Abstract
Background
Irritable bowel syndrome (IBS) with mixed bowel habits (IBS-M) is a heterogeneous subtype with varying symptoms of constipation and diarrhea, and has not been well characterized. We aimed to characterize gastrointestinal (GI) and non-GI symptoms in IBS-M patients from a U.S. community, and to compare them with IBS with constipation (IBS-C) and diarrhea (IBS-D).
Methods
Subjects answering community advertisements and meeting Rome III criteria for IBS completed symptom questionnaires.
Key Results
Of the initial 289 IBS patients identified, one-third (n=51, 32.5%) who met Rome III criteria for IBS-M endorsed having either loose stools or hard stools due to medication. These patients had more severe symptoms and longer duration of flares compared to the rest of the IBS-M group (p = 0.014, p = 0.005). Excluding IBS-M patients with medication-related extremes in stool form who could not be reclassified by medical history, 247 IBS patients were assessed. IBS-M was the most common (44.1%), followed by IBS-C (27.9%), IBS-D (26.3%), and IBS-U (unsubtyped, 1.6%). IBS-M shared symptoms with both IBS-C and IBS-D (p-value range: <0.001–0.002). IBS-M patients reported most bothersome symptoms more similarly to IBS-D, with the most common being irregular bowel habits (27.5%), bloating (26.6%), and abdominal pain (20.2%). There were no differences in non-GI symptoms between subtypes.
Conclusions & Inferences
IBS-M is a heterogeneous symptom group and thus requires that subclassification criteria be better defined. Use of laxative/anti-diarrheal medications adds to the diagnostic complexity in a potentially more severe subset of IBS-M and should be assessed for accurate subclassification.
Keywords: irritable bowel syndrome, diarrhea, constipation, mixed bowel habits, abdominal pain
Introduction
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder (FGID) characterized by abdominal pain or discomfort associated with changes in bowel habit. According to the Rome III bowel habit subclassification, (1) IBS is subtyped into four categories based on stool form alone: IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), IBS with a mixed pattern (IBS-M), and unsubtyped (IBS-U). Studies demonstrate that IBS-M is the most common clinical subtype with a prevalence of 30-63%. (2-5) A recent meta-analysis suggested that the three main IBS subtypes have equal distribution, however it should be noted that these results were drawn from only a small subset of the meta-analysis studies and subtypes were defined using different criteria including Manning, Rome I, Rome II, or Rome III. (6)
Prior to the Rome III subclassification criteria, IBS was usually subgrouped into only IBS-C or IBS-D using a more complicated combination of bowel related symptoms including stool form, stool frequency, urgency, and straining. (7) Patients who did not meet criteria for either were typically defined as non-C, non-D IBS or IBS with alternating bowel habits (IBS-A). In 2006, the Rome III Functional Bowel Disorders committee redefined the IBS bowel habit subgroups and defined IBS-M as having mixed stool forms (i.e., both hard/lumpy and loose/watery at least 25% of evacuations). The current Rome III subclassification is easier to use, as it is based solely on the prevalence of stool form, which correlates with colonic transit time, (8, 9) and has been shown to be the most reliable and sensitive criteria for subtype differentiation. (10)
However, IBS-M remains a heterogeneous group and is characterized by varying symptoms commonly associated with IBS-C and IBS-D, thus posing challenges both in clinical practice and research. While many studies have characterized bowel habits in IBS-C and IBS-D, there are few that have evaluated IBS-M, particularly in those classified by Rome III criteria in a U.S. population. Many that have done so using the Rome III criteria have been performed in non-U.S. based populations and mostly at larger tertiary care centers. (11-13)
While new IBS-specific drugs continue to emerge, most clinical trials have focused primarily on IBS-C or IBS-D. There remains a paucity of treatments specifically for IBS-M given its heterogeneous nature and lack of accepted and valid patient reported outcome measures (PROs) for this group. There are only a few published clinical trials that specifically evaluated pharmaceutical treatments in the IBS-M subtype. (5, 14) However, these studies only used a binary primary endpoint, which is no longer recommended by regulatory agencies including the Food and Drug Administration (FDA). A lack of understanding of the IBS-M subgroup is further demonstrated by the exclusion of enrollment and responder criteria for IBS-M in the recent FDA guidance for IBS clinical trials. (15)
Thus, the main aims of this study are 1) to characterize the IBS-M subtype based on the Rome III criteria in patients recruited from a U.S. community, and 2) to compare clinical characteristics of IBS-M with IBS-C and IBS-D.
