Abstract
Background
Upgrade rates of high-risk breast lesions after screening mammography were examined.
Study design
The Breast Cancer Surveillance Consortium registry was used to identify all BI-RADS 4 assessments followed by needle biopsies with high-risk lesions. Follow-up was performed for all women.
Results
High-risk lesions were found in 957 needle biopsies, with excision documented in 53%. Most (N=685) were atypical ductal hyperplasia (ADH), 173 were lobular neoplasia, and 99 were papillary lesions. Upgrade to cancer varied with type of lesion (18% in ADH, 10% in lobular neoplasia and 2% in papillary). In premenopausal women with ADH, upgrade was associated with family history. Cancers associated with ADH were mostly (82%) ductal carcinoma in situ, those associated with lobular neoplasia were mostly (56%) invasive. During further 2 years of follow-up, cancer was documented in 1% of women with follow-up surgery and in 3% with no surgery.
Conclusion
Despite low rates of surgery, low rates of cancer were documented during follow-up. Benign papillary lesions diagnosed on BI-RADS 4 mammograms among asymptomatic women do not justify surgical excision.
Introduction
Percutaneous image guided needle biopsies have revolutionized the management of suspicious breast imaging findings. The ability to obtain tissue from mammography, ultrasound, or MRI findings enables women with benign pathology to avoid surgery, whereas those diagnosed with cancer can be planned for a definitive one-stage surgery. However, there is one group of women that do not gain from this breakthrough—women diagnosed with high-risk breast lesions on needle biopsy. These lesions include: atypical ducal hyperplasia (ADH), atypical lobular hyperplasia (ALH), lobular carcinoma in situ (LCIS), papillary lesions (benign papilloma, atypical papillary hyperplasia), radial sclerosing lesions, and columnar lesions. Many will undergo surgery after the needle biopsy to achieve a definitive diagnosis and rule out cancer. Those that are upgraded to invasive cancer may need further surgery to achieve negative margins or to stage the axilla. There is great controversy regarding the need for follow-up surgery. Multiple studies and reviews have been published on the surgical results with a wide range of upgrade rates to cancer, and hence different recommendations. These reports are limited by the small numbers of women included with a mix of indications for biopsy, by selection of women for surgery, and by lack of imaging-pathology correlation. To complicate matters, poor inter-observer variability has been reported with these lesions (1). In addition, there is great variation in physician recommendations; in surveys of surgeons (2), radiologists (3) and pathologists (4), there seemed to be more disagreement then agreement on the management of some of these lesions. This problem will only increase with the increased use of newer imaging technologies such as MRI, breast tomosynthesis, and molecular breast imaging. Large, population-based studies with adequate follow-up of both women who did and did not have surgery are needed to resolve these questions. We used data from the Breast Cancer Surveillance Consortium (BCSC) to examine the rates of upgrade of high risk lesions in this population-based cohort.
Methods
We included data from five mammography registries that participate in the National Cancer Institute–funded BCSC (http://breastscreening.cancer.gov/): the Carolina Mammography Registry, Group Health Cooperative in Washington, the New Hampshire Mammography Network, the New Mexico Mammography Project, and the Vermont Breast Cancer Surveillance System. These registries collect information on mammography examinations done in their defined catchment areas. Each mammography registry annually links women in their registry to a state tumor registry or regional Surveillance Epidemiology and End Results program that collects population-based cancer data and pathology databases that collect information on both benign and malignant diagnoses. The BCSC Statistical Coordinating Center (SCC) pooled and analyzed the data. Each mammography registry and the SCC have received Institutional Review Board approval for either active or passive consenting processes or a waiver of consent to enroll participants, link data, and perform analytic studies. All procedures comply with the Health Insurance Portability and Accountability Act, and all registries and the SCC have received a Federal Certificate of Confidentiality and other protection for the identities of women, physicians, and facilities studied by this research.
The study sample included screening mammography examinations and short-interval follow-up examinations done between January 1, 1994 and December 31, 2007 on women ages 40 years and older. To avoid misclassifying diagnostic examinations as screening examinations, we excluded examinations done within 9 months of a prior breast imaging examination. Short interval follow-up examinations were defined by the indication given by the radiologist. BI-RADS 5 exams were excluded from the study. Mammography examinations that occurred after 2007 were not included to ensure at least 12 months for reporting cancers to tumor registries after the most recent mammography examination.
A self-administered questionnaire included questions about family history of breast cancer (first-degree relative), time since previous mammography, menopausal status, and current use of postmenopausal hormone treatment. Time since previous mammography was classified as <1 year (9–11 months), 12–35 months, 36–59 months, and 5 years or more or no previous.
Pathology data included pathology results for the first needle biopsy within 4 months of the mammogram and for all surgical biopsies (including excisional biopsies, lumpectomies and mastectomies) done on the same side within 6 months of the needle biopsy. Fine-needle aspiration specimens were excluded.
