Abstract
In the medical field, attached bacteria can cause infections associated with catheters, incisions, burns, and medical implants especially in immunocompromised patients. The problem is exacerbated by the fact that attached bacteria are ~1000 times more resistant to antibiotics than planktonic cells. The rapid spread of antibiotic resistance in these and other organisms has led to a significant need to find new methods for preventing bacterial attachment. The goal of this research was to evaluate the effectiveness of novel polymer coatings to prevent the attachment of three medically relevant bacteria. Tests were conducted with Pseudomonas aeruginosa, Staphylococcus epidermidis, and Staphylococcus aureus for oligomers derived from modifications of natural rubber (cis 1,4-polyisoprene). The different oligomers were: PP04, with no quaternary ammonium (QA); MV067, one QA; PP06, three QA groups. In almost all experiments, cell attachment was inhibited to various extents as long as the oligomers were used. PP06 was the most effective as it decreased the planktonic cell numbers by at least 50% for all bacteria. Differences between species sensitivity were also observed. P. aeruginosa was the most resistant bacteria tested, S. aureus, the most sensitive. Further experiments are required to understand the full extent and mode of the antimicrobial properties of these surfaces.
Keywords: Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Polyisoprene, Antibacterial, Rubber
1. Introduction
Biofilms are communities of microorganisms growing in a self-made extracellular matrix of polysaccharides, DNA and other compounds. Generally, bacteria attach irreversibly to surfaces, and express different gene profiles in a biofilm compared to those of free living, planktonic bacteria. Once a biofilm has formed, the bacteria are extremely resistant to treatment with antimicrobials. A major problem is biofilms that grow on catheters, incisions, burns, medical implants and in immunocompromised patients [1]. It has been estimated that 80% of all microbial infections are associated with biofilms [2]. Nosocomial infections such as these present a major health burden. Reports have shown that bacteria in a biofilm are up to 1000-fold more resistant to the effects of antibiotics than bacteria growing as planktonic forms [3,4]. It is believed that the bacteria undergo unique phenotypic transformations that confer resistance to conventional therapeutic antibiotics [5]. The rapid spread of antibiotic resistance in these and other organisms mean that there is a great need for other methodologies of fighting bacterial infections, such as altering surfaces where biofilms form.
The three most prevalent bacterial species that develop biofilms causing infections are Pseudomonas aeruginosa, Staphylococcus epidermidis, and Staphylococcus aureus. S. aureus has been identified as the dominant strain associated with biofilms in patients with chronic rhinosinusitis [6]. S. aureus and S. epidermidis are the two Gram positive strains most commonly associated with infections on medical devices such as catheters, orthopedics, artificial heart valves and shunts [7–12]. P. aeruginosa is a Gram negative rod commonly found as aggregates in the lungs of cystic fibrosis patients, as well as on the bandages of burn patients, on contact lens, urinary catheters, ports for cancer patients, and other implanted devices [13–18].
The initial attachment of bacteria to either biotic and abiotic/implanted surfaces can increase their resistance to the natural immune responses of the body and to antibiotic treatments. Most attachment occurs relatively quickly, sometimes in as little as 60 s [19,20]. Although attachment is rapid, there are distinct steps involved in the colonization of bacteria to a surface: the conditioning of the surface via accumulation of organic molecules, a reversible binding stage of cells, followed by irreversible attachment and biofilm development. The first major step is the reversible adherence of single cells to a surface by van der Waals forces and hydrogen bonding. The second step is less likely to be reversible and is mediated by specific interactions between cells and host proteins [21]. Rohde et al. [1] found that infections caused by S. epidermidis were dependent on the organism’s ability to adhere to surfaces prior to assembling into a large biofilm. Both attachment steps occur by a variety of mechanisms including specific receptors, proteins and overall surface charge, which leads to the extensive hydrogel-like extracellular matrix. While the exact mechanism of biofilm formation in terms of specific receptor or other proteins is species dependent, the most effective method of preventing biofilm formation is still to prevent the initial stages of protein and bacterial attachment [5,7,22,23]. Studies have shown that surface charge, hydrophobicity, surface roughness and microtopography can be important in preventing the initial attachment, as well as culture conditions, pH, ionic strength and the species of bacteria involved [24].
