Abstract
Human GABAB receptor is a G-protein coupled receptor central to inhibitory neurotransmission in the brain. It functions as an obligatory heterodimer of GBR1 and GBR2 subunits. Here we present the first crystal structures of a heterodimeric complex between the extracellular domains of GBR1 and GBR2 in the apo, agonist-bound, and antagonist-bound forms. The apo and antagonist-bound structures represent the resting state of the receptor; the agonist-bound complex corresponds to the active state. Both subunits adopt an open conformation at rest, and only GBR1 closes upon agonist-induced receptor activation. The agonists and antagonists are anchored in the interdomain crevice of GBR1 by an overlapping set of residues. An antagonist confines GBR1 to the open conformation of the inactive state, while an agonist induces its domain closure for activation. Our data reveals a unique activation mechanism for GABAB receptor that involves the formation of a novel heterodimer interface between subunits.
GABA (γ-amino butyric acid) is the predominant inhibitory neurotransmitter in the central nervous system. Metabotropic GABAB receptor is a G-protein coupled receptor (GPCR) that mediates slow and prolonged synaptic inhibition through Gi/o protein1,2. Presynaptic GABAB receptor suppresses neurotransmitter release, and postsynaptic GABAB receptor causes hyperpolarization of neurons1,2. Malfunction of GABAB receptor can lead to various neurological disorders, including spasticity, epilepsy, and pain1–3. Baclofen, a selective GABAB agonist, is used clinically to treat muscle spasticity associated with multiple sclerosis, cerebral palsy, and spinal cord injury1–3.
GABAB receptor belongs to the distinct class C GPCR family4. Ligand-binding to these receptors takes place within a large extracellular Venus Flytrap (VFT) module that has sequence homology to bacterial periplasmic amino acid binding proteins (PBPs)4. Unlike metabotropic glutamate receptors (mGluRs) and extracellular calcium sensing receptor, which function as disulfide-tethered homodimers5–8, GABAB and taste receptors act as heterodimers9–16.
GABAB receptor functions as a heterodimeric assembly of GBR1 and GBR2 subunits9–12,14. GBR2 facilitates cell surface expression of GBR1 by masking an endoplasmic reticulum retention signal of GBR117,18. GBR1 is responsible for ligand recognition through its extracellular domain19,20. Although GBR2 does not bind any known GABAB ligand9–11,21, its ectodomain directly interacts with the GBR1 ectodomain to enhance agonist affinity10,11,22–26 and is required for receptor activation22,25,27. Finally, the transmembrane domain of GBR2 is responsible for G-protein coupling22,25,28–32.
Most of the current knowledge about class C GPCR structures derives from homodimeric mGluRs. The ectodomain structures of three mGluR subtypes have been determined with and without ligand33–35. Here we assembled a stable heterodimeric complex of the human GBR1 and GBR2 ectodomains, and determined its crystal structure in the absence of ligand and in the presence of various agonists and antagonists. Together with our mutational data, these structures provide insights into the molecular mechanisms of receptor heterodimerization, ligand recognition, and receptor activation.
Structures of GABAB heterodimer
The extracellular VFT module of human GBR1b (GBR1bVFT) and GBR2 (GBR2VFT) were co-secreted as a heterodimeric complex from insect cells (Supplementary Fig. 1). The GBR1bVFT:GBR2VFT heterodimer binds various agonists and antagonists with the same rank order of affinities as the full-length receptor, indicating that it is physiologically relevant26.
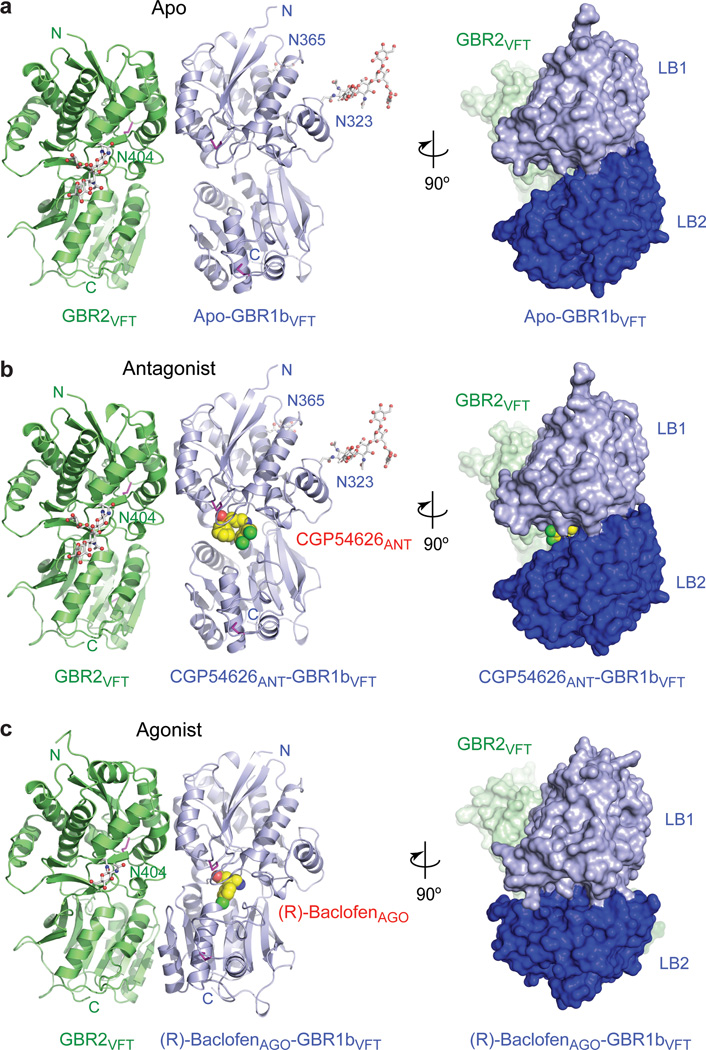
We determined the crystal structure of the GBR1bVFT:GBR2VFT complex in the apo form, bound to six different antagonists (CGP54626ANT, CGP46381ANT, CGP35348ANT, SCH50911ANT, (S)-2-OH-saclofenANT, and (R)-phaclofenANT), and bound to two different agonists (endogenous ligand GABA and clinical drug (R)-baclofenAGO) (Supplementary Table 1). Each structure consists of a non-covalent heterodimer of GBR1bVFT and GBR2VFT, wherein the two subunits “dance cheek-to-cheek”: the protomers are bound to each other such that they are side by side and facing opposite directions (Fig. 1a–c; Supplementary Fig. 2). All of the agonists and antagonists bind in the crevice between the LB1 and LB2 domains of GBR1bVFT.
Figure 1. Crystal structures of the GBR1bVFT:GBR2VFT complex.
a, Apo structure. b, Antagonist CGP54626ANT-bound structure. c, Agonist (R)-baclofenAGO-bound structure. Each complex is shown in two views related by a 90°-rotation about the vertical axis. Front view (left panel) is shown as a ribbon diagram; side view (right panel) is presented as a molecular surface. GBR1bVFT and GBR2VFT are colored blue and green, respectively. The observed carbohydrates are shown as ball-and-stick models in gray. Disulfide bridges are in magenta. The ligands are displayed as space-filling models.
