell invasion induced by NF/c‐Ski‐CM was abolished by Anti‐SDF‐1 antibody (Figure 7A lower left panel, and 7B). Notably, decreased invasion of MDA‐MB‐231 cells induced by CAFs‐sh/c‐Ski‐CM was rescued by SDF‐1 supplementation (Figure 7A lower right panel, and 7B). These findings indicate that c‐Ski utilizes SDF‐1 paracrine signaling to promote the invasion of MDA‐MB‐231 cells.
Figure 7.
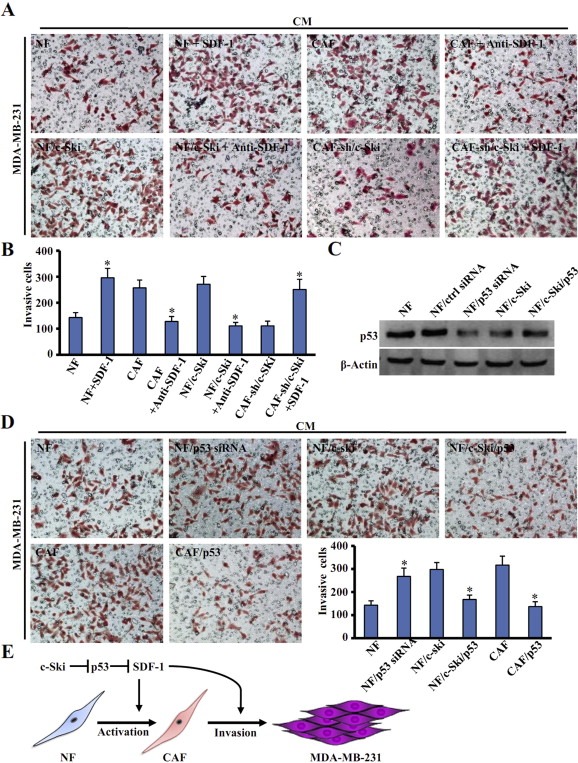
c‐Ski governs invasion of MDA‐MB‐231 cells through SDF‐1 and p53. (A) Transwell assay was employed to test the invasion of MDA‐MB‐231 cells in conditional medium (CM) from each fibroblast. NFs: CM from parental NFs; NFs + SDF‐1: CM from NFs added with SDF‐1; NFs/c‐Ski: CM from NFs transfected with c‐Ski; NFs/c‐Ski + Anti‐SDF‐1: CM from NFs/c‐Ski treated with anti‐SDF1 neutralizing antibody; CAF: CM from parental CAFs; CAFs + Anti‐SDF‐1: CM from CAFs treated with anti‐SDF1 neutralizing antibody; CAF‐sh/c‐Ski: CM from CAFs with silenced c‐Ski by c‐Ski shRNA. CAF‐sh/c‐Ski + SDF‐1: CM from CAF‐sh/c‐Ski treated with human SDF‐1. (B) A quantitative graph of invasive cells measured in (A). Each bar represents the mean ± S.D. of three different experiments. (*P < 0.01, ANOVA followed by Student–Newman–Keuls test). (C) Western blot analysis was used to test p53 level in cells. β‐Actin was used as an internal control. (D) The effect of fibroblast‐associated p53 on cancer cell invasion was tested. CM was added into the transwell lower chamber. The labeled cells were listed as: NF or CAF means parental cells; NF/p53 siRNA: NF with silenced p53 by siRNA; CAF/p53: CAF transfected with p53; NF/c‐Ski: NF transfected with c‐Ski; NF/c‐Ski/p53: NF with overexpressing c‐Ski and p53. The quantitative graph showed the invasive MDA‐MB‐231 cells. (*P < 0.01, ANOVA followed by Student–Newman–Keuls test). (E) A model depicting the role of c‐Ski in activation of NFs into CAFs and promotion of breast cancer invasion via p53 and SDF‐1.
