Abstract
Intrathecal (IT) studies have shown that several voltage sensitive calcium channels (VSCCs), such as the L-, N- and T-type may play roles in nociception and that of these only the N-type regulates primary afferent substance P (SP) release. However, the actions of other VSCCs at the spinal level are not well known. We investigated the roles of spinal P/Q- and R-type VSCCs, by IT administration of R-type (SNX-482) and P/Q-type (ω-agatoxin IVA) VSCC blockers on intraplantar formalin-evoked flinching, SP release from primary afferents and c-Fos expression in spinal dorsal horn. Intraplantar injection of formalin (2.5%, 50 µL) produced an intense, characteristic biphasic paw flinching response. In rats with IT catheters, IT SNX-482 (0.5 µg) reduced formalin-evoked paw flinching in both phase 1 and 2 compared with vehicle. Intraplantar formalin caused robust neurokinin 1 receptor (NK1r) internalization (indicating SP release) and c-Fos expression in the ipsilateral dorsal horn, which were blocked by IT SNX-482. IT ω-agatoxin IVA (0.03, 0.125 and 0.5 µg) did not reduce formalin-evoked paw flinching or c-Fos expression at any doses, with higher doses resulting in motor dysfunction. Thus, we demonstrated that blockade of spinal R-type, but not P/Q type VSCCs attenuated formalin-induced pain behavior, NK1r internalization and c-Fos expression in the superficial dorsal horn. This study supports a role for Cav2.3 in presynaptic neurotransmitter release from peptidergic nociceptive afferents and pain behaviors.
Keywords: Voltage sensitive calcium channels, SNX-482, ω-agatoxin IVA, neurokinin 1 receptor, c-Fos, spinal cord
1. Introduction
Voltage-sensitive calcium channels (VSCCs) facilitate calcium influx and play an important role in the regulation of neurotransmitter release, synaptic transmission and neuronal excitability (Catterall, 2000; Catterall and Few, 2008). The a1 is the pore-forming subunit and dictates the VSCC’s major characteristics of pharmacology, electrophysiology and kinetic activity, representing the basis for the calcium channel subtype. Ten different a1 have been identified, namely Cav1.1–1.4, Cav2.1–2.3 and Cav3.1–3.3. These are distributed into five subgroups, L-(Cav1.1–1.4), P/Q-(Cav2.1), N-(Cav2.2), R-(Cav2.3) and T-type (Cav3.1–3.3). Based on their voltage activation properties, VSCCs are divided into two classes, high voltage-activated channels and low voltage-activated channels (Ertel et al., 2000). High voltage-activated channels include L-, P/Q-, N- and R-types. These are heteromeric complexes consisting of an a1 subunit along with auxiliary subunits such as α2δ, β and γ subunit. Low voltage-activated channels or T-type are activated by much more negative membrane potentials (Carbone and Lux, 1984; Nowycky et al., 1985) and are not known to interact with auxiliary subunits.
VSCCs are expressed in the dorsal root ganglion (DRG) and spinal cord dorsal horn (Murakami et al., 2001; Westenbroek et al., 1998; Yusaf et al., 2001), suggestive of their critical role in nociception. Intrathecal (IT) ziconotide (Prialt®), a selective N-type blocker is approved for treatment of severe chronic pain. Consistent with location of N-type VSCCs on peptidergic primary afferents, N-type VSCC blockade attenuates neurotransmitter release from primary afferents, as defined in in vitro and in vivo models (Evans et al., 1996; Maggi et al., 1990; Santicioli et al., 1992; Takasusuki and Yaksh, 2011). However, the role of other VSCCs subtypes in afferent neurotransmitter release remains unclear. We found previously that intrathecal L- and T-type blockers minimally affected intraplantar formalin-evoked release of substance P (SP) in vivo as measured by neurokinin 1 receptor (NK1r) internalization (Takasusuki and Yaksh, 2011). Those results suggested L- and T-type VSCCs were not involved in stimulus-evoked SP release. In the present study we focus on the role of P/Q- and R-types.
Cav2.1 (P/Q-type) channels contain the a1A subunit and are distributed in the nervous system including DRG and spinal dorsal horn (Catterall and Few, 2008; Kulik et al., 2004; Urban et al., 2005). P/Q-type VSCCs are inhibited by ω-agatoxin IVA, a 48- amino acid peptide isolated from the venom of funnel web spider, Agelenopsis aperta (Mintz et al., 1992). ω-agatoxin IVA blocks P-type with high affinity and Q-type with lower affinity (Adams, 2004). IT ω-agatoxin IVA reduces the number of flinches in formalin phase 2, but did not alter thermal escape latencies (Malmberg and Yaksh, 1994). Cav2.3 (R-type) channels contain the α1E subunit, and are detected in spinal cord and DRG (Murakami et al., 2001; Saegusa et al., 2000; Westenbroek et al., 1998; Yusaf et al., 2001). Cav2.3 knockout mice exhibited reduced pain behaviors in the formalin test (Saegusa et al., 2000). In another study, Cav2.3 was shown to participate in nerve injury-induced hypersensitivity (Matthews et al., 2007). Dense labeling of Cav2.3 was observed in the superficial layers of the dorsal horn (Saegusa et al., 2000). However, whether Cav2.3 is involved in primary afferent neurotransmitter release has not been established.
Here, we investigated intrathecal R- and P/Q-type channel blockers and spinal release of SP evoked by intraplantar formalin. Increase in local extracellular SP due to its release (induced by intraplantar formalin) from small peptidergic, transient receptor potential protein vanilloid 1 (TRPV1) (+) C-fibers evokes NK1r internalization (Kondo et al., 2005; Mantyh, 2002). Thus NK1r internalization provides a powerful tool to evaluate in vivo the effects of VSCCs blockade on afferent terminal release of SP. Furthermore, intraplantar formalin induces c-Fos protein expression in dorsal horn neurons, which receive input from small diameter Aδ and C primary afferents (Bullitt, 1990; Hunt et al., 1987; Long et al., 2012), causing c-Fos expression to be a useful index of spinal activation. This in vivo methodology allows us to assess the effects of R- and P/Q- type VSCCs antagonists upon SP release, dorsal horn neuron activation and assess the covariance of these effects with pain behaviors (flinching) at the corresponding drug doses.
2. Material and Methods
2.1 Animals
Male Holtzman Sprague-Dawley rats (250–300g; Harlan Indianapolis, IN) were individually housed in standard cages and maintained on a 12-h light/dark cycle (lights on at 07:00 h). Testing occurred during the light cycle. Food and water were available ad libitum to all rats in the study. Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 85–23, Bethesda, MD) and as approved by the institutional Animal Care and Use Committee of the University of California, San Diego. All efforts were made to minimize animal suffering, to reduce the number of animals used.
2.2 Intrathecal catheter implantation
Rats were implanted with a single intrathecal catheter for drug delivery (Malkmus and Yaksh, 2004; Yaksh and Rudy, 1976). In brief, rats were anesthetized by induction with 4% isoflurane in a mixture of air and 100% oxygen (1:1). Anesthesia was maintained with 2% isoflurane delivery by mask. The animal was placed on a stereotaxic head holder, a midline incision was made on the back of the occipital bone and the cisternal membrane was exposed by blunt dissection. A small opening was made on the cisternal membrane by a 22 gauge needle, and an 8.5 cm single-lumen polyethylene (OD 0.36 mm) catheter was inserted through the opening and passed into the intrathecal space to the level of the L2 to L3 spinal segments. The other end of the catheter was tunneled subcutaneously to exit through the top of the head, flushed with 10 µL of saline and plugged. Rats were given subcutaneously 5 mL of lactated Ringer’s solution, to which was added 1.25 mg/mL of carprofen and recovered in a warmed chamber. Rats with any motor weakness or signs of paresis on recovery from anesthesia were euthanized immediately. Animals were allowed to recover for 5 to 7 days prior to other studies.