Methods
IBS subject recruitment and screening
IBS patients were recruited between 2007-2012 mainly by advertisements within a diverse urban population and on-campus at an academic university. All participants met Rome III diagnostic criteria (1) and were evaluated by a gastroenterologist with expertise in IBS to confirm that subjects met symptom-based diagnostic criteria during the medical interview. At the time of their initial visit, subjects were asked to complete each of the questionnaires listed below. Informed consent was individually obtained from each patient. This study was approved by the Institutional Review Board and was conducted in accordance with institutional guidelines regulating human subject research.
Bowel Symptom Questionnaire
A bowel symptom questionnaire (BSQ), which included the Rome III diagnostic questions for IBS, bowel habit subtypes, dyspepsia, heartburn, and demographic characteristics, was administered to all subjects. (16) Dyspepsia was assessed by asking, “How often did you have pain or burning in the middle of your abdomen, above your belly button but not in your chest” and “How often did you feel uncomfortably full after a regular-sized meal.” They had to report having either symptom on at least one day per week and for at least six months. The presence of heartburn was determined if the patient answered “yes” to the question, “In the last 3 months, did you have heartburn, a burning pain or discomfort in your chest, for at least 3 weeks (lasting at least one day each week)?” This questionnaire also characterized the severity of symptoms, duration of flares and remissions, description of typical symptoms, and most bothersome symptoms. Choices for typical and most bothersome symptom included: bloating, visible abdominal distension, sensation of fullness in rectum after BMs, urgency, nausea, abdominal pain, and irregular bowel habits. Duration of flares and remissions were also asked with options for flares being: less than 1 hour, less than 24 hours, 1-3 days, 4-7 days, less than 1 month, less than 1 year, and greater than 1 year (coded as 1-7, 1 =less than 1 hour and 7=greater than 1 year). Remissions had the same options minus the choice of answering “greater than 1 year”.
Patients were subclassified based solely on the Rome III classification into the four subtypes of IBS-M, IBS-C, IBS-D, and IBS-U using patient-reported symptoms on the BSQ. Two of the questions ask about the prevalence of stool form: “In the last 3 months, how often did you have loose, mushy, or watery stools?” and “In the last 3 months, how often did you have hard or lumpy stools?” Subjects then had the option of answering “never or rarely”, “about 25% of the time”, “about 50% of the time”, “about 75% of the time”, or “always, 100% of the time”. IBS-C subjects were identified as those answering hard/lumpy stools at least 25% of the time and loose/watery stools less than 25% of the time. IBS-D subjects were identified as having loose/watery stools at least 25% of the time and having hard/lumpy stools less than 25% of the time. IBS-M subjects were identified as answering both loose/watery stools at least 25% of the time and hard/lumpy stools at least 25% of the time. IBS-U subjects did not meet any of the criteria above and were identified as having loose/watery stools less than 25% of the time and hard/lumpy stools less than 25% of the time.
Medication effects on stool form were also assessed. To avoid misclassification of IBS-M, patients who met Rome III subclassification criteria for IBS-M were excluded or reclassified based on additional information from the medical history if they answered “I usually have hard, lumpy stools and develop loose, mushy, or watery stools only after using a laxative” or “I usually have loose, mushy, or watery stools and develop hard, lumpy stools only after taking an antidiarrheal.” To further characterize this subject group (referred to as “IBS-M Medication Group”), symptoms were compared to that of the remaining group of IBS-M patients.