Furthermore, the effects of fibroblast‐associated p53 on MDA‐MB‐231 cell invasion were tested using CM from NFs or CAFs. As shown, p53 knockdown in NFs (Figure 7C) increased the invasion of MDA‐MB‐231 cells (Figure 7D upper left panel), whereas increase of p53 in CAFs reduced the invasion of MDA‐MB‐231 cells towards CM (Figure 7D lower left panel). Similarly, restoration of p53 in c‐Ski‐activated NFs abolished the effect of NFs/c‐Ski‐CM on stimulating the invasion of MDA‐MB‐231 cells (Figure 7D upper right panel). Thus, p53 mediates the effect of CAF‐associated c‐Ski on tumor cells.
4. Discussion
c‐Ski is an evolutionarily conserved protein and involved in many cellular processes, such as proliferation, differentiation, transformation, and tumor progression (Bonnon and Atanasoski, 2012). It has been demonstrated that the dysregulation of c‐Ski expression participates in the initiation and development of some solid tumors. In addition, it was found that c‐Ski is involved in wound healing through promoting proliferation of myofibroblasts (Li et al., 2011). However, the role of c‐Ski in CAFs is less well known. Using cDNA microarrays, we found that the expression of c‐Ski is enhanced in breast cancer CAFs. It thus suggested that c‐Ski might play a role in CAFs. In the present study, c‐Ski was found to be highly expressed in the fibroblasts of breast tumors, especially in invasive breast carcinoma. Compared with normal fibroblasts isolated from adjacent tissue to tumor tissue, c‐Ski expression was higher in CAFs derived from tumor tissue. And high level of c‐Ski increased the proliferation, migration, invasive and contractile activity of CAFs. More interestingly, c‐Ski was demonstrated to play a key role in activation of breast CAFs. This process may be associated with p53 and SDF‐1. To our knowledge, it is the first report to show that c‐Ski up‐regulates SDF‐1 through repressing p53 in breast CAFs and that this pathway is essential for the activation of CAFs.
Studies have shown that local normal fibroblasts could be induced to transdifferentiate into CAFs by the surrounding cancer cells, and these activated CAFs further promote the progression of cancer (Kalluri and Zeisberg, 2006). In the present study, we found that overexpression of c‐Ski in NFs increased the level of α‐SMA and FAP, two of biomarkers of CAFs. Consistent with this, silencing of c‐Ski by RNA interference diminished the expression of α‐SMA and FAP in CAFs. Our data suggest that c‐Ski is one of the factors that promote the activation of NFs into CAFs in breast cancer.
p53 and PTEN, two critical tumor suppressors, are known to be decreased in the stromal cells (Addadi et al., 2010; Trimboli et al., 2009). Previous studies have also shown that c‐Ski could inhibit p53 in several tumor cells (Ding et al., 2012; Inoue et al., 2011). However, there has been no evidence to prove that p53 is involved in the activation of stroma cells in tumor microenvironment. In the current work, we found that p53, but not the PTEN was repressed by c‐Ski in CAFs, which in turn increased the expression of SDF‐1. Several secreted protein, such as TGF‐β (Casey et al., 2008), PDGF (Murata et al., 2011), HGF (Wang et al., 2009) and SDF‐1 (Orimo et al., 2005) derived from local cancer cells may play an important role in the transfer of NFs to CAFs. In this study, up‐regulation of SDF‐1, but not other secreted factors, by c‐Ski via suppression of p53 in the normal breast fibroblasts may refer to activation of CAFs.
CAFs are an important component in the tumor microenvironment and play a key role in cross‐talking with cancer cells to promote cancer growth and invasion (Casey et al., 2009). CAFs could secrete a set of cytokines interacting with cancer cells through paracrine signaling (Ao et al., 2007). We found that p53/SDF‐1 mediates the induction of migration and invasion of MDA‐MB‐231 cells by CAF‐associated c‐Ski. In summary, our findings indicate that c‐Ski elevates SDF‐1 to stimulate the activation of breast CAFs through repressing p53, and c‐Ski‐activated CAFs by c‐Ski further promote the invasion of MDA‐MB‐231 cells (Figure 7E). These findings have important implications not only for understanding the molecular mechanisms underlying the activation of breast CAFs, but also for the regulation of tumor cells by stromal cells. These data also suggest that c‐Ski may be a novel therapeutic target for breast cancer.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
The following are the supplementary data related to this article:
Supplementary data
Figure S1 Characterization of immortalized cancer‐associated fibroblasts (CAFs) and normal fibroblasts (NFs). The primary CAFs and NFs were isolated from the breast tumor tissues and immortalized by transfection with human telomerase reverse transcriptase (hTERT). The universal marker of fibroblasts (FN constructor) and the known hallmarkers of CAFs (α‐SMA and FAP) were examined by immunofluorescence staining. Scale bars: 300 μm.