2.3 Voltage-sensitive calcium channel (VSCC) blockers on formalin-induced paw flinching behavior
Formalin-induced flinching behavior was analyzed by a paw movement detection system (Yaksh et al., 2001). Briefly, a soft metal band was placed around the left hind paw and secured with a drop of adhesive. Animals were allowed to acclimate in individual acrylic glass chambers for 30 min before experimental manipulation. Rats were intrathecally treated with saline, SNX-482 (0.5 µg) or ω-agatoxin IVA (0.03, 0.125 and 0.5 µg) in a volume of 10 µL, followed by 10 µL of saline. 10 min after the IT injection, rats received a subcutaneous injection of 50 µL of formalin (2.5%) into the dorsal side of the banded paw. Rats were immediately, placed into test chambers and paw flinching assessed over the ensuing 60 min interval with an automated device (Department of Anesthesiology, University of California, San Diego, CA) for 60 min.
2.4 VSCC blockers on formalin-induced NK-1r internalization and c-Fos expression
Rats with catheter received IT saline or antagonist ten minutes before intraplantar formalin (50µl, 2.5%) injection. For NK1r internalization, rats were transcardially perfused 10 min after formalin. For c-Fos expression, rats were perfused 120 min after formalin.
2.5 Tissue preparation and Immunohistochemistry
Anesthetized rats were transcardially perfused with NaCl (0.9%) followed by paraformaldehyde (4%) in 0.1 M phosphate buffer (PB), pH 7.4. The lumbar spinal cord was removed and post-fixed in paraformaldehyde (4%) overnight. After cryoprotection in 30% sucrose, coronal sections (30 µm) were cut on a sliding microtome (HM 450; Thermo Scientific, Kalamazoo, Ml).
For NK1r, sections were incubated in a rabbit anti–NK-1r polyclonal antibody ab6 (Abeam, Eugene, OR, 1:3000) and mouse anti-NeuN MAB377 (Millipore, Temecula, CA, 1:500) overnight at room temperature. After the sections were rinsed in PBS, they were incubated for 2 hours at room temperature in a goat-anti-rabbit secondary antibody conjugated with Alexa 488 (Invitrogen, Carlsbad, CA, 1:500) to identify NK-1r and a goat-anti-mouse secondary antibody conjugated with Alexa 555 (Invitrogen, Carlsbad, CA, 1:500) to identify NeuN. All sections were finally rinsed and mounted on glass slides and coversliped with ProLong mounting medium (Invitrogen, Carlsbad, CA).
Immunohistochemistry of c-Fos was performed using the avidin-biotin complex (ABC) method. In short, free-floating spinal cord sections were treated with 3% hydrogen peroxide (Sigma, St. Louis, MO) for 10 min and then incubated in primary antibody solution containing 0.5% Triton X-100, 10% goat serum in phosphate buffered saline, and anti-c-Fos (sc-52, Santa Cruz Biotechnology, Santa Cruz, CA, 1:10,000) overnight at room temperature. After sections were rinsed in PBS, incubated with a biotinylated goat-anti-rabbit secondary antibody (BA-1000, Vector laboratories, Burlingame, CA,1:500) for 120 min. Sections were incubated in ABC solution for 1 hr (PK-6100, Vector laboratories, Burlingame, CA) and subsequently DAB substrate solution (SK-4100, Vector laboratories, Burlingame, CA) for an appropriate amount of time. Following mounting and dehydration, slides were coversliped with DPX (Electron Microscopy Science, Hatfield, PA).
2.6 Quantification of NK1r internalization and c-Fos expression
The amount of NK1r internalization was quantified using a standard method (Abbadie et al., 1997a; Kondo et al., 2005; Mantyh et al., 1995). NK1r-positive neurons in lamina l/ll on both sides of the dorsal horn were counted using an Olympus BX-51 fluorescence microscope (Olympus Optical, Tokyo, Japan) at × 60 magnification. Neurons with 10 or more endosomes in their soma and the conterminous proximal dendrites were deemed as having internalized NK1r. The person counting the neurons was blinded to the experimental treatment. In each segment, three to four randomly selected sections were counted. The sum of the number of NK1r-positive neurons with and without NK1r internalization taken from a segment were used to calculate the percentage of NK1r internalization to represent the particular spinal segment for a given animal. Four animals per each treatment group were used for statistical analysis. The ratio of cells showing NK-1r internalization versus all NK1r-positive cells was reported.
We quantified the c-Fos-immunoreactive (IR) neurons under the Olympus BX-51 microscope at ×10 magnification in the ipsilateral and contralateral spinal dorsal horn. The number of c-Fos-IR neurons in lamina I/II and lamina III/V was determined by a blinded observer. Means counted from six to nine randomly selected sections at L3-L6 level spinal cord were employed for each animal. At least one spinal section was selected for each spinal segment. Five animals per treatment group (for the ω-agatoxin IVA study, two to five animals) were used for statistical analysis. Light microscopic images were taken using MagnaFire SP (Optronics, Goleta, CA) and processed by Photoshop CS5 (Adobe, San Jose, CA).
2.7 Behavioral and motor effects of intrathecal VSCC blockers
Behavioral and motor functions were carefully observed (Malmberg and Yaksh, 1994) after the intrathecal administration of VSCCs blockers. Behavior tests include irritability after stimuli by gently touching the flank and back with a plastic tubing (touch-evoked agitation and touch-evoked vocalization), reflective muscle jerk in any of the extremities evoked by click sound from behind (startle response) and toe pinching (withdrawal response). Motor function was assessed by: i) the placing /stepping reflex (upward lifting of the paw caused by drawing the dorsum of the hindpaw across the edge of the table) and ii) the righting reflex (placing the rat on its back, which induces a quick coordinated twisting. Catalepsy was assessed by placing the forelimb on a horizontal bar kept at 4 cm from a table surface. Failure to move from the bar within 30 sec was judged as a positive. Rats without any behavioral or motor dysfunction were used in the formalin test.
2.8 Antagonists
SNX-482 and ω-agatoxin IVA were purchased from Peptide institute (Osaka, Japan). Both drugs were dissolved in saline and administered in a volume of 10 µL followed by a 10-µL saline flush. Nomenclature for drugs and receptors conforms with the guide to receptors and channels of the British Journal of Pharmacology (Alexander et al., 2008).
2.9 Statistical Analysis
Statistical analysis was performed by Prism GraphPad 5 (GraphPad, La Jolla, CA). Changes in formalin-induced paw-flinching behavior, NK-1r internalization and c-Fos expression were analyzed by t test or one-way ANOVA. Data in table 2 was analyzed with two-way ANOVA with repeated measures. In t test, P value was expressed using the two-tailed test. In all analyses, probability to detect the difference was set at the 5% level (P < 0.05).
Table 2.
