Abstract
Iptakalim is an ATP-sensitive potassium channel opener, as well as an α4β2-containing nicotinic acetylcholine receptor (nAChR) antagonist. Pretreatment with iptakalim diminishes nicotine-induced dopamine (DA) and glutamate release in the nucleus accumbens. This neuropharmacological profile suggests that iptakalim may be useful for treatment of nicotine dependence. Thus, we examined the effects of iptakalim in two preclinical models. First, the impact of iptakalim on the interoceptive stimulus effect of nicotine was evaluated by training rats in a discriminated goal-tracking task that included intermixed nicotine (0.4 mg/kg, SC) and saline sessions. Sucrose was intermittently presented in a response-independent manner only on nicotine sessions. On intervening test days, rats were pretreated with iptakalim (10, 30, 60 mg/kg, IP). Results revealed that iptakalim attenuated nicotine-evoked responding controlled by the nicotine stimulus in a dose-dependent manner. In a separate study, the impact of iptakalim on the reinforcing effects of nicotine was investigated by training rats to lever-press to self-administer nicotine (0.03 mg/kg/infusion). Results revealed that pretreatment with iptakalim (1, 3, 6 mg/kg, IV) decreased nicotine intake (i.e., less active lever responding). Neither behavioral effect was due to a non-specific motor effect of iptakalim, nor to an ability of iptakalim to inhibit DA transporter (DAT) or serotonin transporter (SERT) function. Together, these finding support the notion that iptakalim may be an effective pharmacotherapy for increasing smoking cessation and better understanding its action could contribute medication development.
INTRODUCTION
Over 400,000 people in the United States die annually from tobacco-related diseases (CDC, 2008). It is estimated that $193 billion per year of expenditures are associated with health care cost and loss of productivity related to the consumption of tobacco products (CDC, 2008). Even with a number of smoking cessation treatments available (e.g., Zyban®, Chantix®), only about 20% of users remain abstinent for over a year (Schnoll and Lerman, 2006). Clearly there is still great need for new and effective treatments for nicotine dependence.
Iptakalim is an adenosine triphosphate-sensitive potassium channel opener that is approved for use in treating hypertension in China (Wang et al, 2005a; Wang et al, 2005b). Along with its action on potassium channels, iptakalim has several other neuropharmacological effects that suggest that it could be used to treat nicotine dependence. For example, iptakalim is an α4β2 nicotinic acetylcholine receptor antagonist (Hu et al, 2006). Nicotine increases dopamine and glutamate release in the nucleus accumbens. Pretreatment with iptakalim reduces this nicotine -induced intraccumbal dopamine and glutamate release (Hu et al, 2006; Liu et al, 2006). Behaviorally, iptakalim under some conditions can decreases nicotine-induced hyperactivity (Hu et al, 2006; Liu et al, 2006; Volf et al, 2012). Given these findings, the first goal of the current research was to examine the effect of iptakalim on nicotine in two separate animal models: the discriminated goal-tracking task that assesses the interoceptive stimulus effects of nicotine and the intravenous (IV) drug self-administration task that assess the positive reinforcing effects of nicotine.
The interoceptive stimulus effects of nicotine and the associated learning processes are thought to be important to chronic tobacco use and nicotine dependence (Bevins and Palmatier, 2004). Indeed, the pharmacological effects of nicotine that generate a perceptible internal stimulus (cf. drug intoxication) can come to control reinforced behaviors in rodents and primates—including humans (for reviews see Smith and Stolerman, 2009; Wooters et al, 2009). In the present study, we used the drug discriminated goal-tracking (DGT) task with rats to investigate the effects of iptakalim on the interoceptive stimulus effects of nicotine (Besheer et al, 2004; Bevins et al, 2012). In this task, rats receive intermixed sessions of nicotine and saline. In nicotine sessions, sucrose is available intermittently regardless of behavior; during saline sessions, sucrose is unavailable. After a period of training, nicotine comes to evoke a robust anticipatory food-seeking response, or what is often referred to as goal-tracking. In the DGT, this food-seeking response translates into an increase in head-entries into a dipper receptacle where sucrose has occurred in the past (Farwell and Ayres, 1979). This discriminated goal-tracking response is mediated by centrally located nicotinic acetylcholine receptors (Besheer et al, 2004; Struthers et al, 2009). The α4β2-containing receptors seem to be of particular importance. For example, the α4β2 partial receptor agonist varenicline and the α4β2 agonists ABT-418 fully substitute for the nicotine stimulus in the DGT task (Reichel et al, 2010). Further, the α4β2 antagonist dihydro-β-erythroidine blocks conditioned responding evoked by the nicotine stimulus (Struthers et al, 2009).
Another factor contributing to chronic tobacco use is the reinforcing effect of nicotine (Henningfield and Goldberg, 1983; Le Foll and Goldberg, 2009; Stolerman and Shoaib, 1991). Intravenous nicotine self-administration in rats has been commonly used to assess the reinforcing effects of nicotine. In this task, rats have a catheter implanted into the jugular vein. Rats are then given an opportunity to make one of two responses (e.g., press on the right or left lever). Responding as prescribed by the experimenter on one of the two levers will produce an infusion of nicotine; responding on the other inactive lever has no programmed consequence. Nicotine self-administration, as indicated by greater responding on the drug lever, has been demonstrated in many laboratories (Caggiula et al, 2002; Corrigall and Coen, 1989; DeNoble and Mele, 2006; Donny et al, 1995; Feltenstein et al, 2012; Neugebauer et al, 2006). The α4β2 antagonist DHβE attenuates nicotine self-administration (Grottick et al, 2000; Tobey et al, 2012; Watkins et al, 1999).
We found that iptakalim attenuated responding in the DGT task and decreased the intake of nicotine in the self-administration task. Monoamine transporter inhibition can likewise diminish these behaviors. For example, pretreatment with the selective serotonin reuptake inhibitor citalopram blocks nicotine-evoked responding in the DGT task (Dion et al., 2012). Further, the dopamine reuptake inhibitor bupropion substitutes for the nicotine stimulus (Wilkinson et al., 2010). Given these findings, a second goal of the present research was to examine the effects of iptakalim on DAT and SERT to determine if these transporters contribute to iptakalim’s mechanism of action.
