Abstract
The progression from in situ to invasive breast carcinoma is still an event poorly understood. However, it has been suggested that interactions between the neoplastic cells and the tumor microenvironment may play an important role in this process. Thus, the determination of differential tumor-stromal metabolic interactions could be an important step in invasiveness.
The expression of stromal Caveolin-1 (Cav-1) has already been implicated in the progression from ductal carcinoma in situ (DCIS) to invasive ductal carcinoma (IDC). Additionally, stromal Cav-1 expression has been associated with the expression of stromal monocarboxylate transporter 4 (MCT4) in invasive breast cancer. However, the role of stromal MCT4 in invasiveness has never been explored, neither the association between Cav-1 and MCT4 in the transition from breast DCIS to IDC.
Therefore, our aim was to investigate in a series of breast cancer samples including matched in situ and invasive components, if there was a relationship between stromal Cav-1 and MCT4 in the progression from in situ to invasive carcinoma. We found loss of stromal Cav-1 in the progression to IDC in 75% of the cases. In contrast, MCT4 stromal expression was acquired in 87% of the IDCs. Interestingly, a concomitant loss of Cav-1 and gain of MCT4 was observed in the stroma of 75% of the cases, when matched in situ and invasive carcinomas were compared. These results suggest that alterations in Cav-1 and MCT4 may thus mark a critical point in the progression from in situ to invasive breast cancer.
Keywords: Caveolin-1, DCIS, IDC, MCT4, breast cancer, immunohistochemistry, stroma, tumor progression
Introduction
Breast cancer is a heterogeneous and complex disease, encompassing a variety of pathological entities with distinct clinical behaviors. The development of new technologies has offered the opportunity to explore the molecular complexity of human breast carcinomas.1 However, despite these advances, the mechanisms controlling the transition from an in situ to an invasive carcinoma still remain unclear. Therefore, there is a significant interest in identifying molecular events driving invasive progression, not only to determine at which point the lesion is most likely to progress to malignancy, but also to identify new molecular targets that could trigger the progression at early stages.1 Several studies have evaluated the gene expression profiles of both ductal carcinomas in situ (DCIS) and invasive ductal carcinomas (IDC),2-8 but only few compared the in situ and invasive components within the same breast tumor.5-8 In fact, although some genes have been described as differentially expressed between in situ and invasive components, the majority of the studies failed to demonstrate significant differences between the expression of the codified proteins in the neoplastic epithelial cells of DCIS and IDC.5,9 Recently, our group, using patient-matched DCIS/IDC tumor samples, showed concordance between in situ and invasive molecular profiles in 94% of the cases.10 These results suggested that the alterations in the tumor microenvironment would have a more important role in the progression from an in situ to an invasive phenotype than the biology of the tumor cells per se, which showed a tendency to be maintained between these both components.
Actually, it is widely accepted that any cancer is a complex system composed not only by neoplastic cells but also by a fine-tuned microenvironment. The first reference to the importance of the microenvironment in cancer comes from Paget, with his proposal of the “seed and soil” hypothesis. Unexpectedly, this concept was “forgotten” and only “recovered” several years later. In breast cancer, tumor microenvironment plays a key role in defining tumor behavior and patient outcome.11 Gene expression changes that occur in cancer-associated stroma are known to be implicated in prognosis, as well as in cancer progression.12-14 Ma and colleagues, using gene expression profiling, provided strong evidence that the stroma co-evolves with the epithelial compartments during cancer progression.12 Analyzing 14 patients with matched normal epithelium, normal stroma, tumor epithelium, and tumor-associated stroma, the authors proposed that microenvironment participates in tumorigenesis even before tumor cells invade the stroma, and it may play an important role in the transition from pre-invasive to invasive growth.12
Caveolin-1 (Cav-1), a scaffolding protein mainly involved in vesicular transport, cholesterol homeostasis, and signal transduction, has been associated to the progression from in situ to invasive carcinoma.15,16 Lisanti and colleagues showed that Cav-1 loss in tumor stroma was associated with an increased risk for early recurrence, metastasis, and decreased overall survival in breast cancer, being also a strong prognostic factor for basal-like breast carcinomas.17,18 In DCIS, a loss of stromal Cav-1 was predictive of disease recurrence and progression to invasive cancer, since all the patients with loss of Cav-1 recurred, and 80% of them progressed to invasive disease.16 Moreover, loss of stromal Cav-1 has been related with stromal MCT4 expression in triple-negative breast cancers, also predicting for poor clinical outcome.19 This protein is a major transporter directly responsible for L-lactate efflux from glycolytic cells and a functional marker of oxidative stress and hypoxia.20 In addition, it seems to have a role in stromal breast cancer metabolism, since it has been demonstrated that breast cancer cells induce MCT4 overexpression in stromal fibroblasts.21
Since stromal expression of MCT4 and the association between Cav-1 and MCT4 had never been implicated in the progression from DCIS to IDC, the aim of this study was to better understand the stromal interactions surrounding in situ and invasive components of breast carcinomas, evaluating the stromal expression of Cav-1 and MCT4 using patient-matched DCIS/IDC tumor samples.
