SUMMARY
Over 20 years of evidence indicates a strong association between obstructive sleep apnea (OSA) and cardiovascular disease. Although inflammatory processes have been heavily implicated as an important link between the two, the mechanism for this has not been conclusively established. Atherosclerosis may be one of the mechanisms linking OSA to cardiovascular morbidity. This review addresses the role of circulating adhesion molecules in patients with OSA, and how these may be part of the link between cardiovascular disease and OSA. There is evidence for the role of adhesion molecules in cardiovascular disease risk. Some studies, albeit with small sample sizes, also show higher levels of adhesion molecules in patients with OSA compared to controls. There are also studies that show that levels of adhesion molecules diminish with continuous positive airway pressure therapy. Limitations of these studies include small sample sizes, cross-sectional sampling, and inconsistent control for confounding variables known to influence adhesion molecule levels. There are potential novel therapies to reduce circulating adhesion molecules in patients with OSA to diminish cardiovascular disease. Understanding the role of cell adhesion molecules generated in OSA will help elucidate one mechanistic link to cardiovascular disease in patients with OSA.
Keywords: Cardiovascular diseases, Epidemiological studies, Atherosclerosis, Sleep apnea, Adhesion molecules
Introduction and overview
Obstructive sleep apnea (OSA) is a leading public health problem, affecting 5–15% of adults,1 and it is associated with repetitive episodes of transient oxygen desaturation during sleep (caused by partial or complete obstruction of the airway), resulting in cyclical, intermittent hypoxia and sleep fragmentation. There is growing evidence that OSA is an independent risk factor for cardiovascular disease,2,3 although the pathogenesis is not completely understood. Although the mechanism for the initiation of cardiovascular disease has not been fully established, one theorized mechanism is the intermittent hypoxia produced by the frequent respiratory events.4 The repeated episodes of hypoxia followed by re-oxygenation that occur in the context of OSA are proposed to result in oxidative stress and increased production of reactive oxygen species (ROS).5 The formation of oxygen free radicals from intermittent hypoxia and re-oxygenation is thought to lead to activation of transcriptional factors such as nuclear factor-kappa B (NFκB) that upregulate the expression of adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule (VCAM-1) and cytokines (such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6, IL-8, and chemokines).4,6–8 Inflammation is recognized as playing a role in all stages of the atherosclerotic disease process; for this reason, evaluation of circulating bio-markers of inflammation, including adhesion molecules, has become recognized as a useful tool for identifying patients at high risk for future cardiovascular events.9 Since OSA is associated with elevated cardiovascular risk, and inflammation plays an important role in the development of cardiovascular disease, it is reasonable to suspect that OSA may confer risk through an inflammatory mechanism. As adhesion molecules are a key component of the inflammatory process, it is likely that, if OSA is associated with increased inflammation, OSA will also be associated with increased adhesion molecules. Further, it is possible that these elevations in adhesion molecules could be ameliorated with treatment of OSA.
Cell adhesion molecules are cell surface proteins involved in the binding of cells (usually leukocytes) to endothelial cells or to the extracellular matrix.9 The adhesion of circulating leukocytes to the endothelial cells is believed to be one of the initial steps in the pathogenesis of atherosclerosis.10,11 In both animal and human models of atherosclerosis, the adherence of monocytes and lymphocytes to the intact endothelial lining is one of the earliest detectable events in atherosclerosis.11–13
Adhesion molecules can be measured in the circulation. The shedding of cellular adhesion molecules from the surface of an activated endothelium via proteolytic cleavage allows for measurable plasma levels of soluble cellular adhesion molecules. Although cell-bound adhesion receptors are challenging to study in vivo, circulating levels of soluble VCAM-1 (sVCAM-1) have been correlated with cellular VCAM-1 expression in the human aorta from samples obtained during surgery.14 To our knowledge, there have not been studies in vivo involving circulating levels of soluble ICAM-1 (sICAM-1) and correlation to cellular ICAM-1 in the human aorta. However, the assessment of soluble adhesion molecules may be useful biomarkers for stratifying disease risk and prognosis for atherosclerosis.
A graphical depiction of a proposed model, linking OSA to cardiovascular disease, and the potential role of adhesion molecules, is depicted in Fig. 1. This review will do the following: provide first an overview of the evidence and mechanisms of OSA as an independent cardiovascular risk factor; provide a background on adhesion molecules in atherosclerosis and the process of leukocyte recruitment; synthesize the available literature on adhesion molecules in OSA; identify novel therapeutic modalities that consider cell adhesion molecules as potential therapeutic targets, and also suggest future research directions.
Fig. 1.
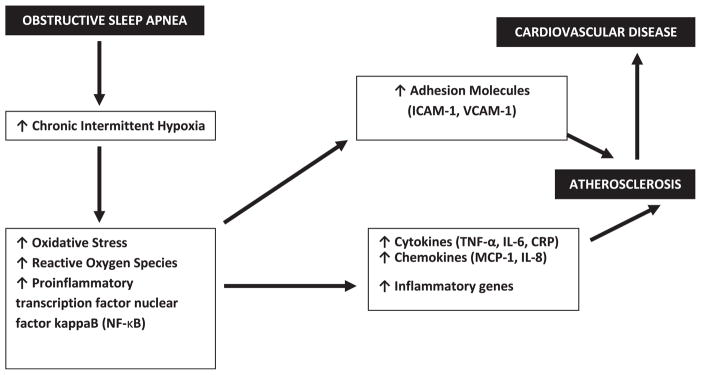
Schematic illustration of obstructive sleep apnea and the link to atherosclerosis and cardiovascular disease, including the role of adhesion molecules. CRP, C-reactive protein; ICAM-1; intercellular adhesion model-1; IL-6, interleukin-6; IL-8, interleuken-8; MCP-1, monocyte chemoattractant protein-1; NF-κB, nuclear factor-kappa B; TNF-α, tumor necrosis factor-α; VCAM-1, vascular cell adhesion model-1.
Obstructive sleep apnea, intermittent hypoxia, and cardiovascular disease
Obstructive sleep apnea and cardiovascular disease
OSA is associated with a number of cardiovascular diseases such as heart failure, myocardial infarction, arrhythmias (including atrial fibrillation), systemic and pulmonary hypertension, and stroke.15–20 It has been suggested that cardiovascular consequences of OSA may appear even in the absence of classical cardiovascular risk factors.21,22 Thus, OSA has emerged as an independent risk factor for cerebrovascular disease and coronary artery disease.2,23,24
Mechanisms for this relationship
There are a number of potential mechanisms for the cardiovascular consequences of OSA, including sleep fragmentation, obesity, and intermittent hypoxia.
Sleep fragmentation
Currently, there is a paucity of studies specifically linking sleep fragmentation to cardiovascular disease risk. Previous studies have found that fragmentation of sleep resulted in increased cortisol secretion,25 which may be associated with increased sympathetic activation and metabolic changes.26–28 In addition, fragmentation of sleep has been shown to increase daytime sleepiness, which is a risk factor for a number of medical conditions.26 Sleep fragmentation was shown to alter responses to airway occlusion,29 which may interact with intermittent hypoxia to elevate cardiovascular risk in the context of OSA.26,30 Further, sleep-related movement disorders may also carry cardiovascular risk. For example, periodic limb movement frequency and sleep fragmentation are associated with incident cardiovascular disease in community-dwelling elderly men.31 Despite these findings, the potential associations between sleep fragmentation and cardiovascular disease risk remain largely unexplored.