GBR1bVFT and GBR2VFT have similar overall structures, in agreement with their sequence homology (33% identity) (Supplementary Fig. 3). Both subunits have a bi-lobed architecture related to that found in mGluRs33–35, natriuretic peptide receptors36,37, ionotropic glutamate receptors38–40 and PBPs41. However, the extracellular domains of GBR1b and GBR2 lack the cysteine-rich region found at the C-terminal end of mGluR ectodomains. Each GABAB subunit contains two distinct domains, LB1 and LB2. The individual LB1 and LB2 domains of the two subunits exhibit high correlation with each other.
Despite similarities, the GBR1bVFT and GBR2VFT structures have different interdomain arrangements, consistent with their disparate ligand-binding characteristics (Fig. 2a, b). The ligand-binding subunit GBR1bVFT can oscillate between open and closed conformations, wherein the more compact closed conformation is associated with agonist binding. In contrast, the non-ligand binding subunit GBR2VFT has nearly identical conformations with and without dimer partner GBR1VFT.
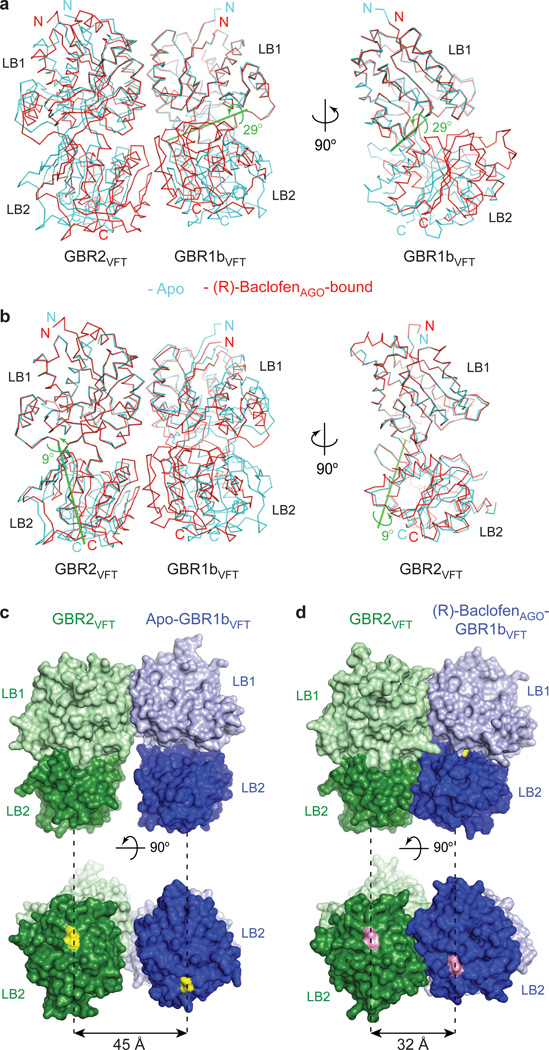
Figure 2. Agonist-induced conformational changes.
a, b, Superposition of apo (cyan) and (R)-baclofenAGO-bound (red) complexes based on the LB1 domain of GBR1bVFT (a), or GBR2VFT (b). Side view is shown on the right for the superimposed GBR1bVFT (a) and GBR2VFT (b) subunits. Green line is the axis of rotation that relates the LB2 domain of GBR1bVFT (rotation χ = 29°, screw translation τχ = 0.2 Å) (a) or GBR2VFT (rotation χ = 9°, screw translation τχ = 0.1 Å) (b) from the apo and agonist-bound structures.
c, d, Surface representation of apo (c) and (R)-baclofenAGO-bound (d) GBR1bVFT:GBR2VFT in front view (top), and bottom view (bottom). Distances between C-termini of the two subunits (yellow in apo structure; pink in (R)-baclofenAGO-bound structure) are marked by dashed lines.
In the crystal structure of apo-GBR1bVFT:GBR2VFT, both subunits adopt an open conformation when compared with the known structures of mGluRs33–35 (Supplementary Fig. 4). All six antagonist-bound structures closely resemble that of the apo complex, both in the arrangement of the heterodimer and in the structures of the individual subunits (Supplementary Fig. 4). The ligand-binding cleft of GBR1bVFT stays open with each bound antagonist. In addition, GBR2VFT remains wide open with an empty interdomain cleft. This open-open configuration of the apo and antagonist-bound structures corresponds to the resting (or inactive) state of the heterodimeric receptor.
Agonist binding causes large conformational changes within the heterodimeric complex. First, both the agonists GABA and (R)-baclofenAGO induce domain closure of GBR1bVFT, as previously predicted42 (Fig. 2a). When the LB1 domains of apo and agonist-bound GBR1bVFT are superimposed, their LB2 domains can be related by a 29°-rotation about a nearly horizontal interdomain axis. Since the rotational axis has a slight vertical offset, this transformation also brings the LB2 domain of GBR1bVFT into close contact with the LB2 domain of GBR2VFT to form a large heterodimer interface unique to the active state.
Second, GBR2VFT remains open in the agonist-bound state, consistent with our previous prediction that GBR2VFT has a constitutively open conformation26. Nevertheless, the LB2 domain of GBR2VFT undergoes a twist motion of 9° around a nearly vertical axis, and moves toward the LB2 domain of GBR1bVFT to form new heterodimeric contacts (Fig. 2b).
Finally, the substantial rearrangement of the LB2 domains from the apo to the agonist-bound state shortens the distance between the C-termini of the two subunits from 45 Å to 32 Å (Fig. 2c, d; Supplementary Fig. 5). This decrease in the separation between membrane proximal LB2 domains may lead to changes in the relative orientation of the transmembrane domains. In summary, both agonist-bound GBR1bVFT:GBR2VFT complexes adopt a closed-open structural arrangement, which corresponds to the active state of the receptor (Supplementary Fig. 4).
Common subunit-subunit interactions
In both the resting and active states, GBR1bVFT and GBR2VFT interact through their LB1 domains (Supplementary Fig. 6, 7). In the apo and antagonist-bound structures, the subunit association is exclusively facilitated by this LB1-LB1 contact. The heterodimer buries over 1,400 Å2 of solvent accessible surface area and exhibits exceptionally high interfacial shape correlation (Supplementary Table 2).
The LB1-LB1 interaction is mediated by the B and C helices of both subunits (Fig. 3a). The heterodimer interface can be divided into three regions (Fig. 3b). Site I is located at the center of the interface, and it is flanked by sites II and III on each side.