We have developed novel polymer formulations derived from natural rubber [25]. The overall goal of this research is to evaluate the effectiveness of the novel polymers in preventing attachment and subsequent biofilm formation of three medically relevant bacteria. The antimicrobial properties of the acrylate and ammonium oligomers are also compared.
2. Materials and methods
2.1. Bacterial cultures
Pseudomonas aeruginosa PA01 (ATCC # BAA-47), Staphylococcus aureus FDA 209 (ATCC # 6538), and Staphylococcus epidermidis RP62A (ATCC # 35984) were the medically relevant organisms chosen for this study. All strains were purchased from the American Type Culture Collection and maintained on Tryptic Soy Agar (TSA). Stock cultures were grown overnight at 37 °C in Tryptic Soy Broth (TSB). For attachment assays, cultures were inoculated with cells of OD600 of approximately 0.05, corresponding to a starting inoculum of 107 cells/mL, as detailed below.
2.2. Growth curves
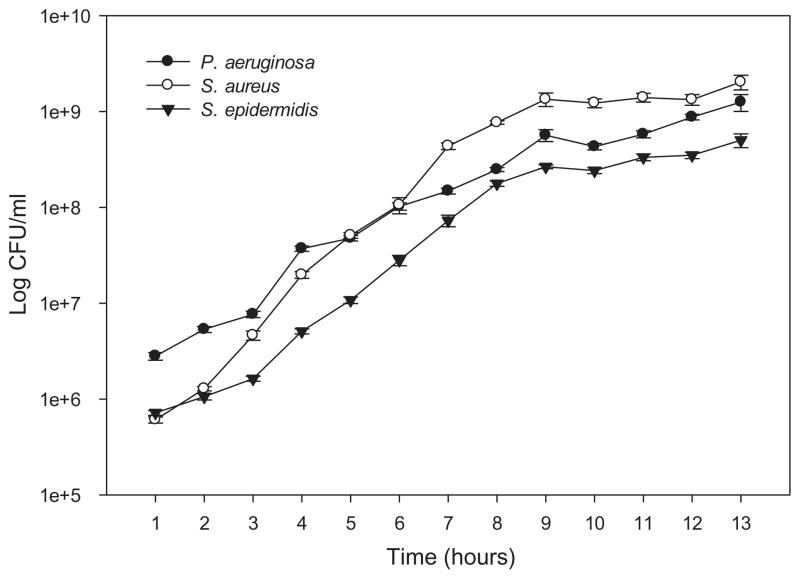
Growth curves were performed for each strain to ascertain the initial inoculation density for the static attachment assays (Fig. 2). They were also used to access the toxicity of the polymers by comparing initial and final microbial numbers. One mL of overnight culture was transferred into 100 mL of fresh TSB medium and incubated at 37 °C and 250 rpm. Initial OD600 and cell counts were carried out at time zero. A one mL sample was removed every hour for 13 h, and an OD600 taken. Cells were then diluted, plated on TSA plates using a spiral plater (Autoplate® Spiral biotech, Bethesda, MD, USA), incubated overnight at 37 °C and colonies counted to determine colony forming units, CFU/ml.
Fig. 2.
Growth curves of S. aureus, S. epidermidis and P. aeruginosa in TSB. Error bars, standard error (n = 3).
2.3. Preparation of polymer surfaces
The syntheses of all the oligomers used in this study have been described in detail [25]. Three different types of oligomers were prepared (Fig. 1: PP04, MV067, PP06) and combined in different proportions (Table 1) to generate the different surfaces. In addition to the acrylate oligomer composed of the simple polyisoprene chain (PP04, Fig. 1) [26], we introduced one quaternary ammonium (QA) group at one chain end (MV067, Fig. 1), or three QA groups in the same oligomer (PP06, Fig. 1). A series of formulations was prepared varying the percentages of the different oligomers. The rationale was to start with 100% of the simple acrylate oligomer, which is expected to have the lowest or no antibacterial action, and to increase the weight percent of a second oligomer containing one or three QA groups, to evaluate QA action and to optimize the composition of the coatings. In some cases HDDA (hexanediol diacrylate), a reactive diluent, was added to reduce pre-polymerization solution viscosity. For each coating, the prepolymerization mixture was applied into each well of a 24 well plate and polymerized [25].
Fig. 1.
