Abstract
Adoptive transfer of antigen-specific T cells has been adapted by investigators for treatment of chronic lymphocytic leukemia (CLL). To overcome issues of immune tolerance which limits the endogenous adaptive immune response to tumor-associated antigens (TAAs), robust systems for the genetic modification and characterization of T cells expressing chimeric antigen receptors (CARs) to redirect specificity have been produced. Refinements with regards to persistence and trafficking of the genetically modified T cells are underway to help improve potency. Clinical trials utilizing this technology demonstrate feasibility, and increasingly, these early-phase trials are demonstrating impressive anti-tumor effects, particularly for CLL patients, paving the way for multi-center trials to establish the efficacy of CAR+ T cell therapy.
Keywords: Chronic lymphocytic leukemia, Chimeric antigen receptor, Gene therapy
Introduction
T cells can target and eliminate malignant B cells. Allogeneic hematopoietic stem-cell transplantation (HSCT) cures a substantial portion of patients with chronic lymphocytic leukemia (CLL) who are incurable with conventional chemotherapy, and underscores the powerful therapeutic effect of the T cell immune response in controlling advanced and high-risk disease [1•, 2, 3]. Polyclonal (non-targeted) T cell therapy in the form of donor lymphocyte infusion (DLI) following HSCT has been used to effectively treat patients with relapsed CLL [3–11]. However, disease relapse and graft-versus-host-disease (GVHD) following HSCT and DLI illustrate the two most significant limitations of non-directed cellular therapy, namely, immune evasion of the tumor leading to relapse, and on-target effects in which donor-derived Tcells target major or minor histocompatibility antigens leading to GVHD.
To achieve remission, infused T cells must recognize and eliminate tumor cells that have arisen in the immunocompetent host and that have evolved a range of passive and active immune evasion strategies to avoid immune-mediated destruction. Passive evasion strategies include the emergence of tumor escape variants that have lost the targeted tumor-associated antigen (TAA) such as described in a report by Vago and colleagues [12]. Active evasion strategies are exemplified by the ability of tumors to adversely modulate the tumor microenvironment that impair T cell effector functions, such as through secretion of TGFβ [13].
Investigators have used genetic tools to overcome the limitation of immune tolerance by genetically modifying T cells to express transgenic T cell receptor (TCR) α and β chains that recognize TAA in context of human leukocyte antigen (HLA), or by expressing a single-chain chimeric antigen receptor (CAR) to redirect T cell specificity to a TAA expressed on the cell surface independent of HLA. In this review, we focus on the design and implementation of CARs for CLL.
CLL Antigens
The most commonly targeted TAA on CLL is CD19. The rationale for redirecting the specificity of T cells for CD19 using a CAR is based on the following: (i) CD19 is a lineage cell-surface antigen expressed on CLL derived from malignant B cells, (ii) CD19 is not expressed on cells other than derived from B-lineage, (iii) CD19 is apparently not shed into circulation to interfere with binding of CAR, and (iv) CD19+ tumor cells are amenable to lysis by T cells expressing CD19-specific CAR [14–16]. Other antigens that are expressed on B-CLL cells and are being studied as targets of CAR are CD20, λ or κ light chains, and the B cell tumor associated antigen ROR1 [17•].
The CAR Structure
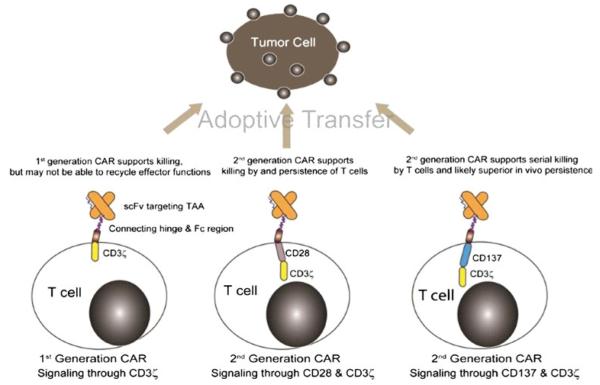
The prototypical CAR uses a mouse monoclonal antibody (mAb) that docks with a designated cell-surface TAA triggering desired T cell activation and effector functions. The specificity of a CAR is achieved by its exodomain which is typically derived from the antigen-binding motif from a mAb that links VH with VL sequences to construct a single-chain fragment variable (scFv) region. In the event that the TAA is itself a receptor, exodomains of CARs have also been fashioned from ligands or peptides (e.g., cytokines) to redirect specificity to receptors (e.g., cytokine receptors), such as the IL-13Rα2–specific “zetakine” [18]. The exodomain is completed by the inclusion of a flexible (hinge), such as from CD8α or immunoglobulin [19, 20] and is expressed on the T cell surface via a trans membrane domain. Upon binding TAA, the CAR activates T cells via an endodomain which typically includes cytoplasmic domains from CD3 or high-affinity receptor FcεRI [21–23]. The docking of CAR to TAA ideally provides the genetically modified T cell with a fully competent activation signal, minimally defined as CAR-dependent killing, proliferation, and cytokine production. Such effector functions are made possible by the design and redesign of CARs, such as to include more than one chimeric activation domain. These iterative modifications to the CAR have resulted in first-, second-, and third-generation CARs designed with one, two, or three signaling motifs within an endodomain. However, most trials for CLL are currently administering T cells genetically modified to express second-generation CAR designs (Fig. 1). These include modifying CAR endodomains with cytoplasmic signaling motifs derived from CD28, CD134, CD137, Lck, ICOS, and DAP10 [14, 15, 20, 24].
Fig. 1.
