Abstract
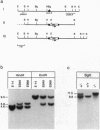
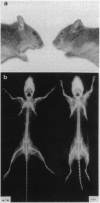
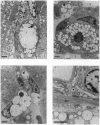
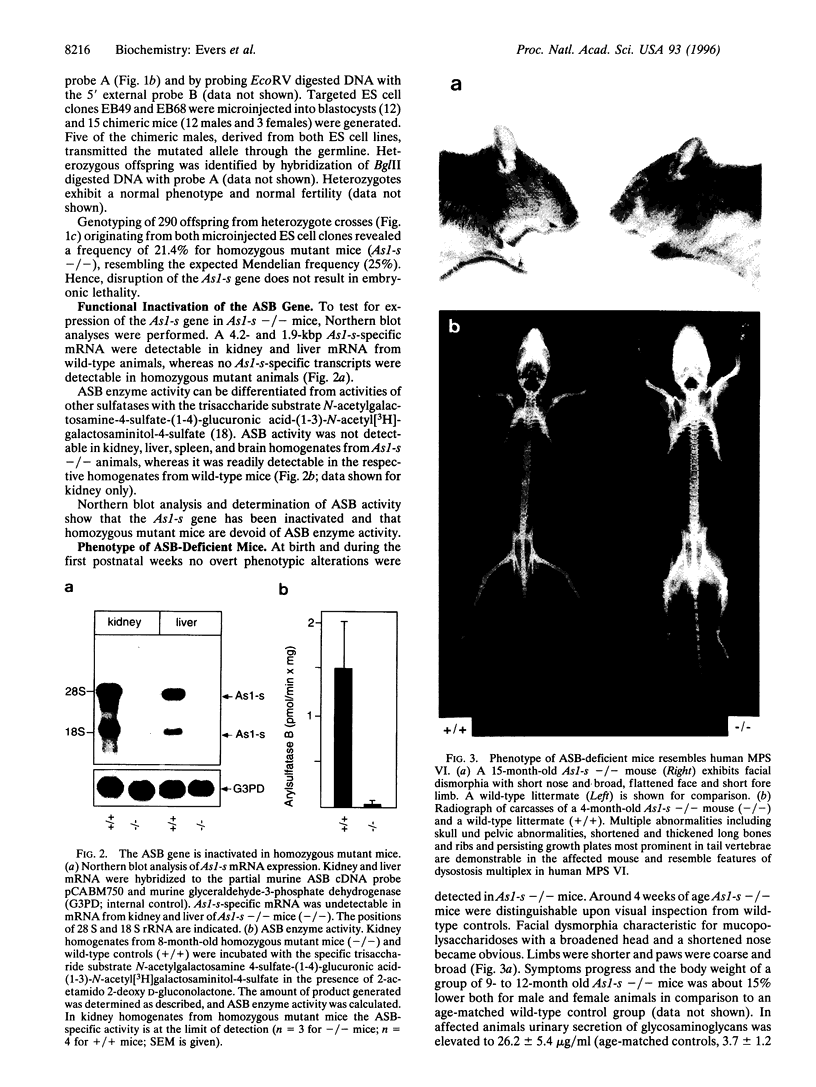
Mucopolysaccharidosis VI (MPS VI) is a lysosomal storage disease with autosomal recessive inheritance caused by a deficiency of the enzyme arylsulfatase B (ASB), which is involved in degradation of dermatan sulfate and chondroitin 4-sulfate. A MPS VI mouse model was generated by targeted disruption of the ASB gene. Homozygous mutant animals exhibit ASB enzyme deficiency and elevated urinary secretion of dermatan sulfate. They develop progressive symptoms resembling those of MPS VI in humans. Around 4 weeks of age facial dysmorphia becomes overt, long bones are shortened, and pelvic and costal abnormalities are observed. Major alterations in bone formation with perturbed cartilaginous tissues in newborns and widened, perturbed, and persisting growth plates in adult animals are seen. All major parenchymal organs show storage of glycosaminoglycans preferentially in interstitial cells and macrophages. Affected mice are fertile and mortality is not elevated up to 15 months of age. This mouse model will be a valuable tool for studying pathogenesis of MPS VI and may help to evaluate therapeutical approaches for lysosomal storage diseases.