Pathology results were classified as ADH, lobular neoplasia (atypical lobular hyperplasia, lobular carcinoma in situ), papillary lesions (intraductal papilloma, multiple papillomas) or cancer (DCIS or invasive cancer). Papillary lesions with atypia were combined with ADH lesions. Data on papillary lesions and radial sclerosing lesions was available from 2 registries. Radial sclerosing lesions were excluded because there were too few. As complete cancer ascertainment is available for the women in the BCSC, to determine if high-risk lesions were upgraded to cancer (within 1 year from needle biopsy), all women, regardless of documented surgery, were included, though cancer was rare in women without follow-up surgery. Two and three-year follow-up were available for 749 (78%) and 678 (71%) of the study subjects respectively.
Cancers were classified according to their grade and American Joint Committee on Cancer 6th edition (5) stage at diagnosis. Invasive cancers were classified according to their histology.
Statistical Analysis
Associations between characteristics of women and the likelihood of having documented follow-up surgery within 6-months on the same side were examined using logistic regression adjusting for registry. Rates of upgrade to cancer were calculated for each lesion type according to age, family and personal breast cancer history, type of mammogram, time since last mammogram, year of mammogram and mammographic breast density. Significance of associations was examined using logistic regression adjusting for BCSC registry. Variables that were associated with upgrade rates at the 0.10 level were included in a multivariable model adjusting for age and registry. As high risk lesions and cancer can be influenced by presence of endogenous and exogenous hormones, data was stratified by menopausal status. We examined the time to cancer diagnosis among women with and without follow-up surgery using Kaplan-Meier survival curves.
Results
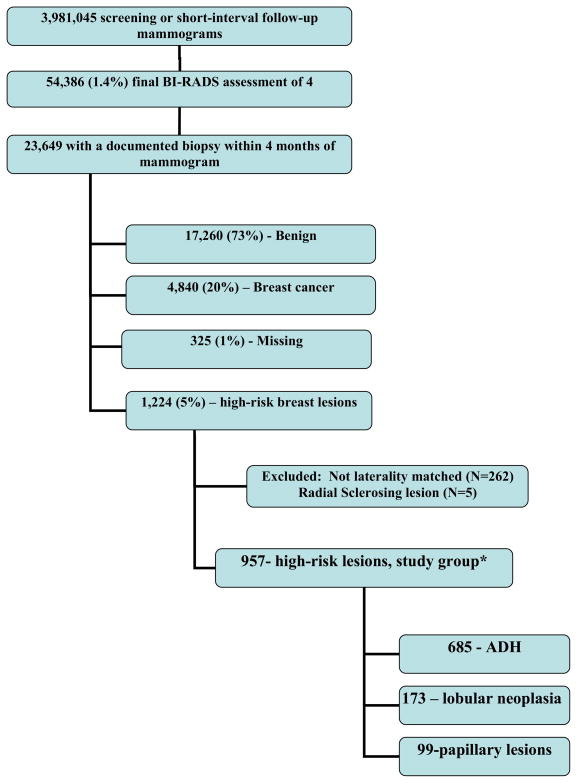
The entire sample included 3,981,045 screening or short-interval follow-up mammograms, 54,386 (1.4%) of which were assigned a BI-RADS 4 final assessment (Figure 1). Of 23,649 women that had a biopsy done within 4 months, 4,840 (20%) were diagnosed with breast cancer, and 1,224 (5%) with a high-risk lesion. After restricting to needle biopsies done on the same side as the positive BI-RADS assessment and exclusion of radial sclerosing lesions, 957 women were included in the study group. Most (N=685) of these were atypical ductal hyperplasia, 173 were diagnosed with lobular neoplasia, and 99 were benign papillary lesions. Five hundred and seven (53%) had a follow-up surgery on the same side documented within 6 months of the needle biopsy (Table 1). Among women with a high-risk lesion, younger women were more likely to undergo surgery. Type of high-risk lesion was associated with undergoing follow-up surgery, with 61% of women with ADH vs. only 19% of women with papillary lesions undergoing surgery within 6 months.
Figure 1.
Flow of women included in the analysis.
BI-RADS-Breast Imaging-Reporting and Data System
ADH- atypical ductal hyperplasia
Table 1.
Characteristics of the women in the study with high-risk lesions, with and without follow-up surgery within 6 months of diagnosis.