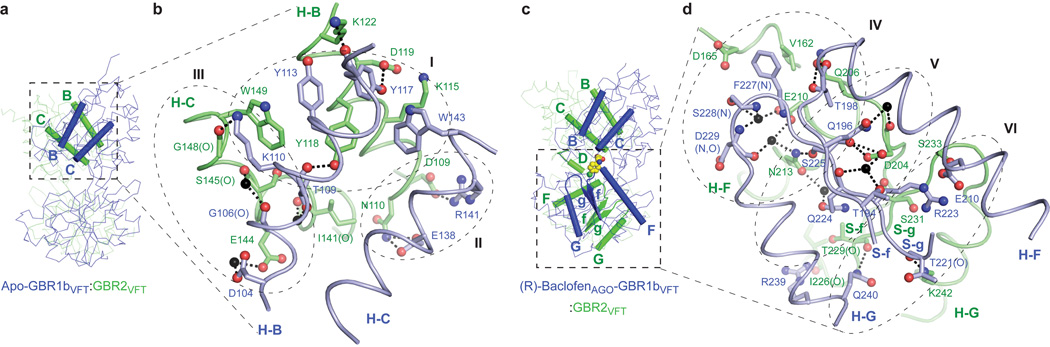
Figure 3. Heterodimer interface.
a, Structure of apo-GBR1bVFT:GBR2VFT with the elements involved in heterodimer formation highlighted by ribbons (LB1-LB1: B and C helices). b, Specific contacts at the LB1-LB1 heterodimer interface of apo-GBR1bVFT:GBR2VFT. The interface area is divided into three regions I, II, and III. Dashed lines indicate hydrogen bonds.
c, Structure of (R)-baclofenAGO-GBR1bVFT:GBR2VFT showing the elements involved in heterodimer formation (LB1-LB1: B and C helices; LB2-LB2: F and G helices, f and g strands, and connecting loops). d, Specific contacts at the LB2-LB2 heterodimer interface of (R)-baclofenAGO-GBR1bVFT:GBR2VFT. The interface area is divided into three regions IV, V, and VI. Dashed lines indicate hydrogen bonds.
Site I is comprised of a central hydrophobic patch surrounded by hydrogen bonds. The heterodimer contacts within this site are highly conserved in all of the GBR1bVFT:GBR2VFT structures. In particular, it features three deeply buried tyrosine residues (Y113 and Y117 of GBR1bVFT, and Y118 of GBR2VFT) that are critical for heterodimer interaction and receptor activation26. These tyrosine residues participate in aromatic stacking interactions, and form interfacial hydrogen bonds. Together with the adjacent lysine and tryptophan residues, they are responsible for the majority of hydrophobic contacts at the LB1-LB1 heterodimer interface.
Site II interactions are mostly hydrogen bonds, and include a universal salt bridge (GBR1bVFT-R141 : GBR2VFT-D109) as well as a conserved hydrogen bond (GBR1bVFT-E138 : GBR2VFT-N110). Site III consists predominantly of water-mediated contacts, and is the most variable part of the LB1-LB1 interface.
Agonist-induced heterodimer interface
Agonist binding induces the formation of an additional heterodimer interface between the LB2 domains of GBR1bVFT and GBR2VFT subunits (Supplementary Fig. 7). This is consistent with our calorimetry measurements showing that GBR2VFT has higher affinity for agonist-bound than antagonist-bound GBR1bVFT26. The LB2-LB2 interface buries over 1,300 Å2 of solvent accessible surface area, has poor shape complementarity, and is dominated by polar interactions (Supplementary Table 2).
The LB2-LB2 interaction is mediated by two strand-loop-helix motifs from each LB2 domain (Fig. 3c). The neighboring strands f and g are part of the central β-sheet in LB2, and helices F and G flank the β-sheet. The heterodimer contacts consist primarily of hydrogen bonds, some of which are mediated by water molecules. The interface can be divided into three adjacent areas (Fig. 3d). Sites IV and V each feature a large cluster of hydrogen bonds, while site VI mostly consists of isolated contacts. The GBR2VFT residue N213 is located at the intersection of sites IV and V, and it bridges the hydrogen bond networks within these two regions. In addition, a minor LB2-LB1 contact involving helix D of GBR2VFT is formed at the edge of site IV.
To confirm the importance of the LB2-LB2 heterodimer interface to receptor activation, we carried out alanine scanning mutagenesis of the interfacial residues. We identified several polar residues from each subunit that are critical to agonist-dependent Gi protein activity (Supplementary Fig. 7). These include the GBR1bVFT residues T198, E201 and S225, and the GBR2VFT residues D204, Q206, N213 and S233. All of these residues are engaged in multiple interfacial hydrogen bonds at the LB2-LB2 interface. This reliance on hydrophilic interactions to form a distinct subunit interface in the active state allows the receptor to readily dissociate upon returning to its resting state. Previous studies have also shown that introduction of a large N-glycan into the LB2 domain of either GABAB subunit inhibits agonist-induced receptor activation43.
Ligand recognition
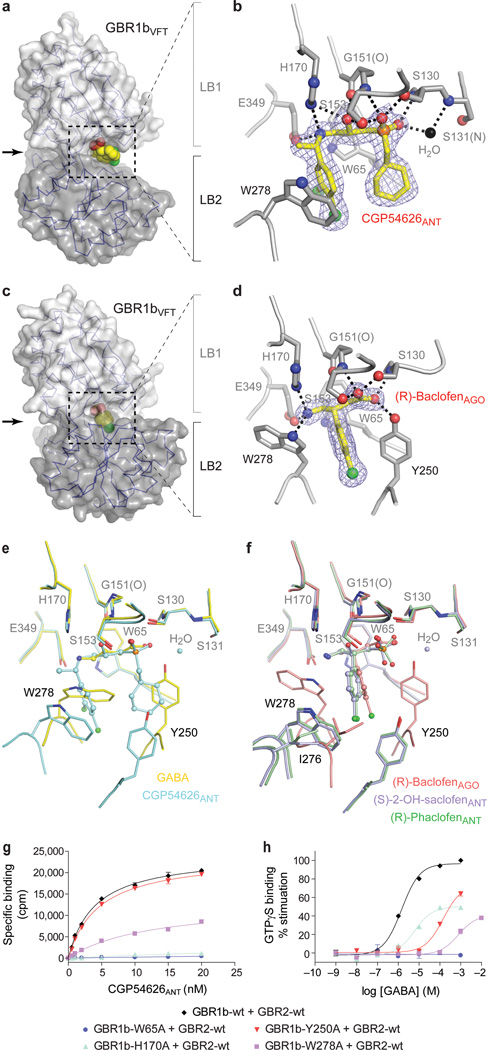
All of the antagonists are derivatives of GABA, and have the general structure of a γ-amino acid. The receptor-antagonist interactions are mediated largely by hydrogen bonds (Fig. 4a, b; Supplementary Fig. 8). First, each antagonist is anchored at the crevice of GBR1bVFT by two sets of hydrogen bonds. The α-acid group at one end forms hydrogen bonds with the LB1 residues S130 and S153; the γ-amino group at the other end is hydrogen-bonded to H170 and E349. Second, W65 makes van der Waals contacts with all of the antagonists. Third, the β-hydroxyl substituent of CGP54626ANT and (S)-2-OH-saclofen makes additional hydrogen bonds with the receptor that are specific to these antagonists. Finally, all of the antagonists except SCH50911ANT and (R)-phaclofenANT participate in water-mediated interaction with S131. These extensive contacts indicate that the LB1 domain is primarily responsible for anchoring antagonist.