IBS-M Subanalysis
Given the heterogeneity of bowel habits in IBS-M, GI symptom characteristics were analyzed to determine if there were subgroups within the IBS-M group. Stool form patterns were evaluated by measuring the prevalence of loose/watery stools (referred to as diarrhea) and hard/lumpy stools (referred to as constipation). Stool form patterns were separated into three subgroups depending on whether they showed a majority of hard/lumpy stools, loose/watery stools, or equal proportions: “constipation > diarrhea”, “diarrhea > constipation”, or “equal constipation & diarrhea”. The distribution of stool frequency was also further categorized into “abnormal BM frequencies” and “normal BM frequencies”. “Abnormal BM frequencies” were defined as having BMs more than 3 times a day, less than 3 times a week, or having both at least 25% of the time. Patients with “normal BM frequencies” did not report any of the above. Interactions between stool form, stool frequency, and most bothersome symptoms were also analyzed.
Non-GI Symptom Questionnaires
Validated questionnaires were administered to assess psychological and somatic symptoms and health-related quality of life (HRQOL). A seven-question subscale of the full Coping Strategies Questionnaire (CSQ) was used to measure catastrophizing as a coping strategy. (17) Scores were averaged with higher scores indicating greater catastrophizing. The Hospital Anxiety and Depression Scale (HAD) is a widely used 14-item questionnaire for assessing current symptoms of anxiety and depression. (19) The Patient Health Questionnaire (PHQ-15) is a somatic symptom subscale of the full PHQ covering the most common outpatient physical complaints and most prevalent somatic symptoms in the DSM-IV somatization disorder. (20) A PHQ-12 questionnaire, excluding three questions on GI symptoms to measure extraintestinal somatic symptoms only, was used in this study. Higher scores indicate greater somatic symptom severity. The Visceral Sensitivity Index (VSI) is a validated 15-item questionnaire developed to assess GI symptom-specific anxiety. (21) Lower scores indicate greater symptom-specific anxiety. The 12-Item Short Form Health Survey (SF-12) is a validated short form of the original 36-Item Short-Form Health Survey (SF-36) used to measure HRQOL. (22, 23) Physical and Mental Component Summary Scores (PCS & MCS) were calculated with 0 being the lowest level of health.
Statistical Analysis
Group comparisons among bowel habits and across IBS-M medication status were evaluated using a Kruskal-Wallis, Chi-square, Fisher's exact test, or multinominal regression. Irregularity of bowel habits, typical symptoms and most bothersome symptom differences among the bowel habits were examined using a multinomial regression while controlling for racial/ethnicity differences. In addition, differences in irregularity of bowel habits, typical symptoms and most bothersome symptom and differences among the IBS-M subgroups based on predominant stool frequency and form and between IBS-M “medication” and “non-medication” groups were evaluated using Chi-square test or Fisher's exact test. Abdominal pain severity, presence of dyspepsia, presence of heartburn, psychological symptoms, or bowel movement frequencies were compared among IBS-C, IBS-D, and IBS-M using a Kruskal-Wallis test. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA) or R version 2.14.1 (http://cran.r-project.org/). A p-value less than 0.05 was considered to be significant for all comparisons. There was no adjustment for multiple testing because the sample size was relatively small and this was considered to be an exploratory study.