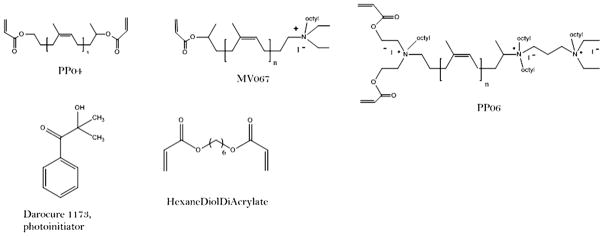
Structure of the natural rubber derived oligomers PP04, MV067, and PP06. Darocur 1173 was used as a photoinitiator and HDDA (hexanedioldiacrylate) as a reactive diluant.
Table 1.
Experimental protocols for testing antimicrobial properties of polymers applied to surfaces. (A) Polymer mixtures with and without reactive diluent (HDDA) in Protocol 1, (B) Polymer mixtures using MV076 in Protocol 2. PPO4 is an acrylate oligomer; PPO6 and MV067 are different ammonium oligomers.
| Formulation | %PPO4 | %PPO6 | %HDDA |
|---|---|---|---|
| (A) Protocol 1 | |||
| S1 | 100 | 0 | 0 |
| S15 | 0 | 100 | 0 |
| S16 | 50 | 50 | 0 |
| S18 | 75 | 25 | 0 |
| S19 | 70 | 0 | 30 |
| S20 | 0 | 70 | 30 |
| S21 | 35 | 35 | 30 |
| S22 | 85 | 0 | 15 |
| Formulation | %PPO4 | %MV067 |
|---|---|---|
| (B) Protocol 2 | ||
| S1 | 100 | 0 |
| S3 | 0 | 100 |
| S5 | 50 | 50 |
2.4. Assaying antibacterial effects of polymers
Growth surfaces of 24-well tissue culture plates were coated with mixtures of polymers, as specified in Table 1. Each plate consisted of a positive and negative control and replicates of each strain tested. Table 1 lists the testing protocol. One mL of TSB was added to each well of the plates. Two μL (1:100 dilution of overnight culture of approximately OD600 = 0.05) of P. aeruginosa, S. aureus, or S. epidermidis were introduced into separate sample wells and incubated at 37 °C for 3 h. Triplicates of controls and samples were used for each bacteria-polymer sample.
The amount of attachment on each polymer surface, as well as planktonic growth (unattached cells in the medium overlying the surface), was assayed at the end of the contact time, by two methods. The first method used 1 μL of growth medium solution for colony counts (CFU/ml), as described in Section 2.2. The second method assessed the amount of loosely attached biofilm. Two mL of fresh TSB medium was added to the washed plates, the wells were sonicated for 8 s, and medium containing the detached cells was removed, diluted and plated on TSA plates, incubated at 37 °C overnight, and colonies counted to obtain CFU/ml of surviving bacteria.
2.5. Statistical analysis
Statistical significance was assessed by a 2-tailed pairwise comparison t-test between the control and sample of each bacteria-polymer combination. Separate statistical analyses were conducted for CFU on the polymer surface and in the medium. Significance was assigned when p ≤ 0.05. Two-tailed comparisons with p ≤ 0.001 were considered highly significant. Error bars in graphs represent standard error.