MATERIALS
Animals
Thirty male Sprague-Dawley rats weighing 275–299 grams upon arrival from Harlan (Indianapolis, IN, USA) were housed individually in clear polycarbonate cages (48.3×26.7×20.3 cm) lined with wood shavings. The temperature- and humidity-controlled colony was on a 12-h light/dark schedule (lights on at 0600); experiments were conducted during the light cycle. Water was freely available in the home cage. Rats’ weight in the DGT task was maintained at 85% of their free-feeding body weight. For rats in the self-administration study, chow was available ad libitum for the day before surgery and the 6 days following surgery. Otherwise, rats were maintained at 90% of their free-feeding body weight. Four weeks into the self-administration study, the target weight was increased by 2 g to adjust for average growth pattern provided by the supplier. Experimental protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
Apparatus
The conditioning chambers (ENV-008CT; Med Associates, Inc.; St. Albans, VT, USA) measuring 30.5×24.1×21.0 cm (l×w×h), were enclosed in a sound- and light-attenuating cubicle equipped with an exhaust fan. Each chamber had aluminum sidewalls, metal rod floors with polycarbonate front, back, and ceiling. A recessed receptacle (5.2×5.2×3.8 cm; l×w×d) was centered on one sidewall. A dipper arm, when raised, provided access to 0.1 ml of 26% (w/v) sucrose solution in the receptacle. Access to the dipper was monitored by an infrared beam mounted 1.2 cm into the receptacle and 3 cm above the floor. A second infrared beam that monitored chamber activity was located 4 cm above the floor and 14.5 cm from the side wall containing the receptacle. Two retractable levers (147 nN required for micro-switch closure) were mounted on each side of the receptacle. A white cue-light (2.54 cm diameter; 28V, 100 mA) was mounted 7 cm above each lever. A house light (two white 28V, 100 mA lamps) was located 10 cm above the conditioning chamber. The infusion pump (PMH-100VS; Med Associates; St. Albans, VT, USA) for each chamber was located outside the sound-attenuating cubicle. A 5-ml syringe mounted on the infusion pump was connected with Tygon® tubing (AAQ04103; VWR; West Chester, PA, USA). The tubing was attached to a swivel coupled with a spring leash (C313C; Plastics One; Roanoke, VA, USA) which were suspended over the ceiling of the chamber on a balanced metal arm. Med Associates interface and software (Med-PC for Windows, version IV) were used to collect data and present programmed events
Drugs
(−)-Nicotine hydrogen tartrate (Sigma; St. Louis, MO, USA) and iptakalim hydrochloride (99.9%; synthesized and provided by the Institute of Pharmacology and Toxicology, Academy of Military Medical Sciences of China) were dissolved in 0.9% saline. The pH of the nicotine solution was adjusted to 7.0±0.2 with a dilute NaOH solution. Nicotine doses (reported as base), iptakalim doses (reported as salt), route of injection, and injection-to-placement intervals (i.e., time between the injection and the onset of behavioral assessment) were selected based on previous research (Charntikov et al, 2012; Murray and Bevins, 2007a, b; Sun et al, 2010). In the DGT study, nicotine was administered subcutaneously (SC) and iptakalim was administered intraperitoneally (IP) at a volume of 1 ml/kg. In the self-administration study, nicotine was infused intravenously (IV) 35.74 μl per infusion across 1 sec; iptakalim was administered IV at 1 ml/kg (see later for more detail).
METHODS
Discriminated goal-tracking task
Rats in this study (n=10) were trained on our standard DGT protocol (as previously described in Bevins et al, 2012; Wilkinson et al, 2010). Before this study, these rats served in a separate experiment investigating how drug combinations, some of which bind to nAChRs (e.g., sazetidine A, nornicotine, bupropion), affected responding. This use of trained animals in the drug discrimination field, and in our lab specifically, is common given that performance is reliable and repeatable across time (Polewan et al, 2013; Smith et al, 2009; Struthers et al, 2009; Wooters et al, 2009). For the present study, all rats were tested on all doses of iptakalim using a repeated 5-day testing cycle. The first 4 days of this standardized testing protocol were training sessions to insure the nicotine/saline discrimination was maintained. For each daily training session, rats were injected SC with either nicotine (0.4 mg/kg) or saline 5 min before placement into the chamber for a 20-min session. There were 2 nicotine and 2 sucrose sessions intermixed per 4-day training cycle. Access to sucrose was initiated between 124 to 152 s from the start of the nicotine session. There were 36 separate 4-sec deliveries of sucrose per nicotine session. Time between sucrose deliveries ranged from 4 to 80 s (mean = 25 s). Sucrose was unavailable during the saline sessions. On day 5 of the test cycle, rats were injected with a randomly assigned dose of iptakalim (0, 10, 30, or 60 mg/kg; IP) 10 min before the start of a 4-min test session; nicotine was administered 5 min before the test. During test sessions, head entries into the dipper receptacle and general chamber activity were recorded, but sucrose was not available. Once all doses of iptakalim in combination with nicotine were tested, we noted that the combination with 60 mg/kg iptakalim decreased chamber activity. To determine if iptakalim impaired spontaneous locomotion in a manner that could account for the decrease in goal-tracking behavior evoked by the nicotine stimulus, we then tested all doses of iptakalim alone (no nicotine). These tests proceeded similar to those previously described except saline replaced nicotine as the solution injected 5 min before the 4-min test session. For all DGT sessions, the house light remained off and both levers were retracted.
