Abstract
Dopamine signaling in the nucleus accumbens (NAc) plays a critical role in the regulation of motivational states. Recent studies in male rodents show that social defeat stress increases the activity of ventral tegmental dopamine neurons projecting to the NAc, and that this increased activity is necessary for stress-induced social withdrawal. Domestic female mice are not similarly aggressive, which has hindered complementary studies in females. Using the monogamous California mouse (Peromyscus californicus), we found that social defeat increased total dopamine, DOPAC, and HVA content in the NAc in both males and females. These results are generally consistent with previous studies in Mus, and suggest defeat stress also increases NAc dopamine signaling in females. However, these results do not explain our previous observations that defeat stress induces social withdrawal in female but not male California mice. Pharmacological manipulations provided more insights. When 500 ng of the D1 agonist SKF38393 was infused in the NAc shell of females that were naïve to defeat, social interaction behavior was reduced. This same dose of SKF38393 had no effect in males, suggesting that D1 receptor activation is sufficient to induce social withdrawal in females but not males. Intra-accumbens infusion of the D1 antagonist SCH23390 increased social approach behavior in females exposed to defeat but not in females naïve to defeat. This result suggests that D1 receptors are necessary for defeat-induced social withdrawal. Overall, our results suggest that sex differences in molecular pathways that are regulated by D1 receptors contribute to sex differences in social withdrawal behavior.
1. Introduction
There is compelling evidence that the mesolimbic dopamine system has important effects on behavior in aversive contexts. Social defeat stress induces an immediate increase in dopamine turnover in ventral striatum (Mos and Van Valkenburg, 1979; Puglisi-Allegra and Cabib, 1990) and dopamine release in the nucleus accumbens (NAc) (Tidey and Miczek, 1996). In addition to these short term responses, defeat stress induces long lasting increases in burst firing of ventral tegmental area (VTA) dopamine neurons (Anstrom et al., 2009; Cao et al., 2010; Krishnan et al., 2007; Razzoli et al., 2011). Withdrawal from social contexts is linked to hyperactivity of VTA dopamine neurons (Krishnan et al., 2007). Inhibition of burst firing by VTA dopamine neurons through overexpressing potassium channels (Krishnan et al., 2007) or direct optogenetic control (Chaudhury et al., 2013) increases social interaction behavior in male Mus musculus exposed to defeat. Increases in activity of VTA neurons projecting to the NAc, but not medial FC were especially critical for inducing social avoidance. This suggests that sustained increases in dopaminergic activity in the NAc are important for inducing social withdrawal behavior.
Social withdrawal is an important component of stress-induced mental disorders including anxiety and depression. These disorders are more commonly diagnosed in women than men, and there are important sex differences in neurobiological and endocrine responses to stress (Trainor, 2011). A handful of studies have examined the effects of social defeat in female rodents (Holly et al., 2012; Huhman et al., 2003; Solomon et al., 2007), but no study has tested whether the mesolimbic dopamine system is affected by defeat in females. The most widely studied rodent model species, M. musculus, is not optimal for studying sex differences because female aggression levels are low (Jacobson-Pick et al., 2013). We addressed this gap in the field through studying the monogamous California mouse (Peromyscus californicus), a species in which both males and females (Silva et al., 2010) exhibit territorial aggression.
Female California mice exposed to three episodes of social defeat stress exhibit social withdrawal behavior whereas this effect is reduced or absent in males (Trainor et al., 2011; Trainor et al., 2013). In females, defeat stress increased the number of phosphorylated CREB (pCREB) positive cells in the NAc shell, and social interaction behavior is negatively correlated with the number of pCREB cells in the NAc shell (Trainor et al., 2011). Activation of dopamine D1 receptors increases cyclic AMP production (Kebabian et al., 1972), which in turn facilitates phosphorylation of CREB (Yamamoto et al., 1988). We hypothesized that increased activation of D1 receptors in the NAc shell would inhibit social interaction behavior and that this effect would be enhanced in females compared to males. We also examined, for the first time, the effects of social defeat on dopamine content and receptor mRNA in the NAc in both males and females. Our results show that activation of D1 receptors is indeed necessary and sufficient to induce social withdrawal in female California mice, but that the mechanism for sex differences in behavior may be downstream of D1-signaling.
2. Materials and Methods
2.1 Animals and housing conditions
Male and female California mice were obtained from our breeding colony at UC Davis. They were group housed (2–3 same-sex animals per cage) unless otherwise stated for each experiment. Animals were maintained in a temperature-controlled room on a 16L-8D cycle with ad libitum water and food (Harlan Teklad 2016, Madison, WI). Cages were polycarbonate plastic with corn-cob bedding, nestlets, and enviro-dri. All procedures were approved by the Internal Animal Care and Use Committee (IACUC) and conformed to NIH guidelines. All efforts were made to minimize animal suffering, to reduce the number of animals used.
2.2 Social defeat
Mice were randomly assigned to social defeat or control handling for three consecutive days (Trainor et al., 2011; Trainor et al., 2013). Mice assigned to social defeat were introduced to the home cage of an aggressive, same-sex sexually-experienced mouse during the dark phase. Episodes of defeat were terminated following either 7 minutes or 10 bites from the resident, whichever occurred first. Control mice were introduced to an empty cage for 7 minutes. This approach more closely resembles methods used in rats (Rattus norvegicus)(Carnevali et al., 2012; Nikulina et al., 2012) and Syrian hamsters (Mesocricetus auratus)(Morrison et al., 2012; Taylor et al., 2011).
2.3 Open field and social interaction test
Social interaction tests consisted of 3 phases, 3 minutes each (Trainor et al., 2013). In the open field phase (OF), animals were introduced into a large open field (89×63×60 cm). Durations within a center zone located 14cm from the sides were recorded using the Any-Maze video tracking system (Stoelting, Wood Dale, IL). During the acclimation phase a small wire cage was introduced against one side of the arena, the amount of time the mouse spent within 8 cm of the empty cage was recorded. During the social interaction phase an unfamiliar, same-sex virgin stimulus mouse was placed into the wire cage. We recorded the amount of time the focal mouse spent interacting with the wire cage and the duration spent in the two corners opposite the wire cage. We also calculated ratios for the interaction zone and corner zones defined as time during social interaction phase/time during acclimation phase × 100, as previously described (Krishnan et al., 2007; Vialou et al., 2010). Total distance traveled during the open field was used as an estimate of total activity.