3. Results and discussion
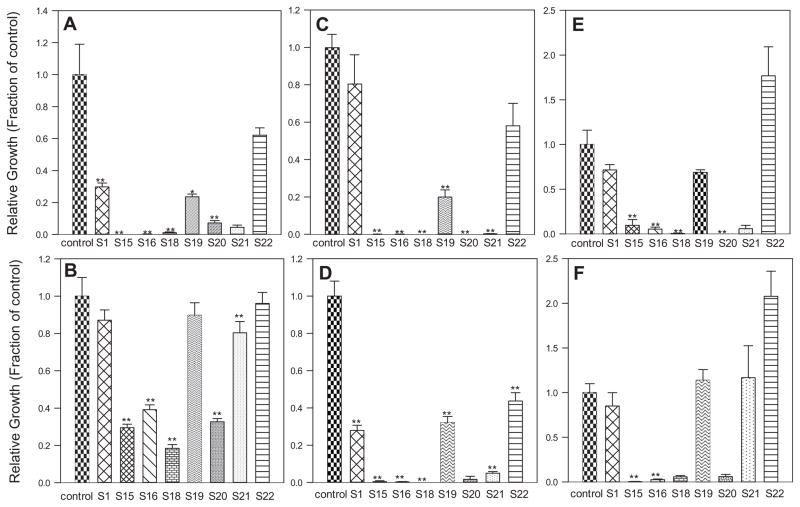
The present study was designed to evaluate the antimicrobial properties in biological settings for three different oligomers: PP04 (no QA), MV067 (one QA), PP06 (3 QAs) (Fig. 1). Two series of attachment assays were performed. The first series used surfaces prepared from a mixture of two oligomers, PP04 and PP06 in various proportions, with or without addition of HDDA. The experimental protocol in terms of proportions of each polymer are listed in Table 1. Each separate surface, composed of different proportions of the oligomers, was given a unique identifier, formulation S1, S3, etc., (Table 1). HDDA is used to create a smoother surface. This allowed us to compare the anti-attachment properties of coatings differing by the proportions of oligomers, and estimating a possible influence of the surface structure on cell growth and attachment. The second series of experiments was designed to compare the attachment properties of surfaces made from acrylate alone (PP04), from oligomer containing one QA (MV067), or a mixture of the two oligomers. For these surfaces HDDA was not added.
Figs. 3 and 4 present results as growth relative to control, on the control or polymer surface and in the growth medium above the surface, for each bacterial strain tested. The average colony forming units/mL (CFU/mL) of each experimental value was divided by the average control value and plotted. Overall, growth of P. aueroginosa was more robust than the other cultures. As shown in Fig. 3B, there were more bacteria in the medium above the surface than on the polymer surface itself (Fig. 3A) for each formulation. The most significant results were with 100% PPO6 (S15). PPO4 (S1) prevented the attachment of P. aeruginosa to a lesser extent. For the mixed polymer formulations, there tended to be fewer colonies on the polymer surfaces with higher PPO6 content. All wells had viable cells in the medium with values ranging from 5.0 × 105 to over 2.1 × 106 CFU/mL. The presence of HDDA did not appear to affect surface properties. S22 (85% PPO4 and 15% HDDA) had more CFU on the surface than the control well. This was not surprising since P. aueroginosa biofilms depend on surface roughness [27], and the polymer coatings were not as smooth as the control polystyrene well.
Fig. 3.
Protocol 1 (see Table 1.A.): (A). P. aeruginosa on the surface. (B). P. aeruginosa in the medium. (C). S. aureus on the surface. (D). S. aureus in the medium. (E). S. epidermidis on the surface. (F). S. epidermidis in the medium. The average of each experimental value was divided by the average control value and plotted. *p < .05, **p < .01 compared to control values.
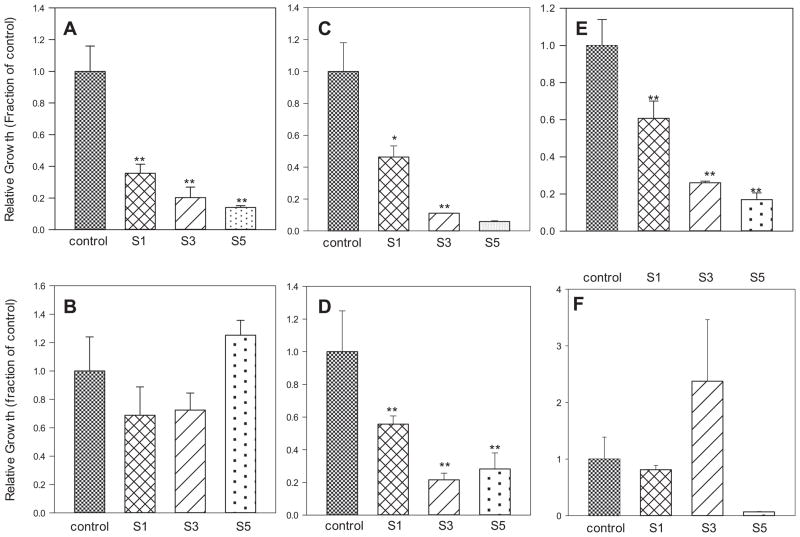
Fig. 4.