Figure S2 Levels of other cytokines have no significant change in c‐Ski silenced CAFs cells. The qRT‐PCR was used to detect the expression of 8 cytokines except SDF‐1 in CAFs, CAFs with c‐Ski knockdown (CAF‐sh/c‐Ski) and its control cells (CAF‐sh/Vec). The relative fold change was compared with CAFs.
Figure S3 Over‐expression of P53 do not significantly change the expression of other cytokines in CAFs. The expression of 8 cytokines except SDF‐1 in parental CAFs, CAFs transfected with pCMV‐HA‐p53 (CAF/p53) or control vector (CAF/Vec) were determined using qRT‐PCR. The relative fold change was compared with parental CAFs.
Acknowledgments
This work was supported in part by National Natural Science Foundation of China (NSFC 81072147, NSFC 30940096); Natural Science Foundation of Chongqing Science & Technology Commission, China (CSTC2010BB5099); the Doctoral Fund of Ministry of Education, China (20105503110001); the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Ministry of Education, China ([2011]508); the Major Program of Chongqing Medical University XBZD201006; and the Scientific Research Foundation for the Returned Overseas Scholars of Chongqing Medical University; and supported from National Institutes of Health (R01CA151610) and Avon Foundation (02‐2010‐068) to Xiaojiang Cui.
Supplementary data 1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2013.08.007.
Wang Liyang, Hou Yixuan, Sun Yan, Zhao Liuyang, Tang Xi, Hu Ping, Yang Jiajia, Zeng Zongyue, Yang Guanglun, Cui Xiaojiang, Liu Manran, (2013), c-Ski activates cancer-associated fibroblasts to regulate breast cancer cell invasion, Molecular Oncology, 7, doi: 10.1016/j.molonc.2013.08.007.
References
- Addadi, Y. , Moskovits, N. , Granot, D. , Lozano, G. , Carmi, Y. , Apte, R.N. , Neeman, M. , Oren, M. , 2010. p53 status in stromal fibroblasts modulates tumor growth in an SDF1-dependent manner. Cancer Res.. 70, 9650–9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderberg, C. , Li, H. , Fredriksson, L. , Andrae, J. , Betsholtz, C. , Li, X. , Eriksson, U. , Pietras, K. , 2009. Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts. Cancer Res.. 69, 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao, M. , Franco, O.E. , Park, D. , Raman, D. , Williams, K. , Hayward, S.W. , 2007. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res.. 67, 4244–4253. [DOI] [PubMed] [Google Scholar]
- Bhowmick, N.A. , Neilson, E.G. , Moses, H.L. , 2004. Stromal fibroblasts in cancer initiation and progression. Nature. 432, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnon, C. , Atanasoski, S. , 2012. c-Ski in health and disease. Cell Tissue Res.. 347, 51–64. [DOI] [PubMed] [Google Scholar]
- Boone, B. , Haspeslagh, M. , Brochez, L. , 2009. Clinical significance of the expression of c-Ski and SnoN, possible mediators in TGF-β resistance, in primary cutaneous melanoma. J. Dermatol. Sci.. 53, 26–33. [DOI] [PubMed] [Google Scholar]
- Bravou, V. , Antonacopoulou, A. , Papadaki, H. , Floratou, K. , Stavropoulos, M. , Episkopou, V. , Petropoulou, C. , Kalofonos, H. , 2009. TGF-β repressors SnoN and Ski are implicated in human colorectal carcinogenesis. Anal. Cell. Pathol.. 31, 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey, T. , Bond, J. , Tighe, S. , Hunter, T. , Lintault, L. , Patel, O. , Eneman, J. , Crocker, A. , White, J. , Tessitore, J. , 2009. Molecular signatures suggest a major role for stromal cells in development of invasive breast cancer. Breast Cancer Res. Treat.. 114, 47–62. [DOI] [PubMed] [Google Scholar]