Obesity
Obesity is highly comorbid with OSA and is the most common predisposing factor for OSA. As OSA interrupts sleep and causes daytime sleepiness, this can encourage inactivity, since self-reported physical activity decreases with increased sleepiness.32 Obesity also induces an inflammatory state, as adipose tissue has resident macrophages33 and is an abundant source of proinflammatory cytokines such as TNF-α and IL-6.34,35 The common co-occurrence of obesity and OSA, and the fact that both states increase oxidative stress and inflammation, make it challenging to determine independent roles of OSA and obesity on inflammation.36 It is possible that the effects of OSA on inflammation might be attenuated because of the effect of obesity itself. Conversely the effects of OSA could be amplified by the increased number of macrophages in fat37 since they are the major source of proinflammatory cytokines. Recent data from the Icelandic sleep apnea cohort suggest that the latter is the case.38 OSA is independently associated with elevated IL-6 levels in obese subjects but not in those with body mass index (BMI) < 30 kg/m2.38
Intermittent hypoxia
Intermittent hypoxia is a proposed mediator of cardiovascular disorders seen in OSA. The repetitive cycling of oxygen desaturation and subsequent re-oxygenation leads to a number of adverse outcomes at the molecular level, including mitochondrial dysfunction and an altered redox state.39,40 This leads to formation of ROS during the intermittent-re-oxygenation that is characteristic of intermittent hypoxia. This can be considered as a less severe variantof reperfusion injury when blood flow is restored following an ischemic episode.39–42 ROS are highly reactive and can cause modifications of lipids, proteins, and other important molecules. They have been implicated in the activation of proinflammatory cascades and hypertension.43–45 In support of this hypothesis, studies have demonstrated enhanced in vivo release of free oxygen radicals (unstable oxygen molecules which can damage cells) from neutrophils and monocytes in patients with OSA.42,46–48 These alterations can cause an activation of redox-sensitive transcription factors such as NFκB, which cause changes in gene expression, leading to activation of an inflammatory process that participates in the development of atherosclerosis.4,49–51 In this way, intermittent hypoxia may play an important role in a pathway that leads to atherosclerosis and, ultimately, the cardiovascular consequences of OSA.
Adhesion molecules
Among molecules that would be anticipated to be upregulated as part of this inflammatory response are adhesion molecules. The process of atherosclerotic plaque development is, however, multi-faceted. Endothelial dysfunction is known to be caused by oxidative stress that precedes the development of atherosclerosis.52,53 Initially, endothelial dysfunction leads to cholesterol accumulating in the artery wall as well as smooth muscle cell proliferation. This leads to a cascading inflammatory process in the plaque, which leads to further accumulation of inflammatory cells, degradation of the extracellular matrix, and unstable lesions. This increases the risk for plaque rupture and thrombosis,52,54 which may be responsible for as many as 50 percent of cases of myocardial infarction and acute coronary syndrome.52,54,55
Adhesion molecules play a role in the process of plaque development. They serve the important function of facilitating the interaction of leukocytes with the endothelium. Circulating leukocytes adhere poorly to the normal endothelium.56 During inflammation, endothelial cells are activated to express adhesion molecules as well as synthesize chemokines and chemoattractants on their luminal surface.57 Selectively recruited to inflamed sites on the endothelium, circulating leukocytes participate in a cascade of events that involves a number of adhesion receptors.57,58
In order to leave the vessel at sites of inflammation, leukocytes roll along the microvascular endothelium prior to arresting and transmigrating into inflamed tissue.57–59 This phenomenon is illustrated in Fig. 2, which depicts the general leukocyte recruitment process.
Fig. 2.

Leukocyte recruitment process. Leukocytes in the bloodstream tether and roll onto an inflamed endothelium via interactions between selectins and their respective ligands. The defined general sequence of events includes tethering, rolling, adhesion, and transmigration. Chemokines and or other proinflammatory mediators are released by various sources within the tissue (e.g., mast cells) and are presented on the endothelium to the rolling leukocytes. This action results in integrin activation and firm adhesion followed by transmigration into the site of inflammation. Reproduced with permission from Kubes and Kerfoot with a modified legend.61
To facilitate this process, endothelial cells and leukocytes have complementary surface adhesion molecules, which briefly tether and release, causing the leukocyte to roll along the endothelium like a tumbleweed until it adheres with endothelial cells.57,60
Types and functions of adhesion molecules
Adhesion molecules are in three general families of proteins specifically involved in leukocyte trafficking: 1) selectins 2) integrins and 3) immunoglobulins.
Selectins
In post-capillary venules of the systemic circulation, leukocyte rolling on an activated endothelium is largely mediated by the selectin family of adhesion molecules.57 Tethering and rolling are mediated by L-selectin, P-selectin, and E-selectin (where L, P and E stand for leukocyte, platelet, and endothelial, respectively). These interact with the P-selectin glycoprotein ligand 1 (PSGL1) and other glycosylated ligands. L-selectin is expressed constitutively by most leukocytes, whereas E-selectin and P-selectin are only expressed by inflamed endothelial cells (thrombin- or histamine-activated).57 PSGL1 is the primary ligand for all three selectins and is expressed by leukocytes and also will interact with endothelial cells at sites of inflammation. Inflammation causes the upregulation of cell adhesion molecules and the interactions of selectins with their ligands enable flowing leukocytes in the blood to adhere to the inflamed endothelium.57,59
Integrins
Once leukocytes adhere to an inflamed endothelium, this allows intimate contact with chemoattractants that come from endothelial cells. This initiates the activation of complex intracellular signaling networks that modulate adhesiveness of leukocytes and increases the expression of the integrins, which participate in slow rolling and mediating firm leukocyte adhesion on the endothelium.58 The integrins are non-covalently-associated, heterodimeric cell adhesion receptors, consisting of α and β subunits. These subunits recognize the extracellular matrix, cell surface glycoproteins, and some soluble molecules such as fibrinogen.57,58
Immunoglobulins
Members of the immunoglobulin (Ig) superfamily such as ICAM-1 and VCAM-1, which are the largest family of endothelial adhesion molecules, also interact with integrins. Leukocyte arrest (halting of rolling) is rapidly triggered by chemokines and other chemoattractants and is mediated by the binding of leukocyte integrins to ICAM-1 and VCAM-1 that are expressed by endothelial cells.9
ICAM-1 is expressed at low levels on non-stimulated endothelial cells and is upregulated after exposure to cytokines. ICAM-1 is an 80–110 kDa glycoprotein and is a ligand for lymphocyte function associated antigen-1α (LFA-1; CD11a/CD18), which is a B2-integrin.62,63 ICAM-1 plays an important role in migration of the leukocyte to the inflamed area, and it is essential for adhesion of any type of leukocyte to the apical surface of the endothelium.59 ICAM-1 is strongly upregulated on endothelial cells by inflammatory mediators such as TNF-α.
VCAM-1 is expressed minimally in a non-activated endothelium but is also induced after cytokine activation with a similar time-course to ICAM-1. VCAM-1 is a 110 kDa glycoprotein. It is a ligand for very late antigen-4, which does not adhere to appropriate ligands until leukocytes are activated. It can be expressed by cell types such as macrophages, myoblasts, and dendritic cells.64 TNF-α also increases expression of VCAM-1,65 which increases the likelihood that monocytes will adhere to the arterial endothelium, transmigrate from the intima to the media, and secrete TNF-α and other inflammatory cytokines.9
Adhesion molecules as biomarkers of inflammation
In addition to being expressed on the cell surface, soluble forms of adhesion molecules have been detected in circulating blood and have been shown to retain their functional ability.66 This facilitates their assessment as a potential biomarker of inflammation. However, there is limited evidence that the changes in soluble levels of adhesion molecules in the plasma reflect levels at the cell membrane.67–71 Despite this, the adhesive events leading to normal diapedesis of mature leukocytes have become better characterized over the last 20 years.57,59,60,72,73 Plasma concentrations of soluble adhesion molecules (sICAM-1 and sVCAM-1) are shown to significantly correlate with carotid intima thickness, which is an index of early atherosclerosis.67,74,75
ICAM-1 is constitutively expressed by a variety of cells and correlates with C-reactive protein (CRP), providing similar predictive information to CRP in primary prevention for cardiovascular disease.9,76,77 VCAM-1 is not expressed in baseline conditions but is induced by pro-atherosclerotic conditions in animals and humans.78,79 Thus, VCAM-1 does not appear as a risk factor in settings of healthy individuals without endothelial dysfunction, but is a significant risk predictor in patients suffering from pre-existing disease.9 This is supported by sVCAM-1 being a significant predictor of future cardiovascular events in coronary artery disease (CAD) patients, diabetic patients, or those with unstable angina.9,80,81
As cellular interactions are critical for the development of atherosclerosis, adhesion molecules serve as direct markers of cell activation and are essential mediators of the cellular associations contributing to the atherogenic process.82 This is important, as cell adhesion molecules have been shown to play a crucial role in the formation of atherosclerosis and cardiovascular pathology (as described above).