Protocol 2 (see Table 1B): (A) P. aeruginosa on the surface. (B) P. aeruginosa in the medium. (C) S. aureus on the surface. (D) S. aureus in the medium. (E) S. epidermidis on the surface. (F) S. epidermidis in the medium. The average of each experimental value was divided by the average control value and plotted. *p < .05, **p < .01 compared to control values.
The first series of assays demonstrated that most of the coatings tested reduce bacterial attachment. As expected the effect is coating- and species-dependent (Fig. 3 and Table 1, Part A). P. aeruginosa was the most resistant to the antimicrobial effects of the polymers, especially in its planktonic form. In contrast, S. epidermidis and S. aureus grown in the presence of PP04, either in contact with the surfaces or in suspension did not show any appreciable difference from controls. The two Staphylococcus strains were much more affected by PP06. S. aureus was the most sensitive to the QA, showing little bacterial attachment and a decrease in cell numbers with as little as 25% PP06 (Fig. 3E). S. aureus was also the only organism to show a significant decrease in planktonic growth for all the surfaces (Figs. 3 and 4).
Pure PPO4 acrylate oligomer (S1), 70:30 PPO4:HDDA (S19) and 85:15 PPO4:HDDA (S22), had one order of magnitude fewer colonies on the polymer surface than in the medium (Fig. 3). Pure PPO6 (S15) had minimal bacteria ~2.22 × 102 CFU/mL on the surface and viable cells in the medium. S18 and S20 were toxic to S. aureus, with 0 CFU/mL for both surface and media counts. S. aureus appeared to be more sensitive to PPO6 with significantly fewer CFU/mL in the medium and on the polymer surface as the PPO6 content of the surface increased.
S. epidermidis showed a trend similar to the other bacterial strains. As the PPO6 content increased, growth on the polymer surface decreased (Fig. 3A). PPO4 also prevented attachment of bacteria, but to a lesser extent. Pure PPO4 (S1) had 4.4 CFU/mL while S18 had 6.6 CFU/mL. PPO6 might have been slightly toxic to S. epidermidis as was evident by only 4.4 × 102 CFU/mL in the media for S15 (pure PPO6). Again, HDDA did not appear to influence microbial attachment.
3.1. Attachment studies investigating contributions of ammonium functional groups
The first experimental set indicated that PPO6 performed best as an antimicrobial surface. This was attributed to PPO6 having three ammonium functional groups. Ozturk et al. [28] found that nitrogen based compounds were effective at preventing initial bacteria attachment for biofilm formation on implanted devices. They also found that surface roughness was a major factor in initial bacterial attachment. The most effective formulation for preventing bacterial attachment was the surface prepared using the three QA bearing oligomer (PP06). To investigate whether a mixture composed of a polymer with no QA (PP04) and a polymer with one acrylate (MV067) had different or additional anti-attachment efficacy, a second series of coatings was prepared. S1 was comprised of 100% PPO4, utilizing a new curing technique to minimize surface roughness. S3 was composed of pure MV067, the new oligomer with one acrylate and one QA group. This was developed to investigate the impact of ammonium on bacterial attachment. S5 was a 50:50 mixture of PPO4 and MV067 (Table 1).
Fig. 4 shows growth, in CFU/mL, on the surface (a) and in the medium (b) when P. aeruginosa was present. Consistent with the first set of experiments, Pseudomonas was more robust and grew to higher numbers than the other two bacterial strains. One possibility is that MV067 may have prevented bacterial attachment by an inherent toxicity. Control plates had more colonies than the polymer surfaces or medium. This was more pronounced for Staphyloccus strains. In Fig. 4, S3 (pure MV067) had ~104 CFU/mL in the medium whereas control medium growth was ~6 × 104 CFU/mL. The difference was less pronounced with S5 (50:50 PPO4:MV067).
One general conclusion is that the more QA groups in the formulation, the greater the antibacterial effect. However it raises questions about the mechanism of action of these coatings. QA salts have been shown to be an effective antibacterial both in solution and when incorporated into a polymer. Jiang et al. [29] showed that polystyrene resins with QA were able to kill S. aureus in suspension; they did not investigate initial bacteria attachment. A similar phenomenon was seen with S. aureus in the media of samples containing both one QA and three QA groups (Fig. 4). Dental research also found that QA based materials were effective in reducing bacterial attachment [30]. One theory for the antibacterial effect is that electrostatic interactions between QAs and the cell cause damage [31]. Pfaffenroth et al. [32] also found that polymers with QA groups disrupt the bacteria membranes. In PP06, two of the three QA groups extend from the surface and it is hypothesized that this facilitates the antimicrobial properties.