CAR structures expressed by clinical-grade genetically modified T cells to target CD19. Most CARs combine an antibody binding domain (scFv) that recognizes a desired tumor associated antigen (TAA) with one or more T cell receptor signaling endodomains. Oligomerization of CAR on the T cell surface (such as by chimeric Fc regions derived from immunoglobulin) leads to activation of T cells through CD3ζ and other chimeric co-stimulatory domains (e.g., CD28 and CD137). Signaling through CAR in addition to CD3ζ appears to enhance the therapeutic potential of the genetically modified T cells. Thus, second-generation CARs are considered superior to a first-generation CAR design that activates T cells solely through CD3ζ. The final choice for design of second-generation CAR structure has not been determined and likely depends on tumor load, TAA density, and the sub-type of genetically modified lymphocytes to be infused. Based on available clinical data it appears that populations of CAR+ T cells that are activated through CD137/CD3ζ and CD28/CD3ζ can both target malignant cells (and normal B cells). However, a CAR that signals through CD137/CD3ζ has resulted in the most impressive anti-tumor effects to date and thus is currently considered by many to be the preferred second-generation design
Implicit in the design of CARs is the desire by investigators to improve the survival of adoptively transferred T cells as persistence correlates with their therapeutic potential. While the optimal CAR design remains to be determined, results from early clinical trials appear to indicate that first-generation technology, in which a CAR signals solely through immunoreceptor tyrosine-based activation motif (ITAM) domains on CD3-ζ, is unlikely to sustain the in vivo persistence of T cells in most patients [16, 25–28]. Second-generation CARs, which have signaling domains in addition to CD3-ζ coupled to co-stimulatory molecules, have improved T cell effector functioning [16, 29, 30]. For this reason, most current clinical trials infusing CAR T cells use the second-generation CAR design. Third-generation CARs include a combination of co-stimulatory endodomains (e.g., combining chimeric CD28 and CD137 (or CD134) with CD3-ζ) which may be capable of supraphysiologic signaling [20].
The persistence of infused CAR+ T cells may be curtailed by endogenous immune response recognizing immunogenic determinants from the genetically modified T cells. Modifications to scFv region may reduce potential immunogenicity by using humanized scFv regions, for example, to target carcinoembryonic antigen (CEA) [31] and ERBB2 [20]. It is anticipated that these humanized CARs may avoid immune-mediated recognition leading to elimination of the genetically modified T cells.
Approaches to Genetic Modification of Clinical-Grade T Cells to Express CAR
Genetic manipulation of T cells for the introduction of CAR transgene often relies on transduction using recombinant retrovirus. As an alternative, we and others are investigating the clinical potential of non-viral approaches to gene transfer. The different approaches to the expression of transgenes are summarized reviews by June and Jena [32, 33]. Recombinant retroviral systems can efficiently and stably genetically modify populations of T cells with the inherent goal to shorten their in vitro time to production and release, since prolonged time in culture can lead to terminal differentiation and replication senescence [34–39]. Despite the theoretical risk for insertional mutagenesis there has been no apparent genotoxicity attributed to CAR+ T cells genetically modified with retrovirus. However, transduction using recombinant clinical grade retroviruses can be cumbersome and expensive as it requires specialized facilities and personnel skilled in current good manufacturing practice (cGMP). Nevertheless, retroviral transduction systems have been extensively studied and validated in the clinical setting. Lentivirus vectors offer some advantages over retroviral vectors, in that, like non-viral gene transfer using the Nucleofector technology, they can transduce non dividing cells [40, 41]. This avoidance of ex vivo activation before transduction may contribute to reducing the activation induced clonal exhaustion and cell death [42]. Lentiviruses when compared to retroviruses also have a higher cargo capacity and reduced susceptibility to gene silencing. Although insertional mutagenesis is still a possibility, there is a reduced possibility of integration in to transcriptionally sensitive sites compared to retroviral vectors [43, 44]. In efforts to determine the superiority of either approach, investigators at Memorial Sloan Kettering and the University of Pennsylvania have designed an NCI funded collaborative study in which patients will receive a 50/50 mix of CARs manufactured with lentivirus and retrovirus. Results of this study will yield important information towards the optimization of the manufacturing process [45].
As an alternative to transduction, electroporation has been adapted as an approach to the nonviral gene transfer of DNA plasmids to generate CAR+ T cells [27, 46, 47]. The electrotransfer and integration of naked plasmid DNA into T cells has been considered inefficient because it depends on illegitimate recombination for stable genomic insertion of nonviral sequences. As a result, lengthy in vitro culturing times were required to select for stably transfected T cells, leading to senescence of some of the T cells and decreased efficacy [48, 49]. The efficiency of integration can be greatly improved leading to shortened time in tissue culture using transposon and transposase systems such as derived from Sleeping Beauty (SB) [46, 50, 51] and piggyBac [52, 53] to stably introduce CAR from electrotransferred DNA plasmids [47, 52, 54–58]. The electroporation of T cells in compliance with cGMP with clinical-grade supercoiled DNA plasmids from the SB system is less costly compared with retrovirus or lentivirus systems. We have shown that the SB system can be used to introduce CAR and other transgenes into primary human T cells with approximately 60-fold improved integration efficiency, compared with electro transfer of DNA transposon plasmid without transposase [50]. After electroporation, T cells can be rapidly expanded in a CAR-dependent manner by recursive culture on γ-irradiated artificial antigen-presenting cells (aAPC) achieving clinically sufficient numbers of cells for infusion within a few weeks after electroporation.
Clinical Trials Infusing Autologous CD19-Specific CAR+ T Cells
CD19-Specific CAR There have been several clinical reports describing the therapeutic potential of targeting CD19 on malignant B cells by CAR+ T cells and six studies are highlighted here. Kalos et al. treated three patients with advanced chemo-refractory CLL with second generation CAR+ CD19-specific T cells. The “CART-19” cells were autologous T cells transduced with a lentivirus construct that expressed a CAR signaling through both CD3-ζand 4-IBB costimulatory domain to target CD19 on CLL cells. All three patients had received extensive prior chemo-immunotherapy and two of the three patients had p53 deletion which has shown to be a poor prognostic factor in CLL. At the time of the CART 19 infusion all three patients had extensive lymphadenopathy, bone marrow (BM) infiltration (40 – 95 %) and one patient had peripheral lymphocytosis. All patients received lymphodepleting chemotherapy prior to a single infusion of CAR+ T cells. The CART-19 cells infusion was administered over 3 days because of prior concerns regarding toxicity due to synchronous activation of a bolus of T cells. In two of three patients delayed release of high levels of cytokines was observed, but classic cytokine storm was not observed presumably because the infusion was given over 3 days and because of use of signaling domains that did not promote secretion of IL-2 and TNF-α [59••, 60••]. Potent antileukemic responses were observed in all three patients as two of three recipients achieved a complete remission and one patient achieved a partial remission. In contrast to previously reported studies the authors were able to show >1,000-fold expansion of CAR+ T cells in vivo and persistence for greater than 6 months. The correlative studies benefited from the application of a monoclonal antibody that detected the scFv region and multi-parameter flow cytometry demonstrated that a sub-population of infused T cells persisted with a memory phenotype.