IV nicotine self-administration task
Preliminary training
Before the start of this study, a separate set of rats (n=20) were handled for a minimum of 2 min per each of three consecutive days. Rats were then trained to lever press. The start of each lever-press session was signaled by illumination of the house light and insertion of a randomly selected lever (right or left). A lever press or a lapse of 15 sec resulted in 4-sec access to sucrose, retraction of the lever, and commencement of a timeout that lasted on average 60 s (range=30 to 89 s). Following the timeout, a randomly selected lever was again inserted into the chamber with the condition that the same lever could not be presented more than 2 times in a row. This protocol was repeated for 60 sucrose deliveries. Daily sessions ranged from 65–80 min, depending on individual performance and were continued until a lever press was made on at least 80% of the lever insertions for two consecutive days (i.e., 3 to 5 training sessions).
Catheter surgery
Following at least 24 h after preliminary training, rats were anesthetized with 1 ml/kg ketamine (100 mg/ml) and xylazine (20 mg/ml) mixture (2:1 ratio; administered intramuscularly; Sigma; St. Louis, MO, USA). Polyurethane catheter (RJVR-23; Strategic Applications Inc.; Lake Villa, IL, USA) with rounded tip and double suture beads (one secured internally and other externally) was implanted into the right external jugular vein. The other end of the catheter was subcutaneously placed around the shoulder and exited below the scapula via subcutaneously implanted polycarbonate back-mount access port (313-000BM; Plastics One Inc.; Roanoke, VA, USA). Immediately following surgery, catheters were flushed with 0.2 ml of streptokinase (2 mg/ml; Sigma; St. Louis, MO, USA) diluted in sterile heparinized saline (30 U/ml; Midwest Veterinary Supply; Lincoln, NE, USA). Atipamezole hydrochloride (0.5 mg/kg; IM; Sigma; St. Louis, MO, USA) diluted in saline was used to terminate anesthesia (Wee et al, 2006). To manage post-surgical pain, buprenorphine hydrochloride (0.1 mg/kg; SC) was administered immediately after the surgery and daily for the next two recovery days. Starting from the day after surgery, catheters were flushed daily with heparinized saline (30 U/ml). Catheter patency was assessed when patency loss was suspected or upon completion of the self-administration study using an infusion of 0.05 ml xylazine (20 mg/ml; IV). This xylazine concentration produces motor ataxia within 5 sec (cf. Bevins, 2005; Reichel et al, 2008). Only the 13 rats with patent catheters were included in the analyses.
Continued preliminary training
Following 7 days of recovery from surgery in the home cage, rats were trained for 3 consecutive daily sessions to lever press for liquid sucrose on a variable ratio (VR3) schedule of reinforcement (i.e., on average every third response was followed by access to sucrose; range=1 to 6 presses). This training was similar to the pre-surgery training except lever pressing was now required to access sucrose. Across the 3 daily sessions, all rats earned at least 80% of the 60 available sucrose deliveries and were thus moved to the self-administration phase (Nb. this protocol insures a high baseline level of lever pressing with both levers having a similar reinforcement history).
Nicotine self-administration
Before the start of each 60-min self-administration session, catheters were flushed with 0.2 ml of heparinized saline. The start of each session was signaled by turning the house light off, priming the catheter with nicotine (ca. 31 μl or 90% of internal catheter volume), and insertion of both levers. Which lever served as the active lever was balanced across the rats. The active lever was reinforced on aVR3 schedule of reinforcement. A VR schedule was used because in preliminary studies in our laboratory, this reinforcement schedule produced more reliable self-administration and lever discrimination than other schedules (e.g., fixed ratio [FR] 1 or FR5). Further, VR schedules typically control high and steady rates of behavior which may provide enhanced sensitivity to experimental manipulations (Williams, 1988). Upon completion of the VR3, there was a 1-sec infusion of nicotine (0.01 mg/kg/infusion), retraction of both levers, and illumination of the house light for a 20-sec timeout. Following the timeout, the house light was turned off and levers were inserted back into the chamber. Inactive lever responding was recorded but there was no programmed consequence. Immediately after each self-administration session, catheters were flushed with the antibiotic cefazolin (10 mg) diluted in 0.2 ml of heparinized saline (30 U/ml). After rats had reached a criterion of 80% discrimination between the active and inactive lever, iptakalim testing commenced.
Iptakalim dose response
Iptakalim (0, 1, 3, or 6 mg/kg) was administered IV 5 min before the start of the self-administration session. The IV route was adopted because of limited drug availability at the time of the experiment. The 10 fold decrease in doses for iptakalim for IV administration was adapted from Bardo et al (1999). All doses were tested in a randomly selected order for each rat. Following each test day there were at least 2 iptakalim-free self-administration sessions. At least 80% responding had to be on the active lever before being tested with the next randomly selected iptakalim dose.
[3H]dopamine and [3H]serotonin uptake
Inhibition of [3H]dopamine and [3H]serotonin uptake by iptakalim was determined using a rat striatal (dopamine) or hippocampal (serotonin) synaptosomal preparation previously described (Hadlock et al, 2011). Briefly, synaptosomes were prepared by homogenizing freshly dissected striatal or hippocampal tissue in ice-cold 0.32 M sucrose pH 7.4, and centrifuged (800 × g, 12 min; 4°C). The supernatants were centrifuged (22,000 × g, 15 min; 4°C) and the resulting pellets were re-suspended in ice-cold assay buffer (in mM: 126 NaCl, 4.8 KCl, 1.3 CaCl2, 16 sodium phosphate, 1.4 MgSO4, 11 glucose and 1 ascobic acid; pH 7.4) and 1 μM pargyline. Iptakalim (1 nM–1 mM) was present in the assay tubes. Samples were incubated for 10 min at 37°C and the assays initiated by the addition of [3H]dopamine or [3H]serotonin (0.5 nM or 5 nM final concentration, respectively). Following incubation for 3 min, samples were placed on ice to stop the reaction and were filtered through GF/B filter paper (Whatman; Florham Park, NJ, USA) soaked previously in 0.05% polyethylenimine. The filter paper was rapidly washed three times with 3 ml of ice-cold 0.32 M sucrose buffer using a filtering manifold (Brandel; Gaithersburg, MD, USA). For [3H]dopamine uptake, nonspecific values were determined in the presence of 50 μM cocaine. For [3H]serotonin uptake, nonspecific values were determined in the presence of 10 μM fluoxetine. Radioactivity trapped in the filter paper was counted using a liquid scintillation counter.