Number of NK1r-positive neurons per section in the superficial dorsal horn of lumbar segments (L3-L6) of rats after unilateral intraplantar formalin injection
For each rat, 3–4 sections from lumbar segment were counted and the mean was taken as representative of that segment. The mean ± SEM of these individual counts for the 4 rats is presented in the table. Two-way ANOVA with repeated measures revealed no significant differences across ipsilateral and contralateral side (p = 0.13) and between drug treatments (p = 0.43).
| Treatment | Number of rats |
L3/4 | L/4/5 | L5/6 | Overall mean ± SEM | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ipsi | Contra | Ipsi | Contra | Ipsi | Contra | Ipsi | Contra | ||
| IT saline | 4 | 8.8 ± 1.6 | 5.7 ± 1.2 | 9.2 ± 1.1 | 7.6 ± 1.5 | 7.0 ± 0.7 | 6.8 ± 0.9 | 8.2 ± 1.1 | 6.7 ± 1.1 |
| IT SNX-482 | 4 | 9.7 ± 1.3 | 8.0 ± 1.1 | 9.6 ± 2.0 | 6.5 ± 1.0 | 9.3 ± 0.4 | 7.5 ± 1.7 | 9.5 ± 1.2 | 7.3 ± 1.2 |
3. Results
3.1 Behavior and motor effects of intrathecal SNX-482
IT administration of R-type VSCCs blocker SNX-482 (0.5 and 4.6 µg) in rats induced various adverse effects such as agitation, spontaneous and touch-evoked vocalization and motor deficits (Table 1). At the low dose (0.5 µg) these effects were minor and the incidence was low. The following behaviors were noticed within 2 hours after the IT drug injection: vocalization evoked by gently touching the flank and back with a plastic tubing (2/14), facilitated startle response (2/14), absence of righting reflex (1/14), pinna reflex (1/14) and cornel reflex (1/14). The adverse effects became more severe and occurred at a higher incidence when a high dose (4.6 µg) of IT SNX-482 was injected. Behaviors observed included agitation (2/3), loss of pinna/cornel reflex (1/3), deficits in the placing /stepping test (2/3), trunk rigidity (1/3), intense whole-body shaking (1/3) and tail biting (1/3). Thus we concluded the highest tolerable dose of IT SNX-482 was 0.5 µg for the current study.
Table 1.
Behavioral and Motor Effects of Intrathecal Voltage-Sensitive Calcium Channel Blockers
SNX-482 (0.5 and 4.6µg) and ω-agatoxin IVA (0.03, 0.125, 0.5 and 1.0µg) were administered intrathecally. Behavior and motor functions (see Material and Methods) were monitored for 2 hours. Percentage of rats with the indicated behavior was shown in the table.
| Drug | Dose (µg) | n | Agitation/ Vocalization |
Minor motor dysfunction |
Severe motor dysfunction |
|---|---|---|---|---|---|
| SNX-482 | 0.5 | 14 | 14% | 7% | 0% |
| 4.6 | 3 | 67% | 0% | 67% | |
| ω-agatoxin IVA | 0.03 | 2 | 0% | 0% | 0% |
| 0.125 | 6 | 0% | 0% | 0% | |
| 0.5 | 5 | 40% | 20% | 20% | |
| 1 | 1 | 100% | 0% | 100% |
3.2 Effects of intrathecal SNX-482 on formalin-induced paw flinching behavior
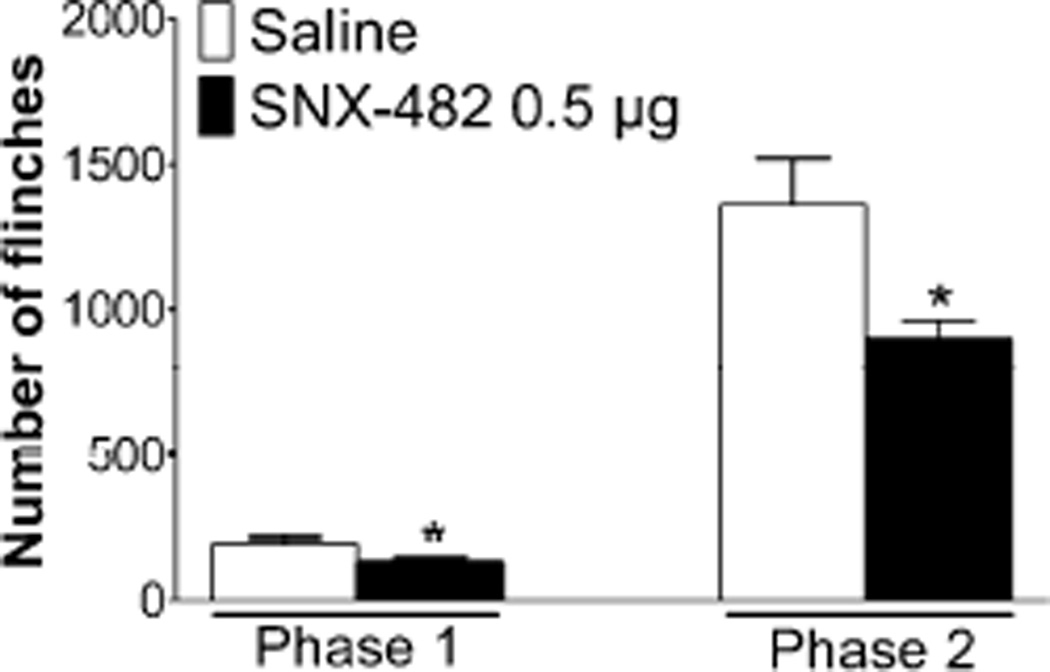
Intraplantar injection of formalin (2.5%, 50 µl) in rats produced a characteristic biphasic paw flinching response over a 60 min period (Figure 1). Pretreatment with IT SNX-482 (0.5 µg) before formalin significantly inhibited the number of flinches in both phase 1 (saline: 191 ± 20 vs. SNX-482: 131 ± 16, P < 0.05) and phase 2 (saline: 1362 ± 159 vs. SNX-482: 902 ± 61, P < 0.05).
Figure 1.
Effects of intrathecal (IT) SNX482 on formalin-induced paw flinching behavior. Rats received IT injection of SNX-482 (0.5 µg) or saline 15 min before intraplantar formalin (2.5%, 50 µL) injection. The evoked flinching behavior was recorded for 60 min by an automated device. The total number of flinches in phase 1 (0–10 min) and phase 2 (11–60 min) were shown in the histogram. Data are expressed as Mean ± SEM. *P < 0.05 compared with saline by t-test. N = 5 rats per group.
3.3 Effects of intrathecal SNX-482 on formalin-induced NK1r internalization
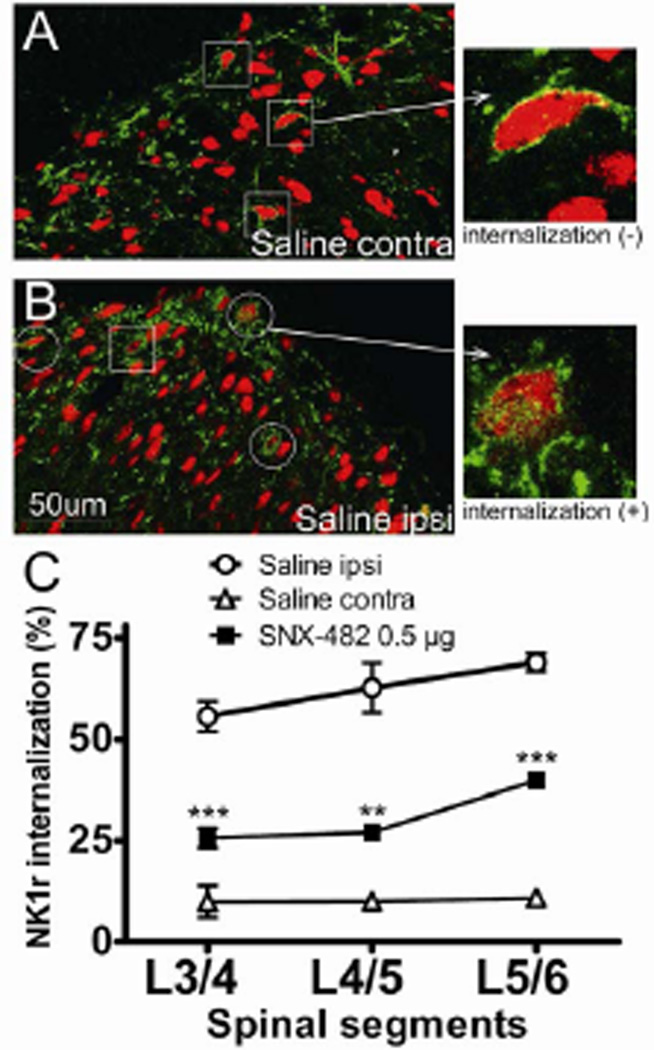
NK1r is readily detected in the superficial dorsal horn neurons (Figure 2A and B). In naïve spinal cord, NK1r immunoreactivity is concentrated on the cell membrane and the incidence of intracellular NK1r is low. The number of NK1r-positive neurons per spinal cord section (showing internalization or not) was shown in table 2. As indicated, on average there were 8.2 ±1.1 and 6.7±1.1 NK1r-positive cells per section in the lumber spinal cord. Two-way ANOVA with repeated measures revealed no difference between the ipsilateral and contralateral sides. Hindpaw intraplantar injection of formalin resulted in a robust NK1r internalization in the ipsilateral laminae I/II at the L3-L6 level (L3/4: 56 ± 4%, L4/5: 63 ± 6% and L5/6: 69 ± 2%, figure 2B and C), but not on the contralateral side (L3/4: 10 ± 4%, L4/5: 10 ± 2% and L5/6: 11 ± 2, figure 2A and C). This formalin-induced NK1r internalization on the ipsilateral side was greatly inhibited by an IT pretreatment of SNX-482 (0.5 µg) (SNX-482, L3/4: 26 ± 2%; P < 0.001, L4/5: 27 ± 2%; P < 0.01 and L5/6: 30 ± 2%; P < 0.001, compared with IT saline ipsilateral, respectively). On the contralateral side, there was no significant difference in percentage of NK1r internalization between SNX-482 (0.5 µg) and saline groups (SNX-482, L3/4: 11 ± 2%; L4/5: 7 ± 3% and L5/6: 12 ± 3% vs. saline: L3/4: 10 ± 4%, L4/5: 10 ± 2% and L5/6: 11 ± 2, figure 2C). In addition, no change was detected in the number of NK1r-positive neurons per section after SNX-482 treatment compared to saline controls.