P. aeruginosa had more robust growth than the Staphylococcus species. P. aeruginosa showed no differences from control with respect to CFU/mL in the medium, but cell numbers were significantly reduced for surface growth (Fig. 4). S. aureus was the most sensitive bacterial strain used in this study. Its growth was greatly inhibited by the presence of QAs. This confirms the importance of QAs in the antibacterial effects of the coatings. It is not surprising that the bacterial strains used here have differential sensitivities to the antimicrobial effects of the polymers. S. epidermidis and S. aureus are both Gram positive strains while P. aeruginosa is a Gram negative. Initially researchers believed that the first step for biofilm formation of Gram positive strains was the production of polysaccharide intracellular adhesion (PIA). However not all biofilm forming strains of S. epidermidis produce PIA [7]. Recent studies have demonstrated that S. epidermidis strains that form biofilms produced Aap and Bhp proteins while S. aureus produces Bap proteins [33]. It is the Bap protein that enables S. aureus to form biofilms on surfaces such as polymers [34]. Although the scope of this research was not to identify the exact bacterial attachment mechanisms, our results help to explain the differences in initial biofilm formation on the different polymer formations. In general, for each polymer, more S. aureus than S. epidermidis grew on the surfaces.
Regarding these three medically important bacteria, each reacted to the surfaces differently. P. aeruginosa was more robust and less affected by the presence of the QA group in the polymer, while S. aureus appeared to be quite sensitive to it, both planktonically and in terms of attachment abilities. Similarly, Litzler et al. [27] showed that attachment behavior of P. aeruginosa and S. epidermidis was affected by pyrolytic carbon surface free energy, while S. aureus was not. Thus there may not be a “one size fits all” solution to the problem of unwanted bacterial attachment. The addition of QA groups, alone or in combination, affected growth and attachment of all three bacterial species tested. MV076 exerted the greatest antibacterial effect, which may be due to the addition of the extra ammonium groups. The difference in cell wall makeup between Gram positive and Gram negative bacteria might account for the varying responses of these organisms to potential antimicrobial surfaces. Gram positive bacteria have a thicker peptidoglycan layer but are less complex than the Gram negative cell wall. The cell wall of Gram negative cells has 2 layers, a much thinner peptidoglycan layer and an outer membrane which is absent in Gram positive cells. This outer membrane is composed of lipopolysaccharides which are amphipathic and proteins like porins, which allow for molecules of a certain size to pass through the membrane. The composition of the bacterial cell wall not only determines how it will respond to antibacterial properties but also its mode of surface attachment.
The use of natural rubber has many benefits beyond its potential antibacterial properties, which include sustainability, economic, and physical advantages. The polymer surfaces used here retain physical properties, like flexibility, similar to those that make natural rubber ideal for so many applications. Our results suggest possible application of these rubber-based coatings in the medical field to avoid biofilm formation on surfaces in contact with patients.
In conclusion, there are many factors involved in the initial attachment of bacteria to a surface. No one factor is solely responsible for attachment, and there is no one universal solution to prevent this attachment. Further studies using mixed cultures, expanding the species tested or extending incubation times might also yield more information as to the antimicrobial mechanisms of these polymers. Determining the reusability of the polymer surfaces and how long they retain their antimicrobial properties will be information that further demonstrates the value of these polymers.
Acknowledgments
Work was supported in part by NIH GM086895. We thank Justin Brantner for reviewing the manuscript.
Contributor Information
Hope T. Badawy, Email: hope.badawy@gmail.com.
Pamela Pasetto, Email: pamela.pasetto@univ-lemans.fr.
Jean-Luc Mouget, Email: jean-luc.mouget@univ-lemans.fr.
Jean-François Pilard, Email: Pilard@univ-lemans.fr.
Teresa J. Cutright, Email: tcutrig@uakron.edu.
Amy Milsted, Email: milsted@uakron.edu.