STATISTICAL ANALYSES
An omnibus analysis of variance (ANOVA) preceded all planned comparisons. Higher-order interactions were further analyzed by two-way ANOVAs or ANCOVA and followed, if necessary, by Tukey’s HSD post-hoc tests. Statistical significance was set at p<0.05. To examine the effect of iptakalim on dipper entry rates and chamber activity, a separate one-way ANOVA with iptakalim dose as the within-subjects factor was performed. To fully assess the potential locomotor effects of iptakalim on nicotine-associated activity, a separate LSD comparisons to a saline control (0 mg/kg iptakalim + saline; dashed line on Figure 1B) were conducted. The magnitude and persistence of nicotine self-administration behavior was assessed using a 2×6 (Lever × Session) repeated measures ANOVA. The effect of iptakalim on nicotine self-administration behavior was analyzed using a 2×4 (Lever × Dose) ANOVA. To examine the effect of iptakalim on general chamber activity during nicotine self-administration, a one way ANOVA with iptakalim doses as independent and activity as dependent variables was performed. To examine the effect of iptakalim and cocaine on striatal and hippocampal synaptosomal [3H]dopamine and [3H]serotonin uptake, respectively, IC50 values were assessed using a least squares, non-linear regression fit with a minimum of seven data points (determined in triplicate) per curve.
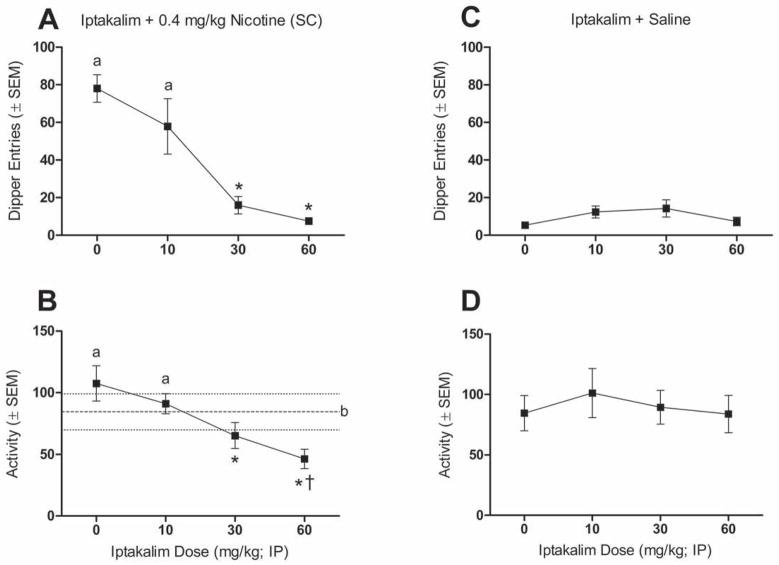
Figure 1.
Data representing discriminated goal-tracking tests. Upper panels show mean (±SEM) dipper entries during iptakalim/nicotine interaction tests (A) and iptakalim alone tests (C). Lower panels show mean (±SEM) number of chamber crosses during iptakalim/nicotine interaction tests (B) and iptakalim alone tests (D). Dashed line represents saline control (0 mg/kg iptakalim + saline) activity baseline with dot lines delineating the SEM boundaries. aSignificantly different from *, and † significantly different from b (p<0.05).
RESULTS
Discriminated goal-tracking task
Figure 1 shows the impact of iptakalim in the DGT task. Iptakalim attenuated nicotine-evoked conditioned responding [main effect of Dose; F(3,27)=20.87, p<0.001; Figure 1A]. Dipper entries following pretreatment with 30 and 60 mg/kg iptakalim were significantly lower than with 0 (saline) or 10 mg/kg iptakalim pretreatment (Tukey HSD tests). Iptakalim also attenuated chamber activity in these nicotine test sessions [main effect of Dose; F(3,27)=12.53, p<0.01; Figure 1B]. Pretreatment with 30 and 60 mg/kg of iptakalim decreased activity relative to saline or 10 mg/kg iptakalim (Tukey HSD tests). When this chamber activity was compared to a saline control value (i.e., both injections were saline; dashed line on Figure 1B) only pretreatment with 60 mg/kg of iptakalim significantly reduced activity (LSD comparisons). Notably, in these brief 4-min test sessions, nicotine did not have a locomotor stimulant effect; no difference in chamber beam breaks between the nicotine and the saline alone condition.
Because iptakalim attenuated activity of nicotine-treated rats, an additional mixed general linear model ANCOVA was performed with dipper entries as a dependent measure, iptakalim dose as the within-subjects variable, and chamber beam breaks as the covariate. This ANCOVA revealed that blockade of nicotine-evoked dipper entries [main effect of Dose; F(3,23)=19.73, p<0.001] cannot be explained by the variance in activity during the test session [no main effect of Activity and no Activity by Dose interaction]. Furthermore, iptakalim did not affect dipper entries or chamber activity (n.s.) in saline-treated rats (Figure 1C and 1D). Combined, these findings suggest that antagonism of the stimulus effects of nicotine by iptakalim cannot be attributed to a non-specific motor effect of iptakalim.