Figure 4. Ligand recognition by GBR1bVFT.
a, c, Molecular surface of GBR1bVFT bound to antagonist CGP54626ANT (a) or agonist (R)-baclofenAGO (c). Ligand is displayed as a space-filling model.
b, d, Specific contacts between GBR1bVFT (gray) and CGP54626ANT (yellow) (b) or (R)-baclofenAGO (d), viewed in the direction of the arrow in a or c. Mesh represents the final 2Fo-Fc electron density map contoured at 1σ. Hydrogen bonds are represented by black dashed lines.
e, Comparison of the binding sites of agonist GABA and antagonist CGP54626ANT. f, Comparison of the binding sites of agonist (R)-baclofenAGO and two related antagonists (S)-2-OH-saclofenANT and (R)-phaclofenANT.
g, h, Dose-dependent [3H]CGP54626ANT binding (g) and GABA-stimulated [35S]GTPγS binding (h) in membranes from cells expressing wild type GABAB receptor (GBR1b-wt + GBR2-wt) or the combination of GBR2-wt and various GBR1b mutants.
In contrast, the interaction between the LB2 domain and bound antagonist is sparse and varies among the different antagonists (Supplementary Fig. 8). Only two antagonists, CGP54626ANT and SCH50911ANT, directly contact W278 of LB2 through a large γ-substituent. As a result of this additional LB2 interaction, both compounds have higher binding affinity to GABAB receptor than the other antagonists reported here3. This suggests that the LB2 domain plays an auxiliary role in antagonist recognition, and enhances the potency of selective antagonists.
GABAB receptor recognizes both the agonists GABA and (R)-baclofenAGO in essentially the same manner (Fig. 4c, d; Supplementary Fig. 9). (R)-baclofenAGO is a derivative of GABA, and contains a chlorophenyl substituent at the β-position. Like the antagonists, each agonist is secured by two hydrogen bond networks, one at each end of the molecule. Furthermore, a common set of LB1 residues are involved in binding the two ends of all of the agonists and antagonists. Unlike the antagonists, both agonists also directly contact two key residues of the LB2 domain, Y250 and W278. In addition, the two tryptophan residues W65 and W278 make extensive van der Waals contacts with both GABA and (R)-baclofenAGO. Therefore, both the LB1 and LB2 domains are required for agonist recognition.
The binding sites of GABA and (R)-baclofenAGO differ in the side chain conformation of the LB2 residue W278 (Supplementary Fig. 9). Relative to its orientation in the GABA-bound complex, the indole ring of W278 is flipped ~170° to accommodate the β-chlorophenyl substituent of (R)-baclofenAGO, which forms aromatic ring-stacking interactions with both Y250 and W278. In contrast, GABA makes van der Waals contact with W278 alone through its aliphatic backbone. The conformational adaptability of W278 provides a mechanism by which the receptor recognizes structurally different ligands while maintaining ligand-binding specificity and affinity.
Agonist versus antagonist action
The function of a GABAB agonist is to stabilize the closed conformation of GBR1bVFT, while that of an antagonist is to confine the GBR1bVFT subunit to the open configuration (Supplementary Fig. 10). Agonist-bound GBR1bVFT has a closed cleft; the agonist is buried and inaccessible to the bulk solvent. In contrast, antagonist-bound GBR1bVFT has an open cleft, and the antagonist is solvent accessible.
The presence of a bulky substituent in each antagonist inhibits domain closure of GBR1bVFT. The highly potent antagonist CGP54626ANT contains an α-cyclohexyl and a γ-dichlorophenyl group. The adverse interactions of these moieties with Y250 and W278 would be expected to prevent the LB1 and LB2 domains from approaching each other (Fig. 4e). Similarly, each of the other antagonists CGP46381ANT, CGP35348ANT, and SCH50911ANT has a bulky substituent at either the α- or γ-position to block GBR1bVFT domain closure (Supplementary Fig. 8, 9). Although the antagonists (S)-2-OH-saclofenANT and (R)-phaclofenANT are structurally analogous to the agonist (R)-baclofenAGO, their α-acid motifs assume a tetrahedral coordination geometry that is incompatible with the active-state conformation of Y250 (Fig. 4f). Furthermore, the α-substituents push the β-chlorophenyl ring toward the γ-amino end of each antagonist, thereby generating potential steric interactions with I276 and W278 to prevent GBR1bVFT domain closure.
All of the residues at the ligand-binding site are conserved within GBR1 sequences across different species (Supplementary Fig. 11). Some of the ligand-binding residues have been implicated by previous studies, including S130, G151, S153 and E349 of GBR1b21,26,44–46.
The LB1 residues are required for both agonist and antagonist recognition. We found that the W65A substitution caused substantial loss of ligand binding and receptor function, (Fig. 4g, h). The H170A mutation essentially abolished antagonist binding, and lowered the maximum agonist-induced [35S]GTPγS binding to half that of wild-type level (Fig. 4g, h). These data indicate that both W65 and H170 are indispensable for ligand recognition.
The LB2 residues are essential for agonist binding. First, the W278A mutant retained the ability to bind the antagonist [3H]CGP54626ANT, although with decreased potency (Fig. 4g). This is consistent with the auxiliary role of W278 in antagonist recognition. On the other hand, this mutation is detrimental to receptor activation, since it not only reduced the maximum GABA-dependent [35S]GTPγS binding, but also increased the half effective concentration (EC50) of GABA by more than 500-fold (Fig. 4h). Second, the Y250A mutation had no effect on antagonist binding, in agreement with our structural observations (Fig. 4g). However, it decreased the agonist response, and increased the EC50 of GABA by more than 100-fold (Fig. 4h). These data indicate that both Y250 and W278 are critical to agonist recognition.
Implications for receptor activation
Structural comparison indicates that the concept of major inter-subunit relocation that holds for the activation of mGluRs cannot be applied to GABAB receptor. The extracellular domains of these receptors share a common mode of dimerization through their LB1 domains (Supplementary Fig. 12, 13). The resting and active configurations of mGluRs differ by a 70°-rotation in dimer orientation33–35. Both closed-open and closed-closed conformations have been reported for activated mGluRs33–35, although full activation requires the closure of both protomers47. In contrast, the heterodimeric LB1-LB1 interface of GABAB receptor undergoes a minor 5°-rearrangement upon agonist binding, and the receptor only adopts a closed-open active conformation. Our data indicate that activation of GABAB receptor involves the formation of a novel LB2-LB2 heterodimer interface.