2.4 Experiment 1: Effects of defeat stress on dopamine content
Males and females were randomly assigned to social defeat or control conditions. Two weeks after defeat or control handling, all mice were tested in the social interaction test. The morning following social interaction testing (during lights on), cages were moved to the necropsy area 30–45 minutes before euthanasia. After a brief increase in activity after transfer, the mice returned to nests and were inactive. Each mouse was then lightly anesthetized and decapitated. It should be noted that the isoflurane anesthesia can inhibit the dopamine transporter and increase dopamine levels after > 15 minutes of anesthesia (Baba et al., 2013; Byas-Smith et al., 2004; Votaw et al., 2003; Votaw et al., 2004). However, because our mice experienced 90 seconds of isoflurane anesthesia we interpret these levels as baseline differences in dopaminergic tone. Brains were rapidly removed and 2 mm slices were dissected using a brain matrix (Trainor et al., 2003). The NAc and medial prefrontal cortex were dissected using a 1 mm punch tool and samples were frozen on dry ice and stored at −40° C. Punch samples were homogenized in 0.3 M perchloric acid and passed through 0.22 μm filters (Ultrafree Millipore, Billerica, MA). Total protein content in each sample was assessed using the Pierce Protein Assay (660 nm). Samples were then frozen at −40 C and then shipped to the Wisconsin National Primate Research Center for high pressure liquid chromatography (HPLC) analysis.
For measurement of norepinephrine, epinephrine, dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), serotonin and homovanillic acid (HVA), 150 μl of perfusate was thawed and aliquotted into polypropylene inserts for HPLC vials. To this, 50 μl of internal standard, 3,4-dihydroxybenzylamine (DHBA), was added at a concentration of 0.1 μg/mL. Samples were loaded onto an autosampler (ESA #542) cooled to 5 °C. The injection volume was 50 μl using 100 μl partial loop onto a 4.6 × 250 mm C18 100A column (#00G-4252-E, Luna, Phenomenex, Torrance, CA). The detection system consisted of ESA (Chelmsford, MA, USA) isocratic pumps with a Coluochem III electrochemical detector. The mobile phase consisted of 10% acetonitrile in phosphate buffer (pH 2.75) containing 1.73 mM 1-octanesulfonic acid. The flow rate was 1.0 mL/min and the pressure at that flow rate was approximately 120 bar. The voltage was as follows: guard at −250 mV, E1 at −250 mV and E2 at +250 mV. The gain for E1 and E2 was set at 500 nA. The analytes were purchased in high purity powder form from Sigma (Sigma-Aldrich, St Louis, MO), and fresh stocks were prepared on a weekly basis (10 μg/mL in 0.2 N perchloric acid). The standard curve was 10-points, ranging from 250 to 0.488 ng/mL also in 0.2N perchloric acid with DHBA as the internal standard. Linearity for each analyte was at least 0.999. The CV was 3.5% for norepinephrine, 4.0% for epinephrine, 4.7% for dopamine, 5.2% for DOPAC, 4.6% for serotonin and 9.1% for HVA.
2.5 Experiment 2: Effects of defeat stress on dopamine receptor expression
Males and females were randomly assigned to social defeat or control conditions. Two weeks after defeat or control handling, all mice were tested in the social interaction test. Immediately after testing each mouse was lightly anesthetized with isoflurane and decapitated. Punch samples of the NAc were collected as in experiment 1.
RNA was extracted from punch samples using RNAqueous kits (Life Technologies,) and reverse transcribed using iScript kits (BioRad). Transcripts were quantified using SYBR Green chemistry on an ABI 7500 Sequencing Detection System. To detect specific dopamine receptor subtypes, primer pairs were based on previously published sequences. Each primer pair was tested with California mouse cDNA and sequenced via Sanger Sequencing to confirm specificity (Table 1). For each sample, dopamine receptor gene expression was normalized to an average of GAPDH and β-actin expression. There were no significant differences in cycle thresholds between groups for GAPDH or β-actin.
Table 1.
Primer pair sequences
| forward | reverse | |
|---|---|---|
|
|
||
| D1 | GGCTCCATCTCCAAGGACTGTA | AGCTTCTCCAGTGGCTTAGCTATTC |
| D2 | GCGTCGGAAGCGGGTCAACA | TCGGCGGGCAGCATCCATTC |
| D3 | TGCGGCTGCATCCCATTCGG | GCTTGGGTGCCATGGTGGGG |
| D5 | GGGCCTTTCGATCACATGTCT | AAGGAAACCTCTTCCTCACAGTCA |
2.6 Experiment 3: Effects of D1 agonist infusion in males and females naïve to defeat
Males and females were anesthetized with isoflurane (3–5% in 1% O2) and implanted with bilateral stainless steel guide cannula (Plastics One, C235I/SPC) aimed at the NAc shell (Fig. 1, AP=0.51 mm, LM=1.1 mm, DV=6.85). The guide cannula (26ga, o.d.=0.46 mm; i.d.=0.24 mm; length=5.85 mm), was lowered into burr holes (#105 dremel bit, 1/16″ tip) and attached to the skull using acrylic dental cement and skull screws (plastics one, 00-96 X 1/16). Guide cannulae were maintained patent using bilateral dummy caps (Plastics One, C235DC). Animals were given 3–7 days for recovery, during which the mice were observed and handled daily.
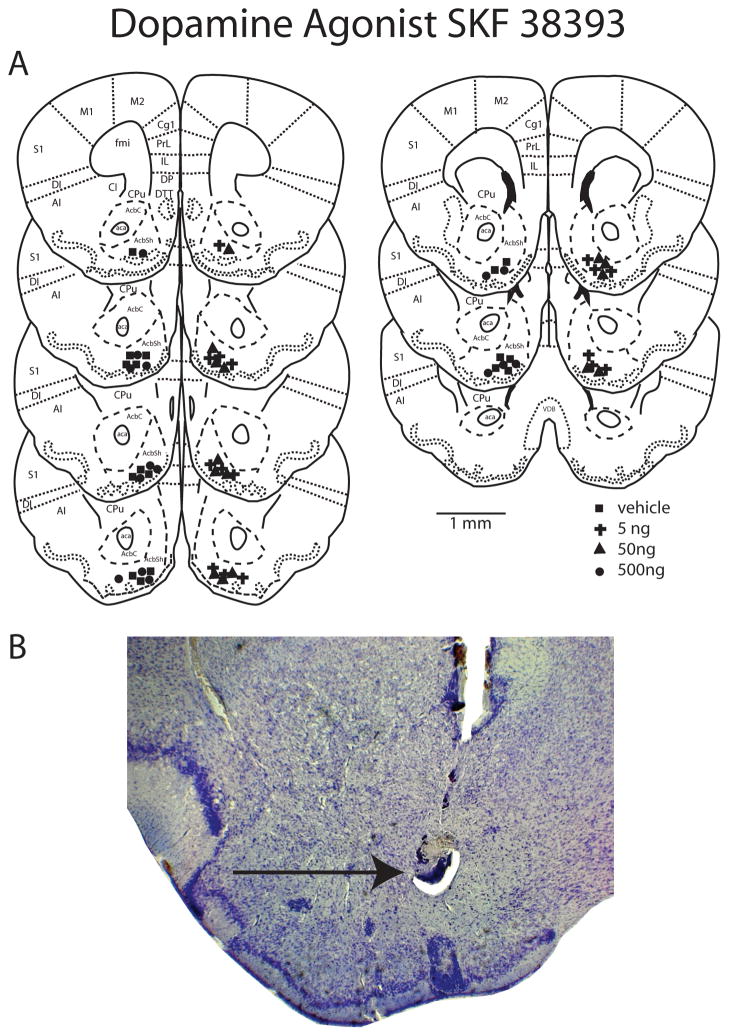
Figure 1.