Adhesion molecules and cardiovascular disease
Circulating sICAM-1 (but not sVCAM-1) is a consistent predictor of coronary heart disease risk.76,81,83 Soluble ICAM-1 appears to be a proinflammatory marker and correlates with acute phase reactants like CRP, providing similar predictive information to CRP in settings of primary prevention.9,76 Elevated levels of ICAM-1 have also been reported in patients with coronary heart disease and myocardial infarction, with high circulating levels shown to be a prognostic risk factor for future cardiovascular events.76,83–85
Adhesion molecules in obstructive sleep apnea
Obstructive sleep apnea and inflammatory markers
Studies have assessed circulating levels of inflammatory markers as independent risk indicators of cardiovascular diseases. Examples include CRP, TNF-α, IL-8, and IL-6.
CRP is a prototypic marker of inflammation and stable downstream marker of the inflammatory process.86 CRP is believed to have an active role in atherogenesis by causing expression of adhesion molecules and mediating monocyte chemoattractant protein-1 (MCP-1) induction.87,88 The role of CRP as a risk marker in OSA is controversial, as it demonstrates a strong relationship to obesity, attenuating its value as a specific marker of OSA-related cardiovascular risk.
TNF-α is recognized as an important proinflammatory cytokine in the development of atherosclerosis.89 Elevated TNF-α levels have been found in OSA patients in comparison to controls,46 an effect that is independent of obesity.90–93
IL-8 is a chemokine, which plays an important pathogenic role in atherogenesis by mediating adhesion of neutrophils and monocytes to the vascular endothelium,94 and enhancing oxidative stress.94,95 IL-8 levels are increased in OSA patients in comparison to controls matched for variables such as age and body mass.90
Il-6 is a cytokine which stimulates production of proinflammatory CRP in the liver and is increased in obesity96 and visceral fat.97,98 This cytokine is also associated with increased carotid intima-media thickness and surrogates of cerebrovascular disease.93,99 Il-6 release has been found to be 2–3 times higher in visceral than subcutaneous fat, and approximately 15–30% of circulating IL-6 levels come from fat tissue.97 Some studies have found positive associations between OSA and IL-6 levels36,38,100 and decrease with continuous positive airway pressure (CPAP) treatment.101 Conflicting studies however, have found no associations between OSA and Il-6 levels88,90 or no change with CPAP treatment.102,103 Further studies are needed to evaluate the clinical value of this biomarker.
Upregulation of adhesion molecules in obstructive sleep apnea
Monocytes from OSA patients adhere avidly to unstimulated endothelial cells in culture, indicating the functional significance of the increased expression of adhesion molecules in OSA.46 Moreover, in vitro studies of monocytes from patients with OSA show that they increased invasive ability compared to monocytes from controls.104 The increased adherence of monocytes from patients with OSA to endothelial cells suggests that the repeated apnea-related hypoxic events result in endothelial and monocyte activation. In a rat model, recurrent OSA led to significant increases in various leukocyte-endothelial cell interactions such as leukocyte rolling and firm adhesion in experimental groups in which repeated obstruction for 3 h (rate: 60/h, length: 5 s) was conducted compared to a control group which was only instrumented, with no obstruction.105 This may lead to endothelial dysfunction, which is the initial step leading to cardiovascular morbidity in OSA patients.
Circulating adhesion molecules in obstructive sleep apnea
Only a few investigators have examined levels of various cellular adhesion molecules and their relation to OSA, hypoxic stress, and cardiovascular risks in OSA patients (see Table 1). Ohga and coworkers measured circulating ICAM-1, VCAM-1, and L-selectin levels before and after sleep in seven male patients with OSA and six age-matched male controls. The circulating ICAM-1, VCAM-1, and L-selectin levels were all significantly increased in the OSA patients before sleep compared with the normal subjects. While this result is compatible with the proposed model, there was no increase in ICAM-1, VCAM-1 or L-selectin over the course of the night (which would be expected if secretion was triggered by hypoxic stress).68 The correlation between adhesion molecule levels and level of hypoxia was not explored.
Table 1.
Epidemiological evidence on adhesion molecules in OSA.
| Authors (year of publication) | Subjects | Method | Findings |
|---|---|---|---|
| Ohga et al.68 1999 |
N = 7 OSAS patients N = 6 controls |
PSG for two consecutive nights (samples obtained 2nd night) Circulating ICAM-1, VCAM-1 and L-selectin before and after PSG Two-time sampling |
After sleep, significantly greater levels of ICAM-1 and L-selectin, but not VCAM-1 were observed in the OSA patients compared to controls ICAM-1: 392.9 ± 48.5 vs. 201.2 ± 55.0 ng/mL, P < 0.05 VCAM-1: 811.0 ± 87.8 vs. 574.2 ± 42.7 ng/mL, P < 0.05 L-selectin: 1386.6 ± 77.9 vs. 1038.8 ± 78.6 ng/mL, P < 0.01 |
| El-Solh et al.71 2002 | N = 15 subjects with angiographically proven CAD deemed to have stable angina | Overnight PSG Circulating ICAM-1 and VCAM-1 L-selectin and E-selectin levels in the serum One-time sampling |
ICAM-1, VCAM-1, and E-selectin were significantly elevated (except for L-selectin) in CAD patients with moderate-to severe OSA compared to those without OSA. ICAM-1: 367.4 ± 85.2 ng/mL vs. 252.8 ± 68.4 ng/mL, p = 0.008 VCAM-1: 961.4 ± 281.7 ng/mL vs. 639.1 ± 294.4 ng/mL, p = 0.004 E-selectin: 81.0 ± 30.4 ng/mL vs. 58.1 ± 23.2 ng/mL, p = 0.03 The concentration of circulating adhesion molecules correlated with severity of sleep apnea and desaturation index. |
| Ursavas et al.70 2007 |
N = 39 moderate-to-severe OSAS N = 34 non-apneic controls |
Overnight PSG Circulating ICAM-1 and VCAM-1 levels in the serum One-time sampling |
ICAM-1 and VCAM-1 significantly higher in OSA patients compared to controls ICAM-1: 480.1 ± 216.7 vs. 303.4 ± 98.6 ng/mL, p < 0.0001 VCAM-1: 1156.6 ± 79.8 vs. 878.8 ± 71.1 ng/mL, p = 0.002 Positive correlation between circulating levels for ICAM-1 and ln of AHI |
| Carpagnano et al.106 2010 |
N = 12 obese OSA (OO) patients N = 10 non-obese OSA (NOO) patients N = 10 obese non-OSA (ONO) subjects N = 8 healthy subjects (HS) |
Overnight PSG ICAM-1 and IL-8 levels in breath condensate and in the plasma and inflammatory cells in induced sputum One-time sampling |
Obesity, not only OSA, cause systemic airway inflammation through adhesion molecule activation ICAM-1 and IL-8 levels higher in OO, NOO, and ONO patients compared to HS (not statistically significant) OO: 83.9 ± 3.9 pg/mL NOO: 62.6 ± 3.6 pg/mL ONO: 77.8 ± 1.8 pg/mL HS: 46.8 ± 1.6 pg/mL; p < 0.001 Positive correlation between ICAM-1 and IL-8 levels in exhaled breath condensate and plasma biomarkers |
AHI, apnea hypopnea index; CAD, coronary artery disease; ICAM-1, intercellular adhesion molecule-1; IL-8, interleukin-8; OSA, obstructive sleep apnea; PSG, poly-somnography; VCAM-1, vascular cell adhesion molecule.