Brentjens et al., treated 10 patients with chemotherapy-refractory CLL or relapsed B cell acute lymphoblastic leukemia (ALL) with autologous T cells modified with retrovirus to express CD19-28z (a second-generation CAR that signals through CD28 and CD3-ζ). In contrast to their earlier report [61•], the infusions of CAR+ T cells split over 2 days was well tolerated in eight of the nine treated patients. Three of four patients with bulky CLL who received prior conditioning with cyclophosphamide exhibited either a significant reduction or a mixed response in lymphadenopathy. The persistence of infused T cells was enhanced by lymphodepleting the recipient with cyclophosphamide administration and was inversely proportional to the peripheral blood tumor burden. The authors showed rapid trafficking of modified T cells to tumor. One patient with bulky CLL developed a syndrome of hypotension, dyspnea, and renal failure following administration of T cells that were delivered as one infusion. He died 4 days after administration of cyclophosphamide and modified T cells. Post mortem studies did not attribute the cause of death to infusion of the CAR+ T cells. Another subject at the −1 treatment T cell dose level exhibited fever and transient hypotensive episode 24 hours after T cell infusion that responded to increased intravenous hydration. This episode rapidly resolved with no evidence of infectious etiology or renal compromise [62].
Kochenderfer et al. achieved lymphodepletion in a patient with advanced non-Hodgkin lymphoma (NHL) with fludarabine and cyclophosphamide preconditioning followed by infusion of autologous T cells modified with retrovirus transduction to express a second generation CAR that recognized CD19 and activated T cells via CD28 and CD3-ζ. Significant regression of the follicular lymphoma was noted which could be attributed to the chemotherapy and/or T cell infusion. Evidence for in vivo activity of administered T cells was based on observation that B-cell precursors were selectively eliminated from the BM and absent in the peripheral blood for at least 39 weeks following infusion [63]. In a follow up publication, Kochenderfer et al. subsequently treated eight patients with advanced, progressive B cell malignancies (CLL 4, NHL 4). Six of the eight patients obtained objective remissions after single infusion of CAR_ T cells. Four of the eight patients had long-term depletion of normal polyclonal CD19+ B-lineage cells. One patient on the study died from a viral infection presumably because of loss of humor immunity [64]. They were able to detect genetically modified T cells containing the CAR transgene in the blood of all patients. Four of the eight treated patients had significant, but reversible, elevations in the serum of IFNγ and TNF after infusion of CAR+ Tcells and the severity of acute toxicities experienced by the recipients correlated with levels of these pro-inflammatory cytokines [65].
Jensen et al. administered autologous T cells expressing a first-generation CAR that activated only through CD3-ζ in patients with recurrent B cell NHL. The CAR was stably expressed after electro-transfer of a DNA plasmid and use of drug selection and extensive ex vivo propagation to retrieve genetically modified T cells. A total of 15 infusions were administered to four patients. Detection of transferred genetically modified T cells in peripheral blood, as measured by quantitative polymerase chain reaction, was short (1–7 days), and immune rejection responses targeting the bacterial-derived drug selection gene were noted in 2 patients [66].
Savaldo et al. treated six patients with B cell NHL administering two autologous T cell products expressing first- and second-generation CARs with specificity for the CD19 TAA. One CAR product encoded only the CD3-ζ, while the other encoded for both CD28 and CD3-ζ. T cells bearing a CAR that signaled through CD28 endodomain showed enhanced expansion and persistence compared with first-generation CAR+ T cells lacking this endodomain [67].
Kebriaei et al. have begun enrolling patients that receive patient-derived CAR+ T cells after autologous HSCT. The T cells are genetically modified using the Nucleofector system to electrotransfer DNA plasmids coding for a second-generation CAR (that activates T cells through CD28 and CD3-ζ) as a SB transposon and a hyperactive SB transposase [68]. Stable integrants expressing CAR could be retrieved and selectively propagated by co-culture on γ-irradiated CD19+_aAPC without the need to co-express a bacterial-derived selection gene in CAR+ T cells [50]. These electroporated and propagated T cells can be produced in compliance with cGMP for infusion in the lymphopenic recipient who is lymphodepleted due to the conditioning regimen employed for HSCT.
Immunotherapy with CAR+ T Cells Targeting Other TAA on Malignant B Cells
Other targets bedside CD19 that have been studied as targets for CAR and these include CD20, immunoglobulin light chain, CD23, and ROR1 [69–71].
CD20-Specific CAR
Till et al. conducted a pilot clinical trial in patients with relapsed indolent B cell and mantle cell lymphomas to evaluate a third-generation CD20-specific CAR that activated T cells through CD28, 4-1BB, and CD3-ζ. The CAR was introduced into T cells by electroporation. Four patients were enrolled, and three received T cell infusions after lymphodepletion with cyclophosphamide. One patient developed transient infusional symptoms. Two patients without evaluable disease remained progression-free for 12 and 24 months. The third patient had an objective partial remission, but relapsed at 12 months after infusions. Genetically modified T cells were detected by quantitative PCR at tumor sites and at low levels for up to 1 year in peripheral blood. No evidence of host immune responses against infused cells was detected. A similar approach could be used in CLL patients [69].
CAR Specific for Immunoglobulin Light Chain
Most B-CLL cells express monoclonal immunoglobulins expressing either kappa or lambda light chains. Vera et al. modified autologous T cells to target the tumor-associated light chain, thus sparing normal B lymphocytes that expressed the reciprocal light chain, and consequently avoid impairment of humoral immunity. They observed that T cells expressing a CAR targeting kappa light chain exhibited in vitro and in vivo cytotoxic activity against Ig kappa+ tumor cell lines and B-CLL cells. Incorporation of the CD28 endodomain within the CAR enhanced the anti-tumor effects. Free Ig kappa did not compromise the ability of redirected T cells to eliminate Ig kappa+ tumors. Adoptive transfer of CAR+ T cells targeting the appropriate light chain could be a useful immunotherapy approach to treat B-CLL and other -lymphocyte malignancies that clonally express immunoglobulin without entirely compromising humoral immunity [70].