Results
IHC quantification for Cav-1 and MCT4 was performed on each set of the 22 TMA slides using patient-matched DCIS/IDC tumor samples. Data on ER, PgR, HER-2, P-cad, CK5, EGFR, Ki-67 status, histological grade, and lymph node metastases were already available and published for this series.10
Cav-1 and MCT4 expression in normal breast
In normal breast, it can be observed that Cav-1 expression was absent from the epithelium, whereas its expression was observed in the stromal component, as previously described.16-18 MCT4 expression was absent in both epithelial and stromal components, as observed in Figure 1A.
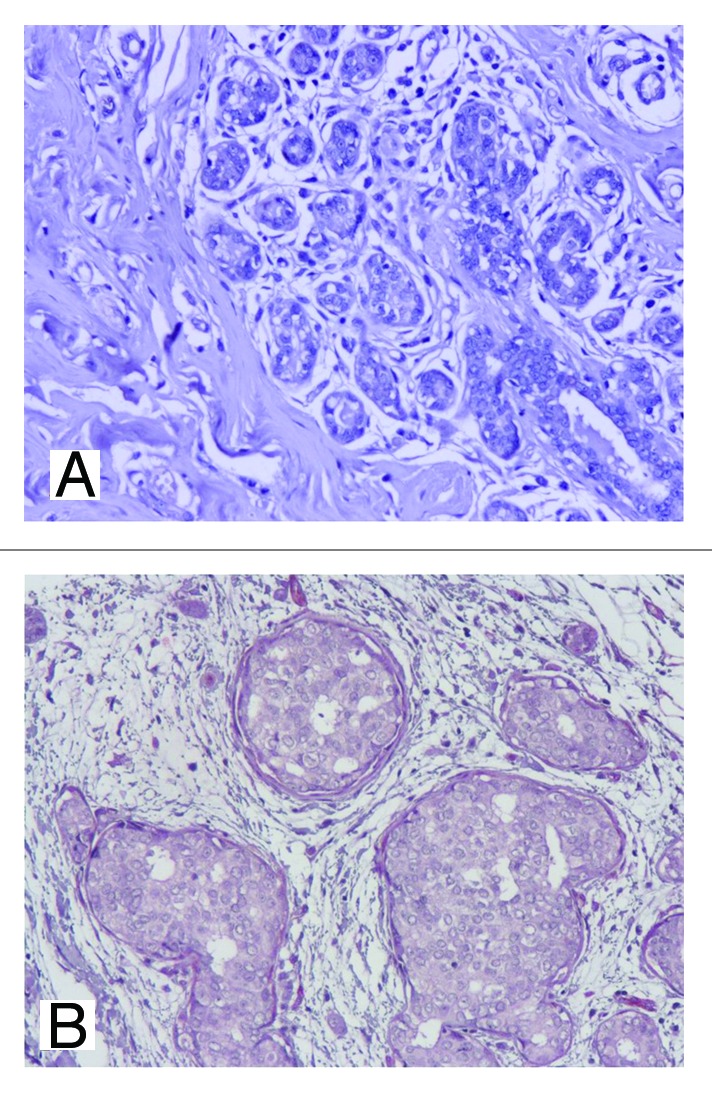
Figure 1. IHC expression of stromal MCT4 in normal and in situ component. Absent stromal MCT4 expression can be observed in normal breast (A) and in in situ component (B), 200×.
Stromal Cav-1 expression in the progression from in situ to invasive carcinoma
In the DCIS component, only 19 cases (13%) showed no Cav-1 expression in the stroma, whereas 55 cases (39%) had moderate expression, and the majority had strong expression of stromal Cav-1 (67 cases, 48%). In the invasive component, the majority (n = 108, 76%) of the cases showed absent Cav-1 expression in the stroma, with only 27 cases (19%) with moderate expression and 7 cases (5%) with strong expression. Figure 2 represents the expression levels of stromal Cav-1 in in situ and invasive components, where a significant decrease of Cav-1 from DCIS to IDC can be observed. An IHC example of Cav-1in in situ and invasive components is shown in Figure 3.