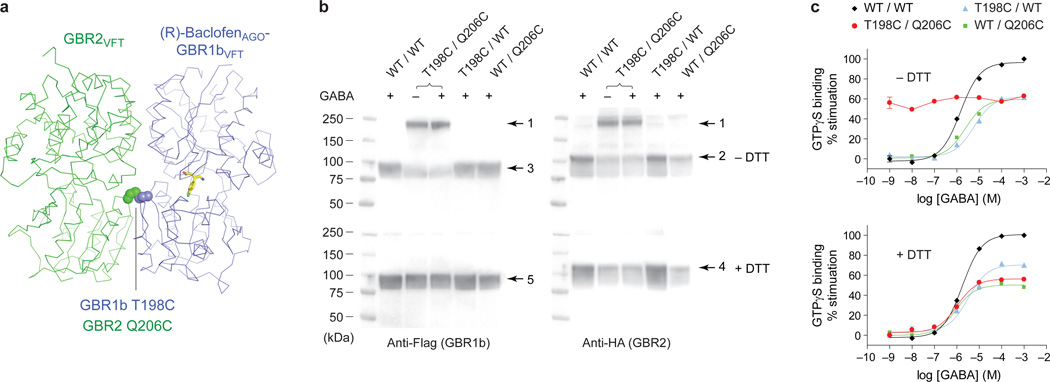
We carried out disulfide crosslinking studies48 to determine the physiological relevance of the LB2-LB2 interaction in full length receptor. Based on the active-state structure of GBR1bVFT:GBR2VFT, we introduced cysteine mutations into a residue pair across the LB2-LB2 dimer interface (GBR1-T198C and GBR2-Q206C), which had the proximity and geometry required for disulfide formation (Fig. 5a). Western blot analysis indicates that co-expression of wild-type GBR1b and GBR2 or the combination of a single cysteine mutant with its wild-type partner in mammalian cells produced monomeric protein bands in the presence of GABA under both reducing and non-reducing conditions (~95 kDa for GBR1b; ~115 kDa for GBR2) (Fig. 5b). In contrast, co-expression of the cysteine mutant pair yielded a heterodimeric protein band (~210 kDa) under non-reducing conditions (Fig. 5b). This band was recognized by both anti-Flag and anti-HA antibodies, which were used to detect differentially tagged GBR1b and GBR2 subunits. Furthermore, it was observed in the absence of ligand and in the presence of the agonist GABA. These observations indicate the spontaneous formation of a disulfide-tethered GBR1b-GBR2 heterodimer, and confirm that the LB2-LB2 interface observed in the active-state GBR1bVFT:GBR2VFT structure is also present in free and agonist-bound native GABAB receptor.
Figure 5. Constitutive activity of disulfide-tethered GBR1b:GBR2 heterodimer.
a, Position of cysteine mutations (spheres) at the LB2-LB2 heterodimer interface of (R)-baclofenAGO-GBR1bVFT:GBR2VFT.
b, Western blot analysis of membranes from cells expressing different combinations of wild-type (WT) and mutant GABAB receptor subunits (GBR1b-T198C, abbreviated as T198C; GBR2-Q206C, abbreviated as Q206C). The samples were assayed in the presence of 10 mM GABA under reducing (+DTT) and non-reducing (−DTT) conditions. The double cysteine mutant (T198C / Q206C) was also analyzed in the absence of ligand. GBR1b and GBR2 were detected by anti-Flag and anti-HA antibodies, respectively. Arrow 1, GBR1b-GBR2 heterodimer; arrow 2 and 4, GBR2 monomer; arrow 3 and 5, GBR1b monomer.
c, GABA-stimulated dose-dependent [35S]GTPγS binding in membranes from cells expressing wild-type or various cysteine mutant receptors in the presence and absence of DTT.
To determine the functional effects of locking the LB2-LB2 interface, we measured agonist-dependent Gi protein activation of different combinations of wild-type and cysteine mutant receptors (Fig. 5c). For the wild-type receptor and single cysteine mutants, application of GABA led to stimulation of [35S]GTPγS binding both in the absence and presence of dithiothreitol (DTT). In contrast, the double cysteine mutant exhibited constitutive activity under non-reducing conditions, and addition of GABA did not further increase its functional activity (Fig. 5c). This indicates that the inter-subunit disulfide bond holds the receptor in a fully active form. Indeed, upon reduction of the disulfide bond, the double cysteine mutant receptor lost its constitutive activity, but regained sensitivity to GABA to a level comparable to that of a single cysteine mutant (Fig. 5c). Our data demonstrate that formation of the LB2-LB2 interface is both necessary and sufficient for GABAB receptor activation.
In the conformational equilibrium of GABAB receptor, an antagonist maintains the inactive conformation of the receptor, while an agonist stabilizes its active conformation (Supplementary Fig. 14). Agonist binding to GABAB receptor induces domain closure in the GBR1 subunit, an expansion of the heterodimer interaction to include a large LB2-LB2 interface, and a decrease in the separation between the membrane-proximal LB2 domains. Since receptor function is not affected by alterations in the peptide linker between the VFT and transmembrane domains of each subunit27, these changes would likely be directly relayed to the transmembrane domains. We expect that the transmembrane domains of the GABAB subunits exist as pre-formed heterodimers on the cell surface because both the extracellular and intracellular components form stable heterodimers12,23,24,26,49. Therefore, agonist-induced conformational changes may lead to a rearrangement of the transmembrane domain heterodimer for signal transduction across the membrane. This novel activation mechanism would be, as of yet, unique to inhibitory GABAB receptor.
Methods
Protein expression and purification
The extracellular domains of human GBR1 and GBR2 were separately cloned into the pFBDM vector50 for expression in baculovirus-infected insect cells. The GBR1 isoform GBR1b19 was used in this study. The GBR1bVFT construct contained residues 48–459, with the signal peptide of baculovirus envelope surface glycoprotein gp67 attached at the N-terminus and a Flag tag at the C-terminus. The GBR2VFT construct contained residues 1–466 and a C-terminal Flag tag, as previously described26.
Sf9 insect cells were co-infected with recombinant GBR1bVFT and GBR2VFT baculoviruses at 23°C for 96 hours. The GBR1bVFT:GBR2VFT complex was purified from cell supernatant by anti-Flag antibody (M2) affinity chromatography followed by gel filtration chromatography (Superdex 200, GE Healthcare). The CGP54626ANT-GBR1bVFT:GBR2VFT complex was produced in the presence of 10 µM CGP54626ANT throughout expression and 20 µM CGP54626ANT during purification. The (R)-baclofenAGO-GBR1bVFT:GBR2VFT complex was expressed and purified in the presence of 100 µM (R)-baclofen, and the GABA-GBR1bVFT:GBR2VFT complex was produced in the presence of 100 µM GABA.
Crystallization and data collection
Crystals of the apo-GBR1bVFT:GBR2VFT complex were grown at 4°C in 10% PEG 3350, 20% glycerol and 0.12 M Na acetate, pH 7.0. Crystals of various antagonist-bound GBR1bVFT:GBR2VFT complexes were obtained under the same condition as the apo complex. Specifically, the CGP54626ANT-bound heterodimer was crystallized using protein that was purified in the presence of CGP54626ANT. The apo-GBR1bVFT:GBR2VFT complex was also co-crystallized with 10 mM of each of the following antagonists: CGP46381ANT, CGP35348ANT, SCH50911ANT, (R, S)-2-OH-saclofenANT, and (R, S)-phaclofenANT. All of the crystals were directly frozen from drops.
The agonist-bound (R)-baclofenAGO-GBR1bVFT:GBR2VFT complex was crystallized at 20°C from 20% PEG 2000, 15% glycerol, 0.2 M NH4Cl, and 0.1 M Na cacodylate, pH 5.2, in the presence of 10 mM (R)-baclofen. Crystals of the GABA-GBR1bVFT:GBR2VFT complex were grown at 20°C from 18% PEG 2000, 5% glycerol, 0.15 M NH4Cl, and 0.1 M Na cacodylate, pH 5.0, in the presence of 10 mM GABA. The crystals were frozen in a cryoprotecting solution containing 20% glycerol and all other components of the crystallization solution.
Native data for the different complexes were collected at the 24ID-C and 24ID-E beamlines of Advanced Photon Source (APS). Diffraction data for the apo, CGP46381ANT-, CGP35348ANT-, SCH50911ANT-, and GABA-bound complexes were integrated using XDS51 and scaled with SCALA52. Data for the CGP54626ANT-, (S)-2-OH-saclofenANT-, (R)-phaclofenANT-, and (R)-baclofenAGO-bound complexes were integrated and scaled using HKL200053.