| P-value (adjusted for registry) | Women without follow-up surgery*, N, (row %) | Women with follow-up Surgery*, N, (row %) | Women with high-risk lesions |
|---|---|---|---|
| N=450 (47) | N=507 (53) | Characteristic (N, column %, N=957) | |
| 0.0026† | Age at mammography, years | ||
| 120 (45) | 148 (55) | 40–49 (268, 28%) | |
| 138 (41) | 195 (59) | 50–59 (333, 35%) | |
| 104 (53) | 94 (48) | 60–69 (198, 21%) | |
| 88 (56) | 70 (44) | 70+ (158, 17%) | |
| 0.48 | Personal history of breast cancer | ||
| 427 (47) | 488 (53) | No (915, 96%) | |
| 23 (55) | 19 (45) | Yes (42,4%) | |
| 0.38 | Family history of breast cancer | ||
| 321 (48) | 351 (52) | No (672, 70%) | |
| 60 (43) | 80 (57) | Yes (140, 15%) | |
| 69 (48) | 76 (52) | Unknown (145, 15%) | |
| 0.86 | Menopausal status | ||
| 117 (46) | 140 (55) | Pre (257, 27%) | |
| 294 (49) | 305 (51) | Post (599, 63%) | |
| 39 (39) | 62 (61) | Missing (101, 11) | |
| 0.62 | Hormone therapy use | ||
| 304 (48) | 330 (52) | No (634, 66%) | |
| 87 (46) | 103 (54) | Yes (190, 20%) | |
| 59 (44) | 74 (56) | Unknown (133, 14%) | |
| 0.58 | Indication for mammogram | ||
| 401 (47) | 451 (53) | Routine screening (852, 89%) | |
| 49 (47) | 56 (53) | Short interval follow-up (105, 11%) | |
| 0.097† | Time since last mammography | ||
| 57 (50) | 58 (50) | <1 year (115, 12%) | |
| 316 (48) | 349 (53) | 12–35 months (665, 70%) | |
| 23 (36) | 41 (64) | 36–59 months (64, 7%) | |
| 44 (52) | 41 (48) | No previous mammo/5+ years ago (85,9%) | |
| 10 (36) | 18 (64) | Unknown (28, 3%) | |
| 0.63† | 15 (52) | BI-RADS breast density | |
| 14 (48) | 157 (49) | Almost entirely fat (29, 3%) | |
| 166 (51) | 234 (57) | Scattered fibroglandular tissue (323, 35%) | |
| 175 (43) | 45 (52) | Heterogeneously dense (409, 43) | |
| 42 (48) | 56 (51) | Extremely dense (87, 9%) | |
| 53 (49) | Missing (109, 11%) | ||
| 0.0061 | 73 (53) | 64 (47) | Exam year |
| 138 (52) | 130 (49) | 1994–1998 (137, 14%) | |
| 165 (42) | 231 (58) | 1999–2001 (268, 28%) | |
| 74 (47) | 82 (53) | 2002–2005 (396, 41%) | |
| 2006–2007 (156, 16%) | |||
| <0.0001 | Lesion type on needle biopsy | ||
| 265 (39) | 420 (61) | ADH (685, 72%) | |
| 105 (61) | 68 (39) | Lobular Neoplasia (173, 18%) | |
| 80 (81) | 19 (19) | Papillary (199, 10%) |
including excisional biopsies, lumpectomies and mastectomies
trend test
ADH-Atypical ductal hyperplasia
Overall 18% (123) of women with ADH, 10% (18) of women with lobular neoplasia, and 2% (2) of women with papillary lesions were upgraded to cancer (Table 2). In women with ADH on needle biopsy, increased upgrade rates were seen in those that had follow-up surgery within 6 months of the needle biopsy (26% vs. 5%) and in women not on hormone treatment. On multivariate analysis, higher rates of upgrade were seen in premenopausal women with a family history of breast cancer (45% vs. 19% in those with no family history), and in postmenopausal women with no use of hormone treatment (18% vs. 11%) (Table 3). In women with lobular neoplasia, upgrade was significantly associated with follow-up surgery done within 6 months (25% in women with documented surgery vs. 2% in those with no documented surgery within 6 months). Only 2 (2%) women with papillary lesions were upgraded to cancer.
Table 2.
Comparison of women with and without upgrade to cancer on follow-up.