CD23-Specific CAR
Giordano et al. cloned and expressed a CAR targeting the CD23 TAA in autologous T cells and in vitro and in vivo cytotoxic activity against CD23+ tumor cell lines and primary CD23+ CLL cells. This effect was obtained without significant toxicity against normal B cells, in contrast to CARs targeting CD19 or CD20 antigens, which are also expressed by normal B lymphocytes leading to their destruction. Thus, a CD23-specific CAR could be used as selective immunotherapy for the elimination of CD23+ CLL cells [71].
Receptor Tyrosine Kinase-Like Orphan Receptor 1 (ROR1)-Specific CAR
One of the major disadvantages of T cells modified to express chimeric antigen receptors specific for B cell lineage surface molecules such as CD20 or CD19 is that they exert antitumor activity, but deplete normal B cells. This results in profound hypogammaglobulinemia in majority of cases. The receptor tyrosine kinase-like orphan receptor 1 (ROR1) is selectively expressed on B cell CLL, but not on normal B cells and may, therefore, serve as a tumor-specific target for immunotherapy. ROR1 has characteristics of an oncofetal gene and is expressed in undifferentiated embryonic stem cells, B-CLL, mantle cell lymphoma, and other tumors [72, 73], but not in major adult tissues (except for low level expression on adipose tissue and at an early stage of B cell development). Hudecek et al. constructed a ROR1-specific CAR that when expressed in T cells from healthy donors or patients with CLL conferred specific recognition of primary B-CLL and mantle cell lymphoma, but not mature normal B cells. T cell therapies targeting ROR1 may, therefore, be effective in B-CLL and are being pursued by us [17].
Allogeneic T Cells
Clinical trials that infuse allogeneic T cells genetically modified to express CAR are also being conducted. Kochenderfer et al. used retroviral vector to enforce expression of CD19-specific CAR in allogeneic T cells. They treated a 65 year-old man with CLL who had relapsed after HLA-matched unrelated donor HSCT. Following the relapse, the patient received four DLIs with a maximum CD3+ cell dose of 2.9×107/kg and then a second HSCT from the original donor without achieving a response. Five months after the second HSCT the patient received an infusion of 6.2×107 (106 cells/kg) allogeneic CD19-specific CAR+ T cells derived from his unrelated transplant donor. The patient did not receive any other therapy in conjunction with the infusion of genetically modified T cells. After infusion, the patient experienced fevers, fatigue, mild hypoxemia, and intermittent mild hypotension and increases in serum electrolytes consistent with tumor lysis syndrome. The patient’s blood B cell count decreased from 286 cells/μL before infusion of CAR+ T cells to 0 cells/μL, 26 days after the T cell infusion. Before the infusion, CLL cells made up 80 – 90 % of the patient’s hypercellular bone marrow and a bone marrow biopsy performed 26 days after the T cell infusion showed a normocellular marrow, nearly absent B lineage cells, and no evidence of CLL. Imaging studies revealed a greater than 50 % decrease in the size of multiple lymph nodes after the delivery of CAR+ T cells. CAR+ T cells were not detected in the patient’s blood by quantitative PCR during the first week after the T cell infusion, but made up 0.98 % of blood mononuclear cells 11 days after administration. These results encourage further development of donor-derived CAR+ T cells as a treatment for malignant relapse after allogeneic HSCT [74].
Bollard et al. hypothesized that a single T cell platform mediating both antiviral and antileukemic activity could benefit Patients who relapse after allogeneic stem cell transplantation. They prepared cytotoxic T-lymphocyes (CTLs) with specificities through native receptors directed towards Epstein-Barr virus /cytomegalovirus /adenovirus (Ad) and then engineered them to express a CAR targeting CD19. They used allogeneic donor-derived antigen presenting cells expressing Ad antigens and transgenic CMVpp65 following transduction with the Ad vector Ad5f35CMVpp65. Multivirus (MV)-specific CTL were then transduced with a retroviral vector encoding CAR-CD19.28 ζ. Six patients (ALL: 2, CLL: 4) were infused with 1.5 to 4.5×107 cells/m2. There was no infusion related toxicity. One CLL patient developed fever, diarrhea, and hypotension 4 weeks post CTL. Findings were consistent with ileitis at known site of disease. Biopsy showed an absence of normal and malignant B cells, and the presence of CAR-CD19.28 ζ T cells. Persistence of CAR cells was documented by their presence in disease sites for up to 9 weeks. One patient had adenovirus positivity in stool, which resolved without any antiviral treatment. No other patient developed viral infections post CTL. Of the four patients with CLL one patient had resolution of lymphadenopathy within 2 weeks of CTL infusion but following the disappearance of CTLs from peripheral blood, progressed and died. The second CLL patient had stable disease for over 6 months with an influx of T cells in his bone marrow. The remaining two CLL patients are still early but by 6 weeks both have had reductions in their CLL counts without toxicity [75].
Improving Persistence and Trafficking of CAR+ T Cells
Limited survival of infused genetically modified T cells may contribute to a lack of clinical responses. Some reasons for poor persistence are (i) incomplete T cell activation through the CAR, (ii) diminished proliferative capacity of the T cell sub-population into which CAR was inserted, (iii) unfavorable environment into which T cells are infused and/or home to, and (iv) immune response by the recipient leading to clearance of infused T cells. The CAR design has been modified to improve the ability of T cells to undergo a fully competent activation signal to ultimately improve efficacy (Fig. 1). The sub-type of T cell into which the CAR is expressed impacts the ability of the T cell to proliferate and survive after adoptive transfer. Initially, pools of T cells directly obtained from peripheral blood were genetically modified to express CAR. However, sub-populations of T cells, such as exhibiting naïve or central memory genetic and protein signatures, may be produced for improved persistence and thus augmented therapeutic effect. Recent data from a pre-clinical model of CARs directed against the tumor antigen mesothelin suggest that a new subset of T cells termed T stem cell memory (TSCM) may perform better naïve or central memory sub-populations [76]. However, the preferred T cell phenotype for expression of CAR generation remains unknown [77, 78]. Additionally, T cells that signal through additional receptor, for example via an endogenous αβ TCR with specificity for known antigen (e.g. viral antigen or allo-antigen) [28, 79, 80] or via enforced expression of a co-stimulatory molecule such as CD80 and CD137L, may exhibit improved persistence [81]. Triggering such TCRs in vivo can lead to T cell proliferation thereby improving antitumor effect delivered by the introduced CAR [80, 82].