- Casey, T.M. , Eneman, J. , Crocker, A. , White, J. , Tessitore, J. , Stanley, M. , Harlow, S. , Bunn, J.Y. , Weaver, D. , Muss, H. , 2008. Cancer associated fibroblasts stimulated by transforming growth factor beta1 (TGF-β1) increase invasion rate of tumor cells: a population study. Breast Cancer Res. Treat.. 110, 39–49. [DOI] [PubMed] [Google Scholar]
- Colmenares, C. , Sutrave, P. , Hughes, S. , Stavnezer, E. , 1991. Activation of the c-ski oncogene by overexpression. J. Virol.. 65, 4929–4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, B. , Sun, Y. , Huang, J. , 2012. Overexpression of SKI oncoprotein leads to p53 degradation through regulation of MDM2 protein sumoylation. J. Biol. Chem.. 287, 14621–14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco, O.E. , Shaw, A.K. , Strand, D.W. , Hayward, S.W. , 2010. Cancer associated fibroblasts in cancer pathogenesis. Semin. Cell Dev. Biol.. 21, 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell, F. , 1994. Fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol.. 124, 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györffy, B. , Lanczky, A. , Eklund, A.C. , Denkert, C. , Budczies, J. , Li, Q. , Szallasi, Z. , 2010. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res. Treat.. 123, 725–731. [DOI] [PubMed] [Google Scholar]
- Heider, T.R. , Lyman, S. , Schoonhoven, R. , Behrns, K.E. , 2007. Ski promotes tumor growth through abrogation of transforming growth factor-β signaling in pancreatic cancer. Ann. Surg.. 246, 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, Y. , Iemura, S.-i. , Natsume, T. , Miyazawa, K. , Imamura, T. , 2011. Suppression of p53 activity through the cooperative action of Ski and histone deacetylase SIRT1. J. Biol. Chem.. 286, 6311–6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri, R. , Zeisberg, M. , 2006. Fibroblasts in cancer. Nat. Rev. Cancer. 6, 392–401. [DOI] [PubMed] [Google Scholar]
- Kim, K.O. , Sampson, E.R. , Maynard, R.D. , O'Keefe, R.J. , Chen, D. , Drissi, H. , Rosier, R.N. , Hilton, M.J. , Zuscik, M.J. , 2012. Ski inhibits TGF-β/phospho-Smad3 signaling and accelerates hypertrophic differentiation in chondrocytes. J. Cell. Biochem.. 113, 2156–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima, Y. , Acar, A. , Eaton, E.N. , Mellody, K.T. , Scheel, C. , Ben-Porath, I. , Onder, T.T. , Wang, Z.C. , Richardson, A.L. , Weinberg, R.A. , 2010. Autocrine TGF-β and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc. Natl. Acad. Sci. U S A. 107, 20009–20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Li, P. , Zhang, Y. , Li, G. , He, F. , Zhou, Y. , Yang, K. , Dai, S. , 2012. Upregulation of ski in fibroblast is implicated in the peroxisome proliferator-activated receptor δ-mediated wound healing. Cell. Physiol. Biochem.. 30, 1059–1071. [DOI] [PubMed] [Google Scholar]
- Li, P. , Liu, P. , Xiong, R.P. , Chen, X.Y. , Zhao, Y. , Lu, W.P. , Liu, X. , Ning, Y.L. , Yang, N. , Zhou, Y.G. , 2011. Ski, a modulator of wound healing and scar formation in the rat skin and rabbit ear. J. Pathol.. 223, 659–671. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Turck, C. , Teumer, J. , Stavnezer, E. , 1986. Unique sequence, ski, in Sloan–Kettering avian retroviruses with properties of a new cell-derived oncogene. J. Virol.. 