| Papillary | Lobular neoplasia | ADH | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N (%) Upgrade | N (%) No Upgrade | p-value | N (%) Upgrade | N (%) No Upgrade | p-value | N (%) Upgrade | N (%) No Upgrade | Characteristic |
|
| ||||||||
| 2 (2) | 97 | 18 (10) | 155 (90) | 123 (18) | 562 (82) | Total (N=957) | ||
|
| ||||||||
| Age at mammography, years | ||||||||
| 17 (100) | 4 (8) | 47 (92) | 44 (22) | 156 (78) | 40–49 (268) | |||
| 35 (100) | 8 (11) | 65 (89) | 31 (14) | 194 (86) | 50–59 (333) | |||
| 1 (4) | 24 (96) | 4 (13) | 26 (87) | 25 (18) | 118 (83) | 60–69 (198) | ||
| 1 (5) | 21 (95) | 0.74 | 2 (11) | 17 (90) | 0.28* | 23 (20) | 94 (80) | 70+ (158) |
|
| ||||||||
| Personal history of breast cancer | ||||||||
| 2 (2) | 91 (98) | 18 (11) | 152 (89) | 116 (18) | 536 (82) | No (915) | ||
| 6 (100) | NE | 3 (100) | 0.77 | 7 (21) | 26 (79) | Yes (42) | ||
|
| ||||||||
| Family history of breast cancer | ||||||||
| 2 (4) | 55 (97) | 14 (11) | 114 (89) | 81 (17) | 406 (83) | No (672) | ||
| 8 (100) | 2 (9) | 21 (91) | 27 (25) | 82 (75) | Yes (140) | |||
| 34 (100) | 0.72 | 2 (9) | 20 (91) | 0.076 | 15 (17) | 74 (83) | Unknown (145) | |
|
| ||||||||
| Menopausal status | ||||||||
| 12 (100) | 5 (9) | 51 (91) | 42 (22) | 147 (78) | Pre (245) | |||
| 2 (5) | 41(95) | 8 (15) | 45 (85) | 41 (18) | 189 (82) | Post : No Hormone treatment (283) | ||
| 21(100) | 2 (6) | 31 (94) | 15 (11) | 121(89) | With hormone treatment (169) | |||
| 23 (100) | 0.61 | 3(10) | 28 (90) | 0.037 | 25 (19) | 105 (81) | Missing (161) | |
|
| ||||||||
| Indication for mammogram | ||||||||
| 2 (2) | 84 (98) | 17 (11) | 142 (89) | 108 (18) | 499 (82) | Routine screening (852) | ||
| 13 (100) | 0.44 | 1 (7) | 13 (93) | 0.78 | 15 (19) | 63 (81) | Short interval f/u (105) | |
|
| ||||||||
| Time since last mammography | ||||||||
| 12 (100) | 3 (14) | 18 (86) | 15 (18) | 67 (82) | <1 year (115) | |||
| 2(3) | 66 (97) | 12 (9) | 118 (91) | 84 (18) | 383 (82) | 1–2 years (665) | ||
| 4 (100) | 0 | 8 (100) | 7 (14) | 45 (87) | 3–4 years (64) | |||
| 11 (100) | 2 (18) | 9 (82) | 14 (22) | 49 (78) | No previous/5+ years (85) | |||
| 4 (100) | 0.16* | 1 (33) | 2 (67) | 0.95* | 3 (14) | 18 (86) | Unknown (28) | |
|
| ||||||||
| BI-RADS breast density | ||||||||
| 3 (100) | 0 (0) | 2 (100) | 4 (17) | 20 (83) | Almost entirely fat (29) | |||
| 1 (2) | 41 (98) | 6 (14) | 36 (86) | 32 (13) | 207 (87) | Scattered fibroglandular tissue (323) | ||
| 21 (100) | 6 (8) | 68 (92) | 62 (20) | 252 (80) | Heterogeneously dense (409) | |||
| 1 (25) | 3 (75) | 4 (15) | 23 (85) | 14 (25) | 42 (75) | Extremely dense (87) | ||
| 29 (100) | 0.94 | 2 (7) | 26 (93) | 0.084* | 11 (21) | 41 (79) | Missing (109) | |
|
| ||||||||
| 17(100) | 0.41* | 0 | 16 (100) | 24 (23) | 80 (77) | Exam year | ||
| 37 (100) | 4 (10) | 38 (91) | 24 (13) | 165 (87) | 1994–1998 (137) | |||
| 2 (5) | 36 (95) | 11 (15) | 64 (85) | 54 (19) | 229 (81) | 1999–2001 (268) | ||
| 7 (100) | 3 (8) | 37 (93) | 0.73* | 21 (19) | 88 (81) | 2002–2005 (396)2006–2007 (156) | ||
|
| ||||||||
| F/u surgery† within 6 months | ||||||||
| 1 (1) | 79 (99) | 2 (2) | 103 (98) | 13 (5) | 252 (95) | No (450) | ||
| 1 (5) | 18 (95) | 0.0006 | 16 (24) | 52 (77) | <.0001 | 110 (26) | 310 (74) | Yes (507) |
trend test continuous.
including excisional biopsies, lumpectomies, and mastectomies
ADH-atypical ductal hyperplasia
BI-RADS- Breast Imaging-Reporting and Data System
Table 3.
Multivariable model of upgrade of ADH to cancer according to clinical and mammographic characteristics.
| Post-menopausal | Pre-menopausal | Characteristic | ||
|---|---|---|---|---|
| * p-value | OR (95% Cl) | * p-value | OR (95% Cl) | |
| 0.78 | 1.01 (0.97, 1.04) | 0.31 | 0.94 (0.84, 1.06) | Age at mammography, continuous in years |
| 0.65 | 1.20 (0.53, 2.59) | 0.0022 | 4.88 (1.80, 14.01) | Family history of breast cancer (yes vs. no) |
| 0.0029 | 0.44 (0.20, 0.90) | N/A | Hormone therapy use (yes vs. no) | |
| 0.20 | 1.39 (0.85, 2.30) | 0.70 | 1.14 (0.58, 2.29) | BI-RADS breast density (one-category change) |
| <0.0001 | 11.6 (4.45, 39.6) | 0.0004 | 10.2 (3.23, 46.40) | Surgery† within 6 months |
included variables significant at 0.10 level from univariate models plus BCSC registry.