Structure determination
The structure of the apo-GBR1bVFT:GBR2VFT complex was solved by molecular replacement. The position of GBR2VFT was identified using the free GBR2VFT structure (PDB code 4F11)26 as the search model. The location of GBR1bVFT was found using the individual LB1 and LB2 domains of GBR2VFT as the search probes. A complete atomic model of the apo-GBR1bVFT:GBR2VFT complex was developed through a succession of manual building and iterative refinement. The final model contained the GBR1bVFT residues 48–368 and 377–459, the GBR2VFT residues 53–292, 300–379 and 385–466, and part of the Flag tag at the C-termini of both subunits. Carbohydrate residues were also attached to Asn323 and Asn365 of GBR1bVFT, and Asn404 of GBR2VFT.
All of the antagonist-bound GBR1bVFT:GBR2VFT structures were solved by molecular replacement using the apo-GBR1bVFT:GBR2VFT structure as the search model. For each complex, the bound antagonist was modeled into the residual electron density map obtained in the final rounds of refinement. All of the antagonist-bound structures contained the GBR1bVFT residues 48–368 and 377–459, the GBR2VFT residues 53–292, 300–379 and 385–466, and part of the Flag tag at the C-termini of both subunits. Carbohydrate residues were also attached to Asn323 and Asn365 of GBR1bVFT, and Asn404 of GBR2VFT. Although a racemic mixture (R, S)-2-OH-saclofenANT was used for crystallization, only the (S)-2-OH-saclofenANT enantiomer was bound to GBR1bVFT in the structure. Our observation is consistent with previous findings that (S)-2-OH-saclofenANT enantiomer is the active antagonist54. Similarly, we found that (R)-phaclofen was the active enantiomer, in agreement with previous studies55.
The structure of the (R)-baclofenAGO-GBR1bVFT:GBR2VFT complex was also determined by molecular replacement. The position of GBR2VFT was found using the GBR2VFT structure from the apo complex as the search model. The (R)-baclofenAGO-bound GBR1bVFT molecule was located using the individual LB1 and LB2 domains of apo-GBR1bVFT as the search probes. A complete model of the (R)-baclofenAGO-GBR1bVFT:GBR2VFT complex was constructed through iterative rounds of manual building and refinement. The GABA-bound GBR1bVFT:GBR2VFT structure was solved using the refined (R)-baclofenAGO-GBR1bVFT:GBR2VFT complex structure as the search model. For each complex, the bound agonist was modeled into the residual electron density map obtained in the final rounds of refinement. The (R)-baclofenAGO-GBR1bVFT:GBR2VFT complex contained the GBR1bVFT residues 50–368 and 377–459; the GABA-GBR1bVFT:GBR2VFT complex contained the GBR1bVFT residues 50–84, 92–337, 344–368 and 377–459. Both agonist-bound structures contained the GBR2VFT residues 50–291 and 302–466, and part of the Flag tag at the C-termini of both GBR1bVFT and GBR2VFT. Carbohydrate residues were attached to Asn404 of GBR2VFT.
Molecular replacement searches were carried out using PHASER56. Model building was performed with COOT57. Structural refinement was executed using BUSTER58. Ramachandran statistics were calculated for each structure using MolProbity59. Pairwise structural comparison was performed using LSQMAN60. Software installation support was provided by SBGrid61.
Cell surface expression
Full-length human GBR1b and GBR2 were individually cloned into a pcDNA3.1(+) vector (Invitrogen) for expression in human embryonic kidney (HEK293) cells. A Flag tag was inserted after the signal peptide of GBR1b, and an HA tag was placed after the signal peptide of GBR2. Mutants of GBR1b and GBR2 were constructed using the QuikChange mutagenesis system (Stratagene).
HEK293 T/17 cells (ATCC) were co-transfected by Lipofectamine 2000 (Invitrogen) with the GBR1b and GBR2 plasmids. Cells permeabilized with 0.5% Triton X100 were used to determine the total expression levels of GBR1b and GBR2 in transfected cells. Untreated cells were used to determine the cell surface expression level of each subunit. The amount of surface protein detected for each construct was normalized to that found in the total cell lysate.
The cells were blocked with 5% milk, and then incubated with mouse anti-Flag M1 antibody (Sigma) as the primary antibody to measure GBR1b expression. Similarly, mouse anti-HA antibody HA.11 clone 16B12 (Covance) was used to detect GBR2. Donkey anti-mouse IRDye 800-labeled antibody (LiCor) was used as the secondary antibody in both cases. Fluorescent signals were measured with an Odyssey Infrared Imager (LiCor). The results of three independent experiments were used for statistical analysis. All of the mutants reported here were expressed on the cell surface at levels comparable to that of wild-type GABAB receptor.
Agonist-stimulated [35S]GTPγS binding
HEK293 T/17 cells were transiently transfected with full length GBR1b and GBR2 plasmids. The cells were harvested in 50 mM Hepes, pH 7.4 to obtain the membrane fraction. Membranes were suspended in an assay buffer containing 50 mM Tris pH 7.7, 100 mM NaCl, 12 mM MgCl2, 1.8 mM CaCl2, and 0.2 mM EGTA to approximately 400 µg protein per ml. The membrane homogenates were incubated with increasing concentrations of GABA in the presence of 10 µM GDP. [35S]GTPγS (1,250 Ci/mmol) was then added to a final concentration of 0.5 nM. After incubation at room temperature for 45 minutes, unbound [35S]GTPγS was removed by centrifugation. The amount of bound [35S]GTPγS was measured using a Beckman LS6500 liquid scintillation counter. Nonspecific binding was measured in the presence of 20 µM unlabeled GTPγS. Basal activity was determined in the absence of GABA. The basal activity of the wild-type receptor was used to calculate the percent stimulation of the double cysteine mutant receptor GBR1b-T198C/GBR2-Q206C under non-reducing conditions. The reduced [35S]GTPγS binding activity of the double cysteine mutant (~60% of the wild-type value) could be attributed to the effect of the mutations themselves, since introduction of a single cysteine mutation into either subunit also caused a decrease in agonist response.
To measure [35S]GTPγS binding under reducing conditions, the membrane homogenates were pre-incubated with 1mM dithiothreitol (DTT) before the addition of various concentrations of GABA and 10 µM GDP. The presence of DTT reduced the basal activity of all different combinations of wild-type and cysteine mutant receptors. The percent stimulation of each receptor mutant was calculated based on the wild-type response obtained under the same condition. Data analysis was performed using the non-linear regression algorithms in Prism (GraphPad Software). Data points represent average ± s.e.m. of triplicate measurements.