(A) Reconstructions of a coronally cut series of sections through the nucleus accumbens showing the histological verification of injection of D1 agonist SKF 38393 placement into the nucleus accumbens shell. AcbC nucleus accumbens core; AcbSh nucleus accumbens shell; aca anterior commissure; AI agranular insula; DI dysgranular insula; S1 primary somatosensory cortex; Cgl cingulate cortex; PrL prelimbic cortex; M1 primary motor cortex; M2 secondary motor cortex; IL infralimbic cortex; DP dorsal peduncular cortex; DTT dorsal tenia tecta; fmi forceps minor or the corpus callosum; CPu caudate putamen; cl claustrum; DEn dorsal endopiriform nucleus; VDB vertical limb of diagonal band nucleus. All images are original work of the authors drawn from California mouse sections. (B) Photomicrograph of a Nissl stained section showing correct cannula placement and microinjection into the nucleus accumbens shell. Injection sites are represented by filled squares for vehicle, plus symbols for 5ng SKF 38393, filled triangles for 50ng SKF 38393 and filled circles for 500ng SKF 38393. Scale bar is 1 mm. Black arrow indicates microinjection site.
Infusions were made using bilateral internals (Plastics One, C235I/SPC, 33ga, o.d.=0.21 mm; i.d.=0.11 mm) that projected 1 mm past the cannula guide (6.85 mm total length). The D1 agonist SKF38393 (Sigma, St. Louis, MO) was dissolved in artificial cerebrospinal fluid (aCSF) and prepared fresh on the day of injection. Males and females were randomly assigned to receive a 200 ul infusion containing either aCSF, 5ng, 50ng, or 500ng of SKF38393. Hamilton syringes were attached to an automatic micropump delivery apparatus (PHD 2000, Harvard Apparatus, Cambridge, MA) set to deliver 100nl/min. Internal guides were kept in place for 1 min after injection to ensure delivery after which dummy guides were placed back into cannula guide. Each mouse was returned to its home cage and after 30 min was tested in social interaction tests as described above. Immediately following testing, each animal received a 200 nl infusion of blue food coloring to visualize both the injection site and fluid diffusion. Each mouse was then anesthetized with isoflurane and decapitated. Brains were removed and fixed in 5% acrolein and processed to confirm needle placement. The brains were immersed in 20% sucrose overnight, frozen and sectioned coronally at 40 μm on a cryostat. In order to confirm needle placement, tracks were assessed in sections stained using cresyl violet (Figure 1B). In addition, diffusion of fluid was examined by visualization of blue dye in pictures of each brain during cryostat sectioning. Data from mice with needle tracks outside of the NAc shell were included in statistical analysis as anatomical controls (Tables 4 and 5).
Table 4.
Social interaction data after D1 agonist infusion.
| anatomical hits | cage area time (s) | corners time (s) | open field | interaction ratio | |||||
|---|---|---|---|---|---|---|---|---|---|
| empty cage | novel mouse | empty cage | novel mouse | center time (s) | distance (m) | cage area | corners | ||
|
|
|||||||||
| females | aCSF (n=10) | 106.01 ± 8.87 | 111.98 ± 8.74 | 4.44 ± 1.61 | 7.12 ± 3.06 | 24.78 ± 5.15 | 22.91 ± 2.73 | 109.09 ± 8.03 | 194.44 ± 61.26 |
| 5ng (n=8) | 87.69 ± 13.85 | 103.20 ± 18.20 | 7.84 ± 1.71 | 6.06 ± 2.57 | 39.76 ± 10.15 | 20.15 ± 2.44 | 153.53 ± 43.10 | 328.68 ± 252.95 | |
| 50ng (n=7) | 90.09 ± 7.37 | 120.23 ± 10.75 | 11.87 ± 3.59 | 6.10 ± 3.84 | 23.23 ± 4.78 | 20.10 ± 4.19 | 135.66 ± 9.92 | 66.33 ± 19.95 | |
| 500ng (n=9) | 79.96 ± 11.43 | 62.99 ± 11.01* | 8.30 ± 2.47 | 10.49 ± 3.12 | 32.59 ± 7.33 | 24.15 ± 4.02 | 84.04 ± 10.22* | 164.24 ± 54.17 | |
| males | aCSF (n=7) | 76.39 ± 11.11 | 88.47 ± 20.18 | 11.07 ± 1.71 | 34.90 ± 22.14 | 31.83 ± 7.99 | 16.20 ± 1.59 | 105.75 ± 17.30 | 263.92 ± 175.41 |
| 5ng (n=8) | 67.84 ± 9.05 | 93.08 ± 17.21 | 15.36 ± 3.64 | 8.89 ± 3.01 | 22.38 ± 4.61 | 20.23 ± 3.01 | 149.52 ± 28.66 | 82.41 ± 27.66 | |
| 50ng (n=8) | 75.15 ± 11.32 | 80.34 ± 19.11 | 8.83 ± 2.82 | 5.56 ± 1.58 | 31.08 ± 3.89 | 15.37 ± 2.32 | 102.97 ± 17.81 | 169.21 ± 94.90 | |
| 500ng (n=6) | 66.60 ± 13.00 | 77.33 ± 16.51 | 19.08 ± 9.54 | 33.33 ± 28.41 | 33.52 ± 5.18 | 14.44 ± 2.32 | 124.36 ± 39.38 | 227.65 ± 116.02 | |
| anatomical misses | |||||||||
| females | aCSF (n=8) | 91.43 ± 8.77 | 115.63 ± 8.94 | 4.26 ± 1.53 | 1.71 ± 0.86 | 25.03 ± 1.96 | 19.01 ± 1.96 | 129.08 ± 6.94 | 105.71 ± 80.94 |
| 5ng (n=5) | 80.90 ± 14.96 | 120.94 ± 19.51 | 11.26 ± 4.99 | 3.22 ± 1.66 | 33.24 ± 5.26 | 19.94 ± 3.72 | 163.85 ± 35.47 | 105.55 ± 86.63 | |
| 50ng (n=5) | 58.46 ± 14.30 | 83.40 ± 15.75 | 9.46 ± 2.10 | 6.12 ± 1.63 | 28.68 ± 6.17 | 25.59 ± 6.39 | 157.64 ± 32.96 | 63.07 ± 17.03 | |
| 500ng (n=3) | 79.53 ± 6.30 | 89.37 ± 42.51 | 10.27 ± 3.50 | 5.03 ± 3.98 | 23.10 ± 3.98 | 16.75 ± 4.25 | 111.84 ± 54.09 | 111.86 ± 101.57 | |
p < 0.05 vs aCSF
Table 5.