ICAM-1, VCAM-1, L-selectin and E-selectin were also assessed in a study of 15 patients with moderate–severe OSA (apnea hypopnea index, AHI >= 20 events/h), relative to a control group without sleep apnea (AHI ≤ 5 events/h), matched for age, gender, BMI and the severity of angiographically-assessed coronary artery disease (CAD). Venous blood samples collected the morning after the sleep study showed significantly higher ICAM-1, VCAM-1, and E-selectin in the OSA group compared to the control subjects. The increased levels of adhesion molecules correlated with OSA severity (AHI) and oxygen desaturation index (desaturations per hour), but not with the severity of hypoxemia (percentage of time spent with oxygen saturation < 90%). Overall, the authors concluded that OSA increases the circulating levels of adhesion molecules.71 By limiting this study to only patients with CAD, it was possible to compare circulating adhesion molecules while controlling for the presence of cardiovascular disease in patients with moderate-to severe OSA.
The effects of OSA-induced hypoxia on circulating inflammatory mediators have also been explored.70 Thirty-nine subjects with moderate-to-severe OSA (Mean AHI = 50.5 ± 23.5) and 34 controls (AHI < 5 events/h) were matched for age, gender, BMI, smoking history, and presence of cardiovascular disease (measured by electrocardiography and blood pressure). Overnight poly-somnography was performed and circulating ICAM-1 and VCAM-1 levels in serum were measured by enzyme linked immunosorbent assay (ELISA). Circulating levels of both ICAM-1 and VCAM-1 were significantly increased in the OSA subjects compared to the control group. There was a significant positive correlation between circulating levels of ICAM-1 and log (ln) of AHI (r = 0.276, P = 0.018). The authors concluded OSA may independently increase circulating levels of adhesion molecules.70 As this study focused on the impact of OSA on the levels of adhesion molecules, results of the study may be confounded by differences existing in asymptomatic cardiovascular disease in the two groups.
In another small study, Carpagnano et al. measured ICAM-1 and IL-8 levels in the 1) breath condensate, 2) plasma, and 3) induced sputum of 12 obese OSA patients, 10 non-obese OSA patients, 10 obese non-OSA subjects, and 8 non-obese, non-OSA healthy subjects using ELISA.106 In the breath condensate, exhaled ICAM-1 concentrations were significantly greater in obese OSA patients, non-obese OSA subjects, and in obese non-OSA subjects, relative to healthy non-obese controls. However, the difference between OSA patients and obese controls was not significantly different. A significant increase in the plasma, IL-8 and ICAM-1 was observed in obese OSA patients, non-obese OSA subjects, and obese non-OSA subjects compared with healthy subjects. When these were assessed in plasma, ICAM-1 and IL-8 were found to follow an upward trend in obese OSA patients, but no significant difference was observed between the three groups of obese OSA subjects, non-obese OSA subjects, and obese non-OSA subjects.106
As Carpagnano and coworkers enrolled obese subjects without OSA and OSA subjects who were not obese, they were able to explore the role of obesity. Their results suggest that obesity itself is responsible for systemic and airway inflammation. The results of this study also suggest the occurrence of a neutrophilic airway inflammation in both OSA and obese patients that potentially involves both ICAM-1 and IL-8. The increase in adhesion molecules was greatest in cases who were both obese and had OSA, which may explain the increased risk of developing cardiovascular events in this group.106 A study in a larger group of OSA patients while controlling for obesity will be needed, however, to confirm these results.
Effect of continuous positive airway pressure (CPAP) on levels of adhesion molecules
CPAP is the most effective and widely used treatment for OSA. In a study by Ohga et al.,69 ICAM-1, IL-8, and MCP-1 were measured in serum before and after CPAP therapy in 20 male, untreated OSA patients with a mean BMI of 29.4 ± 1.4 kg/m2. Subjects had no history of cardiovascular, pulmonary, metabolic or neuromuscular disease. Results were compared with an age-matched control group of 10 males. There were no significant differences in age and BMI between the OSA and control groups, although the apnea index in the OSA group (AHI = 38.9 ± 3.11 events/h) was much greater than that of the control group. CPAP was found to decrease circulating ICAM-1 and IL-8 levels significantly in the OSA patients (P < 0.05) relative to pre-treatment levels; although the study did not assess this statistically, the values following CPAP were comparable to values in the control group.69
In another study, Chin et al. investigated 23 patients with OSA with a mean BMI of 29 ± 5 kg/m2 who were treated with CPAP. ICAM-1 and E-selectin levels significantly decreased after CPAP treatment. 3–4 Days after CPAP had been started, the mean (± SD) soluble E-selectin level had decreased from 91 +/− 45 ng/mL to 69 ± 28 ng/mL (P = 0.002). After 1 mo, ICAM-1 decreased from 311 ± 116 ng/mL to 249 ± 74 ng/mL (P = 0.02). After 6 mo, there was no further change in sVCAM-1 while the mean sICAM-1 decreased further (212 ± 59 ng/mL (P = 0.02) as did E-selectin levels). It is important to note that only six subjects were studied after 6 mo.107
Thus, treatment of OSA with CPAP may decrease the rate of progression of OSA-related cardiovascular disease. Future studies, with larger samples as well as studies in patients with different degrees of obesity with and without cardiovascular disease are needed to provide a full picture of the potential benefit of CPAP therapy with respect to reduction in adhesion molecules.
Limitations of existing studies
The existing studies have several important limitations. First, circulating adhesion molecule levels may not necessarily reflect the actual levels of adhesion molecules in the relevant tissue (the vessel wall).
Second, the status of the cardiovascular system may be an important confounder that has not been optimally addressed. Several clinical studies have demonstrated the release of soluble adhesion molecules into the plasma of patients with coronary artery disease, acute myocardial infarction, coronary artery surgery and transplantation.83,108–110 If the presence of cardiovascular disease is not controlled for, it will not be possible to determine whether, if elevated adhesion molecules are found, they preceded the presence of cardiovascular disease, or whether the cardiovascular disease itself caused the elevation of adhesion molecules. Similarly, alcohol consumption should be adequately controlled for, as moderate alcohol intake has an anti-inflammatory effect on the cardiovascular system and reduces levels of ICAM-1, VCAM-1, and E-selectin.111 Current studies do not consistently control for confounding variables such as obesity, cardiac status, hypertension, smoking, age, and level of exercise, in order to more clearly elucidate the independent relationship between OSA and cardiovascular disease.112 Inconsistencies in covariates analyzed across the studies reduce the generalizability of the conclusions. Moreover, the studies linking adhesion molecules to OSA have very small sample sizes, often leaving studies without the statistical power to adequately test hypotheses and to address adequately confounding variables.
Third, the majority of the studies take plasma samples on one occasion, and thus the temporal variation in concentrations that may be present before to after sleep is not evaluated. This limits the ability to directly assess the impact of the repetitive hypoxia during sleep. Also, the current studies of adhesion molecules are limited by not exploring cardiovascular endpoints related to OSA. Prospective studies that monitor untreated OSA subjects with no overt cardiovascular disease but with high levels of adhesion molecules will be needed. This data may provide important information regarding the predictive value of adhesion molecules to provide prognostic information about cardiovascular disease in patients with OSA.
Potential novel therapies and future research directions
Future research will allow for a more complete understanding of the role of inflammation in general, and adhesion molecules in particular, in the pathogenesis of cardiovascular disease in the context of OSA. This could drive initiatives toward future translational research studies of novel therapeutic approaches to the treatment of cardiovascular risk associated with OSA.
Progenitor cells
Progenitor cells can be isolated from circulating mononuclear cells, bone marrow, and cord blood. They may contribute to vascular regeneration directly via incorporation into newly forming vascular structures or indirectly via the secretion of pro-angiogenic growth factors, which enhance the overall vascular and hemodynamic recovery of ischemic tissues.113,114 Progenitor cells may either help to protect/repair the endothelium by interacting with microenvironment via integrins and promote plaque stabilization, or conversely participate in the pathogenesis of atherosclerosis by contributing to smooth muscle cell accumulation and inducing the narrowing of the lumen.115
Further research is required in order to potentially manipulate vascular progenitor cells to control their trafficking to areas of damaged endothelium.113 A study conducted by Hill and coworkers explored circulating endothelial progenitor cells (EPCs), vascular function and cardiovascular risk.116 There was a strong correlation between the number of circulating EPCs and subjects’ combined Framingham risk factor score (used to predict risk of coronary artery disease in persons free of clinical disease) (r = −0.47, P = 0.001). Overall, EPCs from high-risk subjects are both fewer in number and become senescent (biological aging) more rapidly than those from low-risk subjects. In healthy men, levels of EPCs may be a surrogate biologic marker for vascular function and cumulative cardiovascular risk.116 As endothelial progenitor cells (EPCs) can repair injured vessels by homing to sites of ischemia and neo-vascularization, EPCs may have a cardioprotective role in OSA patients, and this may be more apparent in chronic as opposed to acute OSA subjects in whom neovascularization will more likely have occurred. There is a need for further research, as intracellular signaling pathways that regulate adhesion molecules and their role in the recruitment of progenitor cells to areas of inflammation are not entirely clear. Patients with OSA who are free of overt cardiovascular disease have been noted to have reduced circulating levels of EPCs.117 However, the role of OSA in these processes needs to be clarified.