IV nicotine self-administration task
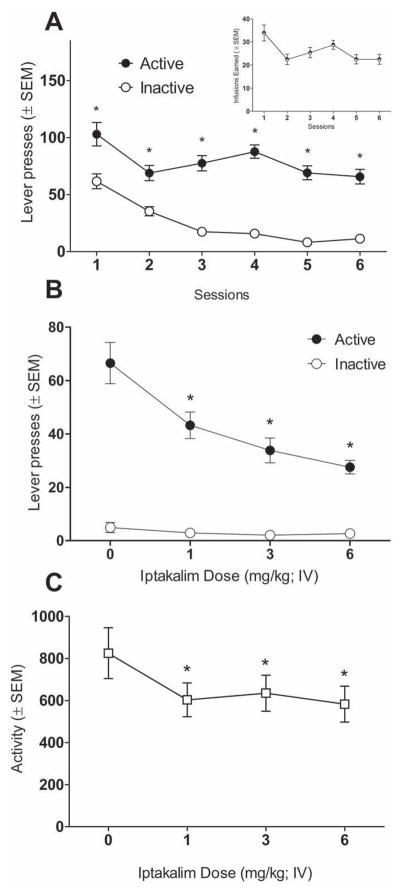
Figure 2 shows the impact of iptakalim in a second preclinical model of nicotine abuse liability—nicotine self-administration. During the first 6 days of self-administration, responding on the active lever was significantly higher than on the inactive lever [main effect of Lever; F(1,216)=265.15, p<0.001; Figure 2A]. This difference was evident from day 1 on [Lever × Session interaction; F(5,216)=3.00, p<0.05; Tukey HSD tests]. Pretreatment with iptakalim significantly attenuated responding on the active lever without affecting responding on the inactive lever [main effect of Lever; F(1,13)=120.10, p<0.001; Lever × Dose interaction; F(3,39)=12.61, p<0.001; Tukey HSD tests; Figure 2B]. Specifically, all iptakalim doses significantly attenuated active lever responding in comparison to saline [separate one-way ANOVA; main effect of Dose; F(3,39)=15.59, p<0.001; Tukey HSD tests; Figure 2B]. Iptakalim also decreased chamber activity [main effect of Dose; F(3,39)=5.96, p<0.01; Figure 2C]. A follow-up mixed general linear model ANCOVA on the active lever responding with the total chamber beam breaks as a covariate revealed that the attenuation of nicotine intake by iptakalim [main effect of Dose; F(3,35)=16.07, p<0.001] cannot be explained by alterations in activity [no main effect of Activity; F(1,35)=0.34, p=0.55; and no Activity by Dose interaction; F(3,35)=1.61, p=0.20]. [3H]dopamine and [3H]serotonin uptake
Figure 2.
Panel A shows mean (±SEM) lever presses during nicotine self-administration phase (*significantly different from inactive lever; inset graph shows total infusions earned during the session). Panel B shows mean (±SEM) lever presses during iptakalim dose-response tests (*significantly different from 0 mg/kg active lever responding). Panel C shows mean (±SEM) chamber crosses during the iptakalim dose-response tests (*significantly different from 0 mg/kg; p<0.05).
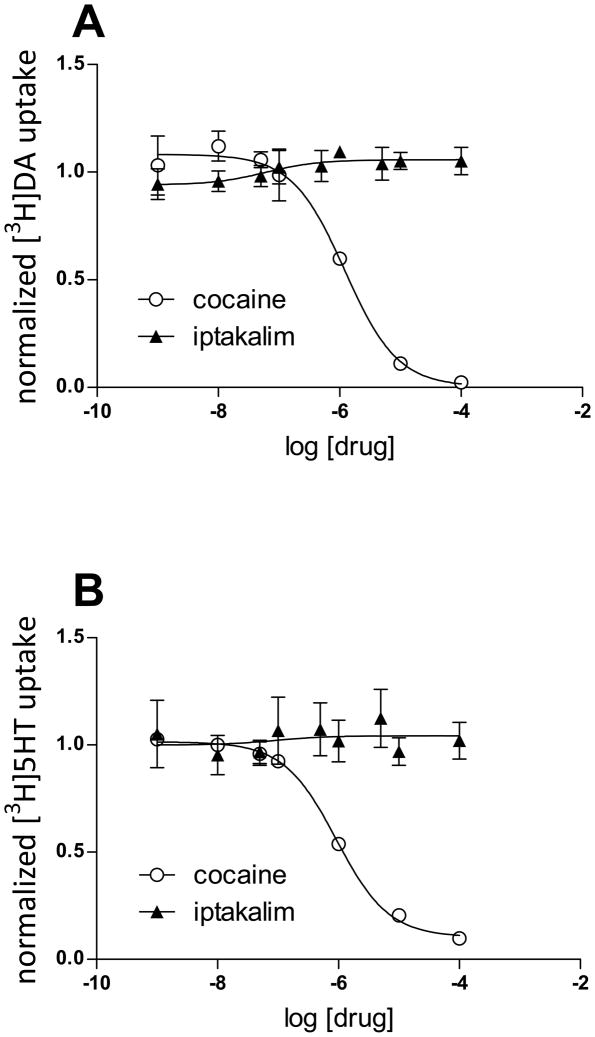
Ligands that act, in part, by inhibiting monoamine transporters interfere with nicotine-evoked goal-tracking and nicotine self-administration (Di Chiara, 2000; Dion et al, 2012; Rauhut et al, 2003; Wilkinson et al, 2010). Thus, the impact of iptakalim per se on dopamine and serotonin transporter function was evaluated. Findings from the present study revealed that iptakalim was without effect, at concentrations ranging from 1 nM – 0.1 mM, on either [3H]dopamine or [3H]serotonin uptake, as assessed in striatal and hippocampal synaptosomes, respectively (see Figure 3). As in internal control to verify the integrity of the assay, IC50 values for cocaine (present in the assay tubes at concentrations of 1 nM–0.1 mM) were also evaluated. Consistent with our previous report (Hadlock et al, 2011), cocaine inhibited [3H]dopamine and [3H]serotonin uptake with IC50 values of 1212 nM and 922 nM, respectively.
Figure 3.
Normalized [3H]DA uptake (A) and normalized [3H]5HTP uptake (B) following cocaine or iptakalim treatment.
DISCUSSION
The known neurobiological and behavioral effects of iptakalim suggest that it may be an effective pharmacotherapy to aid in smoking cessation. A goal of the present research was to test this notion by examining the effect of iptakalim on the stimulus and on the reinforcing effects of nicotine in two preclinical rodent models: a) drug discriminated goal-tracking and b) IV nicotine self-administration. We found that iptakalim, in a dose-dependent manner, attenuated conditioned responding controlled by the nicotine stimulus and attenuated nicotine intake by selectively decreasing active lever pressing.