Factors that modulate the host immune milieu have also been manipulated to improve survival of adoptively transferred T cells. Sustained persistence of genetically modified T cells has been observed when patients received lymphodepleting chemotherapy prior to T cell infusion [26, 63]. Infused T cells may proliferate more efficiently in the lymphopenic host through homeostatic mechanisms mediated by the removal of regulatory and suppressor cells and increased availability of otherwise scarce pro-proliferative cytokines, while immunogenicity against the CAR may be attenuated. Recipients can recognize immunogenic transgenes which might lead to immune-mediated clearance of infused genetically modified T cells. Humoral and cellular anti-CAR T-cell immune responses contributed to the limited peripheral persistence of the transferred T cells in earlier studies [83, 84]. The persistence of T cells can also be augmented by providing the recipient with exogenous cytokine support, such as the infusion of supra-physiologic levels of interleukin (IL)-2. Preclinical models have shown that administration of targeted therapy to induce Treg depletion combined with homeostatic cytokines may be a less toxic approach than delivering cytotoxic, non-specific lymphodepletion [85]. However, systemic administration of cytokines may cause toxicity, activate regulatory T cells, and is expensive. As an alternative, investigators have enforced T cell expression of cytokines such as IL-12 and IL-7 to replace the dependence of T cells on exogenous cytokines for survival [86, 87].
Finally, immune responses to the CAR may be avoided using a humanized CAR [88, 89]. However, the viral vector itself may be a target for an immune response [83]. This anti-vector response may be avoided by using non-viral, electroporation techniques to introduce DNA plasmids to express CAR
To effectively penetrate the tumor to recycle effector functions, the genetically modified T cells must home to the sites of malignancy. Migration may be compromised by the loss of desired chemokine receptors during genetic modification and passage ex vivo, or may result from the selection of T cells that are inherently unable to localize to certain tissues. Panels of tissue-specific homing receptors which are typically composed of integrins, chemokines, and chemokine receptors are associated with T cell migration to anatomic sites of malignancy. Therefore, flow cytometry can be used to describe the potential migration patterns of T cells before infusion [90, 91]. Gene therapy can be used to enforce expression of desired chemokine receptors to administer CAR+ T cells with desired homing abilities.
Safety of CAR+ T Cells
Although several Phase I clinical trials infusing ex vivo propagated autologous T cells are in progress (Table 1) [92], there are still concerns regarding the safety profile. Firstly, toxicity attributable to undesired, on-target effects of the transgene has been observed [83, 93, 94] Hepatic toxicity necessitating discontinuation of treatment in one patient, and dose reduction in two patients was required after infusion of T cells engineered to express CAR specific for CAIX in a patient with renal cell carcinoma (RCC). While CAIX is over-expressed on RCC cells it is also expressed to a reduced extent on epithelial cells lining the digestive tract, including liver bile ducts. Biopsy of the liver in one of these patients indicated an attack of the modified T cells against the bile ducts expressing CAIX resulting in cholangitis [83, 94] Similarly, low-level expression of ERBB2 on normal lung tissue may have been associated with the sudden death of a patient who received a large dose (delivered as a single infusion) of autologous HER2-specific T cells expressing a third-generation CAR [95]. The elimination of normal B cells and profound lymhopenia in the study reported by Kochenderfer and colleagues [63] is an example of an undesired, on-target toxicity. Thus, until B-cell immunity recovers patients that receive CD19-specific CAR+ T cells should receive intravenous immunoglobulin. Two deaths in patients that received CD19-specific CAR+ T cells have not been attributed to the genetically modified T cells.
Table 1.
Published early-phase clinical trials enrolling patients with CLL to receive autologous CD19-specific CAR+ T cells
| Reference | CAR construct and gene transfer approach |
CAR signaling endodomain |
Diagnosis (N) |
Cell dose (CAR+ T cells) |
Chemotherapy prior to CAR+ infusion |
Loss of B cells/Serious Adverse Event |
T-cell persistence |
Anti-tumor response (N) |
|---|---|---|---|---|---|---|---|---|
| Kalos [59••] | CD 19, 2nd gen CAR, lentivirus vector |
CD 137 & CD3-ζ | CLL (3) | 0.014-1.1×109 | yes | Yes | >6 months | CR (2) PR(1) |
| Brentjens [62] | CD 19, 2nd gen CAR, retroviral vector |
CD28 & CD3-ζ | CLL (8) ALL (1) |
0.01-3.2×109 | yes | Yes/One death | 0 to 8 weeks | PD (4) SD (3) NE (1) B-cell aplasia (1) |
| Kochenderfer [65] | CD 19, 2nd gen. CAR, retroviral vector |
CD28 & CD3-ζ | CLL (4) NHL (4) |
0.3-3.0×l07/kg | yes | Yes/One death | <20 days to 6 months |
CR(1) PR (5) SD (1) NE (1) |
Abbreviations: CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin lymphoma: CR, complete remission; PR, partial remission; SD, stable disease; NE, not evaluable; TE, too-early; N, number
There remains a theoretical concern for genotoxicity attributable to the vector [96]. The stable expression of CAR currently requires the introduction of a promoter and the transgene which raises the possibility of insertional mutagenesis leading to autonomous T cell proliferation [97]. To date, there have been no apparent genotoxic events attributed to genetically modified T cells that have been transduced by recombinant virus or electroporation [57, 98]. The risk for insertional mutagenesis may be alleviated to some degree by electro transfer of in vitro transcribed mRNA coding for a CAR [99]. Another area of concern is the type of T cell being infused. In the allogeneic setting, the endogenous αβTCR on donor-derived T cells could target major or minor histocompatibility antigens leading to the development of GVHD. Co-expression of a conditional suicide gene, such as thymidine kinase from herpes simplex virus or dimerizable caspase, may prevent long-term toxicity [100], however, this approach may not control potential CAR-mediated toxicity arising in the acute setting.