Antioxidant therapies
Given the postulated role of ROS, antioxidant therapy may have a potential role in the treatment of OSA. In a study by Christou and coworkers, although there was no significant difference found in the measurement of antioxidant capacity between OSA patients and the healthy sample, patients with severe OSA (AHI > 20 events/h, N = 14) had a linearly negative correlation between antioxidant capacity in blood samples and AHI (R = −0.551, P = 0.041).118 These results suggest that patients with severe OSA syndrome had less antioxidant capacity, and a reduced antioxidant capacity is an index of excessive oxidative stress. In another study, Barcelo and coworkers found decreased plasma levels of total antioxidant status (biological marker from plasma for oxidative stress), antioxidant vitamins A and E, and increased values of γ-glutamyltransferase (a marker for cardiovascular disease) in 47 patients with OSA compared with 37 healthy non-smoking males of similar age and BMI. This study suggests that an impairment of protective systems for oxidative stress exists in patients with OSA.119 Cofta and coworkers assessed E-selectin levels and total antioxidant status in the blood of subjects with different ranges of OSA severity. They found progressively decreased concentrations of total antioxidant status and significantly increased concentrations of E-selectin with increasing severity of OSA subjects.120 These data support the assertion that antioxidants may at least partially ameliorate inflammatory and, subsequently, cardiovascular consequences of OSA.
This concept is supported by evidence from Grebe et al., who studied 10 untreated patients with OSA before and after intravenous injection of antioxidant vitamin C, compared to 10 age and sex-matched controls.121 These investigators found that endothelial dysfunction as measured by flow-mediated dilation of brachial artery assessed by ultrasound in OSA patients can be acutely reversed with vitamin C.121 With vitamin C, the values of endothelial vasoreactivity in patients with OSA rose to a level comparable to those of the control subjects without sleep-disordered breathing. This supports the concept that increased oxidative stress is likely to play a major role in OSA-related endothelial dysfunction.
In summary, it has not been conclusively proven that increasing antioxidant activity will protect against the cardiovascular consequences of OSA. No prospective studies have explored antioxidant intake and the impact on adhesion molecules and cardiovascular outcomes in patients with OSA. However, if the endothelial dysfunction characteristic of OSA is a result of oxidative stress, it can potentially be reversed by administration of antioxidants. This is an area where there is a need for further studies.
Antibodies
Another potential intervention is the use of antibodies to affect specific molecules in the pathway to disease.122 Animal studies have tested the possibility of blocking leukocyte adhesion molecules by antibodies to reduce venous ischemic injury.123–126 More specifically, these studies have used different antibodies specific to different molecules, capable of inhibiting adhesion molecules of interest in order to prevent reperfusion injury in animal models of myocardial infarction. Initial animal studies suggest protective effects on ischemia/reperfusion and a beneficial effect in increasing the viable area of ischemia when treated with appropriate antibodies. However, when this theory was explored in a clinical trial, anti-ICAM-1 antibodies were not successful in ischemic stroke patients who were randomized to placebo or Enlimomab, a murine monoclonal anti-human ICAM-1 antibody.127 The authors concluded that anti-ICAM therapy is not an effective treatment for ischemic stroke in the model studied and, indeed, may significantly worsen stroke outcome.127 There were more adverse events with Enlimomab treatment than placebo that primarily involved infections and fever. Investigators followed up on these negative results with an animal study demonstrating that the administration of murine antibody preparation against ICAM-1 in rats elicits the host antibodies against the protein, activates circulating neutrophils and complement which may explain the mechanisms for central nervous system clinical deterioration that occurred with Enlimomab in acute ischemic stroke.125
Overall, the current research suggests a need to further explore the therapeutic effect of antibodies in relation to preventing cardiovascular disease. Experimental results are variable and are further limited by the fact that upregulation of adhesion molecules following tissue damage may be a physiological response that is important for the repair process. Studies in pre-clinical models such as those involving administering cyclical intermittent hypoxia in rodents would seem a useful approach.
Conclusions
OSA is a known risk factor for the development of cardiovascular disease. One proposed pathway is through intermittent hypoxia, which increases ROS, leading to chronic inflammation (including upregulation of adhesion molecules) – a major risk factor and biomarker for atherosclerosis risk.
During respiratory effort in OSA, it appears intrathoracic pressure is also a mechanism that is causative in the development of coronary artery disease in addition to other factors (i.e., airway collapse, hypoxemia). However, other physiologic mechanisms and their cardiovascular consequences have yet to be studied in the context of adhesion molecules.
The observational studies to date suggest that adhesion molecules are elevated in OSA and hence could contribute to cardiovascular disease. Methodological limitations of current studies, however, must be acknowledged. The results of these observational studies are supported by a small number of CPAP intervention studies, but these investigations have very small sample sizes and do not adequately address obesity and other potential confounders. Larger-scale studies with well-defined patient and control populations and repeated measurements of adhesion molecules before and after sleep are lacking. Prospective studies are needed to clarify the link between the levels of adhesion molecules in OSA subjects and subsequent cardiovascular outcomes.
Cellular interactions are critical for the development of atherosclerosis. Thus, there is a need to understand the precise mechanisms by which adhesion molecules exert effects and enter/exit the bloodstream. Adhesion molecules may serve as a biomarker of cell activation that contributes to atherosclerosis, which may prove useful for risk stratification. Since oxidative stress seems an important part of pathogenesis, OSA subjects who do not tolerate CPAP therapy may potentially benefit from the addition of anti-oxidant supplements. Further research is required to understand the effects of both CPAP treatment and novel therapies reviewed above on the cardiovascular consequences of OSA. Future therapies directed toward inhibiting adhesive interactions may also be useful in order to slow the development of atherosclerotic plaques. Thus, the diagnostic and therapeutic usefulness of focusing on adhesion molecules in OSA remains to be ascertained, and opportunities exist for clinical research in this area with the goal of translating findings to treating patients with OSA.
Practice points.
Adhesion molecules include three general families of proteins known as selectins, integrins, and immunoglobulins, which are involved in leukocyte recruitment. These molecules serve the important purpose of facilitating the function of leukocytes along the endothelium during inflammation. Because of this function, they may play an important role in the development of cardiovascular disease risk, particularly atherogenesis, in patients with OSA.
Few studies have explored adhesion molecules present in OSA, and increased circulating adhesion molecules including ICAM-1 and VCAM-1 have been observed. The small sample sizes of the study and inconsistent control of confounders necessitate further research.
Elevated levels of adhesion molecules in OSA may return to baseline following CPAP treatment.
Research agenda.
Future longitudinal studies that monitor untreated OSA subjects with no overt cardiovascular disease but with high levels of adhesion molecules will be needed.
Additional studies should explore the effectiveness of CPAP on decreasing adhesion molecules and improving cardiovascular outcomes.
The clinical role of assessing adhesion molecules in OSA with respect to the cardiovascular complications of the disorder will need to be clarified.
There is a need for additional studies on novel therapies directed toward inhibiting adhesive interactions in order to affect the development of atherosclerotic plaques and reduce cardiovascular consequences of OSA.
Acknowledgments
This work was supported by NIH grants T32 HL07713 (VMP), K23 HL110216 (MAG) and P01 HL094307 (AIP).