Social interaction data after D1 antagonist infusion in females.
| anatomical hits | cage area time (s) | corners time (s) | open field | interaction ratio | |||||
|---|---|---|---|---|---|---|---|---|---|
| empty cage | novel mouse | empty cage | novel mouse | center time (s) | distance (m) | cage area | corners | ||
|
|
|||||||||
| control | aCSF (n=16) | 91.27 ± 6.65 | 91.97 ± 10.62 | 7.22 ± 1.92 | 16.11 ± 9.31 | 31.58 ± 4.03 | 22.48 ± 3.50 | 95.62 ± 10.22 | 206.26 ± 52.79 |
| 2.5μg (n=9) | 81.80 ± 16.20 | 88.74 ± 16.27 | 9.69 ± 3.55 | 6.57 ± 2.36 | 30.19 ± 6.90 | 15.92 ± 2.24 | 113.09 ± 26.19 | 258.52 ± 121.06 | |
| stress | aCSF (n=19) | 103.17 ± 7.60 | 77.40 ± 10.17 | 5.36 ± 1.33 | 17.98 ± 5.92 | 32.12 ± 2.88 | 22.53 ± 2.26 | 76.22 ± 10.51 | 451.18 ± 111.12 |
| 2.5μg (n=10) | 79.64 ± 15.99 | 93.24 ± 18.37 | 7.00 ± 16.60 | 20.08 ± 17.78 | 28.65 ± 5.59 | 15.22 ± 3.53* | 135.18 ± 19.92** | 108.67 ± 30.39** | |
| anatomical misses | |||||||||
| control | aCSF (n=3) | 136.10 ± 4.39 | 143.83 ± 8.44 | 1.77 ± 1.52 | 0.2 ± 0.06 | 23.1 ± 1.43 | 18.39 ± 3.73 | 105.80 ± 6.06 | 92.36 ± 57.80 |
| 2.5μg (n=4) | 88.18 ± 28.58 | 89.88 ± 20.00 | 1.73 ± 0.92 | 7.5 ± 5.71 | 14.18 ± 4.92 | 20.17 ± 3.63 | 183.02 ± 102.65 | 245.37 ± 245.37 | |
| stress | aCSF (n=3) | 112.25 ± 28.99 | 117.48 ± 16.60 | 7.88 ± 3.76 | 13.25 ± 13.25 | 18.43 ± 3.34 | 18.11 ± 5.07 | 90.40 ± 14.67 | 58.33 ± 44.49 |
| 2.5μg (n=7) | 75.24 ± 14.10 | 73.13 ± 30.44 | 8.77 ± 4.86 | 27.8 ± 6.68 | 27.64 ± 6.68 | 20.43 ± 4.3 | 95.73 ± 29.29 | 186.33 ± 76.81 | |
p<0.05 vs aCSF.
p < 0.01 vs aCSF
2.7 Experiment 4: Effects of D1 antagonist infusion in females exposed to defeat or control conditions
Female California mice were randomly assigned to social defeat or control conditions as described above. Two weeks later each female was implanted with guide cannula aimed at the NAc shell as described in Experiment 3 (Fig. 2). After recovery, each female was randomly assigned to receive an infusion of aCSF or 2.5 μg of the D1 receptor antagonist SCH23390 (Sigma, St. Louis, MO). The infusion procedure and behavioral testing was conducted exactly as described in Experiment 3. After testing, dye infusion and histology were performed as in Experiment 3.
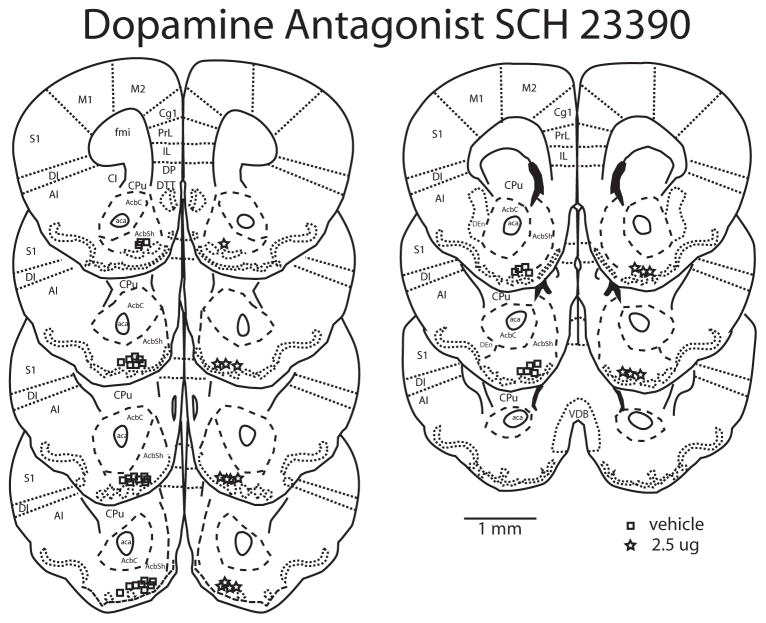
Figure 2.
Reconstructions of a coronally cut series of sections through the nucleus accumbens showing the histological verification of injection placement of D1 antagonist SCH 23390 into the nucleus accumbens shell. AcbC nucleus accumbens core; AcbSh nucleus accumbens shell; aca anterior commissure; AI agranular insula; DI dysgranular insula; S1 primary somatosensory cortex; Cgl cingulate cortex; PrL prelimbic cortex; M1 primary motor cortex; M2 secondary motor cortex; IL infralimbic cortex; DP dorsal peduncular cortex; DTT dorsal tenia tecta; fmi forceps minor or the corpus callosum; CPu caudate putamen; cl claustrum; DEn dorsal endopiriform nucleus; VDB vertical limb of diagonal band nucleus. All images are original work of the authors drawn from California mouse sections. Injection sites are represented by squares for vehicle and stars for 2.5μg SCH 23390. Scale bar is 1 mm.
2.8 Statistical Analyses
In experiments 1 and 2, HPLC and gene expression data were log transformed and analyzed with two way ANOVA (sex and stress). Analyses of Q-Q plots revealed that these data were not normally distributed and so log transformations were used. For experiments 3 and 4 variation in behavioral data was heterogeneous across treatment groups, so nonparametric analyses were used. Specifically, in experiment 3 Kruskal-Wallis tests were used to test for an effect of SKF38393, followed by pair-wise Mann-Whitney U tests. In experiment 4 Mann-Whitney analyses were used to compare the effect of SCH23990 in control and stressed females. In experiment 3 male and female experiments were run at separate times, so male and female data were analyzed separately. In experiments 3 and 4, we also calculated an interaction ratio, defined as the time spent in a zone during the social interaction period divided by the time spent in the zone during the acclimation period (Krishnan et al., 2007; Vialou et al., 2010). Interaction ratios were calculated for both the cage zone and corners opposite the cage.