Radioligand binding assay
HEK293 T/17 cells were transiently transfected with full length GBR1b and GBR2 plasmids. Cell membranes were suspended in an assay buffer containing 20 mM Tris pH 7.4, 118 mM NaCl, 5.6 mM glucose, 1.2 mM KH2PO4, 1.2 mM MgSO4, 4.7 mM KCl, and 1.8 mM CaCl2 to approximately 400 µg protein per ml. [3H]CGP54626ANT (25 Ci/mmol) was added to the reaction mixture to final concentrations ranging from 0.5 nM to 20 nM. After incubation at room temperature for 30 minutes, unbound [3H]CGP54626ANT was removed by centrifugation. The amount of bound [3H]CGP54626ANT was measured by liquid scintillation counting. Nonspecific binding was measured in the presence of 10 mM unlabeled GABA. Data analysis was performed using the non-linear regression algorithms in Prism. Data points represent average ± s.e.m. of triplicate measurements.
Disulfide design and western blot analysis
The structure of (R)-baclofenAGO-GBR1bVFT:GBR2VFT was used for the rational design of disulfide bonds at the LB2-LB2 heterodimer interface. The residue pair, GBR1b-T198 and GBR2-Q206 was identified by the software Disulfide by Design62 to have the proximity and geometry required for disulfide formation when mutated to cysteines. The T198C and Q206C mutations were engineered into full length GBR1b and GBR2 in pcDNA3.1(+), respectively.
HEK293 T/17 cells were transiently transfected with equal amounts of the full length GBR1b and GBR2 plasmids. Cells were harvested in a buffer containing 20 mM Tris, pH 7.5, 150 mM NaCl, and 1% dodecyl-maltoside. After the insoluble materials were removed by centrifugation, the supernatant was analyzed by 4–15% SDS polyacrylamide gel electrophoresis in the absence and presence of 100 mM DTT. In addition, formation of a disulfide-linked heterodimer between the cysteine mutant pair GBR1b-T198C and GBR2-Q206C was analyzed under two different conditions: in the absence of any ligand, and in the presence of 10 mM GABA. Heterodimer formation of all other samples was analyzed in the presence of 10 mM GABA. The samples were transferred to polyvinylidene fluoride (PVDF) membranes. After blocking with 5% milk, the membranes were incubated with a primary antibody. Mouse anti-Flag M1 antibody (Sigma) was used to detect the GBR1b protein. Mouse anti-HA antibody HA.11 clone 16B12 (Covance) was used to probe GBR2. Both were followed by an alkaline phosphatase (AP)-conjugated anti-mouse secondary antibody. Proteins were visualized by colorimetric method.
Supplementary Material
Acknowledgements
We thank Dr. W.A. Hendrickson and Dr. R. Kass for advice and support, Dr. I. Berger for the gift of pFBDM vector, Drs. K. Rajashankar, K. Perry, S. Banerjee, F. Murphy, I. Kourinov and D. Neau at Advanced Photon Source for help with data collection, Y. Chen for technical assistance, and Dr M. Evelyn for reading the manuscript. This work was supported by the American Heart Association grant SDG0835183N, and the National Institute of Health grant R01GM088454 (both to Q.R.F.). Q.R.F. is an Irma Hirschl Career Scientist, Pew Scholar, McKnight Scholar and Schaefer Scholar.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature/com/nature.
Author contributions
Q.R.F. conceived the study and designed the experiments; Y.G., Q.R.F., M.B., L.M. and F.W. performed experiments and analyzed data, Q.R.F. and Y.G. wrote the paper.
Author information
Atomic coordinates and diffraction data are deposited in the RCSB PDB with accession codes 4MQE, 4MQF, 4MR7, 4MR8, 4MR9, 4MRM, 4MS1, 4MS3, and 4MS4.
The authors declare no competing financial interests.
References
- 1.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol. Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 2.Bowery NG, et al. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol. Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- 3.Froestl W. Chemistry and pharmacology of GABAB receptor ligands. Adv. Pharmacol. 2010;58:19–62. doi: 10.1016/S1054-3589(10)58002-5. [DOI] [PubMed] [Google Scholar]
- 4.Pin JP, et al. The activation mechanism of class-C G-protein coupled receptors. Biol. Cell. 2004;96:335–342. doi: 10.1016/j.biolcel.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Romano C, Yang WL, O'Malley KL. Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J. Biol. Chem. 1996;271:28612–28616. doi: 10.1074/jbc.271.45.28612. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto T, et al. Expression and purification of the extracellular ligand binding region of metabotropic glutamate receptor subtype 1. J. Biol. Chem. 1998;273:13089–13096. doi: 10.1074/jbc.273.21.13089. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji Y, et al. Cryptic dimer interface and domain organization of the extracellular region of metabotropic glutamate receptor subtype 1. J. Biol. Chem. 2000;275:28144–28151. doi: 10.1074/jbc.M003226200. [DOI] [PubMed] [Google Scholar]
- 8.Bai M, Trivedi S, Brown EM. Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J. Biol. Chem. 1998;273:23605–23610. doi: 10.1074/jbc.273.36.23605. [DOI] [PubMed] [Google Scholar]
- 9.Jones KA, et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 10.Kaupmann K, et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 11.White JH, et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 12.Kuner R, et al. Role of heteromer formation in GABAB receptor function. Science. 1999;283:74–77. doi: 10.1126/science.283.5398.74. [DOI] [PubMed] [Google Scholar]
- 13.Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br. J. Pharmacol. 2009;158:5–14. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng GY, et al. Identification of a GABAB receptor subunit, gb2, required for functional GABAB receptor activity. J. Biol. Chem. 1999;274:7607–7610. doi: 10.1074/jbc.274.12.7607. [DOI] [PubMed] [Google Scholar]
- 15.Nelson G, et al. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 16.Nelson G, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 17.Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 18.Pagano A, et al. C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(b) receptors. J. Neurosci. 2001;21:1189–1202. doi: 10.1523/JNEUROSCI.21-04-01189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaupmann K, et al. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- 20.Malitschek B, et al. The N-terminal domain of gamma-aminobutyric Acid(B) receptors is sufficient to specify agonist and antagonist binding. Mol. Pharmacol. 1999;56:448–454. doi: 10.1124/mol.56.2.448. [DOI] [PubMed] [Google Scholar]
- 21.Kniazeff J, Galvez T, Labesse G, Pin JP. No ligand binding in the GB2 subunit of the GABA(B) receptor is required for activation and allosteric interaction between the subunits. J. Neurosci. 2002;22:7352–7361. doi: 10.1523/JNEUROSCI.22-17-07352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galvez T, et al. Allosteric interactions between GB1 and GB2 subunits are required for optimal GABA(B) receptor function. EMBO J. 2001;20:2152–2159. doi: 10.1093/emboj/20.9.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, et al. Molecular determinants involved in the allosteric control of agonist affinity in the GABAB receptor by the GABAB2 subunit. J. Biol. Chem. 2004;279:15824–15830. doi: 10.1074/jbc.M313639200. [DOI] [PubMed] [Google Scholar]
- 24.Nomura R, Suzuki Y, Kakizuka A, Jingami H. Direct detection of the interaction between recombinant soluble extracellular regions in the heterodimeric metabotropic gamma-aminobutyric acid receptor. J. Biol. Chem. 2008;283:4665–4673. doi: 10.1074/jbc.M705202200. [DOI] [PubMed] [Google Scholar]