Figure 2.
Effects of intrathecal (IT) SNX-482 on formalin-induced neurokinin 1 receptor (NK1r) internalization. Rats received IT SNX-482 or saline 15 min before intraplantar formalin (2.5%, 50 µL) injection. (A–B) Representative confocal images of formalin-induced NK1r internalization in L5 superficial dorsal horn (green: NK1r; red: NeuN) in an animal received intrathecal saline pretreatment. Squares indicate NK1r immunoreactive neurons without NK1r internalization. Circles indicate neurons showing NK1r internalization. (A, right) NK1r immunoreactivity in the contralateral spinal lamina l/lI. Note the presence of a homogeneous cell membrane and the lack of NK1r-containing endosomes internalizing into the cytoplasm. (B, right) NK1r immunoreactivity in the ipsilateral spinal lamina l/ll. Note the lack of a homogeneous cell membrane and the presence of NK1r-containing endosomes internalizing into the cytoplasm. (C) Line graph depicts percentage of NK1r internalization at each spinal segment. Formalin induced a robust NK1r internalization in the ipsilateral L3-L6 spinal cord dorsal horn, which was significantly blocked by IT SNX-482 pretreatment. Data are expressed as Mean ± SEM. **P < 0.01 and ***P < 0.001 compared with saline by one-way ANOVA. N = 4 rats per group. Scale bar is 50 µm. Ipsi, ipsilateral; contra, contralateral.
3.4 Effects of intrathecal SNX-482 on formalin-induced c-Fos expression
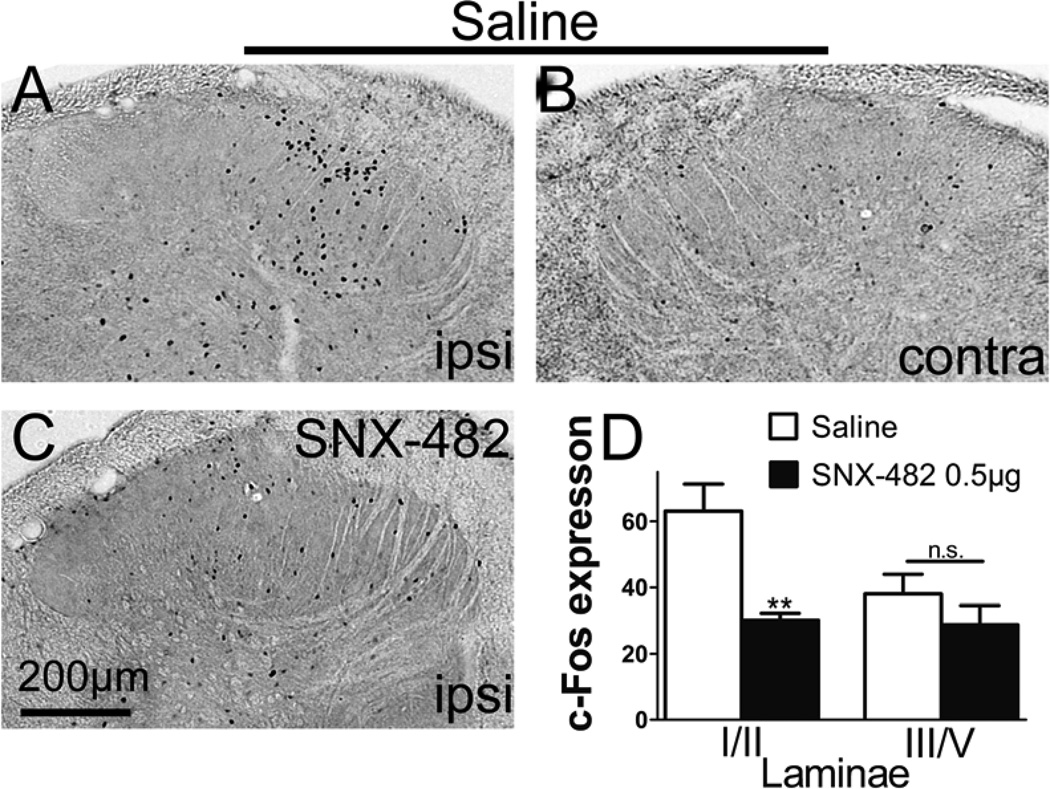
c-Fos is quickly induced in the dorsal horn neurons after peripheral noxious stimuli and often used as a marker for neuronal activation. Two hours following intraplantar formalin, we observed in the ipsilateral lumbar dorsal horn a significant increase in the number of neurons expressing c-Fos, compared to the contralateral side (Figure 3A and B) or naïve tissue (not shown). Pretreatment with IT SNX-482 (0.5 µg) significantly inhibited formalin-induced c-Fos expression in laminae l/II (vehicle: 63 ± 8 vs. SNX-482: 30 ± 2, P < 0.01). There was a trend of c-Fos reduction in lamina III/V although the change did not reach statistical significance (vehicle: 45 ± 7 vs. SNX-482: 29 ± 6, P > 0.05, figure 3D). In the contralateral dorsal horn, there was no significant difference in c-Fos expression between saline and SNX-482 (0.5 µg) groups in laminae l/ll (saline: 11 ± 3 vs. SNX-482: 12 ± 1, P > 0.05) and laminae III/V (saline: 16 ± 4 vs. SNX-482: 14 ± 1, P > 0.05).
Figure 3.
Effects of intrathecal (IT) SNX-482 on formalin-induced c-Fos expression. (A–C) Representative light microscopic images of c-Fos expression in the lumbar spinal cord dorsal horn 2 h following intraplantar formalin (2.5%, 50 µL) injection. Rats were pretreated with IT (A and B) saline or (C) SNX-482 15 min before intraplantar formalin. (D) (D) Histogram represents mean counts of c-Fos positive neurons in lamina l/ll and lamina III/V per spinal section in the ipsilateral dorsal horn. Data from randomly selected slices at L3-L6 spinal segment were employed (Eight to nine slices per rat). Scale bar is 200 µm. Data are expressed as Mean ± SEM. **P < 0.01 compared with saline by t-test. N = 5 rats per group. Scale bar is 50 µm. Ipsi, ipsilateral; contra, contralateral.