Results
Subject clinical characteristics
The demographic and clinical symptom data are shown in Tables 1 and 2, respectively. Figure 1 shows the study subject selection flowchart. Of the initial 289 subjects who met Rome III criteria for IBS, two were excluded because a subtype could not be determined due to incomplete answers. Almost one-half (47.7%) reported having seen a doctor in the past year for their abdominal symptoms. About one-third (51 patients, 32.5%) of the initial 157 IBS-M patients answered positively to having either loose/watery stools or hard stools only when taking laxatives or anti-diarrheal medications. Forty of these patients were excluded from the comparison of IBS subtypes analysis after not being able to definitively conclude a true IBS bowel habit subclassification from review of the medical histories. Of the remaining 11, three remained IBS-M after review of medical histories, seven were reclassified to IBS-C, and one to IBS-D. The final analysis included 247 Rome III positive IBS patients with IBS-M being the most common subtype (109 patients, 44.1%), followed by IBS-C (69 patients, 27.9%), IBS-D (65 patients, 26.3%), and IBS-U (4 patients, 1.6%).
Table 1. Demographic Characteristics.
| Total IBS1 (n=247) | IBS-C (n=69) | IBS-D (n=65) | IBS-M (n=109) | p-value | |
|---|---|---|---|---|---|
| Age, mean (S.D.) | 35.8 (12.6) | 37.2 (12.8) | 34.8 (12.1) | 35.8 (12.7) | 0.589 |
| Gender, female, N (%) | 188 (76.1%) | 54 (78.3%) | 45 (69.2%) | 88 (80.7%) | 0.209 |
| Education, N (%) | 0.201 | ||||
| 8th grade or less | 0 | 0 | 0 | 0 | |
| Some high school | 1 (0.4%) | 0 | 1 (1.5%) | 0 | |
| High school graduate | 16 (6.5%) | 9 (13.0%) | 3 (4.6%) | 4 (3.7%) | |
| Some college | 70 (28.3%) | 22 (31.9%) | 18 (27.7%) | 29 (26.6%) | |
| College graduate, N (%) | 93 (37.7%) | 23 (33.3%) | 26 (40.0%) | 43 (39.4%) | |
| Post-graduate work | 66 (26.7%) | 15 (21.7%) | 17 (26.2%) | 33 (30.3%) | |
| Healthcare Utilization | |||||
| >3 visits/yr, N (%) | 37 (15.0%) | 10 (14.5%) | 11 (16.9%) | 16 (14.7%) | 0.898 |
| Race2, N (%) | 0.036 | ||||
| Hispanic | 38 (15.4%) | 9 (13.0%) | 12 (18.5%) | 17 (15.6%) | |
| Asian | 23 (9.3%) | 2 (2.9%) | 12 (18.5%) | 9 (8.3%) | |
| Black | 25 (10.1%) | 11 (15.9%) | 5 (7.7%) | 9 (8.3%) | |
| White | 137 (55.5%) | 40 (58.0%) | 28 (43.1%) | 66 (60.6%) | |
| Other/Multiracial | 20 (8.1%) | 6 (8.7%) | 7 (10.8%) | 6 (5.5%) | |
| Decline to State | 4 (1.6%) | 1 (1.5%) | 1 (1.5%) | 2 (1.8%) |
IBS-U was not included due to small sample size (n=4).
Statistically significant racial/ethnic differences detected across subtypes (p=0.036)
Table 2. Clinical Characteristics.