- 25.Monnier C, et al. Trans-activation between 7TM domains: implication in heterodimeric GABA(B) receptor activation. EMBO J. 2011;30:32–42. doi: 10.1038/emboj.2010.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng Y, et al. Structure and functional interaction of the extracellular domain of human GABA(B) receptor GBR2. Nat. Neurosci. 2012;15:970–978. doi: 10.1038/nn.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margeta-Mitrovic M, Jan YN, Jan LY. Ligand-induced signal transduction within heterodimeric GABA(B) receptor. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14643–14648. doi: 10.1073/pnas.251554798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margeta-Mitrovic M, Jan YN, Jan LY. Function of GB1 and GB2 subunits in G protein coupling of GABA(B) receptors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14649–14654. doi: 10.1073/pnas.251554498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins MJ, et al. GABA(B2) is essential for g-protein coupling of the GABA(B) receptor heterodimer. J. Neurosci. 2001;21:8043–8052. doi: 10.1523/JNEUROSCI.21-20-08043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duthey B, et al. A single subunit (GB2) is required for G-protein activation by the heterodimeric GABA(B) receptor. J. Biol. Chem. 2002;277:3236–3241. doi: 10.1074/jbc.M108900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Havlickova M, et al. The intracellular loops of the GB2 subunit are crucial for G-protein coupling of the heteromeric gamma-aminobutyrate B receptor. Mol. Pharmacol. 2002;62:343–350. doi: 10.1124/mol.62.2.343. [DOI] [PubMed] [Google Scholar]
- 32.Pin JP, et al. Activation mechanism of the heterodimeric GABA(B) receptor. Biochem. Pharmacol. 2004;68:1565–1572. doi: 10.1016/j.bcp.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 33.Kunishima N, et al. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407:971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- 34.Tsuchiya D, Kunishima N, Kamiya N, Jingami H, Morikawa K. Structural views of the ligand-binding cores of a metabotropic glutamate receptor complexed with an antagonist and both glutamate and Gd3+ Proc. Natl. Acad. Sci. U. S. A. 2002;99:2660–2665. doi: 10.1073/pnas.052708599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muto T, Tsuchiya D, Morikawa K, Jingami H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3759–3764. doi: 10.1073/pnas.0611577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Akker F, et al. Structure of the dimerized hormone-binding domain of a guanylyl-cyclase-coupled receptor. Nature. 2000;406:101–104. doi: 10.1038/35017602. [DOI] [PubMed] [Google Scholar]
- 37.He X, Chow D, Martick MM, Garcia KC. Allosteric activation of a spring-loaded natriuretic peptide receptor dimer by hormone. Science. 2001;293:1657–1662. doi: 10.1126/science.1062246. [DOI] [PubMed] [Google Scholar]
- 38.Jin R, et al. Crystal structure and association behaviour of the GluR2 amino-terminal domain. EMBO J. 2009;28:1812–1823. doi: 10.1038/emboj.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karakas E, Simorowski N, Furukawa H. Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J. 2009;28:3910–3920. doi: 10.1038/emboj.2009.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar J, Schuck P, Jin R, Mayer ML. The N-terminal domain of GluR6-subtype glutamate receptor ion channels. Nat. Struct. Mol. Biol. 2009;16:631–638. doi: 10.1038/nsmb.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sack JS, Saper MA, Quiocho FA. Periplasmic binding protein structure and function. Refined X-ray structures of the leucine/isoleucine/valine-binding protein and its complex with leucine. J. Mol. Biol. 1989;206:171–191. doi: 10.1016/0022-2836(89)90531-7. [DOI] [PubMed] [Google Scholar]
- 42.Kniazeff J, et al. Locking the dimeric GABA(B) G-protein-coupled receptor in its active state. J. Neurosci. 2004;24:370–377. doi: 10.1523/JNEUROSCI.3141-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rondard P, et al. Functioning of the dimeric GABA(B) receptor extracellular domain revealed by glycan wedge scanning. EMBO J. 2008;27:1321–1332. doi: 10.1038/emboj.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galvez T, et al. Mutagenesis and modeling of the GABAB receptor extracellular domain support a venus flytrap mechanism for ligand binding. J. Biol. Chem. 1999;274:13362–13369. doi: 10.1074/jbc.274.19.13362. [DOI] [PubMed] [Google Scholar]
- 45.Galvez T, et al. Mapping the agonist-binding site of GABAB type 1 subunit sheds light on the activation process of GABAB receptors. J. Biol. Chem. 2000;275:41166–41174. doi: 10.1074/jbc.M007848200. [DOI] [PubMed] [Google Scholar]
- 46.Galvez T, et al. Ca(2+) requirement for high-affinity gamma-aminobutyric acid (GABA) binding at GABA(B) receptors: involvement of serine 269 of the GABA(B)R1 subunit. Mol. Pharmacol. 2000;57:419–426. doi: 10.1124/mol.57.3.419. [DOI] [PubMed] [Google Scholar]
- 47.Kniazeff J, et al. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat. Struct. Mol. Biol. 2004;11:706–713. doi: 10.1038/nsmb794. [DOI] [PubMed] [Google Scholar]
- 48.Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- 49.Kammerer RA, et al. Heterodimerization of a functional GABAB receptor is mediated by parallel coiled-coil alpha-helices. Biochemistry. 1999;38:13263–13269. doi: 10.1021/bi991018t. [DOI] [PubMed] [Google Scholar]
Methods references
- 50.Berger I, Fitzgerald DJ, Richmond TJ. Baculovirus expression system for heterologous multiprotein complexes. Nat. Biotechnol. 2004;22:1583–1587. doi: 10.1038/nbt1036. [DOI] [PubMed] [Google Scholar]
- 51.Kabsch W. Xds. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evans P. Scaling and assessment of data quality. Acta Crystallogr. D. Biol. Crystallogr. 2006;62:72–82. doi: 10.1107/S0907444905036693. [DOI] [PubMed] [Google Scholar]
- 53.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 54.Kerr DI, Ong J, Doolette DJ, Schafer K, Prager RH. The (S)-enantiomer of 2-hydroxysaclofen is the active GABAB receptor antagonist in central and peripheral preparations. Eur. J. Pharmacol. 1995;287:185–189. doi: 10.1016/0014-2999(95)00641-9. [DOI] [PubMed] [Google Scholar]
- 55.Frydenvang K, et al. GABAB antagonists: resolution, absolute stereochemistry, and pharmacology of (R)- and (S)-phaclofen. Chirality. 1994;6:583–589. doi: 10.1002/chir.530060712. [DOI] [PubMed] [Google Scholar]
- 56.McCoy AJ, et al. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 58.Roversi P, Blanc E, Vonrhein C, Evans G, Bricogne G. Modelling prior distributions of atoms for macromolecular refinement and completion. Acta Crystallogr. D. Biol. Crystallogr. 2000;56:1316–1323. doi: 10.1107/s0907444900008490. [DOI] [PubMed] [Google Scholar]
- 59.Chen VB, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Novotny M, Madsen D, Kleywegt GJ. Evaluation of protein fold comparison servers. Proteins. 2004;54:260–270. doi: 10.1002/prot.10553. [DOI] [PubMed] [Google Scholar]
- 61.Morin A, et al. Collaboration gets the most out of software. Elife. 2013;2:e01456. doi: 10.7554/eLife.01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dombkowski AA. Disulfide by Design: a computational method for the rational design of disulfide bonds in proteins. Bioinformatics. 2003;19:1852–1853. doi: 10.1093/bioinformatics/btg231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.