Conclusions and Future Directions
The initial results of early phase trials using current CAR technology demonstrate the feasibility and therapeutic potential of genetically modified T cells for patients with CLL and other B-cell malignancies. Further investigation into optimal manufacturing processes to improve T cell persistence, which may be TAA or disease specific, is needed. Future directions will include combination therapies as CAR+ T cells may benefit from concomitant therapy with therapeutic monoclonal antibodies [101] or immunocytokine support [102]. As the technology associated with gene transfer and manufacturing adapts to improve potency, the regulatory oversight for the production and release of the T cells and the safety of the recipient may become more complex. Most pilot trials, including those accruing patients to receive cell and gene therapy, enroll research participants with advanced disease and it is not unexpected for a subset of these medically fragile patients to unfortunately expire during the trial. While it is incumbent on the clinical team to safeguard patient well-being, it is also important for regulatory bodies to continue to monitor gene therapy trials to maintain safety while maintaining progress. The technology to manufacture CAR+ T cells has reached a point of “mass-production” so that many investigators can readily participate in the design and implementation of clinical trials. As the field of adoptive immunotherapy progresses clinical trials will require streamlining the current regulatory processes governing T cell manufacturing so that multi-center trials powered for efficacy can be efficiently conducted to definitively establish the therapeutic potential of CAR+ T cells.
Acknowledgments
Support from: CLL Alliance Global Foundation, MD Anderson Cancer Center Core Grant (CA16672); PO1 (CA100265); RO1 (CA124782, CA120956, CA141303, CA116127); R33 (CA116127); DOD PR064229;
Footnotes
Disclosure C. Hosing: Received grants from Celgene, Sanofi, honoraria from Sanofi, payment for development of educational presentations including service on speakers’ bureaus from Schering-Plough and Genzyme, travel/accommodations expenses covered or reimbursed by Schering-Plough, Genzyme, Mesoblast. P. Kebriaei: nothing to disclose; W. Wierda: nothing to disclose; B. Jena: nothing to disclose; L. Cooper: received grants from NIH and CLL Global Research Foundation and he is the founder of InCellerate, Inc., which seeks to commercialize immune-based therapies; E. Shpall received a grant from Mesoblast.
Contributor Information
Chitra Hosing, Department of Stem Cell Transplantation, University of Texas MD Anderson Cancer Center, Unit 423, 1515 Holcombe Blvd, Houston, TX 77030, USA.
Partow Kebriaei, Department of Stem Cell Transplantation, University of Texas MD Anderson Cancer Center, Unit 423, 1515 Holcombe Blvd, Houston, TX 77030, USA.
William Wierda, Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Bipulendu Jena, Division of Pediatrics, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Laurence J. N. Cooper, Division of Pediatrics, University of Texas MD Anderson Cancer Center, Houston, TX, USA
Elizabeth Shpall, Department of Stem Cell Transplantation, University of Texas MD Anderson Cancer Center, Unit 423, 1515 Holcombe Blvd, Houston, TX 77030, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1 •.Dreger P, Dohner H, Ritgen M, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116:2438–47. doi: 10.1182/blood-2010-03-275420. This is a prospective multicenter phase 2 study investigating the long-term outcome of reduced-intensity conditioning allogeneic stem cell transplantation in patients with poor-risk chronic lymphocytic leukemia.
- 2.Kharfan-Dabaja MA, Pidala J, Kumar A, et al. Comparing efficacy of reduced-toxicity allogeneic hematopoietic cell transplantation with conventional chemo-(immuno) therapy in patients with relapsed or refractory CLL: a Markov decision analysis. Bone Marrow Transplant. 2012 doi: 10.1038/bmt.2012.71. [DOI] [PubMed] [Google Scholar]
- 3.Sorror ML, Storer BE, Sandmaier BM, et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol. 2008;26:4912–20. doi: 10.1200/JCO.2007.15.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khouri IF, Saliba RM, Admirand J, et al. Graft-versus-leukaemia effect after non-myeloablative haematopoietic transplantation can overcome the unfavourable expression of ZAP-70 in refractory chronic lymphocytic leukaemia. Br J Haematol. 2007;137:355–63. doi: 10.1111/j.1365-2141.2007.06591.x. [DOI] [PubMed] [Google Scholar]
- 5.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–83. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 6.Cullis JO, Jiang YZ, Schwarer AP, et al. Donor leukocyte infusions for chronic myeloid leukemia in relapse after allogeneic bone marrow transplantation. Blood. 1992;79:1379–81. [PubMed] [Google Scholar]
- 7.Drobyski WR, Keever CA, Roth MS, et al. Salvage immunotherapy using donor leukocyte infusions as treatment for relapsed chronic myelogenous leukemia after allogeneic bone marrow transplantation: efficacy and toxicity of a defined T-cell dose. Blood. 1993;82:2310–8. [PubMed] [Google Scholar]
- 8.Ferster A, Bujan W, Mouraux T, et al. Complete remission following donor leukocyte infusion in ALL relapsing after haploidentical bone marrow transplantation. Bone Marrow Transplant. 1994;14:331–2. [PubMed] [Google Scholar]
- 9.Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–5. [PubMed] [Google Scholar]
- 10.Lokhorst HM, Schattenberg A, Cornelissen JJ, et al. Donor leukocyte infusions are effective in relapsed multiple myeloma after allogeneic bone marrow transplantation. Blood. 1997;90:4206–11. [PubMed] [Google Scholar]
- 11.Pati AR, Godder K, Lamb L, et al. Immunotherapy with donor leukocyte infusions for patients with relapsed acute myeloid leukemia following partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 1995;15:979–81. [PubMed] [Google Scholar]
- 12.Vago L, Perna SK, Zanussi M, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361:478–88. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 13.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Jensen M, Lin Y, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007;18:712–25. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- 15.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–23. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–1004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 17 •.Hudecek M, Schmitt TM, Baskar S, et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood. 2010;116:4532–41. doi: 10.1182/blood-2010-05-283309. Study targeting the novel B-cell tumor associated antigen ROR 1 with chimeric antigen receptor expressing T-cells.