3. Results
3.1 Experiment 1: Effects of defeat stress on dopamine content
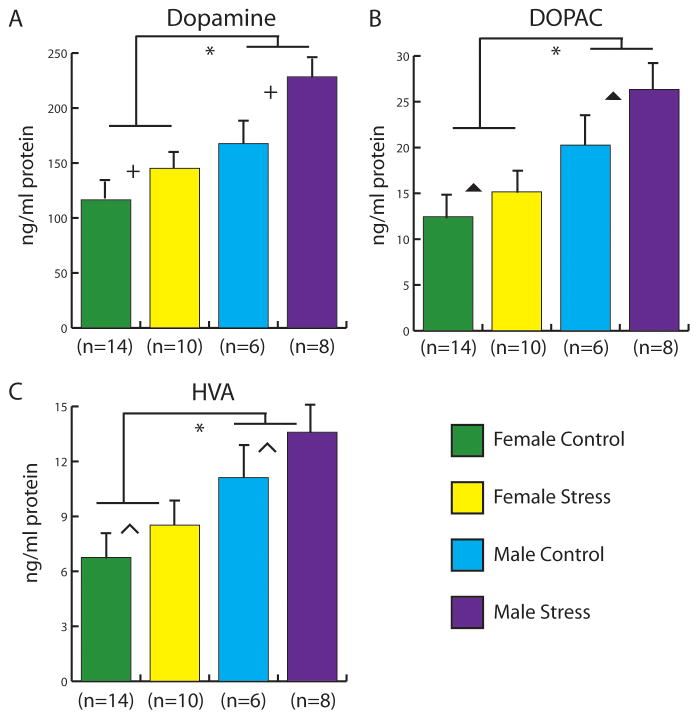
In the NAc, males had higher dopamine (Fig. 3A, F1,31=16.5, p < 0.001), DOPAC (Fig. 3B, F1,31=10.8, p < 0.01), and HVA (Fig. 3C, F1,31=12.5, p < 0.001) content compared to females. In addition, defeat stress induced a significant increase in dopamine (F1,31=8.6, p < 0.01), and modest increases in DOPAC (F1,31=3.7, p = 0.06) and HVA (F1,31=3.2, p < 0.08) content. There were no sex x stress interactions (all p’s >0.4). Norepinephrine levels were significantly higher in males (Table 2, F1,31=17.3, p < 0.001) but there were no effects of stress or interaction. There were no significant differences in 5-HT content (all p’s > 0.15). In the mPFC, females had higher 5-HT content than males (F1,31=9.8, p < 0.01) but there was no effect of stress or interaction. There were no significant differences in dopamine, NE, DOPAC, or HVA.
Figure 3.
Effect of stress and sex on dopamine (A), DOPAC (B), and HVA (C) levels in the nucleus accumbens of control and stressed mice in both females and males using HPLC. Green bars represent control females. Yellow bars represent stressed females. Blue bars represent control males. Purple bars represent stressed males. Error bars are SEM. * indicates main effect of sex, p<0.05. † indicates main effect of stress, p<0.05. Caret indicates a trend for effect of stress, DOPAC p=0.06; HVA p=0.08.
Table 2.
Neurotransmitter protein (ng/ml) measured by HPLC (mean ± SEM).
| Nucleus Accumbens | DOPAC/DA | HVA/DA | DA | DOPAC | Frontal Cortex | ||||
|---|---|---|---|---|---|---|---|---|---|
| NE | Epi | 5HT | HVA | ||||||
|
|
|||||||||
| female | control | 7.41 ± 2.26 | .23 ± .08 | 4.93 ± .61 | .11 ± .02 | .06 ± .01 | 1.02 ± .12 | .56 ± .08 | .25 ± .03 |
| stress | 7.05 ± 2.16 | .31 ± .07 | 5.39 ± .90 | .11 ± .005 | .06 ± .005 | 1.01 ± .24 | .38 ± .12 | .21 ± .03 | |
| male | control | 15.03 ± 2.92** | .39 ± .10 | 3.27 ± .93 | .12 ± .01 | .06 ± .005 | 1.37 ± .34 | .45 ± .05 | .24 ± .03 |
| stress | 14.98 ± 2.53** | .48 ± .08 | 6.10 ± 1.43 | .11 ± .01 | .06 ± .005 | .97 ± .28 | .36 ± .06 | .25 ± .08 | |
main effect of sex
p < 0.05,
p < 0.01
3.2 Experiment 2: Effects of defeat stress on dopamine receptor expression
In the NAc, males had higher levels of D3 mRNA expression in NAc compared to females (Table 3, F1,50 = 10.34, p < 0.01). However, there was no effect of stress or interaction on D3 expression. There were no significant differences in D1, D2, or D5 gene expression.
Table 3.
Dopamine receptor mRNA relative expression.
| D1R | D2R | D3R | D5R | Prodynorphin | ||
|---|---|---|---|---|---|---|
|
|
||||||
| female | control | 2.24 ± .36 | 1.44 ± .20 | 3.22 ± .87 | 1.80 ± .21 | 1.72 ± .25 |
| stress | 1.57 ± .36 | 1.12 ± .20 | 2.61 ± .87 | 1.20 ± .21 | 1.14 ± .25 | |
| male | control | 2.17 ± .41 | 1.38 ± .23 | 6.37 ± 1.00** | 1.18 ± .24 | 1.51 ± .28 |
| stress | 1.47 ± .45 | 1.03 ± .25 | 5.99 ± 1.10** | 1.30 ± .27 | 1.32 ± .31 | |
main effect of sex p < 0.01
3.3 Experiment 3: Effects of D1 agonist infusion in males and female naïve to defeat
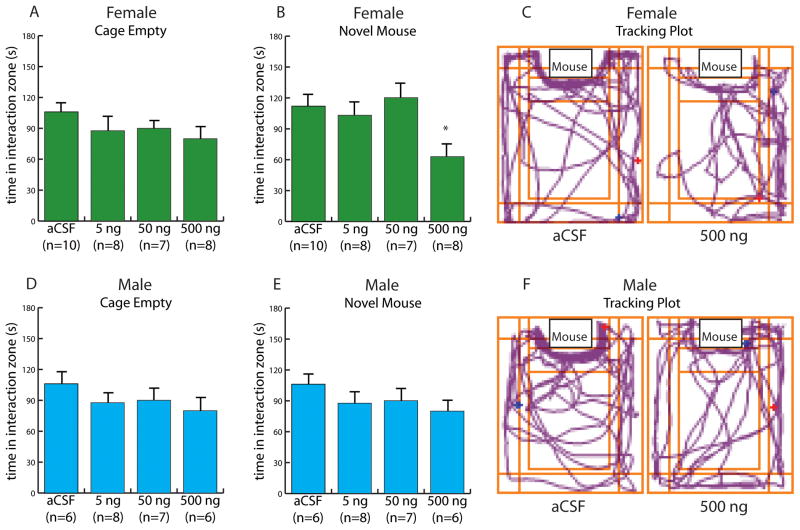
In females, SKF38393 infusions reduced time spent in the cage zone during the social interaction phase in a dose dependent fashion (Fig. 4B, Kruskal-Wallis H3=9.44, p = 0.02). Females treated with 500 ng of SKF38393 spent significantly less time in the interaction zone in the presence of a novel mouse compared to females treated with aCSF (Mann-Whitney U=2.60, p < 0.01), the low dose (Mann-Whitney U=1.99, p < 0.05), or the medium dose (Mann-Whitney U=2.65, p < 0.01). Similar differences were observed in the social interaction ratio (Table 4, Kruskal-Wallis H3=8.76, p < 0.03). The effects of SKF38393 were specific to social contexts because there were no differences in time spent in the cage zone during the acclimation phase (Fig. 4A). There were also no effects on locomotor behavior or time spent in the center of the arena during the open field phase (all p’s > 0.62). In males, there were no significant effects on time spent in the interaction zone during the social interaction phase or during the acclimation phase (p’s > 0.50), and there were no differences in the social interaction ratio. Furthermore, there were no significant differences in locomotor behavior or time spent in the center during the open field phase (Table 4, all p’s > 0.43). No significant difference on any behavioral measure was observed in the anatomical misses (Table 4, all p’s >0.50).