Abbreviations
- AHI
apnea hypopnea index
- BMI
body mass index
- CAD
coronary artery disease
- CAMS
cell adhesion molecules
- CPAP
continuous positive airway pressure
- CRP
C-reactive protein
- ELISA
enzyme linked immunosorbent assay
- EPCs
endothelial progenitor cells
- FMD
flow-mediated dilation
- ICAM
intercellular adhesion molecule
- IL
interleukin
- MCP
monocyte chemoattractant protein
- NF-κB
nuclear factor-kappa B
- OSA
obstructive sleep apnea
- OSAS
obstructive sleep apnea syndrome
- PECAM
platelet endothelial cell adhesion molecule
- PMN
polymorphonuclear neutrophil
- PSGL
P-selectin glycoprotein ligand
- ROS
reactive oxygen species
- sICAM
soluble ICAM
- SMC
smooth muscle cells smooth muscle cells
- sVCAM
soluble VCAM
- TNF
tumor necrosis factor
- VCAM
vascular cell adhesion molecule
Footnotes
The most important references are denoted by an asterisk.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis. 2009;51:434–51. doi: 10.1016/j.pcad.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 4.Yamauchi M, Kimura H. Oxidative stress in obstructive sleep apnea: putative pathways to the cardiovascular complications. Antioxid Redox Signal. 2008;10:755–68. doi: 10.1089/ars.2007.1946. [DOI] [PubMed] [Google Scholar]
- 5.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27:123–8. [PubMed] [Google Scholar]
- 6.Kim CD, Kim YK, Lee SH, Hong KW. Rebamipide inhibits neutrophil adhesion to hypoxia/reoxygenation-stimulated endothelial cells via nuclear factor-kappaB-dependent pathway. J Pharmacol Exp Ther. 2000;294:864–9. [PubMed] [Google Scholar]
- 7.Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–23. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 8.Sawa Y, Sugimoto Y, Ueki T, Ishikawa H, Sato A, Nagato T, et al. Effects of TNF-alpha on leukocyte adhesion molecule expressions in cultured human lymphatic endothelium. J Histochem Cytochem. 2007;55:721–33. doi: 10.1369/jhc.6A7171.2007. [DOI] [PubMed] [Google Scholar]
- *9.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 10.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- *11.Galkina E, Ley K. Leukocyte influx in atherosclerosis. Curr Drug Targets. 2007;8:1239–48. doi: 10.2174/138945007783220650. [DOI] [PubMed] [Google Scholar]
- *12.Cybulsky MI, Lichtman AH, Hajra L, Iiyama K. Leukocyte adhesion molecules in atherogenesis. Clin Chim Acta. 1999;286:207–18. doi: 10.1016/s0009-8981(99)00102-3. [DOI] [PubMed] [Google Scholar]
- 13.Fotis L, Agrogiannis G, Vlachos IS, Pantopoulou A, Margoni A, Kostaki M, et al. Intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 at the early stages of atherosclerosis in a rat model. In Vivo. 2012;26:243–50. [PubMed] [Google Scholar]
- 14.Nakai K, Itoh C, Kawazoe K, Miura Y, Sotoyanagi H, Hotta K, et al. Concentration of soluble vascular cell adhesion molecule-1 (VCAM-1) correlated with expression of VCAM-1 mRNA in the human atherosclerotic aorta. Coron Artery Dis. 1995;6:497–502. [PubMed] [Google Scholar]
- 15.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea–hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336:261–4. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- 19.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320:479–82. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sajkov D, Cowie RJ, Thornton AT, Espinoza HA, McEvoy RD. Pulmonary hypertension and hypoxemia in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1994;149:416–22. doi: 10.1164/ajrccm.149.2.8306039. [DOI] [PubMed] [Google Scholar]
- 21.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:613–8. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 22.Baguet JP, Hammer L, Levy P, Pierre H, Launois S, Mallion JM, et al. The severity of oxygen desaturation is predictive of carotid wall thickening and plaque occurrence. Chest. 2005;128:3407–12. doi: 10.1378/chest.128.5.3407. [DOI] [PubMed] [Google Scholar]
- 23.Kasasbeh E, Chi DS, Krishnaswamy G. Inflammatory aspects of sleep apnea and their cardiovascular consequences. South Med J. 2006;99:58–67. doi: 10.1097/01.smj.0000197705.99639.50. [DOI] [PubMed] [Google Scholar]
- 24.Kohli P, Balachandran JS, Malhotra A. Obstructive sleep apnea and the risk for cardiovascular disease. Curr Atheroscler Rep. 2011 Apr;13:138–46. doi: 10.1007/s11883-011-0161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spath-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry. 1991;29:575–84. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- 26.Bonnet MH, Arand DL. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med Rev. 2003;7:297–310. doi: 10.1053/smrv.2001.0245. [DOI] [PubMed] [Google Scholar]
- 27.Blasi A, Jo J, Valladares E, Morgan BJ, Skatrud JB, Khoo MC. Cardiovascular variability after arousal from sleep: time-varying spectral analysis. J Appl Physiol. 2003;95:1394–404. doi: 10.1152/japplphysiol.01095.2002. [DOI] [PubMed] [Google Scholar]
- 28.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24:775–84. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks D, Horner RL, Kimoff RJ, Kozar LF, Render-Teixeira CL, Phillipson EA. Effect of obstructive sleep apnea versus sleep fragmentation on responses to airway occlusion. Am J Respir Crit Care Med. 1997;155:1609–17. doi: 10.1164/ajrccm.155.5.9154865. [DOI] [PubMed] [Google Scholar]
- 30.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koo BB, Blackwell T, Ancoli-Israel S, Stone KL, Stefanick ML, Redline S, et al. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation. 2011;124:1223–31. doi: 10.1161/CIRCULATIONAHA.111.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basta M, Lin HM, Pejovic S, Sarrigiannidis A, Bixler E, Vgontzas AN. Lack of regular exercise, depression, and degree of apnea are predictors of excessive daytime sleepiness in patients with sleep apnea: sex differences. J Clin Sleep Med. 2008;4:19–25. [PMC free article] [PubMed] [Google Scholar]
- 33.Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, Busse R, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–92. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 34.Arner P. Regional differences in protein production by human adipose tissue. Biochem Soc Trans. 2001;29:72–5. doi: 10.1042/bst0290072. [DOI] [PubMed] [Google Scholar]
- 35.Dusserre E, Moulin P, Vidal H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim Biophys Acta. 2000;1500:88–96. doi: 10.1016/s0925-4439(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 36.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32:447–70. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–71. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 38.Arnardottir ES, Maislin G, Schwab RJ, Staley B, Benediktsdottir B, Olafsson I, et al. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic sleep apnea cohort. Sleep. 2012;35:921–32. doi: 10.5665/sleep.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng Y, Yuan G, Overholt JL, Kumar GK, Prabhakar NR. Systemic and cellular responses to intermittent hypoxia: evidence for oxidative stress and mitochondrial dysfunction. Adv Exp Med Biol. 536:559–64. doi: 10.1007/978-1-4419-9280-2_71. [DOI] [PubMed] [Google Scholar]
- 40.Lavie L. Obstructive sleep apnoea syndrome – an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- *41.McNicholas WT, Bonsigore MR. Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 42.Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162:566–70. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 43.Sedeek MH, Llinas MT, Drummond H, Fortepiani L, Abram SR, Alexander BT, et al. Role of reactive oxygen species in endothelin-induced hypertension. Hypertension. 2003;42:806–10. doi: 10.1161/01.HYP.0000084372.91932.BA. [DOI] [PubMed] [Google Scholar]
- 44.Khodr B, Khalil Z. Modulation of inflammation by reactive oxygen species: implications for aging and tissue repair. Free Radic Biol Med. 2001;30:1–8. doi: 10.1016/s0891-5849(00)00378-6. [DOI] [PubMed] [Google Scholar]