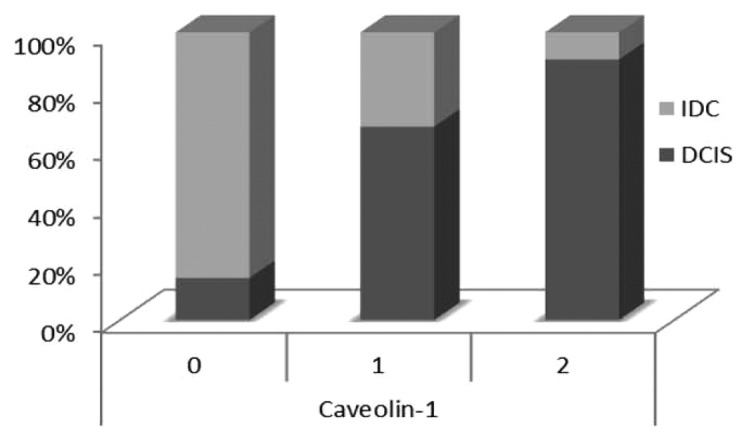
Figure 2. Expression levels of stromal Cav-1 in in situ and invasive components of breast carcinomas. It is possible to notice a significant decrease of Cav-1 stromal expression from DCIS to IDC.
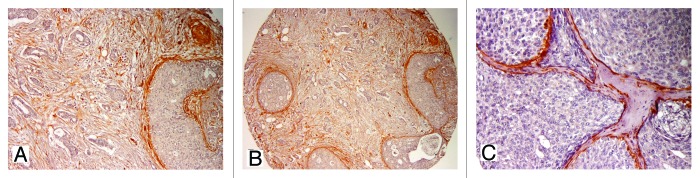
Figure 3. IHC expression of stromal Cav-1 in in situ and invasive components. Note the strong expression of Cav-1 in DCIS, from low (A and B, 100× and 200×, respectively) to higher magnification (C, 400×).
Regarding the progression from in situ to invasive carcinoma, analyzing each case for both matched components, 106 cases (75%) showed loss of stromal Cav-1 expression, whereas 35 (25%) cases maintained protein expression. None of the cases showed gain of stromal Cav-1 expression.
Stromal MCT4 expression in the progression from in situ to invasive carcinoma
Considering the DCIS component, the majority of the cases were negative (n = 131, 93%) (Fig. 1B), 10 cases (7%) showed moderate expression, and 5 cases (3%) were classified as strong for stromal MCT4. In the invasive component, a strong expression of MCT4 in the stroma of the majority of the cases (n = 73, 50%) was observed, whereas moderate expression was observed in 63 (43%) cases; in the remaining 11 cases (7%), no expression of stromal MCT4 was observed.
Figure 4 depicts the expression levels of stromal MCT4 in situ and invasive components, showing an increased expression of stromal MCT4 in the invasive component. Figure 5 represents by IHC the strong MCT4 stromal expression in invasive component.
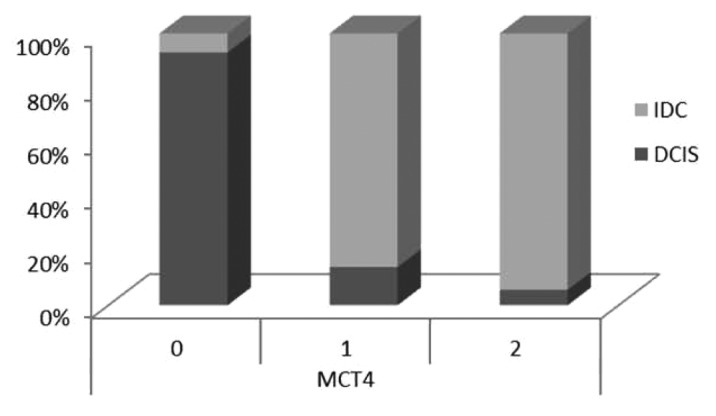
Figure 4. Expression levels of stromal MCT4 in in situ and invasive carcinomas. There is a significant increased expression of stromal MCT4 in the invasive component of breast carcinomas, when compared with DCIS.
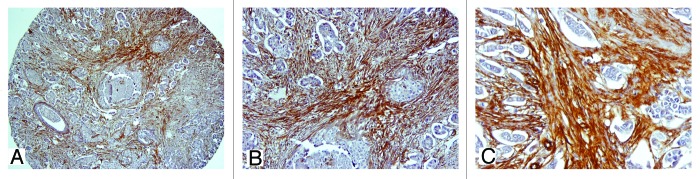
Figure 5. IHC expression of stromal MCT4 in in situ and invasive components. Note the strong MCT4 stromal expression in invasive component, from low (A and B, 100× and 200×, respectively) to high magnification (C, 400×).