including excisional biopsies, lumpectomies and mastectomies
CI-confidence interval
One hundred fifty three women were diagnosed with cancer within 1 year of the needle biopsy (Table 4). Most of the women with ADH on needle biopsy that were upgraded to cancer were found to have DCIS (101, 82%). However, more than half (10, 56%) of the women with lobular neoplasia that were upgraded to cancer were found to have invasive carcinoma, with lobular cancer found in 60% of these women. Most women with invasive carcinoma had grade 1 or 2 cancers, and most were diagnosed at an early stage (stage I or II). Lymph nodes were involved more often in the women that were first diagnosed with lobular neoplasia when compared to those with ADH (20% vs. 5%). The two women with papillary lesions upgraded to cancer were diagnosed with DCIS.
Table 4.
Characteristics of cancers following high-risk breast lesions.
| Initial needle biopsy result | |||
|---|---|---|---|
|
| |||
| Papillary N=2 (%) |
Lobular neoplasia N=18 (%) |
ADH N=123 (%) |
Characteristic |
|
| |||
| Type of cancer | |||
| 2 (100) | 8 (44) | 101 (82) | Ductal carcinoma in situ |
| 10 (56) | 22 (18) | Invasive | |
|
| |||
| DCIS Grade | |||
| 1 (13) | 22 (22) | Grade 1 | |
| 2 (25) | 29 (29) | Grade 2 | |
| 2 (25) | 25 (25) | Grade 3 or 4 | |
| 2 (100) | 3 (38) | 25 (25) | Missing |
|
| |||
|
Invasive cancer characteristics
| |||
| Histology | |||
| 2 (20) | 12 (55) | Ductal | |
| 6 (60) | 2 (9) | Lobular | |
| 1 (10) | 3 (14) | Mixed | |
| 0 | 2 (9) | Other | |
| 1 (10) | 3 (14) | Missing | |
|
| |||
| Grade | |||
| 4 (40) | 6 (28) | Grade 1 | |
| 1 (10) | 6 (28) | Grade 2 | |
| 1 (10) | 3 (14) | Grade 3 | |
| 4 (40) | 7 (32) | Missing | |
|
| |||
| AJCC stage 6th edition | |||
| 4 (40) | 14 (64) | I | |
| 2 (20) | 3 (14) | II | |
| 1 (10) | 1 (5) | III | |
| 3 (30) | 4 (18) | Missing | |
|
| |||
| Lymph nodes | |||
| 5 (50) | 18 (82) | Negative | |
| 2 (20) | 1 (5) | Positive | |
| 3 (30) | 3 (14) | Unknown | |
ADH-atypical ductal hperplsia
DCIS-ductal carcinoma in situ
AJCC-American Joint Committee on Cancer
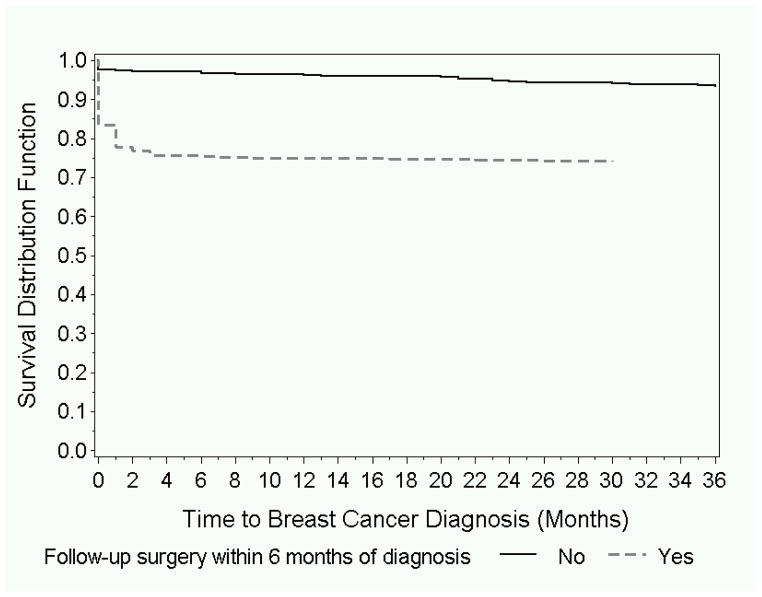
Women that did not undergo surgery as well as those that did remained at low risk of developing cancer during follow-up (Figure 2). During the first year, breast cancer was documented in 25% of all women with high-risk lesions undergoing excisional biopsy and in 4% of those with no excisional biopsy documented. Among women with no cancer within 1 year of the needle biopsy, during an additional 2 years follow-up, cancers were documented in 4 (1%) women that had follow-up surgery and in 11 (3%) women who had no documented surgery in the first 6 months. Most of the cancers diagnosed after the first year were invasive (9, 60%).