3.5 Effects of intrathecal ω-agatoxin IVA on behavior, formalin-induced paw flinching and c-Fos expression
Next we studied the effects of P/Q type VSCC antagonist ω-agatoxin IVA after intrathecal administration. At low doses (0.03 and 0.125 µg), ω-agatoxin IVA did not induce any abnormal behavioral or motor problem during the 2 hours observation period (Table 1). In contrast, 0.5 µg of ω-agatoxin IVA caused behavioral changes such as agitation (2/5), startle response (1/5), trunk rigidity (1/5) and motor dysfunction in the placing/stepping response (2/5) and righting reflex (2/5). Only one rat received the highest dose (1.0 µg). We observed significant side effects, including intense whole-body shaking, motor coordination problems, self-biting, circling behavior and serpentine-like movements of its tail. We concluded the highest tolerable dose of IT ω-agatoxin IVA for this study was 0.5 µg.
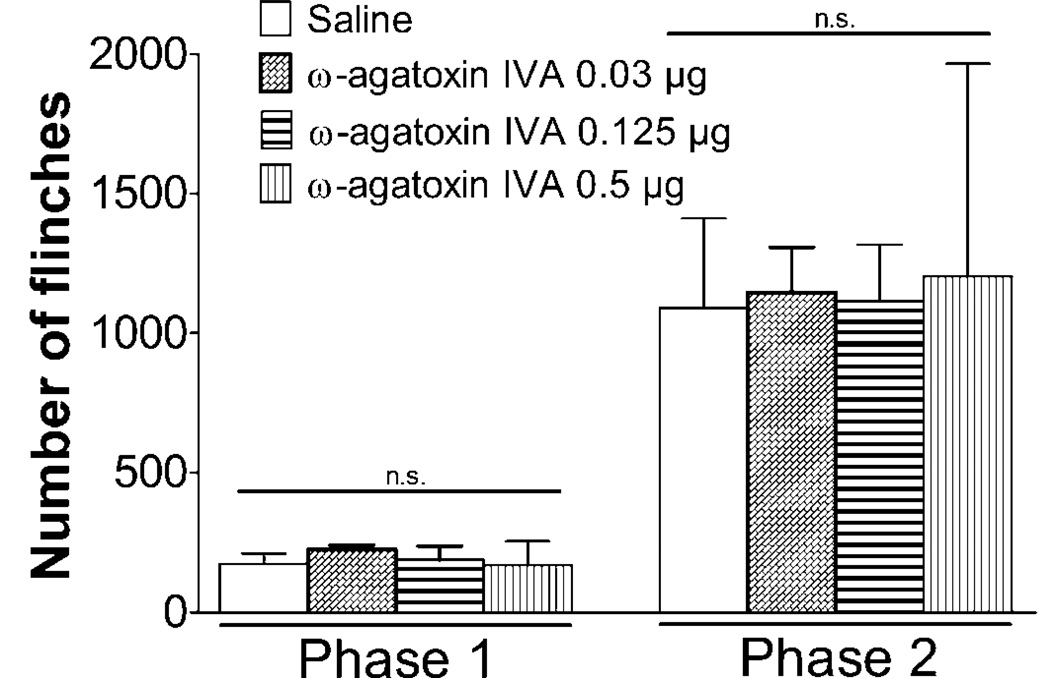
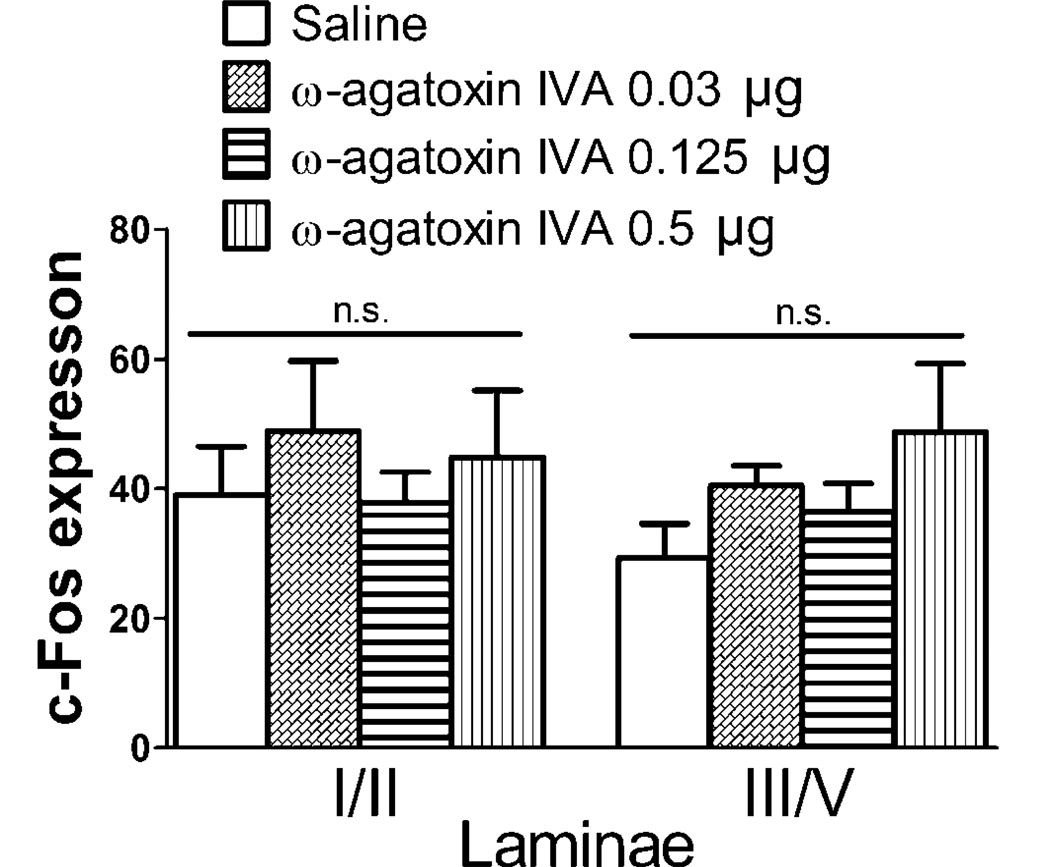
The effects of IT ω-agatoxin IVA on formalin-induced paw flinching were shown in Figure 4. Essentially ω-agatoxin IVA had no effects on the number of flinches at any of the doses (0.03, 0.125 and 0.5 µg) tested.
Figure 4.
Effects of intrathecal (IT) ω-agatoxin IVA on formalin-induced paw flinching behavior. Rats received IT injection of ω-agatoxin IVA (0.03, 0.125 or 0.5 µg) or saline 15 min before intraplantar formalin (2.5%, 50 µL) injection. The evoked flinching behavior was recorded for 60 min by an automated device. The total number of flinches in phase 1 (0–10 min) and phase 2 (11–60 min) were shown in the histogram. Data are expressed as Mean ± SEM. N = 2–5 rats per group.
Consistent with the flinching behavior results, ω-agatoxin IVA did not change the number of c-Fos expressing neurons in the lumbar spinal cord dorsal horn after formalin (Figure 5). We note that these studies shown in Figure 3D were not run concurrently with the studies in Figure 5 and for that reason separate vechile controls were performed for the respective comparsions. The reason for differences in baseline counts is not known.
Figure 5.