- 18.Kahlon KS, Brown C, Cooper LJ, et al. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–6. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 19.Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–84. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Wang QJ, Yang S, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–74. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letourneur F, Klausner RD. T-cell and basophil activation through the cytoplasmic tail of T-cell-receptor zeta family proteins. Proc Natl Acad Sci U S A. 1991;88:8905–9. doi: 10.1073/pnas.88.20.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yun CO, Nolan KF, Beecham EJ, et al. Targeting of T lymphocytes to melanoma cells through chimeric anti-GD3 immunoglobulin T-cell receptors. Neoplasia. 2000;2:449–59. doi: 10.1038/sj.neo.7900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abken H, Hombach A, Heuser C. Immune response manipulation: recombinant immunoreceptors endow T-cells with predefined specificity. Curr Pharm Des. 2003;9:1992–2001. doi: 10.2174/1381612033454289. [DOI] [PubMed] [Google Scholar]
- 24.Yvon E, Del Vecchio M, Savoldo B, et al. Immunotherapy of metastatic melanoma using genetically engineered GD2-specific T cells. Clin Cancer Res. 2009;15:5852–60. doi: 10.1158/1078-0432.CCR-08-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 16:1245–56. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–71. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JR, Digiusto DL, Slovak M, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–33. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 28.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finney HM, Lawson AD, Bebbington CR, et al. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161:2791–7. [PubMed] [Google Scholar]
- 30.Hombach A, Wieczarkowiecz A, Marquardt T, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001;167:6123–31. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 31.Hombach A, Schneider C, Sent D, et al. An entirely humanized CD3 zeta-chain signaling receptor that directs peripheral blood t cells to specific lysis of carcinoembryonic antigen-positive tumor cells. Int J Cancer. 2000;88:115–20. [PubMed] [Google Scholar]
- 32.June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol. 2009;9:704–16. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 116:1035–44. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg SA, Aebersold P, Cornetta K, et al. Gene transfer into humans–immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570–8. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 35.Rischer M, Pscherer S, Duwe S, et al. Human gammadelta T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br J Haematol. 2004;126:583–92. doi: 10.1111/j.1365-2141.2004.05077.x. [DOI] [PubMed] [Google Scholar]
- 36.Turatti F, Figini M, Alberti P, et al. Highly efficient redirected anti-tumor activity of human lymphocytes transduced with a completely human chimeric immune receptor. J Gene Med. 2005;7:158–70. doi: 10.1002/jgm.647. [DOI] [PubMed] [Google Scholar]
- 37.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naldini L, Blomer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–7. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 39.Varela-Rohena A, Carpenito C, Perez EE, et al. Genetic engineering of T cells for adoptive immunotherapy. Immunol Res. 2008;42:166–81. doi: 10.1007/s12026-008-8057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh H, Figliola MJ, Dawson MJ, et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Cancer Res. 2011;71:3516–27. doi: 10.1158/0008-5472.CAN-10-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu WS, Pathak VK. Design of retroviral vectors and helper cells for gene therapy. Pharmacol Rev. 2000;52:493–511. [PubMed] [Google Scholar]
- 42.Cavalieri S, Cazzaniga S, Geuna M, et al. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102:497–505. doi: 10.1182/blood-2003-01-0297. [DOI] [PubMed] [Google Scholar]
- 43.Montini E, Cesana D, Schmidt M, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–96. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 44.Newrzela S, Cornils K, Li Z, et al. Resistance of mature T cells to oncogene transformation. Blood. 2008;112:2278–86. doi: 10.1182/blood-2007-12-128751. [DOI] [PubMed] [Google Scholar]
- 45.Frantz S. Engineered T-cell therapy shows efficacy in blood cancer. Nat Biotechnol. 2011;29:853–5. doi: 10.1038/nbt1011-853. [DOI] [PubMed] [Google Scholar]
- 46.Geurts AM, Yang Y, Clark KJ, et al. Gene transfer into genomes of human cells by the sleeping beauty transposon system. Mol Ther. 2003;8:108–17. doi: 10.1016/s1525-0016(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 47.Huang X, Guo H, Kang J, et al. Sleeping Beauty transposon-mediated engineering of human primary T cells for therapy of CD19+ lymphoid malignancies. Mol Ther. 2008;16:580–9. doi: 10.1038/sj.mt.6300404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen MC, Clarke P, Tan G, et al. Human T lymphocyte genetic modification with naked DNA. Mol Ther. 2000;1:49–55. doi: 10.1006/mthe.1999.0012. [DOI] [PubMed] [Google Scholar]
- 49.Cooper LJ, Topp MS, Serrano LM, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637–44. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 50.Singh H, Manuri PR, Olivares S, et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–71. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xue X, Huang X, Nodland SE, et al. Stable gene transfer and expression in cord blood-derived CD34+ hematopoietic stem and progenitor cells by a hyperactive Sleeping Beauty transposon system. Blood. 2009;114:1319–30. doi: 10.1182/blood-2009-03-210005. [DOI] [PubMed] [Google Scholar]
- 52.Manuri PV, Wilson MH, Maiti SN, et al. piggyBac transposon/transposase system to generate CD19-specific T cells for the treatment of B-lineage malignancies. Hum Gene Ther. 21:427–37. doi: 10.1089/hum.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakazawa Y, Huye LE, Dotti G, et al. Optimization of the PiggyBac transposon system for the sustained genetic modification of human T lymphocytes. J Immunother. 2009;32:826–36. doi: 10.1097/CJI.0b013e3181ad762b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hackett PB, Ekker SC, Largaespada DA, et al. Sleeping beauty transposon-mediated gene therapy for prolonged expression. Adv Genet. 2005;54:189–232. doi: 10.1016/S0065-2660(05)54009-4. [DOI] [PubMed] [Google Scholar]
- 55.Ivics Z, Hackett PB, Plasterk RH, et al. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997;91:501–10. doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- 56.Izsvak Z, Chuah MK, Vandendriessche T, et al. Efficient stable gene transfer into human cells by the Sleeping Beauty transposon vectors. Methods. 2009;49:287–97. doi: 10.1016/j.ymeth.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Hackett PB, Largaespada DA, Cooper LJ. A transposon and transposase system for human application. Mol Ther. 18:674–83. doi: 10.1038/mt.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Izsvak Z, Hackett PB, Cooper LJ, et al. Translating Sleeping Beauty transposition into cellular therapies: victories and challenges. Bioessays. 32:756–67. doi: 10.1002/bies.201000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59 ••.Kalos M, Levine BL, Porter DL, et al. Tcells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. Trial showing impressive clinical activity in 3 patients with relapsed/refractory chronic lymphocytic leukemia who received treatment with chimeric antigen receptor modified T cells.
- 60 ••.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. Study showing long-term persistence of engineered T cells in-vivo.
- 61 •.Brentjens R, Yeh R, Bernal Y, et al. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–8. doi: 10.1038/mt.2010.31. Unforseen adverse event in a patient treated with genetically engineered T-cells.