Figure 4.
Effect of the D1 agonist SKF 38393 in the nucleus accumbens shell on time spent in the cage zone in females (A–C) and males (D–F) naïve to defeat. (A) Time spent in the cage zone during the acclimation phase by females. (B) Time spent in the cage zone during the social interaction phase by females. (C) Tracking plot of a representative female mouse path when a same-sex novel mouse was present after aCSF (left) or 500ng SKF 38393 (right) microinjection. (D) Time spent in the cage zone during the acclimation phase by males. (E) Time spent in the cage zone during the social interaction phase by male mice. (C) Tracking plot of a representative male mouse path during the social interaction phase after aCSF (left) or 500ng SKF 38393 (right) microinjection.* indicates p<0.05 vs. aCSF.
3.4 Experiment 4: Effects of D1 antagonist infusion in females exposed to defeat or control conditions
Although there were no significant differences in the absolute amount of time spent in the interaction zone in this study (Table 5), significant differences were observed for ratio scores (social interaction phase/acclimation phase) for the interaction and corner zones. In stressed females, infusions of the D1 antagonist SCH23390 significantly increased the ratio of time spent in the interaction zone in the presence of a novel mouse (Fig. 5A, Mann-Whitney U = 152, p < 0.01) and significantly decreased the ratio of time spent in the corner zones (Fig. 5B, Mann-Whitney U=42, p = 0.01) compared to aCSF treatment. In control females there was no effect of SCH23390 infusions on the ratio of time spent in the interaction zone in the presence of a novel mouse or the corner zones (p’s > 0.5). In contrast to our study with SKF38393, infusions of SCH23390 did have an inhibitory effect on locomotor behavior. Infusion of SCH23390 reduced locomotor activity in the open field for stressed females (Mann-Whitney U= 41, p = 0.03). A similar trend was observed in control females but was not statistically significant. No significant difference on any behavioral measure was observed in the anatomical misses (Table 5, all p’s >0.50).
Figure 5.
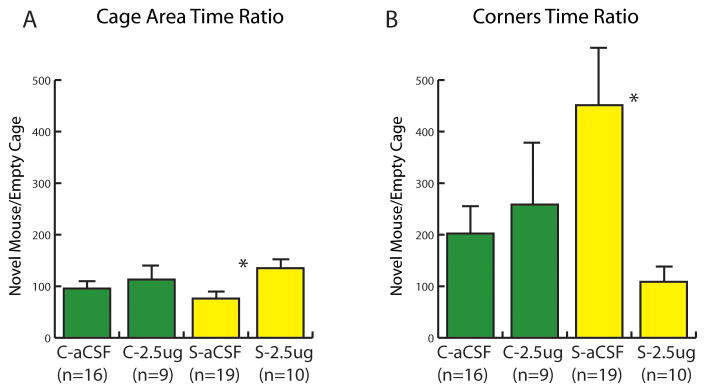
Effect of the D1 antagonist SCH 23390 in the nucleus accumbens shell on the interaction ratios (social interaction/acclimation × 100) for the cage zone (A) and corner zones (B). Infusions of SCH 23390 had no effect on control (C) mice but increased social interaction behavior in stressed (S) mice. ** indicates p<0.01 vs. aCSF.
4. Discussion
Our results indicate that although defeat stress increases dopaminergic signaling in both male and female California mice, dopamine D1-like receptor signaling induces social withdrawal in females but not males. These data suggest that previous observations that defeat stress increases the activity of VTA dopamine neurons (Anstrom et al., 2009; Krishnan et al., 2007; Razzoli et al., 2011) generalize not only to different species of rodents, but also to females. The sex difference in sensitivity to D1- like receptors does not appear to occur at the level of receptor expression in the NAc, because there were no sex differences in dopamine receptor gene expression. We hypothesize that sex differences in the behavioral effects of D1-like receptors are mediated downstream of D1 neurons.
4.1 Effects of Defeat Stress on Dopamine Content and Receptor Expression
In male rats and Mus, social defeat induces a long term increase in dopamine activity. Both male and female California mice exposed to social defeat stress had elevated levels of dopamine and DOPAC in the NAc, two weeks after the last episode of defeat stress. Punch samples were collected during the inactive phase, which suggests that these increases are due to an increase in baseline dopaminergic tone. Three weeks after defeat, baseline rates of in vivo VTA neuronal burst firing are increased in male Mus (Razzoli et al., 2011). In Mus, there is strong evidence that this increase in neuronal activity inhibits social approach behavior. In vivo recordings showed that the baseline activity of VTA neurons is negatively correlated with social interaction behavior, and that the activity of VTA neurons can be normalized by chronic antidepressant treatment (Cao et al., 2010). Reducing burst firing of VTA dopamine neurons by overexpressing potassium channels (Krishnan et al., 2007) or direct optogenetic control (Chaudhury et al., 2013) also increases social interaction behavior in male mice exposed to defeat. Interestingly, studies in Mus follow a standardized protocol of 10 episodes of defeat combined with prolonged sensory contact (Golden et al., 2011). In our studies, only three relatively brief episodes of defeat were sufficient to increase dopamine and DOPAC levels in both males and females. However, this raises the question of why social withdrawal was not observed in male California mice.