Concerning the transition from in situ to invasive carcinoma in terms of gains and losses of MCT4 in the stroma, we found that 126 cases (87%) gained expression in the invasive component, 19 cases (13%) maintained, and none lose the expression.
Combining stromal Cav-1/MCT4 in the progression from in situ to invasive carcinoma
Analyzing matched in situ and invasive components for stromal expression of Cav-1 and MCT4 (Table 1), it was possible to observe a statistically significant association between the loss of stromal Cav-1 and the concomitant gain of MCT4 in the same case (P < 0.0001). Interestingly, 75% of the cases that lost Cav-1 stromal expression in the transition from in situ to invasive cancer also gained MCT4 expression in the stroma. There were only 4 cases (3%) with loss of Cav-1 in the stroma that maintained MCT4 expression and 16 cases (12.5%) that gained MCT4 and maintained Cav-1 stromal expression. In 12 cases (10%), there was the maintenance of stromal expression for both markers. Figure 6 represents an IHC array with the expression levels of these proteins in the progression from in situ to invasive carcinoma.
Table 1. Association between stromal Cav-1 and MCT4 expression levels in the transition from in situ to invasive breast carcinoma.
| MCT4 (in situ to invasive) | ||||
|---|---|---|---|---|
| |
|
Loss of expression N (%) |
Maintenance of expression N (%) |
Gain of expression N (%) |
| Cav-1 (in situ to invasive) | Loss of expression N (%) |
0 (0%) |
4 (3%) |
94 (75%) |
| Maintenance of expression N (%) |
0 (0%) |
12 (10%) |
16 (12.5%) |
|
| Gain of expression N (%) | 0 (0%) | 0 (0%) | 0 (0%) | |
P value ≤ 0.001
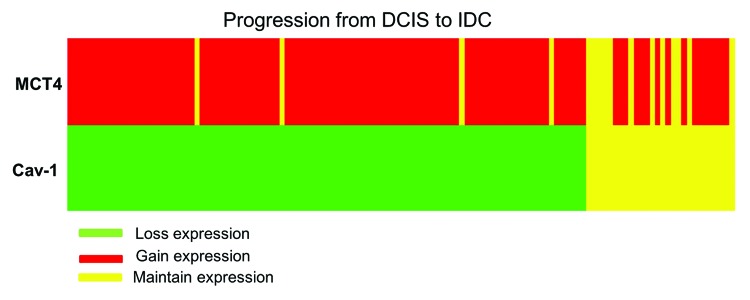
Figure 6. Immunohistochemistry array showing protein expression levels of stromal Cav-1 and MCT4 in the progression from in situ to invasive carcinomas. Cases are arranged along the x-axis and proteins are arranged along the y-axis. Within the heat map, red represents gain of expression, green represents loss of expression, and yellow represents maintained expression from in situ to invasive carcinoma within the same case.
Discussion
The mechanisms that mediate the progression from DCIS to IDC in the breast are still largely unknown. However, it is now widely acknowledged that accumulation of genetic anomalies contributes to the acquisition of an increasingly aggressive, invasive, or therapy-resistant tumor phenotype.1 Nevertheless this knowledge did not improve the predictive power of standard pathological parameters for breast cancer, nor did it explain the mechanisms of invasiveness.
Cav-1 plays an important role in tumor stroma, and recent studies demonstrate that the loss of stromal Cav-1 is associated with advanced tumor and nodal stage, lymphovascular invasion, metastasis, early recurrence, tamoxifen resistance, and reduced progression-free survival in invasive breast cancer.23-25 Additionally, loss of stromal Cav-1 also has prognostic value in a particularly aggressive subgroup of breast cancers, namely the triple-negative and basal-like breast carcinomas, whereas high levels of this protein were correlated with reduced tumor size, low grade, reduced metastasis, and improved survival.18,25,26
Interestingly, loss of stromal Cav-1 also predicts for recurrence and early disease progression in DCIS patients. Witkiewicz et al. reported that 80% of the DCIS patients, which underwent surgical excision and recurred with invasive breast cancers, showed reduced or absent levels of stromal Cav-1 in these tumors.16 In our series, using patient-matched DCIS/IDC tumor samples, it was observed that the majority of the cases showed strong expression of Cav-1 expression in the stroma of DCIS, whereas 76% of the cases showed absent expression for this marker in the stroma of the invasive counterpart. Thus, regarding the progression to invasiveness, it seems that the loss of Cav-1 expression in the stroma is important for tumor invasion.