Effects of intrathecal (IT) ω-agatoxin IVA on formalin-induced c-Fos expression. Histogram represents the number of c-Fos positive neurons in lamina I/II and lamina III/V of ipsilateral dorsal horn. Data from L3-L6 spinal segments were pooled. There was no significant difference between treatment groups. Data are expressed as Mean ± SEM. N = 2–5 rats per group.
4. Discussion
Voltage sensitive calcium channels have received much attention in pain research. A well-defined role for VSCCs, specifically N-type VSCCs, is to mediate neurotransmitter release from primary sensory neurons in response to noxious stimuli (Evans et al., 1996; Maggi et al., 1990; Santicioli et al., 1992). Spinal blockade of N-type VSCCs results in potent analgesia in preclinical models (Chaplan et al., 1994; Lewis et al., 2000), which led to the development of ziconotide (Prialt®), an FDA approved drug to treat severe chronic pain. However, N-type VSCCs is widely distributed in the nervous system and its antagonism often causes adverse side effects, which directly limits the therapeutic window for ziconotide. Although other subtypes of VSCCs are detected in the primary sensory neurons, their involvement in presynaptic neurotransmitter release is less characterized. In this study, we used SNX-482 and ω-agatoxin IVA, selective antagonists for R- and P/Q-type VSCCs respectively, to investigate their contribution to afferent transmitter release and tissue injury-induced pain. We report here for the first time that spinal SNX-482 attenuated formalin-induced NK1r internalization, an indicator for substance P release. This effect was observed in parallel with an inhibition of formalin-evoked flinching behavior and c-Fos expression in the lumbar spinal dorsal horn. ω-agatoxin IVA, on the other hand, showed no effects in the above experiments.
4.1 Dorsal horn NK1 receptor internalization
Small unmyelinated fibers are a principal route for the input initiated by high intensity stimuli and local chemical irritants. A subgroup of the small fibers or peptidergic fibers, release glutamate and small peptides such as calcitonin gene-related peptide (CGRP) and SP in response to high intensity or irritant stimuli. In the spinal cord dorsal horn, SP mainly signals through NK1 receptors, which are heavily expressed on projection neurons (Todd et al., 2002). NK1r belongs to the G protein-coupled receptor (GPCR) superfamily. Agonist (i.e. SP) binding to NK1r may lead to activation of protein kinase A, protein kinase C, extracellular signal-regulated kinase and/or non-selective cation channels (Barber and Vasko, 1996; Fehrenbacher et al., 2003; Ito et al., 2002) and render the neurons more excitable. Events blocking spinal SP release usually correlate with reduced afferent/dorsal horn excitability and attenuated pain behaviors (Yaksh et al., 1980). Selective ablation of spinal NK1r-positive neurons by intrathecal SP-saporin blocked hyperpathia following tissue or nerve injury (Mantyh et al., 1997; Nichols et al., 1999; Suzuki et al., 2002). These data emphasize a critical role for the SP-NK1r pathway nociceptive processing. SP release therefore represents a well-defined model system for the study of factors governing the excitability of a defined population of high threshold afferents.
Typical for a GPCR, ligand (i.e. SP) binding to NK1r induces rapid endosomal internalization of the receptor, which can be reliably visualized and quantified by immunohistochemistry. Several lines of evidence support the generally accepted assertion that the extent of NK1r internalization reflects extracellular SP deriving from primary afferents in dorsal horn (Kondo et al., 2005; Marvizon et al., 2003). 1) Capsaicin pretreatment, which depletes SP in TRPV1-positive primary afferents, prevented NK1r internalization evoked by noxious stimuli (Kondo et al., 2005). 2) Intrathecal opiates, known to reduce SP release from primary afferents via presynaptic mechanisms, attenuated NK1r internalization following noxious stimuli (Marvizon et al., 2003; Yaksh et al., 1980). 3) There was a positive relationship ex vivo between the topical concentration of SP and NK1r internalization in spinal dorsal horn (Marvizon et al., 2003). From these examinations, we regard spinal NK1r internalization as a suitable marker for the measurement of SP release from primary afferent terminals. Further, this in situ method allows us to map the site of release (e.g. lamina I/II vs. lamina V; L4 vs. L6) and establish the anatomy of pain transmission pathway at the spinal level.
4.2 R-type calcium channels and release
Cav2.3, the major subunit of the R-type VSCCs, is readily detectable in small-, medium- and large-diameter primary sensory neurons in adult rodent DRG (Murakami et al., 2001; Westenbroek et al., 1998; Yusaf et al., 2001). Although six splice variants of Cav2.3 (Cav2.3a to Cav2.3f) have been identified in mammalian tissues, only Cav2.3a and Cav2.3e are detected in trigeminal and DRG neurons, with Cav2.3e being the predominant isoform (Fang et al., 2007; Fang et al., 2010). Single-cell RT-PCR revealed that the majority of Cav2.3e mRNA was confined to tyrosine kinase receptor A (tkrA) positive/isolectin B4 (IB4)-negative and TRPV1-positive neurons (Fang et al., 2007; Fang et al., 2010). These neurons are known to be peptidergic nociceptors. The same laboratory further demonstrated that Cav2.3e mRNA expression was associated with SNX-482-sensitive R-type calcium currents in trigeminal neurons (Fang et al., 2007). Although a direct link between Cav2.3e and neurotransmitter release from peptidergic nociceptors has not been established, the previous evidence strongly supported such a possibility. In this study, we use intraplantar formalin to elicit spinal release of SP in the spinal cord, which leads to significant NK1r internalization in dorsal horn neurons. Our results clearly showed that SNX482, the selective R-type VSCC blocker, strongly attenuated NK1r internalization. The data suggested there is a high probability that Cav2.3e, as does Cav 2.2 in previous work, plays a major role in noxious stimuli-induced presynaptic neurotransmitter release from peptidergic primary afferents.
4.3 Formalin evoked flinching
Formalin injected subcutaneously into the paw results in an immediate and intense increase in the primary afferents activity and paw flinching behavior. The flinching displays a biphasic time course. Phase 1 (0 – 10min) is considered to reflect the direct effect of algogen on nociceptors and phase 2 (11 – 60 min) is a reflection of a spinal sensitization (Dubuisson and Dennis, 1978; Puig and Sorkin, 1996; Tjolsen et al., 1992). In the current study, IT SNX-482 produced a significant inhibitory effect upon both phase 1 and phase 2 flinching, at a dose that produced minimal motor and behavior side effects. The result is in agreement with previous mouse studies that Cav2.3 knockout (Saegusa et al., 2000) or intrathecal SNX-482 (Murakami et al., 2004) attenuated formalin-induced pain behavior in phase 2. Further, spinal SNX-482 attenuated thermal and mechanical pain hypersensitivity in a neuropathic pain model (Matthews et al., 2007). However, the role of Cav2.3 in pain could be complicated. In the same paper, Murakami et al. also demonstrated that SNX-482 increased phase 1 flinching (Murakami et al., 2004). The hypersensitivity was only observed at high doses and thought to reflect the attenuation of the inhibitory effects of antinociceptive pathways. In certain neurons, Cav2.3 could act to attenuate neuronal excitability by inhibiting activation of N-methyl-D-aspartate (NMDA) receptors (Bloodgood and Sabatini, 2007). Cav2.3 might also reduce the induction of long-term potentiation by producing trains of back propagating action potentials (Yasuda et al., 2003). More studies are required to elucidate the role of Cav2.3 in pain.