57, 1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M. , Casimiro, M.C. , Wang, C. , Shirley, L.A. , Jiao, X. , Katiyar, S. , Ju, X. , Li, Z. , Yu, Z. , Zhou, J. , 2009. p21CIP1 attenuates Ras-and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proc. Natl. Acad. Sci. U S A. 106, 19035–19039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M. , Sakamaki, T. , Casimiro, M.C. , Willmarth, N.E. , Quong, A.A. , Ju, X. , Ojeifo, J. , Jiao, X. , Yeow, W.S. , Katiyar, S. , 2010. The canonical NF-κB pathway governs mammary tumorigenesis in transgenic mice and tumor stem cell expansion. Cancer Res.. 70, 10464–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, R. , Li, H. , Liu, L. , Yu, J. , Ren, X. , 2012. Fibroblast activation protein: a potential therapeutic target in cancer. Cancer Biol. Ther.. 13, 123–129. [DOI] [PubMed] [Google Scholar]
- Lu, Y. , Yang, W. , Qin, C. , Zhang, L. , Deng, J. , Liu, S. , Qin, Z. , 2009. Responsiveness of stromal fibroblasts to IFN-γ blocks tumor growth via angiostasis. J. Immunol.. 183, 6413–6421. [DOI] [PubMed] [Google Scholar]
- Marcelain, K. , Armisen, R. , Aguirre, A. , Ueki, N. , Toro, J. , Colmenares, C. , Hayman, M.J. , 2012. Chromosomal instability in mouse embryonic fibroblasts null for the transcriptional co-repressor Ski. J. Cell. Physiol.. 227, 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micke, P. , 2004. Tumour-stroma interaction: cancer-associated fibroblasts as novel targets in anti-cancer therapy?. Lung Cancer. 45, S163–S175. [DOI] [PubMed] [Google Scholar]
- Moskovits, N. , Kalinkovich, A. , Bar, J. , Lapidot, T. , Oren, M. , 2006. p53 attenuates cancer cell migration and invasion through repression of SDF-1/CXCL12 expression in stromal fibroblasts. Cancer Res.. 66, 10671–10676. [DOI] [PubMed] [Google Scholar]
- Murata, T. , Mizushima, H. , Chinen, I. , Moribe, H. , Yagi, S. , Hoffman, R.M. , Kimura, T. , Yoshino, K. , Ueda, Y. , Enomoto, T. , Mekada, E. , 2011. HB-EGF and PDGF mediate reciprocal interactions of carcinoma cells with cancer-associated fibroblasts to support progression of uterine cervical cancers. Cancer Res.. 71, 6633–6642. [DOI] [PubMed] [Google Scholar]
- Nomura, T. , Khan, M.M. , Kaul, S.C. , Dong, H.D. , Wadhwa, R. , Colmenares, C. , Kohno, I. , Ishii, S. , 1999. Ski is a component of the histone deacetylase complex required for transcriptional repression by Mad and thyroid hormone receptor. Genes Dev.. 13, 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura, T. , Tanikawa, J. , Akimaru, H. , Kanei-Ishii, C. , Ichikawa-Iwata, E. , Khan, M.M. , Ito, H. , Ishii, S. , 2004. Oncogenic activation of c-Myb correlates with a loss of negative regulation by TIF1β and Ski. J. Biol. Chem.. 279, 16715–16726. [DOI] [PubMed] [Google Scholar]
- Orimo, A. , Gupta, P.B. , Sgroi, D.C. , Arenzana-Seisdedos, F. , Delaunay, T. , Naeem, R. , Carey, V.J. , Richardson, A.L. , Weinberg, R.A. , 2005. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 121, 335 [DOI] [PubMed] [Google Scholar]
- Orimo, A. , Weinberg, R.A. , 2006. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 5, 1597–1601. [DOI] [PubMed] [Google Scholar]
- Östman, A. , Augsten, M. , 2009. Cancer-associated fibroblasts and tumor growth-bystanders turning into key players. Curr. Opin. Genet. Dev.. 19, 67–73. [DOI] [PubMed] [Google Scholar]