Previous research from our laboratory has implicated the DAT and SERT in the stimulus effects of nicotine using the DGT task. For example, bupropion, a dopamine transporter inhibitor, fully substitutes for the stimulus effects of nicotine. Conversely, the SERT inhibitor, citalopram, blocks the conditioned response controlled by the nicotine stimulus (Dion et al, 2012). However, since iptakalim did not appreciably inhibit SERT or DAT function, another mechanism(s) likely underlies its effects on the stimulus and reinforcing effects of nicotine.
Very little data are available concerning the affinity of iptakalim on various receptor subtypes. In fact, one novel finding from the current study is that iptakalim does not inhibit dopamine or serotonin transporter function. Instead, antagonism of α4β2 receptors likely contributes to the efficacy of iptakalim demonstrated in Figures 1 and 2. In fact, heteromeric nAChRs are established contributors to the stimulus effects of nicotine. Evidence of the importance of these receptors includes the finding that agonists like ABT-418, ABT-594, A85380, TC2559, epibatidine, nornicotine, and 5-IA that bind to α4β2–containing nAChRs fully substitute for the nicotine stimulus (Brioni et al, 1995; Cohen et al, 2003; Reichel et al, 2010; Smith et al, 2007). Partial α4β2 agonists like cytisine and varenicline substitute for nicotine stimulus either partially or fully depending on the study (Chandler and Stolerman, 1997; Jutkiewicz et al, 2011; LeSage et al, 2009; Reichel et al, 2010; Smith et al, 2007). The α4β2 antagonist DHβE fully blocks the stimulus effects of nicotine (Struthers et al, 2009; Zaniewska et al, 2006). In addition, α4β2-containing nAChRs appear necessary for nicotine self-administration (Picciotto et al, 1998). Further, selective activation of α4β2-containing nAChRs is sufficient to establish nicotine place preference demonstrating critical involvement of these receptors in the reinforcing/rewarding effects of nicotine (Tapper et al, 2004). Finally, iptakalim inhibits the function of dopaminergic neurons dissociated from substantia nigra by non-competitively antagonizing α4-containing (α4β2 and α4β4) nAChRs localized on these neurons (Hu et al, 2006).
The brief overview in the previous paragraph suggests a likely role for α4-containing nAChRs in effects of iptakalim in the present experiments. It is worth noting, however, that iptakalim’s actions on KATP channels may also contribute to blockade of the reinforcing and stimulus effect of nicotine. For example, the KATP channels is located in brain areas implicated in both reward and learning processes – ventral tegmental area, substantia nigra, the prefrontal cortex, and hippocampus (Ross et al, 2006). KATP channels located in these areas play an important role in regulating glutamatergic, dopaminergic, and GABAergic neurotransmission (Ross et al, 2006), all of which play a role in the behavioral and neural effects of nicotine (Dwoskin et al, 2009; Wooters et al, 2009).
In the discriminated goal-tracking and the self-administration task, iptakalim reduced chamber activity. At first, these finding might suggests that its antagonism of the stimulus and reinforcing effects of nicotine are the by-product of non-specific motor effects by iptakalim. There are several finding that make this account less tenable. First, an effect on activity was not observed after iptakalim per se, but only when iptakalim was administered along with nicotine at the highest test dose (60 mg/kg) in the DGT task. In fact, the stimulus effects of nicotine in the DGT task were also blocked by iptakalim at 30 mg/kg; a dose that did not significantly alter activity. Second, the ANCOVAs that used chamber activity as a covariate revealed that the reduction in locomotion did not significantly contribute to the decrease in nicotine-evoked goal-tracking or the reduction in nicotine-maintained active lever pressing. Finally, in other published experiments, iptakalim increases locomotor activity when administered alone (10 mg/kg IP; Schmidt et al, 2012) or decreases locomotion when administered with stimulants like nicotine or amphetamine (Sun et al, 2010; Volf et al, 2012). We did not observe this increase in activity when iptakalim was given alone (recall Figure 1D). Perhaps the brief 4-min test sessions used here were not sufficiently long to detect this enhanced activity (cf. 60 min in Schmidt et al, 2012). Of course, there are many other potential methodological differences that could account for this discrepancy (e.g., size of apparatus, how activity was indexed, experimental history, rat strain, etc.). Regardless, iptakalim attenuated the stimulus and positive reinforcing effects of nicotine with motor impairment seeming an unlikely account of this blockade.
Nicotine is the primary addictive component of tobacco and purportedly its reinforcing effects are important to the addiction process (Balfour, 2002; Pomerleau and Pomerleau, 1992). However, recent research has highlighted the relative import of its reward or incentive-enhancing effects. For example, low levels of responding can be maintained in rats by a change in visual stimuli as the consequence for completing a schedule of reinforcement. Responding maintained by this visual stimulus is increased significantly when nicotine is administered by the experimenter before the session or by the rat during the session (Caggiula et al, 2001; Chaudhri et al, 2006, 2007; Palmatier et al, 2007). In our self-administration protocol, we used cued timeouts (see Methods) following each nicotine infusion. One might justifiably speculate that the nicotine self-administration seen in the present study reflects the primary reinforcing effects of nicotine plus its reward-enhancing effects on the visual stimulus that signaled the infusion and subsequent timeout. If so, then the decrease in nicotine intake seen with iptakalim may reflect blockade of either of these effects of nicotine, or both.
Although we did not design the present experiments to distinguish between the import of the primary reinforcing effects of nicotine or its reward-enhancing effects on the visual stimulus, the potential implication of the research in this report remains the same. The demonstrated behavioral effects here on the nicotine-evoked goal-tracking and nicotine intake suggest that it should be further investigated as a pharmacotherapy for smoking cessation. The known neural effects of iptakalim on nicotine-induced dopamine release and binding to α4β2-containing nAChRs are consistent with this suggestion. Given the limited research on iptakalim within the context of nicotine dependence and smoking cessation, much more research is needed. For example, will iptakalim blunt reinstatement (relapse) of self-administration behavior triggered by an environmental stressor, drug-associated cues, or priming dose of nicotine? Will iptakalim have an impact on nicotine withdrawal or its associated cognitive deficits? Does the action of iptakalim at ATP-sensitive potassium channels contribute in a unique manner to its potential utility as a smoking cessation aid? Although the present research eliminated inhibition of DAT or SERT function as a potential mechanism, what other central nervous system effects of iptakalim contribute to its potential efficacy and are these localized to particular brain regions? Addressing these questions, and others, in future research will no doubt provide insight into iptakalim as a potential medication for aiding in smoking cessation, as well as suggest potential new targets for medication development.