- 45.Fresquet F, Pourageaud F, Leblais V, Brandes RP, Savineau JP, Marthan R, et al. Role of reactive oxygen species and gp91phox in endothelial dysfunction of pulmonary arteries induced by chronic hypoxia. Br J Pharmacol. 2006;148:714–23. doi: 10.1038/sj.bjp.0706779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–9. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 47.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol. 1989;67:2101–6. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]
- 48.Somers VK, Mark AL, Zavala DC, Abboud FM. Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J Appl Physiol. 1989;67:2095–100. doi: 10.1152/jappl.1989.67.5.2095. [DOI] [PubMed] [Google Scholar]
- 49.Yamauchi M, Tamaki S, Tomoda K, Yoshikawa M, Fukuoka A, Makinodan K, et al. Evidence for activation of nuclear factor kappaB in obstructive sleep apnea. Sleep Breath. 2006;10:189–93. doi: 10.1007/s11325-006-0074-x. [DOI] [PubMed] [Google Scholar]
- 50.Ryan S, Nolan GM, Hannigan E, Cunningham S, Taylor C, McNicholas WT. Cardiovascular risk markers in obstructive sleep apnoea syndrome and correlation with obesity. Thorax. 2007;62:509–14. doi: 10.1136/thx.2006.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryan S, McNicholas WT, Taylor CT. A critical role for p38 map kinase in NF-kappaB signaling during intermittent hypoxia/reoxygenation. Biochem Biophys Res Commun. 2007;355:728–33. doi: 10.1016/j.bbrc.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:419–20. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 53.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 54.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–8. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 55.Falk E. Plaque vulnerability and disruption. Rev Clin Esp. 1996;196:6–12. [PubMed] [Google Scholar]
- 56.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- *57.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 58.Zarbock A, Kempf T, Wollert KC, Vestweber D. Leukocyte integrin activation and deactivation: novel mechanisms of balancing inflammation. J Mol Med. 2012;90:353–9. doi: 10.1007/s00109-011-0835-2. [DOI] [PubMed] [Google Scholar]
- 59.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev. 2007;218:178–96. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 60.Walzog B, Gaehtgens P. Adhesion molecules: the path to a new understanding of acute inflammation. News Physiol Sci. 2000;15:107–13. doi: 10.1152/physiologyonline.2000.15.3.107. [DOI] [PubMed] [Google Scholar]
- *61.Kubes P, Kerfoot SM. Leukocyte recruitment in the microcirculation: the rolling paradigm revisited. News Physiol Sci. 2001;16:76–80. doi: 10.1152/physiologyonline.2001.16.2.76. [DOI] [PubMed] [Google Scholar]
- 62.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–101. [PubMed] [Google Scholar]
- 63.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–9. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 64.Matheny HE, Deem TL, Cook-Mills JM. Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J Immunol. 2000;164:6550–9. doi: 10.4049/jimmunol.164.12.6550. [DOI] [PubMed] [Google Scholar]
- 65.Couffinhal T, Duplaa C, Moreau C, Lamaziere JM, Bonnet J. Regulation of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in human vascular smooth muscle cells. Circ Res. 1994;74:225–34. doi: 10.1161/01.res.74.2.225. [DOI] [PubMed] [Google Scholar]
- 66.Gearing AJ, Hemingway I, Pigott R, Hughes J, Rees AJ, Cashman SJ. Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1, and VCAM-1: pathological significance. Ann N Y Acad Sci. 1992;667:324–31. doi: 10.1111/j.1749-6632.1992.tb51633.x. [DOI] [PubMed] [Google Scholar]
- 67.Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24:2166–79. doi: 10.1016/j.ehj.2003.08.021. [DOI] [PubMed] [Google Scholar]
- *68.Ohga E, Nagase T, Tomita T, Teramoto S, Matsuse T, Katayama H, et al. Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndrome. J Appl Physiol. 1999;87:10–4. doi: 10.1152/jappl.1999.87.1.10. [DOI] [PubMed] [Google Scholar]
- *69.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol. 2003;94:179–84. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- *70.Ursavas A, Karadag M, Rodoplu E, Yilmaztepe A, Oral HB, Gozu RO. Circulating ICAM-1 and VCAM-1 levels in patients with obstructive sleep apnea syndrome. Respiration. 2007;74:525–32. doi: 10.1159/000097770. [DOI] [PubMed] [Google Scholar]
- *71.El-Solh AA, Mador MJ, Sikka P, Dhillon RS, Amsterdam D, Grant BJ. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest. 2002;121:1541–7. doi: 10.1378/chest.121.5.1541. [DOI] [PubMed] [Google Scholar]
- 72.Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci U S A. 1998;95:14423–8. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 74.Peter K, Nawroth P, Conradt C, Nordt T, Weiss T, Boehme M, et al. Circulating vascular cell adhesion molecule-1 correlates with the extent of human atherosclerosis in contrast to circulating intercellular adhesion molecule-1, E-selectin, P-selectin, and thrombomodulin. Arterioscler Thromb Vasc Biol. 1997;17:505–12. doi: 10.1161/01.atv.17.3.505. [DOI] [PubMed] [Google Scholar]
- 75.Rohde LE, Lee RT, Rivero J, Jamacochian M, Arroyo LH, Briggs W, et al. Circulating cell adhesion molecules are correlated with ultrasound-based assessment of carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 1998;18:1765–70. doi: 10.1161/01.atv.18.11.1765. [DOI] [PubMed] [Google Scholar]
- 76.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 77.Ridker PM. C-reactive protein in 2005. Interview by Peter C. Block J Am Coll Cardiol. 2005;46:CS2–5. [PubMed] [Google Scholar]
- 78.O’Brien K, Allen M, McDonald T, Chait A, Harlan J, Fishbein D. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–51. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–91. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 80.Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, et al. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104:1336–42. doi: 10.1161/hc3701.095949. [DOI] [PubMed] [Google Scholar]
- 81.Malik I, Danesh J, Whincup P, Bhatia V, Papacosta O, Walker M, et al. Soluble adhesion molecules and prediction of coronary heart disease: a prospective study and meta-analysis. Lancet. 2001;358:971–6. doi: 10.1016/S0140-6736(01)06104-9. [DOI] [PubMed] [Google Scholar]
- 82.Price DT, Loscalzo J. Cellular adhesion molecules and atherogenesis. Am J Med. 1999;107:85–97. doi: 10.1016/s0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- 83.Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the atherosclerosis risk in communities (ARIC) study. Circulation. 1997;96:4219–25. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 84.Zeitler H, Ko Y, Zimmermann C, Nickenig G, Glanzer K, Walger P, et al. Elevated serum concentrations of soluble adhesion molecules in coronary artery disease and acute myocardial infarction. Eur J Med Res. 1997;2:389–94. [PubMed] [Google Scholar]
- 85.Papayianni A, Alexopoulos E, Giamalis P, Gionanlis L, Belechri AM, Koukoudis P, et al. Circulating levels of ICAM-1, VCAM-1, and MCP-1 are increased in haemodialysis patients: association with inflammation, dys-lipidaemia, and vascular events. Nephrol Dial Transplant. 2002;17:435–41. doi: 10.1093/ndt/17.3.435. [DOI] [PubMed] [Google Scholar]
- 86.Westhuyzen J, Healy H. Review: biology and relevance of C-reactive protein in cardiovascular and renal disease. Ann Clin Lab Sci. 2000;30:133–43. [PubMed] [Google Scholar]
- 87.Pasceri V, Cheng JS, Willerson JT, Yeh ET. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001;103:2531–4. doi: 10.1161/01.cir.103.21.2531. [DOI] [PubMed] [Google Scholar]
- 88.Kim J, Lee CH, Park CS, Kim BG, Kim SW, Cho JH. Plasma levels of MCP-1 and adiponectin in obstructive sleep apnea syndrome. Arch Otolaryngol Head Neck Surg. 2010;136:896–9. doi: 10.1001/archoto.2010.142. [DOI] [PubMed] [Google Scholar]