Figure 2.
Proportion of women without a breast cancer diagnosis in women undergoing surgery within 6 months of biopsy and women not undergoing surgery.
Discussion
Based on our findings, approximately 24 of every 100,000 screening mammograms will lead to a needle biopsy with a diagnosis of a high-risk breast lesion. Our estimates may be slightly low because in the earlier years of the study, many of the first biopsies were open biopsies, and only needle biopsies were included in this study. More than half of these women will subsequently undergo surgery, with approximately four (0.004%) upgraded to cancer.
ADH
We found that premenopausal women with family history of breast cancer were more likely to have an upgrade to cancer than those without a family history. In postmenopausal women, not using hormone treatment was associated with upgrade to cancer. The reverse association between hormone treatment use and upgrade to cancer is not easily explained and could be a chance finding. As hormone use is associated with increased risk of ADH and breast cancer (6) we cannot recommend that women on hormone treatment diagnosed with ADH on needle biopsy not undergo follow up surgery. We were unable to characterize women that can safely avoid surgery after a needle biopsy diagnosis of ADH. Multiple studies report on rates of upgrade in women with a needle biopsy diagnosis of ADH with rates ranging widely between 7–87% (7). Several studies attempted to identify factors associated with increased rates of upgrade to malignancy. In one study age was associated with upgrade (8). There is an association between the extent of sampling (determined by type of needle, number of specimens procured and residual findings on post biopsy imaging) and rate of upgrade to cancer (9). Pathological characteristics associated with upgrade are the extent of ADH (10), presence of severe atypia (11), micropapillary pattern (10) and associated necrosis (11). Most studies recommend surgery following an ADH diagnosis; however, only 61% of our ADH cohort underwent surgery with overall upgrade rates of 18%. Therefore, these women should be managed individually, based on their family history, imaging findings, extent of sampling and pathology findings.
Lobular neoplasia
In this study, limited to asymptomatic women with BI-RADS 4 mammograms, upgrade rates were 10% for lobular neoplasia; however, more than half were upgraded to invasive carcinoma. The literature is inconclusive in recommending follow-up surgery for these lesions. There are several reasons for this lack of a consistent recommendation. Studies are limited by their retrospective design, usually including only small numbers of cases that had follow-up surgery. It is not clear if ALH and LCIS should be approached separately (12), or as one entity as similar rates of upgrade were reported (13). To add to the confusion, there is a debate on the ability of lobular neoplasia to cause an imaging abnormality such as calcifications or mass. Classically, lobular neoplasia was considered an incidental finding with no imaging correlate (14–15). Recently, this dogma has been questioned (3). Surgery rates are lower for lobular neoplasia when compared to ADH; in this report 39% had follow surgery, although rates of 71% were reported in a multicenter study (13). In most studies there is no follow-up available for the women not undergoing surgery (16–17). In early studies, where no imaging-pathologic correlation was done, high rates of upgrade were reported (14–50%) (12); in recent years several studies reported very low rates of upgrade in cases where the imaging abnormality was correctly biopsied and the highest abnormality was lobular neoplasia (18–19). Our results show that physicians are correctly selecting women for surgery, as most do not undergo surgery, and follow-up rates of cancer are low.
Papillary lesions
In this study, papillary lesions (without atypia) found on needle biopsy of mammographic abnormalities were associated with a 2% upgrade to cancer, which in both cases was DCIS. This rate falls within the acceptable range of the BI-RADS 3 assessment, recommending short interval follow-up (20–21). Moreover, in the studies that define the BI-RADS 3 assessment, upgrade to cancer was defined only when invasive cancer was diagnosed during follow-up, not carcinoma in situ or atypical hyperplasia (22). Other studies reported rates of upgrade ranging between 0 and 29% (12). There are several reasons for these large variations. The literature is a mix of mostly retrospective studies of both symptomatic and screening-associated findings diagnosed on different imaging modalities using different biopsy techniques with no or limited follow-up of the women not undergoing surgery. Several studies found an association with age (23–24) or size (25) of the lesion. We included all cases of benign papillomas diagnosed in asymptomatic women undergoing screening mammography, most of which (81%) did not have an excisional biopsy. However as follow-up was available for most of the study group, we are able to show that the rate of cancer after 3 years of follow-up was low. Of 89 papillary lesions with 3 years of follow-up, one was diagnosed with cancer in 1 year and 2 additional were diagnosed within 3 years. Similarly, Sohn reported 1.1% of benign papillomas developing into cancer during mean follow-up of 53 months (26). It is important to understand that these results are limited to asymptomatic women with a benign papillary lesion diagnosed on a needle biopsy of a BIRADS 4 mammogram and cannot be generalized to all women with a needle biopsy diagnosis of benign papilloma.