- 62.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooper LJ, Jena B, Bollard CM. Good T cells for bad B cells. Blood. 2012;119:2700–2. doi: 10.1182/blood-2011-12-398719. [DOI] [PubMed] [Google Scholar]
- 65.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16:1245–56. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kebriaei P, Huls H, Jena B, et al. Infusing CD19-directed T cells to augment disease control in patients undergoing autologous hematopoietic stem-cell transplantation for advanced B-lymphoid malignancies. Hum Gene Ther. 2012;23:444–50. doi: 10.1089/hum.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Till BG, Jensen MC, Wang J, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–50. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vera J, Savoldo B, Vigouroux S, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–7. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giordano Attianese GM, Marin V, Hoyos V, et al. In vitro and in vivo model of a novel immunotherapy approach for chronic lymphocytic leukemia by anti-CD23 chimeric antigen receptor. Blood. 2011;117:4736–45. doi: 10.1182/blood-2010-10-311845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang S, Chen L, Cui B, et al. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS One. 2012;7:e31127. doi: 10.1371/journal.pone.0031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamaguchi T, Yanagisawa K, Sugiyama R, et al. NKX2-1/TITF1/TTF-1-Induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell. 2012;21:348–61. doi: 10.1016/j.ccr.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 74.Kochenderfer JN, AffiliationsNational Cancer Institute B, MD, MED et al. Dramatic Regression of Chronic Lymphocytic Leukemia in the First Patient Treated With Donor-Derived Genetically-Engineered Anti-CD19-Chimeric-Antigen-Receptor-Expressing T Cells After Allogeneic Hematopoietic Stem Cell Transplantation. BBMT. 2011;17:S158. [Google Scholar]
- 75.Bollard C, Cruz C, Savoldo B, et al. Outcomes of CD19-directed multivirus specific cytotoxic T lymphocyte therapy for patients with relapsed B cell malignancies after allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplantation. 2012;47:S56. [Google Scholar]
- 76.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–7. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berger C, Berger M, Anderson D, et al. A non-human primate model for analysis of safety, persistence, and function of adoptively transferred T cells. J Med Primatol. doi: 10.1111/j.1600-0684.2010.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hinrichs CS, Borman ZA, Cassard L, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009;106:17469–74. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rossig C, Bollard CM, Nuchtern JG, et al. Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 2002;99:2009–16. doi: 10.1182/blood.v99.6.2009. [DOI] [PubMed] [Google Scholar]
- 80.Cooper LJ, Al-Kadhimi Z, Serrano LM, et al. Enhanced antilymphoma efficacy of CD19-redirected influenza MP1-specific CTLs by cotransfer of T cells modified to present influenza MP1. Blood. 2005;105:1622–31. doi: 10.1182/blood-2004-03-1208. [DOI] [PubMed] [Google Scholar]
- 81.Stephan MT, Ponomarev V, Brentjens RJ, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–9. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 82.Pule MA, Straathof KC, Dotti G, et al. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–41. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 83.Lamers CH, Willemsen R, van Elzakker P, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo engineered T cells. Blood. 2010 doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 84.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–15. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cui Y, Zhang H, Meadors J, et al. Harnessing the physiology of lymphopenia to support adoptive immunotherapy in lymphoreplete hosts. Blood. 2009;114:3831–40. doi: 10.1182/blood-2009-03-212134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pegram HJ, Lee JC, Hayman EG, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–41. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoyos V, Savoldo B, Quintarelli C, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–70. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manuri SO PR, Dara N, Dawson MJ, Huls H, Lee DA, Shpall EJ, Champlin RE, Cooper LJN. A fully-human chimeric antigen receptor for redirecting specificity of T cells to B-lineage tumors American Society for Blood and Marrow Transplantation, Tandem Meetings; San Diego, California. 2008. pp. 13–14. [Google Scholar]
- 89.Willemsen RA, Debets R, Hart E, et al. A phage display selected fab fragment with MHC class I-restricted specificity for MAGE-A1 allows for retargeting of primary human T lymphocytes. Gene Ther. 2001;8:1601–8. doi: 10.1038/sj.gt.3301570. [DOI] [PubMed] [Google Scholar]
- 90.Palendira U, Chinn R, Raza W, et al. Selective accumulation of virus-specific CD8+ T cells with unique homing phenotype within the human bone marrow. Blood. 2008;112:3293–302. doi: 10.1182/blood-2008-02-138040. [DOI] [PubMed] [Google Scholar]
- 91.Brown CE, Vishwanath RP, Aguilar B, et al. Tumor-derived chemokine MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred T cells. J Immunol. 2007;179:3332–41. doi: 10.4049/jimmunol.179.5.3332. [DOI] [PubMed] [Google Scholar]
- 92.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–76. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–2. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 95.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chime-ric antigen receptor recognizing ERBB2. Mol Ther. 18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–42. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baum C, Kustikova O, Modlich U, et al. Mutagenesis and onco-genesis by chromosomal insertion of gene transfer vectors. Hum Gene Ther. 2006;17:253–63. doi: 10.1089/hum.2006.17.253. [DOI] [PubMed] [Google Scholar]
- 98.Bonini C, Grez M, Traversari C, et al. Safety of retroviral gene marking with a truncated NGF receptor. Nat Med. 2003;9:367–9. doi: 10.1038/nm0403-367. [DOI] [PubMed] [Google Scholar]
- 99.Choi Y, Yuen C, Maiti SN, et al. A high throughput microelectroporation device to introduce a chimeric antigen receptor to redirect the specificity of human T cells. Biomed Microdevices. 12:855–63. doi: 10.1007/s10544-010-9440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoyos V, Savoldo B, Quintarelli C, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 24:1160–70. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.James SE, Orgun NN, Tedder TF, et al. Antibody-mediated B-cell depletion before adoptive immunotherapy with T cells expressing CD20-specific chimeric T-cell receptors facilitates eradication of leukemia in immunocompetent mice. Blood. 2009;114:5454–63. doi: 10.1182/blood-2009-08-232967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singh H, Serrano LM, Pfeiffer T, et al. Combining adoptive cellular and immunocytokine therapies to improve treatment of B-lineage malignancy. Cancer Res. 2007;67:2872–80. doi: 10.1158/0008-5472.CAN-06-2283. [DOI] [PubMed] [Google Scholar]