| Total IBS (n=247) | IBS-C (n=69) | IBS-D (n=65) | IBS-M (n=109) | p-value | |
|---|---|---|---|---|---|
| Abdominal Pain Severity | |||||
| 0-20pt scale, mean (S.D.) | 9.82 (4.65) | 9.69 (4.96) | 9.65 (4.95) | 9.94 (4.28) | 0.961 |
| Mild to moderate, N (%) | 169 (68.4%) | 44 (63.8%) | 46 (70.8%) | 75 (68.8%) | 0.815 |
| Bloating Pain Severity | |||||
| 0-20pt scale, mean (S.D.) | 11.45 (5.17) | 12.09 (5.35) | 11.14 (5.35) | 11.24 (4.98) | 0.627 |
| Presence of dyspepsia, N (%) | 123 (49.8%) | 32 (46.4%) | 37 (56.9%) | 51 (46.8%) | 0.384 |
| Presence of heartburn, N (%) | 60 (24.3%) | 14 (20.3%) | 16 (24.6%) | 30 (27.5%) | 0.528 |
| Psychological symptoms, mean (S.D.) | |||||
| CSQ (0-6) | 1.44 (1.24) | 1.54 (1.48) | 1.25 (0.99) | 1.53 (1.21) | 0.459 |
| HAD Anxiety (0-21) | 7.48 (4.35) | 6.65 (4.33) | 7.55 (4.21) | 8.06 (4.44) | 0.113 |
| HAD Depression (0-21) | 3.82 (3.58) | 4.10 (4.14) | 3.42 (3.15) | 3.96 (3.46) | 0.617 |
| PHQ-12 (0-24) | 6.49 (4.18) | 6.65 (4.86) | 6.13 (3.63) | 6.68 (4.06) | 0.753 |
| VSI (0-75) | 36.39 (16.25) | 34.93 (15.46) | 35.29 (16.93) | 38.83 (15.93) | 0.281 |
| HRQOL(SF-12) | |||||
| PCS (0-100) | 48.51 (9.50) | 48.82 (9.84) | 47.53 (11.04) | 49.07 (8.37) | 0.825 |
| MCS (0-100) | 45.93 (10.53) | 47.57 (9.91) | 45.13 (10.18) | 44.91 (11.15) | 0.391 |
Abbreviations: CSQ, Coping Strategies Questionnaire; HAD, Hospital Anxiety and Depression Scale; PHQ12, Patient Health Questionnaire; SF-12, 12-Item Short Form Health Survey; VSI, Visceral Sensitivity Index; HRQOL, health related quality of life; PCS, physical composite score; MCS, mental composite score.
Figure 1.
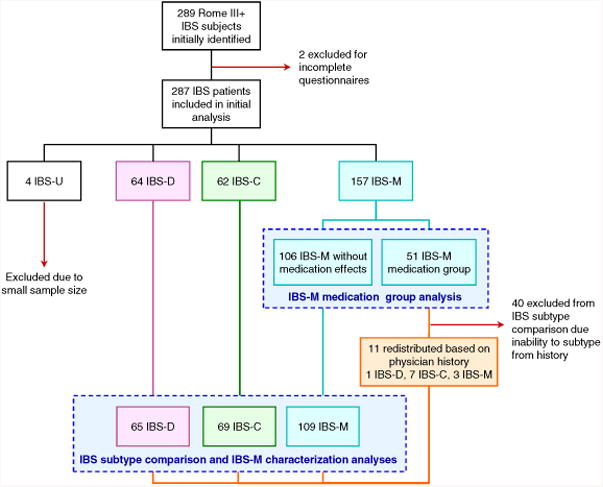
Flowchart depicting subjects selection and sample sizes for each analysis. Dashed boxes indicate groups included in each of the separate analyses: IBS-M medication group assessment and final IBS subtype characterizations.
Mean age, prevalence of women, and education did not differ between bowel habit subtypes. The mean age overall was 35.8 ± 12.6 (range: 18-70 yr). Women comprised the majority of the group, about 76% of the subjects. Race/ethnicity across IBS bowel habit subtypes with more African-Americans comprising the IBS-C group and more Asians and less Caucasians in the IBS-D group (p=0.036).
IBS-M Medication Group
Compared with the 106 IBS-M patients without extreme stool forms only from medications, the 51 patients in the IBS-M medication group had greater severity of GI symptoms in the past week (11.62 ± 4.26 vs. 10.14 ± 3.84, p=0.014) with longer periods of flares (3.76 ± 1.76+ 1.76vs. 3.02 ± 1.64, p=0.005). There was also a higher prevalence of dyspepsia (66.7% vs. 46.2%, p=0.018). Furthermore, a greater proportion of the medication group reported sensation of incomplete evacuation (100% vs. 87.7%, p=0.010), severity of bloating (12.9 ± 5.26 vs. 11.22 ± 4.99, p=0.022), and infrequent BMs (BM < 3×/wk) (48.8% vs. 36.8%, p=0.011). There were no differences in gender, age, race, or psychological symptoms.