Increased reuptake could induce resistance to increased dopamine signaling. Studies using the visible burrow system showed that subordinate rats had reduced dopamine transporter (DAT) binding in NAc shell (Lucas et al., 2004). If DAT activity was elevated in stressed males, we would expect defeat stress to have little or no effect on dopamine metabolites (Huotari et al., 2002). However, DOPAC and HVA were elevated in stressed males. Alternatively, resistance to increased dopamine activity could be achieved via reduced expression of dopamine receptors. While male rats have been reported to have more intense D1 receptor binding in NAc compared to females (Andersen and Teicher, 2000) this effect has not been observed in every study (Ferris et al., 2007). We observed no sex differences in D1-like receptor (D1 or D5) expression, nor did we observe any effects of stress. Thus there is little support for the hypothesis that sex differences in behavioral responses to defeat stress are mediated by differences in dopamine receptor expression. Sex differences in behavior might instead be mediated by mechanisms downstream of receptor expression. Indeed, most evidence suggests that the behavioral effects of psychostimulants (which increase dopamine transmission) are stronger in females than males (Carroll and Anker, 2010). For example, amphetamine injections have stronger effects on rotational behavior in female rats compared to males (Robinson et al., 1980). Similarly, female rats form cocaine-based conditioned place preferences (CPP) at lower doses and with fewer conditioning sessions than males (Russo et al., 2003). These data suggest that behavioral effects of dopaminergic signaling may be stronger in females compared to males.
4.2 Sex differences in effects of D1 receptors on social withdrawal
Two observations suggested that D1 receptors would have important effects on stress-induced social withdrawal. First, D1 receptors are more likely than D2 receptors to be expressed in a low affinity state (Richfield et al., 1989), and several studies suggest that D1 activation occurs primarily in the presence of high dopamine levels (Cheer et al., 2007; Di Chiara and Bassareo, 2007). Second, defeat stress increases the number of phospo-CREB cells in the NAc shell of females but not males (Trainor et al., 2011). Thus, we used D1 receptor agonists and antagonists to test whether D1 receptors mediated stress-induced social withdrawal in California mice. Increased dopamine D1 signaling induced social aversion in females naïve to defeat, suggesting that D1 receptor activation is sufficient to induce social withdrawal behavior in females. Inhibition of D1 receptors in stressed females increased social approach behavior, suggesting that D1 signaling plays an important role in mediating the social withdrawal phenotypes. However, this effect appeared to be more subtle than what was observed in experiment 3 (D1 agonist), because only social interaction ratios were significantly changed. If social defeat induces a sustained increase in dopamine signaling in the NAc, this might induce neuroadaptations that would reduce the sensitivity of the NAc to dopaminergic signaling (Self, 2004).
Although D1 receptors in the NAc have been found to modulate locomotor behavior (Dreher and Jackson, 1989; Essman et al., 1993; Smith-Roe and Kelley, 2000), our results can not be explained by simple changes in locomotor behavior. The dose of SKF that reduced social interaction behavior in females was lower than those doses found to increase locomotor behavior, and we observed no changes in locomotor behavior of California mice in the open field test. While SCH infusions did reduce locomotor behavior in control and stressed females, only stressed females showed an increase in social interaction behavior. Furthermore, in stressed females SCH increased the relative time spent in the interaction zone and decreased the relative time spent in the corners opposite the interaction zone during the social interaction phase of the test. Together, these data indicate that D1 receptors in NAc shell have important effects on social withdrawal behavior.
Systemic injection of D1 agonists reduces social interaction behavior in males (Sams-Dodd, 1998), but only a few studies have considered whether D1 receptors regulate neural circuits controlling social behavior. Dopamine D1 receptors in the NAc shell play an intriguing role in the formation and maintenance of pair bonds between male and female prairie voles (Aragona and Wang, 2009). In males, activation of D1 receptors in the NAc shell prevents the formation of new pair bonds (Aragona et al., 2006). However, once a pair bond is formed, D1 receptor expression in the NAc is enhanced and facilitates the maintenance of the bond by increasing aggressive behavior towards unfamiliar females (Aragona et al., 2006). This effect appears to be mediated by endogenous opioid signaling. Medium spiny neurons in the NAc that express D1 receptor also express dynorphin (Hara et al., 2006), the primary endogenous ligand for the kappa opioid receptor (KOR). Infusion of the KOR antagonist norbinaltorphimine (nor-BNI) into the NAc shell of pair-bonded prairie voles reduces aggressive behavior (Resendez et al., 2012). These results suggest the possibility that effects of D1 receptors on social withdrawal are mediated in part by KOR signaling. However, we did not examine D1 receptor affinity or efficacy which could differ between the sexes and be differentially altered by social stress between the sexes, thus the effects could still be at the level of the D1 receptor.
Stress-induced anxiety and depression disorders are more common in women versus men (Kessler et al., 1993). Rodent studies have demonstrated that females have exaggerated glucocorticoid responses to stress (Weiser and Handa, 2009). Chronic stressors also lead to stronger inductions of depression-like behaviors, such as anhedonia, in females compared to males (Dalla et al., 2005; Dalla et al., 2008; Konkle et al., 2003). Less is known about potential sex differences in neural circuits that may mediate these behavioral responses. Our findings indicate that sex differences in the strength of D1 receptor signaling in the NAc may contribute to increased vulnerability to psychosocial stress in females. These results suggest that further study of molecular pathways downstream of D1 receptor expressing neurons, particularly the dynorphin-KOR pathway, will provide new insights to understanding sex differences in the behavioral effects of psychosocial stress.
Highlights.
Sex differences in D1 receptor activation on social withdrawal behavior were examined.
D1 receptor activation is sufficient to induce social withdrawal in females but not males.
D1 receptor antagonism increased social approach behavior in defeated females.
Result suggests D1 receptors are necessary for defeat-induced social withdrawal.