Despite low overall rates of follow-up surgery (53%), rates of cancer documented during an additional 2 years of follow-up, for women with no cancer diagnosis during the first year after needle biopsy, were low (3% in women with no documented follow-up surgery within 6 months of biopsy vs. 1% in those undergoing surgery). These rates are comparable to those calculated using the Gail model risk calculator (27) for a 55 year old woman with atypical hyperplasia on biopsy --3% risk over 5 years compared to 1.5% in an average woman.
This study has several limitations. Data in the BCSC registry on family history is limited to first degree relatives with breast cancer. We were unable to individually correlate radiology findings with pathology results, or review pathology slides. No information was available on number of cores, size of needle, size of the targeted lesion, amount of atypia present in the specimen, or the criteria for excision. Follow-up surgery rates are probably an underestimation as some cancers were documented within 6 months of the needle biopsy in women with no documented follow-up surgery via linkage with cancer registries.
The design of this study is unique in that it is population and screening-based, from a consortium of registries from several states, including detailed data which allowed us to examine the association of upgrade with personal risk factors. As cancer catchment is accurate for the entire study group, we were able to examine the actual rates of cancer development in this population during follow-up.
Although published surveys show large variability in management of high-risk lesions, our results show that physicians are selectively managing these lesions, and judging by 3 year follow-up cancer rates, they appear to be able to identify women at low risk of breast cancer after a high-risk breast biopsy. Therefore physicians should continue to manage these lesions on a case by case manner with continuous communication between surgery, radiology and pathology specialties. There cannot be a general recommendation that will fit all cases based on this study.
Acknowledgments
This work was supported by the National Cancer Institute-funded Breast Cancer Surveillance Consortium (U01CA63740, U01CA86076, U01CA86082, U01CA63736, U01CA70013, U01CA69976, U01CA63731, U01CA70040, HHSN261201100031C). The collection of cancer and vital status data used in this study was supported in part by several state public health departments and cancer registries throughout the US. For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. We thank the participating women, mammography facilities, and radiologists for the data they have provided for this study. A list of the BCSC investigators and procedures for requesting BCSC data for research purposes are provided at: http://breastscreening.cancer.gov/.
Abbreviations
- BCSC
Breast Cancer Surveillance Consortium
- BI-RADS
Breast Imaging-Reporting and Data System
- ADH
atypical ductal hyperplasia
- ALH
atypical lobular hyperplasia
- DCIS
ductal carcinoma in situ
- LCIS
lobular carcinoma in situ
- AJCC
American Joint Committee on Cancer
- SCC
Statistical Coordinating Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wells WA, Carney PA, Eliassen MS, et al. Statewide study of diagnostic agreement in breast pathology. J Natl Cancer Inst. 1998;90(2):142–5. doi: 10.1093/jnci/90.2.142. [DOI] [PubMed] [Google Scholar]
- 2.Nizri E, Schneebaum S, Klausner JM, Menes TS. Current management practice of breast borderline lesions--need for further research and guidelines. Am J Surg. 2012;203(6):721–5. doi: 10.1016/j.amjsurg.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 3.Georgian-Smith D, Lawton TJ. Variations in physician recommendations for surgery after diagnosis of a high-risk lesion on breast core needle biopsy. AJR Am J Roentgenol. 2012;198(2):256–63. doi: 10.2214/AJR.11.7717. [DOI] [PubMed] [Google Scholar]
- 4.Lawton TJ, Georgian-Smith D. Excision of high-risk breast lesions on needle biopsy: is there a standard of core? AJR Am J Roentgenol. 2009;192(5):W268. doi: 10.2214/AJR.08.2227. [DOI] [PubMed] [Google Scholar]
- 5.Greene FL, Page DL, Fleming ID, Fritz A, editors. AJCC cancer staging manual. 6. Philadelphia (PA): Lippincott-Raven; 2002. [Google Scholar]