References
- 1.Rhode H, Frankenberger S, Zahringer U, Mack D. Structure, function, and contribution of polysaccharide intercellular adhesion (PIA) to Staphylococcus epidermidis biofilm formation and pathogenesis of biomaterial-associated infections. Eur J Cell Biol. 2010;89:103–111. doi: 10.1016/j.ejcb.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Yakandawala N, Gawande PV, LoVeteri K, Madhyastha S. Effects of ovotransferrin, protamine sulfate and EDTA combination on biofilm formation by catheter-associated bacteria. J Appl Microbiol. 2007;102:722–727. doi: 10.1111/j.1365-2672.2006.03129.x. [DOI] [PubMed] [Google Scholar]
- 3.Hess DJ, Henry-Stanley MJ, Wells CL. Interplay of antibiotics and bacterial inoculum on suture associated biofilms. J Surg Res. 2012;177:334–340. doi: 10.1016/j.jss.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan JB, Jabbouri S, Sadovskaya I. Extracellular DNA-dependent biofilm formation by Staphylococcus epidermidis RP62A in response to subminimal inhibitory concentrations. Res Microbiol. 2011;162:535–541. doi: 10.1016/j.resmic.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank KL, Patel R. Intravenously administered pharmaceuticals impact biofilm formation and detachment of Staphylococcus lugdunesis and other staphylococci. Diagn Microbiol Infect Dis. 2008;60:9–16. doi: 10.1016/j.diagmicrobio.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Foreman A, Wormald PJ. Different biofilms, different disease? A clinical outcomes study. Laryngoscope. 2010;120:1701–1706. doi: 10.1002/lary.21024. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick F, Humphreys H, O’Gara JP. The genetics of Staphylococcal biofilm formation- will a greater understanding of pathogenesis lead to a better management of device related infection? Clin Microbiol Infec. 2005;11:967– 973. doi: 10.1111/j.1469-0691.2005.01274.x. [DOI] [PubMed] [Google Scholar]
- 8.Jabbouri S, Sadovshaya I. Mini-Review: characteristics of the biofilm matrix and its role as a possible target for the detection and eradication of Staphylococcus epidermidis associated with medical implant infections. FEMS Immunol Med Microbiol. 2010;59:280–291. doi: 10.1111/j.1574-695X.2010.00695.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Kaplan JB, Lee WY. Microfluidic devices for studying growth and detachment of Staphylococcus epidermidis biofilms. Biomed Microdevices. 2008;10:489–498. doi: 10.1007/s10544-007-9157-0. [DOI] [PubMed] [Google Scholar]
- 10.Myllymaa K, Levon J, Tiaien VM, et al. Formation and retention of Staphylococcal biofilms on DLC and its hybrids compared to metals used as biomaterials. Colloids Surf B Biointerfaces. 2013;101:290–297. doi: 10.1016/j.colsurfb.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Stroh P, Gunther F, Meyle E, et al. Host defense against Staphylococcus aureus biofilms by polymorphonuclear neutrophyls: oxygen radical productions but not phagocytocytosis depends on opsonisation with immunoglobin G. Immunobiology. 2011;216:351–357. doi: 10.1016/j.imbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Toba FA, Akashi H, Arrecubieta C, Lowy FD. Role of biofilm in Staphylococcus aureus and Staphylococcus epidermidis ventricular assist device driveline infections. J Thorac Cardiovasc Surg. 2010;141:1259–1264. doi: 10.1016/j.jtcvs.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies JC. Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr Respir Rev. 2002;3:128–134. doi: 10.1016/s1526-0550(02)00003-3. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg J. Biofilms and antibiotic resistance: a genetic linkage. Trends Microbiol. 2002;10:264. [Google Scholar]
- 15.Hammond A, Dertien J, Colmer-Hamood J, Griswold JA, Hamood AN. Serum inhibits P. aeruginosa biofilm formation on plastic surfaces and intravenous catheters. J Surg Res. 2010;159:735–746. doi: 10.1016/j.jss.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Hoiby N. Prospects for the prevention and control of Pseudomonal infection in children with cystic fibrosis. Pediatr Drugs. 2000;2:451–463. doi: 10.2165/00128072-200002060-00004. [DOI] [PubMed] [Google Scholar]