Actually, it has been already described that loss of Cav-1 in stromal cells may also increase angiogenesis and tumor growth.15 Goetz et al. demonstrated that in vivo and in vitro expression of Cav-1 in cancer-associated fibroblasts facilitates tumor cells invasion and accelerates the in vitro proliferation and in vivo tumorigenesis.27,28
Recent data reveals that loss of Cav-1 induces a metabolic reprogramming of stromal cells to support the growth of adjacent epithelial tumor cells—the “reverse Warburg effect”, where cancer cells induce upregulation of multiple glycolytic enzymes in neighboring stromal fibroblasts.23,29,30 Cav-1 is degraded resulting in a loss of stromal Cav-1 expression.19 At the same time, the breast cancer cells induce MCT4 overexpression in stromal fibroblasts.19
MCT4 is a monocarboxylate transporter that functions as a shuttle to extrude L-lactate from cells using aerobic glycolysis for energy metabolism.20 Although the transporter role of MCT4 has been widely accepted in cancer epithelium, the prognostic value of MCT4 expression is highly compartment-specific and restricted to the tumor stroma, high stromal MCT4 levels being associated to poor patient overall survival.21,31,32 In our series, analyzing DCIS and IDC separately, an increase of MCT4 expression was observed, since in DCIS the majority of the cases were negative, whereas, in the invasive counterpart, 50% of the cases showed strong expression for MCT4. Considering the progression from in situ to invasive breast carcinoma, using matched DCIS/IDC tumor samples, 87% cases gained MCT4 expression, whereas none showed loss of expression, suggesting that the gain of stroma MCT4 provides evidence for the existence of a stromal–epithelial lactate shuttle which fuels the tumor growth.21
Regarding the relation between MCT4 and Cav-1 expression, Witkiewicz et al.,19 using 164 invasive breast cancer samples, verified that stromal MCT4 and stromal Cav-1 levels were inversely related, high levels of stromal MCT4 being directly correlated with a loss of stromal Cav-1 immunostaining.19 Most notably, cases with absent stromal Cav-1 are most likely to present strong stromal staining for MCT4, and, in contrast, cases with strong expression for Cav-1 are most likely to be stromal MCT4 absent.
Nevertheless, studies regarding the role of Cav-1 and MCT4 in the transition from in situ to invasive breast carcinoma were still lacking. In our series, using matched DCIS/IDC and analyzing the concomitant expression of stromal Cav-1 and MCT4, 75% of the cases showed loss of Cav-1 with simultaneous gain of MCT4 in the stroma, suggesting that these events are important for tumor cells to progress and invade.
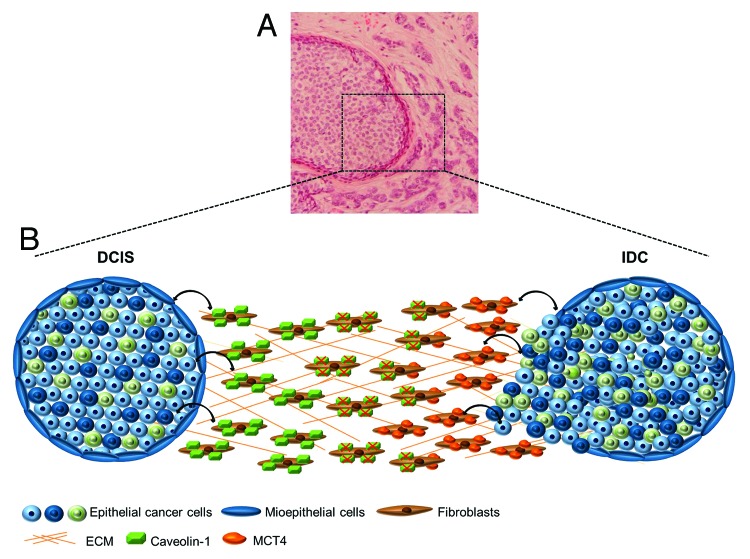
Our results are explained by the recent “two-compartment tumor metabolism” model and the “reverse Warburg effect”, suggesting that the loss of Cav-1 causes the metabolic reprogramming of stromal cells to support the growth of adjacent epithelial tumor cells.23 In Figure 7, a hypothetical model summarizing he alterations in Cav-1 and MCT4 in the stroma of matched in situ and invasive breast carcinoma is shown.
Figure 7. Alterations in Cav-1 and MCT4in the stroma of matched in situ and invasive breast carcinoma. (A) H&E-stained tissue section of human breast cancer, showing in situ and invasive components of breast carcinoma (100×). (B) Hypothetical model summarizing the importance of Cav-1 and MCT4 in the progression from DCIS to IDC. During the progression to invasive carcinoma, Cav-1 is degraded by oxidative stress-induced autophagy in cancer-associated fibroblasts, resulting in a loss of Cav-1. At the same time, the loss of Cav-1 induces a metabolic reprogramming of stromal cells, where cancer cells induce upregulation of MCT4 by stromal fibroblasts, in invasive counterpart.