Iptakalim blocks responding evoked by the stimulus effects of nicotine
IV nicotine self-administration is blunted by pretreatment with iptakalim
Motor impairment cannot account for these effects
Iptakalim’s behavioral effects not due to SERT and DAT inhibition
Acknowledgments
This work was in part supported by NIH research grant DA018114 and DA034389 to RAB and DA019447 to AEF, as well as a grant from the Nebraska Department of Health and Human Services awarded to Ming Li. All MED-PC programs used in the present article are available upon request.
Footnotes
DISCLOSURE
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balfour DJ. The neurobiology of tobacco dependence: a commentary. Respiration. 2002;69(1):7–11. doi: 10.1159/000049362. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Valone JM, Bevins RA. Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology (Berl) 1999;143(1):39–46. doi: 10.1007/s002130050917. [DOI] [PubMed] [Google Scholar]
- Besheer J, Palmatier M, Metschke D, Bevins R. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology (Berl) 2004;172(1):108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bevins RA. The Reference-Dose Place Conditioning Procedure Yields a Graded Dose- Effect Function. International Journal of Comparative Psychology. 2005;18(2) [Google Scholar]
- Bevins RA, Barrett ST, Polewan RJ, Pittenger ST, Swalve N, Charntikov S. Disentangling the nature of the nicotine stimulus. Behavioural Processes. 2012;90(1):28–33. doi: 10.1016/j.beproc.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev. 2004;3(3):143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Kim DJ, Brodie MS, Decker MW, Arneric SP. ABT-418: discriminative stimulus properties and effect on ventral tegmental cell activity. Psychopharmacology (Berl) 1995;119(4):368–375. doi: 10.1007/BF02245851. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70(4):515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163(2):230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- CDC. Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses—United States, 2000–2004. Morbidity and Mortality Weekly Report. 2008;2010 [PubMed] [Google Scholar]
- Chandler CJ, Stolerman IP. Discriminative stimulus properties of the nicotinic agonist cytisine. Psychopharmacology (Berl) 1997;129(3):257–264. doi: 10.1007/s002130050188. [DOI] [PubMed] [Google Scholar]
- Charntikov S, Tracy ME, Zhao C, Li M, Bevins RA. Conditioned Response Evoked by Nicotine Conditioned Stimulus Preferentially Induces c-Fos Expression in Medial Regions of Caudate-Putamen. Neuropsychopharmacology. 2012;37(4):876–884. doi: 10.1038/npp.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, et al. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 2006;189(1):27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, et al. Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology (Berl) 2007;190(3):353–362. doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Bergis OE, Galli F, Lochead AW, Jegham S, Biton B, et al. SSR591813, a novel selective and partial alpha4beta2 nicotinic receptor agonist with potential as an aid to smoking cessation. J Pharmacol Exp Ther. 2003;306(1):407–420. doi: 10.1124/jpet.103.049262. [DOI] [PubMed] [Google Scholar]
- Corrigall W, Coen K. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99(4):473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- DeNoble VJ, Mele PC. Intravenous nicotine self-administration in rats: effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology (Berl) 2006;184(3–4):266–272. doi: 10.1007/s00213-005-0054-z. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393(1–3):295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Dion AM, Sanderson SC, Murrin LC, Bevins RA. Diminished conditioned responding to the nicotine stimulus by antidepressant drugs with differing specificity for the serotonin and norepinephrine transporter. Pharmacol Biochem Behav. 2012;100(3):419–424. doi: 10.1016/j.pbb.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122(4):390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Smith AM, Wooters TE, Zhang Z, Crooks Pa, Bardo MT. Nicotinic receptor-based therapeutics and candidates for smoking cessation. Biochemical pharmacology. 2009;78:732–43. doi: 10.1016/j.bcp.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell BJ, Ayres JJB. Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (‘goal tracking’) in rats. Learning and Motivation. 1979;10:295–312. [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121(3):240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, et al. Evidence that nicotinic alpha(7) receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther. 2000;294(3):1112–1119. [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, et al. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339(2):530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR. Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacol Biochem Behav. 1983;19(6):989–992. doi: 10.1016/0091-3057(83)90405-7. [DOI] [PubMed] [Google Scholar]
- Hu J, DeChon J, Yan KC, Liu Q, Hu G, Wu J. Iptakalim inhibits nicotinic acetylcholine receptor-mediated currents in dopamine neurons acutely dissociated from rat substantia nigra pars compacta. Neurosci Lett. 2006;403(1–2):57–62. doi: 10.1016/j.neulet.2006.04.060. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Brooks EA, Kynaston AD, Rice KC, Woods JH. Patterns of nicotinic receptor antagonism: nicotine discrimination studies. J Pharmacol Exp Ther. 2011;339(1):194–202. doi: 10.1124/jpet.111.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Effects of nicotine in experimental animals and humans: an update on addictive properties. Handb Exp Pharmacol. 2009;(192):335–367. doi: 10.1007/978-3-540-69248-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Ross JT, Carroll FI, Corrigall WA. Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacol Biochem Behav. 2009;91(3):461–467. doi: 10.1016/j.pbb.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Li Z, Ding JH, Liu SY, Wu J, Hu G. Iptakalim inhibits nicotine-induced enhancement of extracellular dopamine and glutamate levels in the nucleus accumbens of rats. Brain Res. 2006;1085(1):138–143. doi: 10.1016/j.brainres.2006.02.096. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. 2007a;561(1–3):91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. The conditional stimulus effects of nicotine vary as a function of training dose. Behav Pharmacol. 2007b;18(8):707–716. doi: 10.1097/FBP.0b013e3282f14ec6. [DOI] [PubMed] [Google Scholar]
- Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Effect of a novel nicotinic receptor antagonist, N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide, on nicotine self-administration and hyperactivity in rats. Psychopharmacology (Berl) 2006;184(3–4):426–434. doi: 10.1007/s00213-005-0163-8. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Matteson GL, Donny EC, Caggiula AR, Sved AF. Conditioned reinforcement in rats established with self-administered nicotine and enhanced by noncontingent nicotine. Psychopharmacology (Berl) 2007;195(2):235–243. doi: 10.1007/s00213-007-0897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391(6663):173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Polewan RJ, Savala SA, Bevins RA. Interoceptive conditioning with the nicotine stimulus: extinction learning as a method for assessing stimulus similarity across doses. Behav Pharmacol: England. 2013;24:45–54. doi: 10.1097/FBP.0b013e32835d5278. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF. Euphoriant effects of nicotine in smokers. Psychopharmacology (Berl) 1992;108(4):460–465. doi: 10.1007/BF02247422. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology (Berl) 2003;169(1):1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Bupropion differentially impacts acquisition of methamphetamine self-administration and sucrose-maintained behavior. Pharmacol Biochem Behav. 2008;89(3):463–472. doi: 10.1016/j.pbb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Barr JD, Bevins RA. Extinction with varenicline and nornicotine, but not ABT-418, weakens conditioned responding evoked by the interoceptive stimulus effects of nicotine. Neuropharmacology. 2010;58(8):1237–1245. doi: 10.1016/j.neuropharm.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Margolis RL, Reading SAJ, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–53. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Schmidt E, Lecavalier D, Wu C, Yang K, Wu J. Dual Effects of Iptakalim on Nicotine-induced Rat Behavioral Sensitization. Pharmacologia. 2012;3(10):506–512. [Google Scholar]
- Schnoll RA, Lerman C. Current and emerging pharmacotherapies for treating tobacco dependence. Expert Opin Emerg Drugs. 2006;11(3):429–444. doi: 10.1517/14728214.11.3.429. [DOI] [PubMed] [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, et al. Ligands selective for alpha4beta2 but not alpha3beta4 or alpha7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology (Berl) 2007;190(2):157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- Smith JW, Stolerman IP. Recognising nicotine: the neurobiological basis of nicotine discrimination. Handb Exp Pharmacol. 2009;(192):295–333. doi: 10.1007/978-3-540-69248-5_11. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Shoaib M. The neurobiology of tobacco addiction. Trends Pharmacol Sci. 1991;12(12):467–473. doi: 10.1016/0165-6147(91)90638-9. [DOI] [PubMed] [Google Scholar]
- Struthers AM, Wilkinson JL, Dwoskin LP, Crooks PA, Bevins RA. Mecamylamine, dihydro-beta-erythroidine, and dextromethorphan block conditioned responding evoked by the conditional stimulus effects of nicotine. Pharmacol Biochem Behav. 2009;94(2):319–328. doi: 10.1016/j.pbb.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Zhao C, Hu G, Li M. Iptakalim: a potential antipsychotic drug with novel mechanisms? Eur J Pharmacol. 2010;634(1–3):68–76. doi: 10.1016/j.ejphar.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, et al. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306(5698):1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Tobey KM, Walentiny DM, Wiley JL, Carroll FI, Damaj MI, Azar MR, et al. Effects of the specific α4β2 nAChR antagonist, 2-fluoro-3-(4-nitrophenyl) deschloroepibatidine, on nicotine reward-related behaviors in rats and mice. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volf N, Hu G, Li M. Iptakalim Preferentially Decreases Nicotine-induced Hyperlocomotion in Phencyclidine-sensitized Rats: A Potential Dual Action against Nicotine Addiction and Psychosis. Clin Psychopharmacol Neurosci. 2012;10(3):168–179. doi: 10.9758/cpn.2012.10.3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Long CL, Zhang YL. A new ATP-sensitive potassium channel opener reduces blood pressure and reverses cardiovascular remodeling in experimental hypertension. J Pharmacol Exp Ther. 2005a;312(3):1326–1333. doi: 10.1124/jpet.104.078220. [DOI] [PubMed] [Google Scholar]
- Wang H, Tang Y, Zhang YL. Hypoxic pulmonary hypertension (HPH) and iptakalim, a novel ATP-sensitive potassium channel opener targeting smaller arteries in hypertension. Cardiovasc Drug Rev. 2005b;23(4):293–316. doi: 10.1111/j.1527-3466.2005.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62(4):743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Wee S, Wang Z, He R, Zhou J, Kozikowski AP, Woolverton WL. Role of the increased noradrenergic neurotransmission in drug self-administration. Drug Alcohol Depend. 2006;82(2):151– 157. doi: 10.1016/j.drugalcdep.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Carroll FI, Bevins RA. An investigation of bupropion substitution for the interoceptive stimulus effects of nicotine. J Psychopharmacol. 2010;24(6):817–828. doi: 10.1177/0269881109102518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BA. Reinforcement, choice, and response strength. In: Atkinson RC, Herrnstein RJ, editors. Stevens’ handbook of experimental psychology. 2. Vol. 1. Wiley; New York: 1988. pp. 167–244. Perception and motivation; Vol. 2: Learning and cognition. [Google Scholar]
- Wooters TE, Bevins RA, Bardo MT. Neuropharmacology of the interoceptive stimulus properties of nicotine. Curr Drug Abuse Rev. 2009;2(3):243–255. doi: 10.2174/1874473710902030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Przegaliński E, Filip M. Evaluation of the role of nicotinic acetylcholine receptor subtypes and cannabinoid system in the discriminative stimulus effects of nicotine in rats. Eur J Pharmacol. 2006;540(1–3):96–106. doi: 10.1016/j.ejphar.2006.04.034. [DOI] [PubMed] [Google Scholar]