Comparison of IBS-M to IBS-C and IBS-D
Prevalence of IBS Symptoms
As shown in Figure 2, there were significant differences among IBS bowel habit subtypes for each of the following symptoms: abnormal BM frequencies (<3 BMs/week or >3BMs/day), straining with defecation, incomplete emptying after defecation, the need for manual evacuation after defecation, and difficulty relaxing or letting go to allow the stool to come out during defecation (p-value range: <0.001 – 0.002). Straining, incomplete evacuation, and the need for manual evacuation were higher in IBS-C and IBS-M than in IBS-D. IBS-M had the largest proportion of patients reporting the need for manual evacuation (43.1%, p=0.003). In terms of bowel movement frequencies, IBS-M tended to be in between IBS-C and IBS-D with approximately 40% reporting <3 BMs/week and >3 BMs/day. The majority of IBS patients reported mild to moderate abdominal pain severity (68.4%). There were no significant differences in abdominal pain and bloating symptom severity ratings, presence of dyspepsia or heartburn symptoms among the IBS bowel habit subtypes. Psychological symptoms and HRQOL measures also did not differ among IBS subtypes (Table 2).
Figure 2.
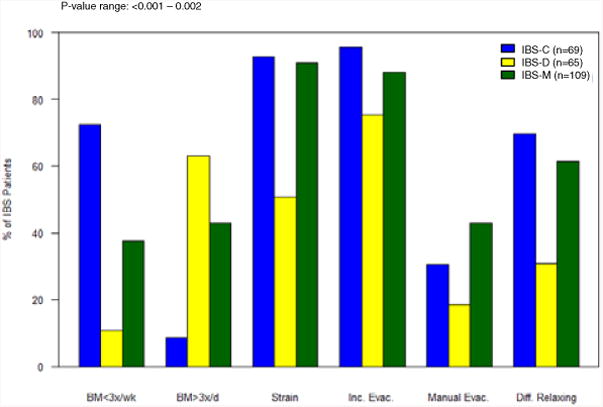
Frequency of associated bowel habit symptoms compared across subtypes (IBS-C, IBS-D, and IBS-M). All symptoms included were found to be significantly different among subtypes (p-value range =<0.001 to 0.002). Bowel movements less than three times a week, bowel movements more than three times a day, straining with defecation, incomplete evacuation, need for manual evacuation after defecation, and difficulty relaxing to let stool come out.
Prevalence of Typical IBS Symptoms
When asked to characterize their “typical symptoms”, the three most common in all IBS subtypes were irregular bowel habits, bloating, and abdominal pain with over 85% reporting each symptom (Figure 3). All typical symptoms were reported similarly between subtypes except for urgency and nausea. Urgency was more common in IBS-D (76.9%) and IBS-M (75.2%) and significantly less in IBS-C (34.8%) (p<0.001). Nausea was significantly more common in IBS-M (43.1%) than in IBS-C (24.6%) and IBS-D (26.2%) (p=0.014).
Figure 3.
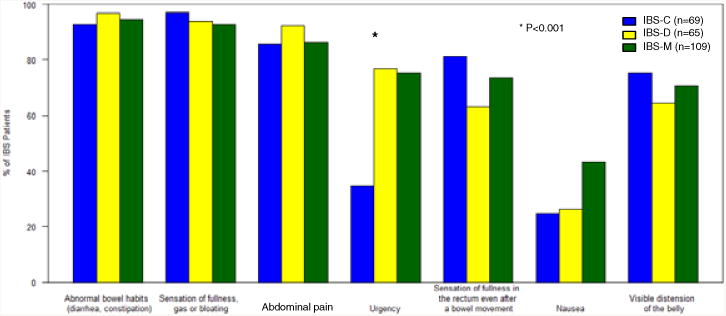
Frequency of typical symptoms IBS subjects reported to experiencing on a usual basis. There was a significant difference among subtypes for reporting urgency (p<0.001) and a trend for a difference in nausea (p = 0.014).