- 6.Menes TS, Kerlikowske K, Jaffer S, et al. Rates of atypical ductal hyperplasia have declined with less use of postmenopausal hormone treatment: findings from the Breast Cancer Surveillance Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2822–8. doi: 10.1158/1055-9965.EPI-09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allison KH, Eby PR, Kohr J, et al. Atypical ductal hyperplasia on vacuum-assisted breast biopsy: suspicion for ductal carcinoma in situ can stratify patients at high risk for upgrade. Hum Pathol. 2011;42(1):41–50. doi: 10.1016/j.humpath.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Chae BJ, Lee A, Song BJ, Jung SS. Predictive factors for breast cancer in patients diagnosed atypical ductal hyperplasia at core needle biopsy. World J Surg Oncol. 2009;7:77. doi: 10.1186/1477-7819-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnamurthy S, Bevers T, Kuerer H, Yang WT. Multidisciplinary considerations in the management of high-risk breast lesions. AJR Am J Roentgenol. 2012;198(2):W132–40. doi: 10.2214/AJR.11.7799. [DOI] [PubMed] [Google Scholar]
- 10.Wagoner MJ, Laronga C, Acs G. Extent and histologic pattern of atypical ductal hyperplasia present on core needle biopsy specimens of the breast can predict ductal carcinoma in situ in subsequent excision. Am J Clin Pathol. 2009;131(1):112–21. doi: 10.1309/AJCPGHEJ2R8UYFGP. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen CV, Albarracin CT, Whitman GJ, et al. Atypical ductal hyperplasia in directional vacuum-assisted biopsy of breast microcalcifications: considerations for surgical excision. Ann Surg Oncol. 2011;18(3):752–61. doi: 10.1245/s10434-010-1127-8. [DOI] [PubMed] [Google Scholar]
- 12.Georgian-Smith D, Lawton TJ. Controversies on the management of high-risk lesions at core biopsy from a radiology/pathology perspective. Radiol Clin North Am. 2010;48(5):999–1012. doi: 10.1016/j.rcl.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim N, Bessissow A, Lalonde L, et al. Surgical outcome of biopsy-proven lobular neoplasia: is there any difference between lobular carcinoma in situ and atypical lobular hyperplasia? AJR Am J Roentgenol. 2012;198(2):288–91. doi: 10.2214/AJR.11.7212. [DOI] [PubMed] [Google Scholar]
- 14.Kopans DB. LCIS found at core needle biopsy may not need surgical excision. AJR Am J Roentgenol. 2008;191(3):W152. doi: 10.2214/AJR.07.3984. [DOI] [PubMed] [Google Scholar]
- 15.Beute BJ, Kalisher L, Hutter RV. Lobular carcinoma in situ of the breast: clinical, pathologic, and mammographic features. AJR Am J Roentgenol. 1991;157(2):257–65. doi: 10.2214/ajr.157.2.1853802. [DOI] [PubMed] [Google Scholar]
- 16.Brem RF, Lechner MC, Jackman RJ, et al. Lobular neoplasia at percutaneous breast biopsy: variables associated with carcinoma at surgical excision. AJR Am J Roentgenol. 2008;190(3):637–41. doi: 10.2214/AJR.07.2768. [DOI] [PubMed] [Google Scholar]
- 17.Hwang H, Barke LD, Mendelson EB, Susnik B. Atypical lobular hyperplasia and classic lobular carcinoma in situ in core biopsy specimens: routine excision is not necessary. Mod Pathol. 2008;21(10):1208–16. doi: 10.1038/modpathol.2008.134. [DOI] [PubMed] [Google Scholar]
- 18.Nagi CS, O’Donnell JE, Tismenetsky M, et al. Lobular neoplasia on core needle biopsy does not require excision. Cancer. 2008;112(10):2152–8. doi: 10.1002/cncr.23415. [DOI] [PubMed] [Google Scholar]
- 19.Menon S, Porter GJ, Evans AJ, et al. The significance of lobular neoplasia on needle core biopsy of the breast. Virchows Arch. 2008;452(5):473–9. doi: 10.1007/s00428-008-0607-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American College of Radiology. Breast imaging and reporting system (BI-RADS) 4. Reston VA: American College of Radiology; 2003. [Google Scholar]
- 21.Kerlikowske K, Smith-Bindman R, Abraham LA, et al. Breast cancer yield for screening mammographic examinations with recommendation for short-interval follow-up. Radiology. 2005;234(3):684–92. doi: 10.1148/radiol.2343031976. [DOI] [PubMed] [Google Scholar]
- 22.Sickles EA. Periodic mammographic follow-up of probably benign lesions: results in 3,184 consecutive cases. Radiology. 1991;179(2):463–8. doi: 10.1148/radiology.179.2.2014293. [DOI] [PubMed] [Google Scholar]
- 23.Cheng TY, Chen CM, Lee MY, et al. Risk factors associated with conversion from nonmalignant to malignant diagnosis after surgical excision of breast papillary lesions. Ann Surg Oncol. 2009;16(12):3375–9. doi: 10.1245/s10434-009-0637-8. [DOI] [PubMed] [Google Scholar]
- 24.Holley SO, Appleton CM, Farria DM, et al. Pathologic Outcomes of Nonmalignant Papillary Breast Lesions Diagnosed at Imaging-guided Core Needle Biopsy. Radiology. 2012;265(2):379–84. doi: 10.1148/radiol.12111926. [DOI] [PubMed] [Google Scholar]
- 25.Chang JM, Moon WK, Cho N, et al. Risk of carcinoma after subsequent excision of benign papilloma initially diagnosed with an ultrasound (US)-guided 14-gauge core needle biopsy: a prospective observational study. Eur Radiol. 2010;20(5):1093–100. doi: 10.1007/s00330-009-1649-2. [DOI] [PubMed] [Google Scholar]
- 26.Sohn V, Keylock J, Arthurs Z, et al. Breast papillomas in the era of percutaneous needle biopsy. Ann Surg Oncol. 2007;14(10):2979–84. doi: 10.1245/s10434-007-9470-0. [DOI] [PubMed] [Google Scholar]
- 27. [accessed January 27th, 2013]; http://www.cancer.gov/bcrisktool/Default.aspx.