The oxidative stress promoted by the tumor cells induces autophagy in cancer-associated fibroblasts (CAFS) that degrade Cav-1 in the in situ stromal compartment and also secrete energy-rich metabolites, such as L-lactate, ketone bodies, and pyruvate as a consequence of metabolic alterations. During the progression to invasive carcinoma, the loss of Cav-1 induces MCT4 expression due to the amount of energy metabolites, used to promote cancer cell glycolysis, aggressive tumor growth, and, ultimately, invasion of breast cancer cells.
Many of the cited studies quantify one or both markers in breast cancer stroma. However, one potential limitation of the quantification methodologies used is the lack of a clear and reproducible definition of stroma, especially regarding DCIS cases. In our case, since all IHC scoring was performed by the same experienced pathologist, we consider this does not affect internal validity and therefore does not affect the results obtained and conclusions drawn.
In summary, it was shown that the loss of stromal Cav-1 and the concomitant gain of stromal MCT4 have a putative role in the transition from in situ to invasive carcinoma of the breast. Therefore, we propose that Cav-1 and MCT4 may represent valuable biomarkers for breast cancer progression. Thus, determining the nature of the cooperation between tumor cells and the microenvironment that leads to invasion could identify therapeutic strategies to prevent the transition from in situ to invasive breast carcinoma.
Material and Methods
Case selection and TMA (tissue microarray) construction
Formalin-fixed and paraffin-embedded samples from 189 tumors, harboring in situ and invasive carcinoma areas in the same block, were consecutively retrieved from our archives. Available data included patient’s age and clinicopathological features, such as tumor size and lymph nodes status. Representative areas of the in situ and invasive breast carcinomas were selected on H&E-stained sections and marked on the correspondent individual paraffin block. Two tissue cores (2 mm in diameter) were obtained from each specimen for TMA construction with each TMA block (donor block) and deposited into a paraffin block (receptor block) using a TMA workstation (TMA builder ab1802, Abcam). In each TMA block, non-neoplastic breast and liver tissue cores were also included as controls and TMA guide, respectively. An H&E-stained section from each TMA block was reviewed to confirm the presence of morphological representative areas of the original lesions.
All morphological and IHC assessments were conducted by a pathologist (FS). The study was conducted under the national regulative law for the handling of biological specimens from tumor banks, the samples being exclusively available for research purposes in retrospective studies.
Cav-1 and MCT4 immunohistochemistry
IHC was performed using the HRP labeled polymer (DakoCytomation) for Cav-1 and with the Ultravision Detection System Anti-polyvalent HRP (Lab Vision Corporation) for MCT4. Antigen unmasking was performed using a dilution of 1:100 from a commercially available solution of citrate buffer, pH = 6.0 (Vector Laboratories) at 98 °C. After the antigen retrieval procedure, the slides were washed in a phosphate buffer solution (PBS) and submitted to blockage of the endogenous peroxidase activity by incubation of the slides in a 3% hydrogen peroxide (Panreac) in methanol (Sigma-Aldrich). The slides were further incubated with the primary antibodies for Cav-1 (2297; BD Biosciences, diluted 1:50) and for MCT4 (H-90; Santa Cruz Biotechnology, diluted 1:500), as previously described.24 All reactions were revealed with diaminobenzidine (DAB) chromogen (DakoCytomation).
For both IHC assays, positive controls were included in each run, in order to guarantee the reliability of the assays. Non-neoplastic breast tissues, as well as normal breast surrounding the neoplastic cells, were considered internal controls.
Cav-1 and MCT4 immunohistochemistry evaluation
Cav-1 and MCT4 expression in stroma were evaluated using the previously described methodology.16-19,21 In summary, Cav-1 and MCT4 were semi-quantitatively scored as negative (0, no staining), weak (1, either diffuse weak or strong staining in less than 30% of stromal cells per core), or strong (2, defined as strong staining in 30% or more of the stromal cells).21
Statistical analysis
Statistical analyses were conducted using StatView 5.0 sofware (SAS Institute Inc). The associations between categorical variables were tested for statistical significance using the chi-square test. A two-tailed significance level of 5% was considered as statistically significant (P < 0.05).
Acknowledgments
Martins D was involved in the construction and characterization of the breast cancer series used in the study and performed the majority of the experimental work and drafted the manuscript. Beça FF and Sousa B have made substantial contributions to the analysis and interpretation of the data. Baltazar F performed some of the immunoassays. Schmitt F was the pathologist that evaluated the immunohistochemical reactions. Paredes J and Schmitt F participated in the design of the study and its coordination and helped to draft the manuscript. All authors had final approval of the submitted and published versions.