- 17.Rao J, DiGiandomenico AD, Artamonov M, et al. Host derived inflammatory phospholipids regulate rahU (PA0122) gene, protein, and biofilm formation in Pseudomonas aeruginosa. Cell Immunol. 2011;270:95–102. doi: 10.1016/j.cellimm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 19.Flint SH, Brooks JD, Bremer PJ. The influence of cell surface properties of thermophilic Streptococci on attachment to stainless steel. J Appl Microbiol. 1997;83:508–517. doi: 10.1046/j.1365-2672.1997.00264.x. [DOI] [PubMed] [Google Scholar]
- 20.Schwab U, Hu Y, Wiedmann M, Boor KJ. Alternative sigma factor σB is not essential for Listeria monocytogenes surface attachment. J Food Prot. 2005;68:311–317. doi: 10.4315/0362-028x-68.2.311. [DOI] [PubMed] [Google Scholar]
- 21.Xu LC, Siedlecki CA. Submicron-textured biomaterial surface reduces staphylococcal bacterial adhesion and biofilm formation. Acta Biomater. 2012;8:72–81. doi: 10.1016/j.actbio.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Lawson MC, Hoth KC, DeForest CA, et al. Inhibition of Staphylococcus epidermidis biofilms using polymerizable vancomycin derivatives. Clini Orthop. 2010;468:2081–2091. doi: 10.1007/s11999-010-1266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukla A, Fleming KE, Chuang HF, et al. Controlling the release of peptide antimicrobial agents form surfaces. Biomaterials. 2010;31:2348– 2357. doi: 10.1016/j.biomaterials.2009.11.082. [DOI] [PubMed] [Google Scholar]
- 24.Palmer J, Flint S, Brooks J. Bacterial cell attachment, the beginning of a biofilm. J Ind Microbiol Biotechnol. 2007;34:577–588. doi: 10.1007/s10295-007-0234-4. [DOI] [PubMed] [Google Scholar]
- 25.Jellali R, Campistron I, Laguerre A, et al. Synthesis of new photocurable oligoisoprenes and kinetic studies of their radical photopolymerization. J Appl Polym Sci. 2013;127:1359–1368. [Google Scholar]
- 26.Bunel C, Campriston I, Hellio C, et al., inventors. Centre National de la Recherche Scientifique (CNRS); Universite du Maine, assignee. United States Patent Application Pub. No.: US 2011/0268689. Photo-crosslinkable antifouling compositions, films obtained from said compositions, and corresponding uses. 2011 Nov 3;:A1.
- 27.Litzler PY, Benard L, Barbier-Frebourg N, et al. Biofilm formation on pyrolytic carbon heart valve: influence of surface free energy, roughness, and bacterial species. Cardiopul Supp Physiol. 2007;134:1025–1032. doi: 10.1016/j.jtcvs.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Ozturk S, Sudagidan M, Turkan M. Biofilm formation by Staphylococcus epidermidis on nitrogen implanted CoCrMo alloy metal. J Biomed Mater Res. 2006;A:664–668. doi: 10.1002/jbm.a.31037. [DOI] [PubMed] [Google Scholar]
- 29.Jiang S, Wang L, Yu H, Chen Y. Preparation of cross-linked polystyrenes with quaternary ammonium and their antibacterial behavior. React Funct Polym. 2005;62:209–213. [Google Scholar]
- 30.Antonucci JM, Zeiger DN, Tang K, et al. Synthesis and characterization of dimethyl acrylates containing quaternary ammonium functionalities for dental applications. Dent Mater. 2012;28:219–228. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kugler R, Bouloussa O, Rondelez F. Evidence of a charge-density threshold for optimum efficiency of biocidal cationic surfaces. Microbiology. 2005;151:1341–1348. doi: 10.1099/mic.0.27526-0. [DOI] [PubMed] [Google Scholar]
- 32.Pfaffenroth C, Winkel A, Dempwolf W, et al. Self-assembled antimicrobial and biocompatible copolymer films on titanium. Macromol Biosci. 2011;11:1515–1525. doi: 10.1002/mabi.201100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fey PD, Olson ME. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol. 2010;5:917–933. doi: 10.2217/fmb.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cucarella C, Solano S, Valle J, et al. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J Bacteriol. 2001;183:2888–2896. doi: 10.1128/JB.183.9.2888-2896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]