4.4 Formalin-evoked dorsal horn c-Fos
The proto-oncogene c-Fos is rapidly induced in spinal dorsal horn neurons following noxious stimuli, such as formalin injection to the paw, and commonly used as a maker for neural activation (Hunt et al., 1987). There is a positive correlation between c-Fos expression and formalin-evoked pain behavior (Gogas et al., 1996). Two hours after formalin injection, c-Fos was observed throughout the ipsilateral lumbar dorsal horn. This pattern of neuron activation correlated with peripheral input from both C-fiber and A-fiber, the majority of which terminate in the superficial and deep dorsal horn respectively. It is well established that paw formalin activates both groups of afferents (Puig and Sorkin, 1996). In the current study, we noted for the first time that intrathecal SNX-482 reduced formalin-evoked c-Fos in ipsilateral dorsal horn in laminae I/II, but not laminae III/V. Our observation supports a role of Cav2.3 in nociceptors but not in the deeply projecting large afferents A-fibers. This result is also in agreement with an electrophysiology study in which SNX-482 inhibited spinal neuronal responses evoked by monosynaptic C- and Aδ-fiber, but not Aβ-fiber, in neuropathic rat (Matthews et al., 2007).
In the current study, intrathecal SNX-482 at the analgesic dose (0.5 µg) produced some minor motor dysfunction and adverse effects, which occurred at a very low rate and disappeared within 2 hours. However, a higher dose (4.6 µg) produced severe motor dysfunction and other adverse behaviors. These effects could be attributed to Cav2.3 in the spinal motor neurons in the ventral horn (Murakami et al., 2004; Saegusa et al., 2000; Westenbroek et al., 1998) and/or neurons in supraspinal sites. Indeed, Cav2.3 knockout mice exhibited reduced spontaneous locomotor activities and signs of “timidity” (Saegusa et al., 2000a).
4.5 Spinal P/Q-type calcium channels
Although there have been some reports regarding P/Q-type calcium channels, the analgesic effects of the blockers are controversial. Previous studies have shown that intrathecal delivery of the antagonist for P/Q-type VSCCs, ω-agatoxin IVA, produced anti-nociceptive effects on formalin-evoked pain behavior (Malmberg and Yaksh, 1994; Murakami et al., 2004) and burn-induced hyperalgesia. Conversely, several spinal ω-agatoxin IVA studies have failed to note anti-nociceptive effects on high-threshold thermal stimuli (Malmberg and Yaksh, 1994), tactile allodynia (Chaplan et al., 1994) or in a hyperalgesia model (Yamamoto and Sakashita, 1998). Despite an early report by our group (Malmberg and Yaksh, 1994), we could not in the present detect any effects of ω-agatoxin IVA on formalin-evoked flinching behavior or c-Fos expression. Whether this was due to differences in animal strain, method of data collection, difference in the ω-agatoxin IVA used and/or a narrow therapeutic window of ω-agatoxin IVA is not known. Correspondence with the supplier indicated data showing purity and appropriate amino acid content (Dr. Watanabe from Peptide Institute, Inc.). We noticed that doses exceeding 0.5 µg caused severe side effects such as involuntary spasm, sudden burst of scratching, jumping followed by motor weakness and depression of breathing, symptoms also reported in previous studies (Malmberg and Yaksh, 1994; Sorkin et al., 2008). The motor side effects clearly show that the test article had biological activity and are consistent with the distribution of P/Q-type (α1A) calcium channel subunits in the ventral horn motor neurons (Kim et al., 2001; Murakami et al., 2004).
In conclusion, we demonstrate that spinal administration of R-type calcium channel blocker attenuated formalin-induced pain behavior, NK1r internalization and c-Fos expression in the superficial dorsal horn. This study strongly supports a role for Cav2.3 in presynaptic neurotransmitter release from peptidergic nociceptive afferents. Our study does not exclude a role for Cav2.3 in regulating post-synaptic dorsal horn neurons. Some conflicts with previous reports warrant further study for the involvement of VSCCs in pain.
Highlights.
IT SNX-482 (R-type antagonist) blocked formalin-evoked flinches in phase 1 and 2.
IT SNX-482 blocked formalin-induced spinal dorsal horn NK1 receptor internalization.
IT SNX-482 blocked formalin-induced spinal dorsal horn c-Fos expression.
Acknowledgments
The authors thank Arbi Nazarian, Ph.D. for his assistance in setting up the internalization protocol. The work was supported by DA02110.
Funding Support:
This research was supported by funding from the National Institutes of Health: NIH-DA02110, Bethesda, MD (TLY).
Abbreviations
- CGRP
calcitonin gene-related peptide
- GPCR
G protein-coupled receptor
- DRG
dorsal root ganglia
- IB4
isolectin B4
- IT
Intrathecal
- NK1r
neurokinin 1 receptor
- NMDA
N-methyl-D-aspartate
- SP
substance P
- trkA
tropomyosin receptor kinase A
- TRPV1
transient receptor potential protein vanilloid 1
- IR
immunoreactive
- VSCCs
voltage-sensitive calcium channels
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
Contributor Information
Tetsuji Terashima, Assistant Professor, Department of Anesthesiology, Dokkyo Medical University, School of Medicine, Kitakobayashi 880, Mibu, Tochigi 321-0293, Japan Department of Anesthesiology,University of California, San Diego, 9500 Gilman Drive,San Diego, La Jolla, California. 92093-0818, youtei3@gmail.com.
Qinghao Xu, Postdoctoral scholar, Department of Anesthesiology, University of California, San Diego, 9500 Gilman Drive,San Diego, La Jolla, California. 92093-0818, q1xu@ucsd.edu
Shigeki Yamaguchi, Professor and Chairman, Department of Anesthesiology, Dokkyo Medical University, School of Medicine, Kitakobayashi 880, Mibu, Tochigi 321-0293, Japan, shigeki@dokkyomed.ac.jp
Tony L. Yaksh, Professor of Anesthesiology and Pharmacology, Department of Anesthesiology, University of California, San Diego, 9500 Gilman Dnve,San Diego, La Jolla, California. 92093-0818, tyaksh@ucsd.edu
Reference
- Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI. Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. J Neurosci. 1997;17:8049–8060. doi: 10.1523/JNEUROSCI.17-20-08049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams ME. Agatoxins: ion channel specific toxins from the american funnel web spider, Agelenopsis aperta. Toxicon. 2004;43:509–525. doi: 10.1016/j.toxicon.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) British journal of pharmacology. (3rd edition) 2008;153(Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber LA, Vasko MR. Activation of protein kinase C augments peptide release from rat sensory neurons. J Neurochem. 1996;67:72–80. doi: 10.1046/j.1471-4159.1996.67010072.x. [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Nonlinear regulation of unitary synaptic signals by CaV(2.3) voltage-sensitive calcium channels located in dendritic spines. Neuron. 2007;53:249–260. doi: 10.1016/j.neuron.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Expression of C-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. The Journal of comparative neurology. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Carbone E, Lux HD. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984;310:501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Pogrel JW, Yaksh TL. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J Pharmacol Exp Ther. 1994;269:1117–1123. [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1978;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- Evans AR, Nicol GD, Vasko MR. Differential regulation of evoked peptide release by voltage-sensitive calcium channels in rat sensory neurons. Brain Res. 1996;712:265–273. doi: 10.1016/0006-8993(95)01447-0. [DOI] [PubMed] [Google Scholar]