Financial Disclosure Statemens
This work was partially supported by research grants from Martins D (FCT-SFRH/BD/66152/2009); Sousa B (SFRH/BD/69353/2010); Paredes J (Ciência 2007: Contratação de Doutorados para o SCTN - financiamento pelo POPH - QREN - Tipologia 4.2 - Promoção do Emprego Científico, comparticipado pelo Fundo Social Europeu e por fundos nacionais do MCTES). IPATIMUP is an Associate Laboratory of the Portuguese Ministry of Science, Technology and Higher Education and is partially supported by FCT.
Glossary
Abbreviations:
- DCIS
ductal carcinoma in situ
- IDC
invasive ductal carcinoma
- Cav-1
caveolin-1
- MCT4
monocarboxylate transporter 4
- TMA
tissue microarray
- H&E
hematoxylin-eosin
- ER
estrogen receptor
- PR
progesterone receptor
- EGFR
epidermal growth factor receptor
- CK
cytokeratin
- P-cad
p-cadherin
- IHC
immunohistochemistry
- FISH
fluorescence in situ hybridization
Disclose of Potential Conflicts of interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/25794
References
- 1.Knudsen ES, Ertel A, Davicioni E, Kline J, Schwartz GF, Witkiewicz AK. Progression of ductal carcinoma in situ to invasive breast cancer is associated with gene expression programs of EMT and myoepithelia. Breast Cancer Res Treat. 2012;133:1009–24. doi: 10.1007/s10549-011-1894-3. [DOI] [PubMed] [Google Scholar]
- 2.Castro NP, Osório CA, Torres C, Bastos EP, Mourão-Neto M, Soares FA, et al. Evidence that molecular changes in cells occur before morphological alterations during the progression of breast ductal carcinoma. Breast Cancer Res. 2008;10:R87. doi: 10.1186/bcr2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 5.Schuetz CS, Bonin M, Clare SE, Nieselt K, Sotlar K, Walter M, et al. Progression-specific genes identified by expression profiling of matched ductal carcinomas in situ and invasive breast tumors, combining laser capture microdissection and oligonucleotide microarray analysis. Cancer Res. 2006;66:5278–86. doi: 10.1158/0008-5472.CAN-05-4610. [DOI] [PubMed] [Google Scholar]
- 6.Hanneman J, Velds A, Halfwerk JB, Kreike B, Peterse JL, van de Vijver MJ. Classification of ductal carcinoma in situ by gene expression profiling. Breast Cancer Res. 2006;8:R-61. doi: 10.1186/bcr1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagaraja GM, Othman M, Fox BP, Alsaber R, Pellegrino CM, Zeng Y, et al. Gene expression signatures and biomarkers of noninvasive and invasive breast cancer cells: comprehensive profiles by representational difference analysis, microarrays and proteomics. Oncogene. 2006;25:2328–38. doi: 10.1038/sj.onc.1209265. [DOI] [PubMed] [Google Scholar]
- 8.Ma XJ, Salunga R, Tuggle JT, Gaudet J, Enright E, McQuary P, et al. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci U S A. 2003;100:5974–9. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castellana B, Escuin D, Peiró G, Garcia-Valdecasas B, Vázquez T, Pons C, et al. ASPN and GJB2 Are Implicated in the Mechanisms of Invasion of Ductal Breast Carcinomas. J Cancer. 2012;3:175–83. doi: 10.7150/jca.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martins D, Sousa B, Lopes N, Gomes M, Veronese L, Albergaria A, et al. Molecular phenotypes of matched in situ and invasive components of breast carcinomas. Hum Pathol. 2011;42:1438–46. doi: 10.1016/j.humpath.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Beck AH, Sangoi AR, Leung S, Marinelli RJ, Nielsen TO, van de Vijver MJ, et al. Systematic analysis of breast cancer morphology uncovers stromal features associated with survival. Sci Transl Med. 2011;3:ra113. doi: 10.1126/scitranslmed.3002564. [DOI] [PubMed] [Google Scholar]
- 12.Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11:R7. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vargas AC, McCart Reed AE, Waddell N, Lane A, Reid LE, Smart CE, et al. Gene expression profiling of tumour epithelial and stromal compartments during breast cancer progression. Breast Cancer Res Treat. 2012;135:153–65. doi: 10.1007/s10549-012-2123-4. [DOI] [PubMed] [Google Scholar]
- 14.Schnitt SJ. Molecular biology of breast tumor progression: a view from the other side. Int J Surg Pathol. 2010;18(Suppl):170S–3S. doi: 10.1177/1066896910370773. [DOI] [PubMed] [Google Scholar]
- 15.Patani N, Martin LA, Reis-Filho JS, Dowsett M. The role of caveolin-1 in human breast cancer. Breast Cancer Res Treat. 2012;131:1–15. doi: 10.1007/s10549-011-1751-4. [DOI] [PubMed] [Google Scholar]