Acknowledgments
The authors thank S. Resendez for technical advice, C. J. Clayton for animal care, and D. Nguyen for technical assistance. This work supported by NIH grant NCRR000167 to the Wisconsin National Primate Research Center, and R21 MH090391, R01 MH097714 to BCT.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24:137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuorscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Dopamine regulation of social choice in a monogamous rodent species. Front Neurosci. 2009;3:15. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba JS, Endres CJ, Foss CA, Nimmagadda S, Jung H, Goddard JS, Lee S, McKisson J, Smith MF, Stolin AV, Weisenberger AG, Pomper MG. Molecular imaging of conscious, unrestrained mice with AwakeSPECT. J Nucl Med. 2013;54:969–976. doi: 10.2967/jnumed.112.109090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byas-Smith MG, Li J, Szlam F, Eaton DC, Votaw JR, Denson DD. Isoflurane induces dopamine transporter trafficking into the cell cytoplasm. Synapse. 2004;53:68–73. doi: 10.1002/syn.20037. [DOI] [PubMed] [Google Scholar]
- Cao J-L, Covington HE, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, Han M-H. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 2010;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevali L, Mastorci F, Graiani G, Razzoli M, Trombini M, Pico-Alfonso MA, Arban R, Grippo AJ, Quaini F, Sgoifo A. Social defeat and isolation induce clear signs of a depression-like state, but modest cardiac alterations in wild-type rats. Physiol Behav. 2012;106:142–150. doi: 10.1016/j.physbeh.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Aragona BJ, Heien MLAV, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007;54:237–244. doi: 10.1016/j.neuron.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z. Chronic mild stress impact: Are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S, Daskas S, Papadopoulou-Daifoti Z. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiology & Behavior. 2008;93:595–605. doi: 10.1016/j.physbeh.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Dreher JK, Jackson DM. Role of D1 and D2 dopamine receptors in mediating locomotor activity elicited from the nucleus accumbens of rats. Brain Res. 1989;487:267–277. doi: 10.1016/0006-8993(89)90831-7. [DOI] [PubMed] [Google Scholar]
- Essman WD, McGonigle P, Lucki I. Anatomical differentiation within the nucleus accumbens of the locomotor stimulatory actions of selective dopamine agonists and D-amphetamine. Psychopharmacology (Berl) 1993;112:233–241. doi: 10.1007/BF02244916. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Silvers JM, Hasselrot U, Beaudin SA, Strupp BJ, Booze RM. Sex mediates dopamine and adrenergic receptor expression in adult rats exposed prenatally to cocaine. International Journal of Developmental Neuroscience. 2007;25:445–454. doi: 10.1016/j.ijdevneu.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Convington HEI, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protocols. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Yakovleva T, Bakalkin G, Pickel VM. Dopamine D1 receptors have subcellular distributions conducive to interactions with prodynorphin in the rat nucleus accumbens shell. Synapse. 2006;60:1–19. doi: 10.1002/syn.20273. [DOI] [PubMed] [Google Scholar]
- Holly EN, Shimamoto A, DeBold JF, Miczek KA. Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology. 2012;224:179–188. doi: 10.1007/s00213-012-2846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrianhamsters. Horm Behav. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Huotari M, Santha M, Lucas LR, Karayiorgou M, Gogos JA, Mannisto PT. Effects of dopamine uptake and inhibition on brain catecholamine levels and locomotion in catechol-O-methyltransferase disrupted mice. J Pharmacol Exp Therapeutics. 2002;303:1309–1316. doi: 10.1124/jpet.102.043042. [DOI] [PubMed] [Google Scholar]
- Jacobson-Pick S, Audet MC, McQuaid RJ, Kalvapalle R, Anisman H. Social agonistic distress in male and female mice: changes of behavior and brain monoamine functioning in relation to acute and chronic challenges. PLOS One. 2013;8:e60133. doi: 10.1371/journal.pone.0060133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian JW, Petzold GL, Greengard P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the “dopamine receptor”. Proc Natl Acad Sci U S A. 1972;69:2145–2149. doi: 10.1073/pnas.69.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and Depression in the National Comorbidity Survey .1. Lifetime Prevalence, Chronicity and Recurrence. Journal of Affective Disorders. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Konkle ATM, Baker SL, Kentner AC, Barbagallo LSM, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, LaPlant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Celen Z, Tamashiro KLK, Blanchard RJ, Blanchard DC, Markham C, Sakai RR, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124:449–457. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Morrison KE, Curry DW, Cooper MA. Social status alters defeat-induced neural activation in Syrian hamsters. Neuroscience. 2012;210:168–178. doi: 10.1016/j.neuroscience.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mos J, Van Valkenburg CFM. Specific effect on social stress and aggression on regional dopamine metabolism in rat brain. Neurosci Lett. 1979;15:325–327. doi: 10.1016/0304-3940(79)96134-2. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Lacagnina MJ, Fanous S, Wang J, Hammer RP., Jr Intermittent social defeat stress enhances mesocorticolimbic ΔfosB/BDNF co-expression and persistently activates corticotegmental neurons: implication for vulnerability to psychostimulants. Neuroscience. 2012;212:38–48. doi: 10.1016/j.neuroscience.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Cabib S. Effects of defeat experiences on dopamine metabolism in different brain areas of the mouse. Aggressive Behavior. 1990;16:271–284. [Google Scholar]
- Razzoli M, Andreoli M, Michielin F, Quarta D, Sokal DM. Increased phasic activity of VTA dopamine neurons in mice 3 weeks after repeated social defeat. Behavioural Brain Research. 2011;218:253–257. doi: 10.1016/j.bbr.2010.11.050. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. κ-opioid receptor within the nucleus accumbens shell mediate pair bond maintenance. J Neurosci. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Ramirez VD. Sex differences in amphetamine-elicited rotational behavior and the lateralization of striatal dopamine in rats. Brain Res Bull. 1980;5:539–545. doi: 10.1016/0361-9230(80)90260-9. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian MR, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Effects of dopamine agonists and antagonists on PCP-induced stereotyped behaviour and social isolation in the rat social interaction test. Psychopharmacology. 1998;135:182–193. doi: 10.1007/s002130050500. [DOI] [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47(Suppl 1):242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Silva AL, Fry WHD, Sweeney C, Trainor BC. Effects of photoperiod and experience on aggressive behavior in female California mice. Behavioural Brain Research. 2010;208:528–534. doi: 10.1016/j.bbr.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Roe SL, Kelley AE. Coincident Activation of NMDA and Dopamine D1Receptors within the Nucleus Accumbens Core Is Required for Appetitive Instrumental Learning. J Neurosci. 2000;20:7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Karom MC, Huhman KL. Sex and estrous cycle differences in the display of conditioned defeat in Syrian hamsters. Hormones and Behavior. 2007;52:211–219. doi: 10.1016/j.yhbeh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Taylor SL, Stanek LM, Ressler KJ, Huhman KL. Differential brain-derived neurotrophic factor expression in limbic brain regions following social defeat or territorial aggression. Behavioral Neuroscience. 2011;125:911–920. doi: 10.1037/a0026172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimibic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Trainor BC. Stress responses and the mesolimbic dopamine system: social contexts and sex differences. Horm Behav. 2011;60:457–469. doi: 10.1016/j.yhbeh.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Bird IM, Alday NA, Schlinger BA, Marler CA. Variation in aromatase activity in the medial preoptic area and plasma progesterone is associated with the onset of paternal behavior. Neuroendocrinology. 2003;78:36–44. doi: 10.1159/000071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus) PLOS One. 2011;6:e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, Steinman MQ. Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm Behav. 2013;63:543–550. doi: 10.1016/j.yhbeh.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, Iniguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolanos CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votaw J, Byas-Smith M, Hua J, Voll R, Martarello L, Levey AI, Bowman FD, Goodman M. Interaction of isoflurane with the dopamine transporter. Anesthesiology. 2003;98:404–411. doi: 10.1097/00000542-200302000-00021. [DOI] [PubMed] [Google Scholar]
- Votaw JR, Byas-Smith MG, Voll R, Halkar R, Goodman MM. Isoflurane alters the amount of dopamine transporter expressed on the plasmamembrane in humans. Anesthesiology. 2004;101:1128–1135. doi: 10.1097/00000542-200411000-00012. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto KK, Gonzalez GA, Biggs WH, Montminy MR. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988;334:494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]