- Fang Z, Hwang JH, Kim JS, Jung SJ, Oh SB. R-type Calcium Channel Isoform in Rat Dorsal Root Ganglion Neurons. Korean J Physiol Pharmacol. 2010;14:45–49. doi: 10.4196/kjpp.2010.14.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Park CK, Li HY, Kim HY, Park SH, Jung SJ, Kim JS, Monteil A, Oh SB, Miller RJ. Molecular basis of Ca(v)2.3 calcium channels in rat nociceptive neurons. J Biol Chem. 2007;282:4757–4764. doi: 10.1074/jbc.M605248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–141. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Gogas K, Cho H, Botchkina G, Levine J, Basbaum A. Inhibition of noxious stimulus-evoked pain behaviors and neuronal fos-like immunoreactiivity in the spinal cord of the rat by supraspinal morphine. Pain. 1996;65:9–15. doi: 10.1016/0304-3959(95)00141-7. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Ito K, Rome C, Bouleau Y, Dulon D. Substance P mobilizes intracellular calcium and activates a nonselective cation conductance in rat spiral ganglion neurons. Eur J Neurosci. 2002;16:2095–2102. doi: 10.1046/j.1460-9568.2002.02292.x. [DOI] [PubMed] [Google Scholar]
- Kim DS, Yoon CH, Lee SJ, Park SY, Yoo HJ, Cho HJ. Changes in voltage-gated calcium channel α1 gene expression in rat dorsal root ganglia following peripheral nerve injury. Molecular brain research. 2001;96:151–156. doi: 10.1016/s0169-328x(01)00285-6. [DOI] [PubMed] [Google Scholar]
- Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:3651–3660. doi: 10.1523/JNEUROSCI.0252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik A, Nakadate K, Hagiwara A, Fukazawa Y, Luján R, Saito H, Suzuki N, Futatsugi A, Mikoshiba K, Frotscher M. Immunocytochemical localization of the α1A subunit of the P/Q-type calcium channel in the rat cerebellum. European Journal of Neuroscience. 2004;19:2169–2178. doi: 10.1111/j.0953-816X.2004.03319.x. [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Nielsen KJ, Craik DJ, Loughnan ML, Adams DA, Sharpe IA, Luchian T, Adams DJ, Bond T, Thomas L, Jones A, Matheson JL, Drinkwater R, Andrews PR, Alewood PF. Novel omega-conotoxins from Conus catus discriminate among neuronal calcium channel subtypes. J Biol Chem. 2000;275:35335–35344. doi: 10.1074/jbc.M002252200. [DOI] [PubMed] [Google Scholar]
- Long I, Ahmad AH, Ismail Z. The differential response of acute swim stress on c-Fos expression on the ipsilateral and contralateral sides in the rat spinal cord after formalin-induced pain. J Physiol. 2012;25:13–18. [Google Scholar]
- Maggi CA, Giuliani S, Santicioli P, Tramontana M, Meli A. Effect of omega conotoxin on reflex responses mediated by activation of capsaicin-sensitive nerves of the rat urinary bladder and peptide release from the rat spinal cord. Neuroscience. 1990;34:243–250. doi: 10.1016/0306-4522(90)90318-x. [DOI] [PubMed] [Google Scholar]
- Malkmus SA, Yaksh TL. Intrathecal catheterization and drug delivery in the rat. Methods in molecular medicine. 2004;99:109–121. doi: 10.1385/1-59259-770-X:011. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Voltage-sensitive calcium channels in spinal nociceptive processing: blockade of N- and P-type channels inhibits formalin-induced nociception. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:4882–4890. doi: 10.1523/JNEUROSCI.14-08-04882.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW. Neurobiology of substance P and the NK1 receptor. The Journal of clinical psychiatry. 2002;63:6. [PubMed] [Google Scholar]
- Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:2622–2626. doi: 10.1073/pnas.92.7.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Marvizon J, Wang X, Matsuka Y, Neubert J, Spigelman I. Relationship between capsaicin-evoked substance P release and neurokinin 1 receptor internalization in the rat spinal cord. Neuroscience. 2003;118:535–545. doi: 10.1016/s0306-4522(02)00977-6. [DOI] [PubMed] [Google Scholar]
- Matthews EA, Bee LA, Stephens GJ, Dickenson AH. The Cav2.3 calcium channel antagonist SNX-482 reduces dorsal horn neuronal responses in a rat model of chronic neuropathic pain. Eur J Neurosci. 2007;25:3561–3569. doi: 10.1111/j.1460-9568.2007.05605.x. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Venema VJ, Swiderek KM, Lee TD, Bean BP, Adams ME. P-type calcium channels blocked by the spider toxin ω-Aga-IVA. 1992 doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Murakami M, Nakagawasai O, Suzuki T, Mobarakeh II, Sakurada Y, Murata A, Yamadera F, Miyoshi I, Yanai K, Tan-No K, Sasano H, Tadano T, Iijima T. Antinociceptive effect of different types of calcium channel inhibitors and the distribution of various calcium channel alpha 1 subunits in the dorsal horn of spinal cord in mice. Brain Res. 2004;1024:122–129. doi: 10.1016/j.brainres.2004.07.066. [DOI] [PubMed] [Google Scholar]
- Murakami M, Suzuki T, Nakagawasai O, Murakami H, Murakami S, Esashi A, Taniguchi R, Yanagisawa T, Tan-No K, Miyoshi I, Sasano H, Tadano T. Distribution of various calcium channel alpha(1) subunits in murine DRG neurons and antinociceptive effect of omega-conotoxin SVIB in mice. Brain Res. 2001;903:231–236. doi: 10.1016/s0006-8993(01)02427-1. [DOI] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1996;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Puig S, Sorkin L. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Saegusa H, Kurihara T, Zong S, Minowa O, Kazuno A, Han W, Matsuda Y, Yamanaka H, Osanai M, Noda T, Tanabe T. Altered pain responses in mice lacking alpha 1E subunit of the voltage-dependent Ca2+ channel. Proc Natl Acad Sci U S A. 2000;97:6132–6137. doi: 10.1073/pnas.100124197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santicioli P, Del Bianco E, Tramontana M, Geppetti P, Maggi CA. Release of calcitonin gene-related peptide like-immunoreactivity induced by electrical field stimulation from rat spinal afferents is mediated by conotoxin-sensitive calcium channels. Neurosci Lett. 1992;136:161–164. doi: 10.1016/0304-3940(92)90039-a. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Doom CM, Maruyama KP, Nanigian DB. Secondary hyperalgesia in the rat first degree burn model is independent of spinal cyclooxygenase and nitric oxide synthase. European journal of pharmacology. 2008;587:118–123. doi: 10.1016/j.ejphar.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- Takasusuki T, Yaksh TL. Regulation of Spinal Substance P Release by Intrathecal Calcium Channel Blockade. Anesthesiology. 2011;115:153–164. doi: 10.1097/ALN.0b013e31821950c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Puskar Z, Spike RC, Hughes C, Watt C, Forrest L. Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance p-containing afferents and respond to noxious stimulation. J Neurosci. 2002;22:4103–4113. doi: 10.1523/JNEUROSCI.22-10-04103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MO, Ren K, Sablad M, Park KT. Medullary N-type and P/Q-type calcium channels contribute to neuropathy-induced allodynia. Neuroreport. 2005;16:563. doi: 10.1097/00001756-200504250-00009. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci. 1998;18:6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Jessell TM, Gamse R, Mudge AW, Leeman SE. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980;286:155–157. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, Yaksh MC. An automated flinch detecting system for use in the formalin nociceptive bioassay. Journal of applied physiology. 2001;90:2386–2402. doi: 10.1152/jappl.2001.90.6.2386. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiology & behavior. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sakashita Y. Differential effects of intrathecally administered N-and P-type voltage-sensitive calcium channel blockers upon two models of experimental mononeuropathy in the rat. Brain research. 1998;794:329–332. doi: 10.1016/s0006-8993(98)00306-0. [DOI] [PubMed] [Google Scholar]
- Yasuda R, Sabatini BL, Svoboda K. Plasticity of calcium channels in dendritic spines. Nat Neurosci. 2003;6:948–955. doi: 10.1038/nn1112. [DOI] [PubMed] [Google Scholar]
- Yusaf SP, Goodman J, Pinnock RD, Dixon AK, Lee K. Expression of voltage-gated calcium channel subunits in rat dorsal root ganglion neurons. Neurosci Lett. 2001;311:137–141. doi: 10.1016/s0304-3940(01)02038-9. [DOI] [PubMed] [Google Scholar]