Dear editor
It is with great interest that we read the publication entitled “Critical appraisal of ranibizumab in the treatment of diabetic macular edema” by Stewart.1 The author emphasized the importance of the vascular endothelial growth factor (VEGF) in the pathophysiology of diabetic macular edema (DME). As highlighted in that article, the anti-VEGF ranibizumab is a superior treatment compared to traditional argon photocoagulation, leading to better anatomical and functional results.
In April 2013, the National Institute for Health and Care Excellence (NICE) of the UK approved the use of ranibizumab as a treatment option to treat diabetic macular edema of the eye if it has a central macular thickness (CMT) of 400 μm or more at the beginning of the treatment.2 The guidelines did not specify which optical coherence tomography (OCT) device(s) should be used for this assessment. This is important as, although good consistency has been shown in using the same instrument, there is a known divergence in CMT measurements between different instruments.3–6 For example, the Spectralis® OCT (Heidelberg Engineering; Carsbad, CA, USA) generally shows higher values of mean CMT in a normal eye compared to most other instruments, in part due to the retinal segmentation algorithm that it employs.4 We hypothesized that similar (or increased) differences might be observed in DME, and that for those countries (such as the UK) where a fixed CMT is used to define eligibility for treatment, the “lottery” of OCT instruments may influence eligibility.
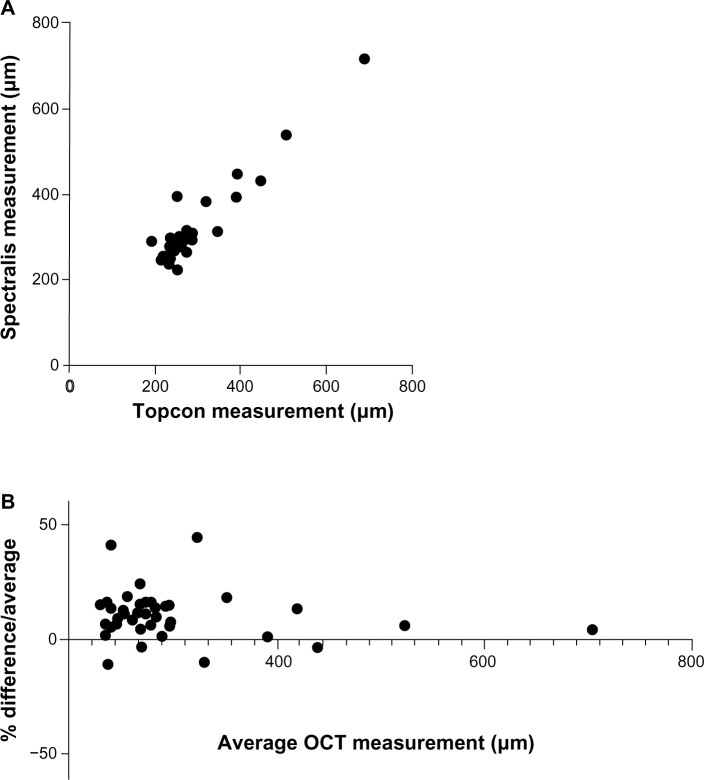
In light of this hypothesis, we conducted a preliminary analysis of 24 patients (48 eyes) with suspected DME who had OCT scans performed on the same day using both 3D OCT-1000 (Topcon; Itabashi, Tokyo, Japan) and Spectralis OCT. Matched macular-centered scans were obtained in 42 eyes; scans were not possible in 6 eyes due to media opacity or problems with patient fixation. The mean (standard deviation) CMT in this cohort was 282.0 (89.0) μm with a range of 191–689 μm using the Topcon OCT, and 312.4 (88.8) μm with a range of 224–719 μm using the Spectralis OCT (Figure 1A). Comparing the two instruments in our cohort using a Bland–Altman analysis, there was a bias of +10.73 μm to the Spectralis with a standard deviation of 10.32, and 95% limits of agreement of −9.497 to 30.96 μm (Figure 1B).
Figure 1.
Comparison of CMT measurements acquired on Topcon 3D OCT-1000 versus Spectralis® OCT for patients with DME. Direct comparison (A) and Bland– Altman plot (B).
Notes: Spectralis® OCT manufactured by Heidelberg Engineering (Carsbad, CA, USA); 3D OCT-1000 manufactured by Topcon (Itabashi, Tokyo, Japan).
Abbreviations: CMT, central macular thickness; DME, diabetic macular edema; OCT, optical coherence tomography.
Recognizing this issue is important for all those involved in care of patients in countries or institutions where the entry to treatment is limited by a defined CMT level. In the specific example considered here, this finding has a direct clinical impact on patients who have DME with a central macular thickness of 390–410 μm. In our small cohort of matched scans from 42 eyes, there were three whose CMT was >400 μm on the Topcon and four whose CMT was >400 μm on the Spectralis: ie, even in this small study, a patient’s eligibility for treatment depended on which scan was used. “Real-world” studies of OCT will become increasingly important if defined CMT levels are to be used as the “gate-keeper” for treatment, and should include repeatability and inter-instrument variability in defined patient cohorts.
Footnotes
Disclosure
Dr Keane is funded by the Department of Health’s NIHR Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital and UCL Institute of Ophthalmology. The views expressed in the publication are those of the author and not necessarily those of the Department of Health. None of the authors have any other conflicts of interest to declare.
References
- 1.Stewart MW. Critical appraisal of ranibizumab in the treatment of diabetic macular edema. Clin Ophthalmol. 2013;7:1257–1267. doi: 10.2147/OPTH.S36443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranibizumab for treating diabetic macular oedema (rapid review of technology appraisal guidance 237) [webpage on the Internet] London: National Institute for Health and Clinical Excellence; 2013. [Accessed August 31, 2013]. Available from: http://publications.nice.org.uk/ranibizumab-for-treating-diabeticmacular-oedema-rapid-review-of-technology-appraisal-guidance-ta274. [Google Scholar]
- 3.Grover S, Murthy RK, Brar VS, Chalam KV. Comparison of retinal thickness in normal eyes using Stratus and Spectralis optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(5):2644–2647. doi: 10.1167/iovs.09-4774. [DOI] [PubMed] [Google Scholar]
- 4.Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2009;50(7):3432–3437. doi: 10.1167/iovs.08-2970. [DOI] [PubMed] [Google Scholar]
- 5.Leung CK, Cheung CY, Weinreb RN, et al. Comparison of macular thickness measurements between time domain and spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2008;49(11):4893–4897. doi: 10.1167/iovs.07-1326. [DOI] [PubMed] [Google Scholar]
- 6.Bentaleb-Machkour Z, Jouffroy E, Rabilloud M, Grange JD, Kodjikian L. Comparison of central macular thickness measured by three OCT models and study of interoperator variability. Scientific World Journal. 2012;2012:842795. doi: 10.1100/2012/842795. [DOI] [PMC free article] [PubMed] [Google Scholar]