Full text
PDF

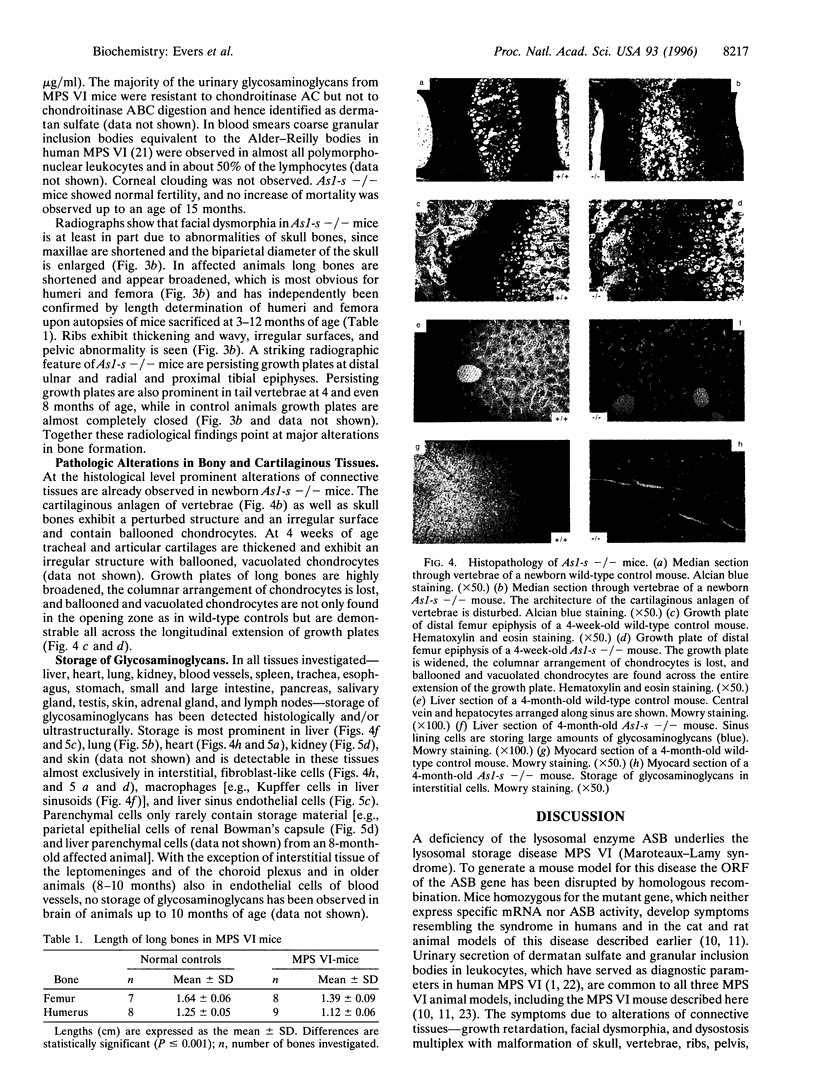


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton N. W., Brady R. O., Dambrosia J. M., Di Bisceglie A. M., Doppelt S. H., Hill S. C., Mankin H. J., Murray G. J., Parker R. I., Argoff C. E. Replacement therapy for inherited enzyme deficiency--macrophage-targeted glucocerebrosidase for Gaucher's disease. N Engl J Med. 1991 May 23;324(21):1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Barker J. E., Vogler C. A., Kyle J. W., Sly W. S., Gwynn B., Levy B., Pegors C. Increased life span and correction of metabolic defects in murine mucopolysaccharidosis type VII after syngeneic bone marrow transplantation. Blood. 1991 Dec 1;78(11):3081–3092. [PubMed] [Google Scholar]
- Gieselmann V. Lysosomal storage diseases. Biochim Biophys Acta. 1995 Apr 24;1270(2-3):103–136. doi: 10.1016/0925-4439(94)00075-2. [DOI] [PubMed] [Google Scholar]
- Gold E. W. A simple spectrophotometric method for estimating glycosaminoglycan concentrations. Anal Biochem. 1979 Oct 15;99(1):183–188. doi: 10.1016/0003-2697(79)90061-7. [DOI] [PubMed] [Google Scholar]
- Haskins M. E., Aguirre G. D., Jezyk P. F., Patterson D. F. The pathology of the feline model of mucopolysaccharidosis VI. Am J Pathol. 1980 Dec;101(3):657–674. [PMC free article] [PubMed] [Google Scholar]
- Hermanns W., Liebig K., Schulz L. C. Postembedding immunohistochemical demonstration of antigen in experimental polyarthritis using plastic embedded whole joints. Histochemistry. 1981 Dec;73(3):439–446. doi: 10.1007/BF00495658. [DOI] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H., Muller V. J., Saccone G. T. Diagnosis of Maroteaux-Lamy syndrome by the use of radiolabelled oligosaccharides as substrates for the determination of arylsulphatase B activity. Biochem J. 1986 Mar 15;234(3):507–514. doi: 10.1042/bj2340507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbrandt D., Arlt G., Brooks D. A., Hopwood J. J., von Figura K., Peters C. Mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): six unique arylsulfatase B gene alleles causing variable disease phenotypes. Am J Hum Genet. 1994 Mar;54(3):454–463. [PMC free article] [PubMed] [Google Scholar]
- Jezyk P. F., Haskins M. E., Patterson D. F., Mellman W. J., Greenstein M. Mucopolysaccharidosis in a cat with arylsulfatase B deficiency: a model of Maroteaux-Lamy syndrome. Science. 1977 Nov 25;198(4319):834–836. doi: 10.1126/science.144321. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Woo S. L. Gene therapy for metabolic disorders. Trends Genet. 1994 Jul;10(7):253–257. doi: 10.1016/0168-9525(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Köster A., Saftig P., Matzner U., von Figura K., Peters C., Pohlmann R. Targeted disruption of the M(r) 46,000 mannose 6-phosphate receptor gene in mice results in misrouting of lysosomal proteins. EMBO J. 1993 Dec 15;12(13):5219–5223. doi: 10.1002/j.1460-2075.1993.tb06217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons K., Graycar J. L., Lee A., Hashmi S., Lindquist P. B., Chen E. Y., Hogan B. L., Derynck R. Vgr-1, a mammalian gene related to Xenopus Vg-1, is a member of the transforming growth factor beta gene superfamily. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4554–4558. doi: 10.1073/pnas.86.12.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon R., Arbogast B., Dorfman A. Deficiency of chondroitin sulfate N-acetylgalactosamine 4-sulfate sulfatase in Maroteaux-Lamy syndrome. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1450–1457. doi: 10.1016/s0006-291x(74)80446-8. [DOI] [PubMed] [Google Scholar]
- Modaressi S., Rupp K., von Figura K., Peters C. Structure of the human arylsulfatase B gene. Biol Chem Hoppe Seyler. 1993 May;374(5):327–335. doi: 10.1515/bchm3.1993.374.1-6.327. [DOI] [PubMed] [Google Scholar]
- O'Brien J. F., Cantz M., Spranger J. Maroteaux-Lamy disease (mucopolysaccharidosis VI), subtype A: deficiency of a N-acetylgalactosamine-4-sulfatase. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1170–1177. doi: 10.1016/0006-291x(74)90435-5. [DOI] [PubMed] [Google Scholar]
- Peters C., Schmidt B., Rommerskirch W., Rupp K., Zühlsdorf M., Vingron M., Meyer H. E., Pohlmann R., von Figura K. Phylogenetic conservation of arylsulfatases. cDNA cloning and expression of human arylsulfatase B. J Biol Chem. 1990 Feb 25;265(6):3374–3381. [PubMed] [Google Scholar]
- Sands M. S., Birkenmeier E. H. A single-base-pair deletion in the beta-glucuronidase gene accounts for the phenotype of murine mucopolysaccharidosis type VII. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6567–6571. doi: 10.1073/pnas.90.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands M. S., Vogler C., Kyle J. W., Grubb J. H., Levy B., Galvin N., Sly W. S., Birkenmeier E. H. Enzyme replacement therapy for murine mucopolysaccharidosis type VII. J Clin Invest. 1994 Jun;93(6):2324–2331. doi: 10.1172/JCI117237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchman E. H., Jackson C. E., Desnick R. J. Human arylsulfatase B: MOPAC cloning, nucleotide sequence of a full-length cDNA, and regions of amino acid identity with arylsulfatases A and C. Genomics. 1990 Jan;6(1):149–158. doi: 10.1016/0888-7543(90)90460-c. [DOI] [PubMed] [Google Scholar]
- Spranger J. W., Koch F., McKusick V. A., Natzschka J., Wiedemann H. R., Zellweger H. Mucopolysaccharidosis VI (Maroteaux-Lamy's disease). Helv Paediatr Acta. 1970 Oct;25(4):337–362. [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Wolfe J. H., Sands M. S., Barker J. E., Gwynn B., Rowe L. B., Vogler C. A., Birkenmeier E. H. Reversal of pathology in murine mucopolysaccharidosis type VII by somatic cell gene transfer. Nature. 1992 Dec 24;360(6406):749–753. doi: 10.1038/360749a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Noguchi J., Ikadai H., Takahashi M., Nagase S. Arylsulfatase B-deficient mucopolysaccharidosis in rats. J Clin Invest. 1993 Mar;91(3):1099–1104. doi: 10.1172/JCI116268. [DOI] [PMC free article] [PubMed] [Google Scholar]