- 89.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252:283–94. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 90.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–30. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 91.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 92.Alberti A, Sarchielli P, Gallinella E, Floridi A, Floridi A, Mazzotta G, et al. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J Sleep Res. 2003;12:305–11. doi: 10.1111/j.1365-2869.2003.00361.x. [DOI] [PubMed] [Google Scholar]
- 93.Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Tanaka A, Oda N, et al. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:625–30. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 94.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–23. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 95.Aukrust P, Halvorsen B, Yndestad A, Ueland T, Oie E, Otterdal K, et al. Chemokines and cardiovascular risk. Arterioscler Thromb Vasc Biol. 2008;28:1909–19. doi: 10.1161/ATVBAHA.107.161240. [DOI] [PubMed] [Google Scholar]
- 96.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 97.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 98.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–82. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 99.Saletu M, Nosiska D, Kapfhammer G, Lalouschek W, Saletu B, Benesch T, et al. Structural and serum surrogate markers of cerebrovascular disease in obstructive sleep apnea (OSA): association of mild OSA with early atherosclerosis. J Neurol. 2006;253:746–52. doi: 10.1007/s00415-006-0110-6. [DOI] [PubMed] [Google Scholar]
- 100.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 101.Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 102.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Collins B, Basta M, et al. Selective effects of CPAP on sleep apnoea-associated manifestations. Eur J Clin Invest. 2008;38:585–95. doi: 10.1111/j.1365-2362.2008.01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carneiro G, Togeiro SM, Ribeiro-Filho FF, Truksinas E, Ribeiro AB, Zanella MT, et al. Continuous positive airway pressure therapy improves hypoadiponectinemia in severe obese men with obstructive sleep apnea without changes in insulin resistance. Metab Syndr Relat Disord. 2009;7:537–42. doi: 10.1089/met.2009.0019. [DOI] [PubMed] [Google Scholar]
- 104.Tamaki S, Yamauchi M, Fukuoka A, Makinodan K, Koyama N, Tomoda K, et al. Nocturnal hypoxic stress activates invasive ability of monocytes in patients with obstructive sleep apnoea syndrome. Respirology. 2009;14:689–94. doi: 10.1111/j.1440-1843.2009.01540.x. [DOI] [PubMed] [Google Scholar]
- 105.Nacher M, Serrano-Mollar A, Farre R, Panes J, Segui J, Montserrat JM. Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea. Respir Physiol Neurobiol. 2007;155:93–6. doi: 10.1016/j.resp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 106.Carpagnano GE, Spanevello A, Sabato R, Depalo A, Palladino GP, Bergantino L, et al. Systemic and airway inflammation in sleep apnea and obesity: the role of ICAM-1 and IL-8. Transl Res. 2010;155:35–43. doi: 10.1016/j.trsl.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 107.Chin K, Nakamura T, Shimizu K, Mishima M, Nakamura T, Miyasaka M, et al. Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. Am J Med. 2000;109:562–7. doi: 10.1016/s0002-9343(00)00580-5. [DOI] [PubMed] [Google Scholar]
- 108.Kerner T, Ahlers O, Reschreiter H, Buhrer C, Mockel M, Gerlach H. Adhesion molecules in different treatments of acute myocardial infarction. Crit Care. 2001;5:145–50. doi: 10.1186/cc1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grunenfelder J, Zund G, Schoeberlein A, Schmid ER, Schurr U, Frisullo R, et al. Expression of adhesion molecules and cytokines after coronary artery bypass grafting during normothermic and hypothermic cardiac arrest. Eur J Cardiothorac Surg. 2000;17:723–8. doi: 10.1016/s1010-7940(00)00401-2. [DOI] [PubMed] [Google Scholar]
- 110.Weigel G, Grimm M, Griesmacher A, Seebacher G, Sichrovsky T, Wolner E, et al. Adhesion molecule behavior during rejection and infection episodes after heart transplantation. Clin Chem Lab Med. 2000;38:403–8. doi: 10.1515/CCLM.2000.058. [DOI] [PubMed] [Google Scholar]
- 111.Sacanella E, Estruch R. The effect of alcohol consumption on endothelial adhesion molecule expression. Addict Biol. 2003;8:371–8. doi: 10.1080/13556210310001656376. [DOI] [PubMed] [Google Scholar]
- 112.Kiely JL, McNicholas WT. Cardiovascular risk factors in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2000;16:128–33. doi: 10.1034/j.1399-3003.2000.16a23.x. [DOI] [PubMed] [Google Scholar]
- 113.Chavakis E, Dimmeler S. Homing of progenitor cells to ischemic tissues. Antioxid Redox Signal. 2011;15:967–80. doi: 10.1089/ars.2010.3582. [DOI] [PubMed] [Google Scholar]
- 114.Alev C, Ii M, Asahara T. Endothelial progenitor cells: a novel tool for the therapy of ischemic diseases. Antioxid Redox Signal. 2011;15:949–65. doi: 10.1089/ars.2010.3872. [DOI] [PubMed] [Google Scholar]
- 115.Campagnolo P, Wong MM, Xu Q. Progenitor cells in arteriosclerosis: good or bad guys? Antioxid Redox Signal. 2011;15:1013–27. doi: 10.1089/ars.2010.3506. [DOI] [PubMed] [Google Scholar]
- 116.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 117.Jelic S, Le Jemtel TH. Inflammation, oxidative stress, and the vascular endothelium in obstructive sleep apnea. Trends Cardiovasc Med. 2008;18:253–60. doi: 10.1016/j.tcm.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 118.Christou K, Moulas AN, Pastaka C, Gourgoulianis KI. Antioxidant capacity in obstructive sleep apnea patients. Sleep Med. 2003;4:225–8. doi: 10.1016/s1389-9457(02)00253-8. [DOI] [PubMed] [Google Scholar]
- 119.Barcelo A, Barbe F, de la Pena M, Vila M, Perez G, Pierola J, et al. Antioxidant status in patients with sleep apnoea and impact of continuous positive airway pressure treatment. Eur Respir J. 2006;27:756–60. doi: 10.1183/09031936.06.00067605. [DOI] [PubMed] [Google Scholar]
- 120.Cofta S, Wysocka E, Michalak S, Piorunek T, Batura-Gabryel H, Torlinski L. Endothelium-derived markers and antioxidant status in the blood of obstructive sleep apnea males. Eur J Med Res. 2009;14:49–52. doi: 10.1186/2047-783X-14-S4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grebe M, Eisele HJ, Weissmann N, Schaefer C, Tillmanns H, Seeger W, et al. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med. 2006;173:897–901. doi: 10.1164/rccm.200508-1223OC. [DOI] [PubMed] [Google Scholar]
- 122.Manikwar P, Tejo BA, Shinogle H, Moore DS, Zimmerman T, Blanco F, et al. Utilization of I-domain of LFA-1 to target drug and marker molecules to leukocytes. Theranostics. 2011;1:277–89. doi: 10.7150/thno/v01p0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fukushima S, Coppen SR, Varela-Carver A, Yamahara K, Sarathchandra P, Smolenski RT, et al. A novel strategy for myocardial protection by combined antibody therapy inhibiting both P-selectin and intercellular adhesion molecule-1 via retrograde intracoronary route. Circulation. 2006;114:I251–6. doi: 10.1161/CIRCULATIONAHA.105.000794. [DOI] [PubMed] [Google Scholar]
- 124.Demirseren ME, Sarici M, Gokrem S, Yenidunya S. Protective effects of monoclonal antibody to intercellular adhesion molecule-1 in venous ischemia-reperfusion injury: experimental study in rats. J Reconstr Microsurg. 2007;23:41–4. doi: 10.1055/s-2006-958701. [DOI] [PubMed] [Google Scholar]
- 125.Furuya K, Takeda H, Azhar S, McCarron RM, Chen Y, Ruetzler CA, et al. Examination of several potential mechanisms for the negative outcome in a clinical stroke trial of enlimomab, a murine anti-human intercellular adhesion molecule-1 antibody: a bedside-to-bench study. Stroke. 2001;32:2665–74. doi: 10.1161/hs3211.098535. [DOI] [PubMed] [Google Scholar]
- 126.Ma XL, Lefer DJ, Lefer AM, Rothlein R. Coronary endothelial and cardiac protective effects of a monoclonal antibody to intercellular adhesion molecule-1 in myocardial ischemia and reperfusion. Circulation. 1992;86:937–46. doi: 10.1161/01.cir.86.3.937. [DOI] [PubMed] [Google Scholar]
- 127.Enlimomab Acute Stroke Trial Investigators. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–34. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]