- Peng, Q. , Zhao, L. , Hou, Y. , Sun, Y. , Wang, L. , Luo, H. , Peng, H. , Liu, M. , 2013. Biological characteristics and genetic heterogeneity between carcinoma-associated fibroblasts and their paired normal fibroblasts in human breast cancer. PLoS ONE. 8, e60321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter, M. , Kattmann, D. , Teichler, S. , Hartmann, O. , Samuelsson, M. , Burchert, A. , Bach, J. , Kim, T. , Berwanger, B. , Thiede, C. , 2006. Inhibition of retinoic acid receptor signaling by Ski in acute myeloid leukemia. Leukemia. 20, 437–443. [DOI] [PubMed] [Google Scholar]
- Shimoda, M. , Mellody, K.T. , Orimo, A. , 2010. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin. Cell Dev. Biol.. 21, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa, T. , Nomura, T. , Colmenares, C. , Ohira, M. , Nakagawara, A. , Ishii, S. , 2001. Increased susceptibility to tumorigenesis of ski-deficient heterozygous mice. Oncogene. 20, 8100 [DOI] [PubMed] [Google Scholar]
- Takahata, M. , Inoue, Y. , Tsuda, H. , Imoto, I. , Koinuma, D. , Hayashi, M. , Ichikura, T. , Yamori, T. , Nagasaki, K. , Yoshida, M. , 2009. SKI and MEL1 cooperate to inhibit transforming growth factor-β signal in gastric cancer cells. J. Biol. Chem.. 284, 3334–3344. [DOI] [PubMed] [Google Scholar]
- Trimboli, A.J. , Cantemir-Stone, C.Z. , Li, F. , Wallace, J.A. , Merchant, A. , Creasap, N. , Thompson, J.C. , Caserta, E. , Wang, H. , Chong, J.-L. , 2009. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 461, 1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Li, Q. , Yamada, T. , Matsumoto, K. , Matsumoto, I. , Oda, M. , Watanabe, G. , Kayano, Y. , Nishioka, Y. , Sone, S. , 2009. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin. Cancer Res.. 15, 6630–6638. [DOI] [PubMed] [Google Scholar]
- Wu, J.W. , Krawitz, A.R. , Chai, J. , Li, W. , Zhang, F. , Luo, K. , Shi, Y. , 2002. Structural mechanism of Smad4 recognition by the nuclear oncoprotein Ski: insights on Ski-mediated repression of TGF-β signaling. Cell.. 111, 357–367. [DOI] [PubMed] [Google Scholar]
- Yang, S. , Xie, N. , Cui, H. , Banerjee, S. , Abraham, E. , Thannickal, V.J. , Liu, G. , 2012. miR-31 is a negative regulator of fibrogenesis and pulmonary fibrosis. FASEB J.. 26, 3790–3799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhao, L. , Sun, Y. , Hou, Y. , Peng, Q. , Wang, L. , Luo, H. , Tang, X. , Zeng, Z. , Liu, M. , 2012. MiRNA expression analysis of cancer-associated fibroblasts and normal fibroblasts in breast cancer. Int. J. Biochem. Cell Biol.. 44, 2051–2059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary data
Figure S1 Characterization of immortalized cancer‐associated fibroblasts (CAFs) and normal fibroblasts (NFs). The primary CAFs and NFs were isolated from the breast tumor tissues and immortalized by transfection with human telomerase reverse transcriptase (hTERT). The universal marker of fibroblasts (FN constructor) and the known hallmarkers of CAFs (α‐SMA and FAP) were examined by immunofluorescence staining. Scale bars: 300 μm.
Figure S2 Levels of other cytokines have no significant change in c‐Ski silenced CAFs cells. The qRT‐PCR was used to detect the expression of 8 cytokines except SDF‐1 in CAFs, CAFs with c‐Ski knockdown (CAF‐sh/c‐Ski) and its control cells (CAF‐sh/Vec). The relative fold change was compared with CAFs.
Figure S3 Over‐expression of P53 do not significantly change the expression of other cytokines in CAFs. The expression of 8 cytokines except SDF‐1 in parental CAFs, CAFs transfected with pCMV‐HA‐p53 (CAF/p53) or control vector (CAF/Vec) were determined using qRT‐PCR. The relative fold change was compared with parental CAFs.