- 16.Witkiewicz AK, Dasgupta A, Nguyen KH, Liu C, Kovatich AJ, Schwartz GF, et al. Stromal caveolin-1 levels predict early DCIS progression to invasive breast cancer. Cancer Biol Ther. 2009;8:1071–9. doi: 10.4161/cbt.8.11.8874. [DOI] [PubMed] [Google Scholar]
- 17.Witkiewicz AK, Kline J, Queenan M, Brody JR, Tsirigos A, Bilal E, et al. Molecular profiling of a lethal tumor microenvironment, as defined by stromal caveolin-1 status in breast cancers. Cell Cycle. 2011;10:1794–809. doi: 10.4161/cc.10.11.15675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witkiewicz AK, Dasgupta A, Sammons S, Er O, Potoczek MB, Guiles F, et al. Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and basal-like breast cancers. Cancer Biol Ther. 2010;10:135–43. doi: 10.4161/cbt.10.2.11983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witkiewicz AK, Whitaker-Menezes D, Dasgupta A, Philp NJ, Lin Z, Gandara R, et al. Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle. 2012;11:1108–17. doi: 10.4161/cc.11.6.19530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimmer KS, Friedrich B, Lang F, Deitmer JW, Bröer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350:219–27. doi: 10.1042/0264-6021:3500219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, Ertel A, Flomenberg N, Witkiewicz AK, et al. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10:1772–83. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miranda-Gonçalves V, Honavar M, Pinheiro C, Martinho O, Pires MM, Pinheiro C, et al. Monocarboxylate transporters (MCTs) in gliomas: expression and exploitation as therapeutic targets. Neuro Oncol. 2013;15:172–88. doi: 10.1093/neuonc/nos298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sotgia F, Martinez-Outschoorn UE, Howell A, Pestell RG, Pavlides S, Lisanti MP. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol. 2012;7:423–67. doi: 10.1146/annurev-pathol-011811-120856. [DOI] [PubMed] [Google Scholar]
- 24.Witkiewicz AK, Dasgupta A, Sotgia F, Mercier I, Pestell RG, Sabel M, et al. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol. 2009;174:2023–34. doi: 10.2353/ajpath.2009.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savage K, Lambros MB, Robertson D, Jones RL, Jones C, Mackay A, et al. Caveolin 1 is overexpressed and amplified in a subset of basal-like and metaplastic breast carcinomas: a morphologic, ultrastructural, immunohistochemical, and in situ hybridization analysis. Clin Cancer Res. 2007;13:90–101. doi: 10.1158/1078-0432.CCR-06-1371. [DOI] [PubMed] [Google Scholar]
- 26.Sloan EK, Ciocca DR, Pouliot N, Natoli A, Restall C, Henderson MA, et al. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am J Pathol. 2009;174:2035–43. doi: 10.2353/ajpath.2009.080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetz JG, Minguet S, Navarro-Lérida I, Lazcano JJ, Samaniego R, Calvo E, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–63. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartholomew JN, Volonte D, Galbiati F. Caveolin-1 regulates the antagonistic pleiotropic properties of cellular senescence through a novel Mdm2/p53-mediated pathway. Cancer Res. 2009;69:2878–86. doi: 10.1158/0008-5472.CAN-08-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonuccelli G, Whitaker-Menezes D, Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, et al. The reverse Warburg effect: glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle. 2010;9:1960–71. doi: 10.4161/cc.9.10.11601. [DOI] [PubMed] [Google Scholar]
- 30.Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, et al. Transcriptional evidence for the “Reverse Warburg Effect” in human breast cancer tumor stroma and metastasis: similarities with oxidative stress, inflammation, Alzheimer’s disease, and “Neuron-Glia Metabolic Coupling”. Aging (Albany NY) 2010;2:185–99. doi: 10.18632/aging.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinheiro C, Longatto-Filho A, Azevedo-Silva J, Casal M, Schmitt FC, Baltazar F. Role of monocarboxylate transporters in human cancers: state of the art. J Bioenerg Biomembr. 2012;44:127–39. doi: 10.1007/s10863-012-9428-1. [DOI] [PubMed] [Google Scholar]
- 32.Pinheiro C, Longatto-Filho A, Ferreira L, Pereira SM, Etlinger D, Moreira MA, et al. Increasing expression of monocarboxylate transporters 1 and 4 along progression to invasive cervical carcinoma. Int J Gynecol Pathol. 2008;27:568–74. doi: 10.1097/PGP.0b013e31817b5b40. [DOI] [PubMed] [Google Scholar]