Most Bothersome Symptom
Across the three bowel habit subgroups, when asked, “If you could get rid of your single most bothersome symptom, which one would you choose?”, the prevalence of the most bothersome symptoms significantly differed (p<0.001). As shown in Figure 4, the three most commonly reported most bothersome symptoms in IBS-M and IBS-D were irregular bowel habits (27.5% IBS-M, 27.7% IBS-D), bloating (26.6%, 20.0%), and abdominal pain (20.2%, 23.1%). Interestingly, while IBS-C patients also endorsed irregular bowel habits (39.1%) and bloating (31.9%) as the most bothersome symptoms, abdominal pain (7.2%) was chosen less often.
Figure 4.
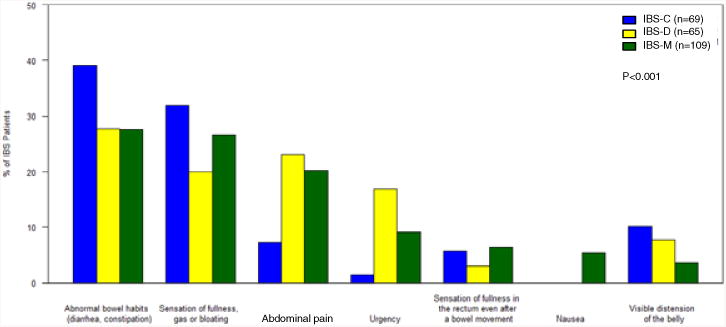
Frequency of the single most bothersome symptom identified by each subject and compared across subtypes (IBS-C, IBS-D, IBS-M). There were differences among IBS subtypes across all symptoms (p<0.001).
Additional Symptom Characterization in IBS-M
Duration of Symptom Flares and Remission
The majority of IBS-M flares and remissions were of short duration, with flares tending to be shorter than remissions. Over 85% of IBS-M patients reported having flares lasting less than one week with three-fourths of patients (75.2%) endorsing flares lasting less than 3 days. Remissions lasting less than one week were reported by 84.4% of IBS-M patients. Most IBS-M patients experienced at least two flares per year (79.8%). Flare and remission patterns were not statistically different between IBS-M and the other IBS subtypes.
Predominant bowel habit patterns within IBS-M patients
Within the IBS-M subtype, stool form distribution showed a trend of having a higher proportion with 42.2% reporting more diarrhea than constipation, 24.8% reporting more constipation than diarrhea and 33.0% reporting equal amounts of diarrhea and constipation (p=0.082).
The distribution of BM frequency in IBS-M showed that most IBS-M patients (63.3%) experienced abnormal BM frequencies, with 20.2% of IBS-M patients only having BMs less than 3 times per a week, 25.7% only having BM more than 3 times per a day, and 17.4% having both extremes of BM frequencies. However, it is notable that over one-third of IBS-M patients had normal BM frequencies (36.7%).
Comparison of bowel habit symptoms in IBS-M subgroups based on most bothersome symptom
In order to determine if symptom patterns differed within the IBS-M group based on the predominant symptom, we compared symptom characteristics in IBS-M patients who reported irregular bowel habits, abdominal pain, or bloating as the most bothersome symptom (Supplemental Figure 1). Eighty percent of IBS-M patients who were most bothered by irregular bowel habits reported having abnormal BM frequency compared to 20% who had normal frequency (p=0.001) (Supplemental Figure 2). However, similar proportions of patients reported abnormal or normal BM frequencies within the patient groups who were most bothered by abdominal pain or bloating. There were no statistically significant differences in stool form distribution in the three IBS-M subgroups based on the most bothersome symptom.
Discussion
IBS-M by nature is a highly heterogeneous subgroup due to a range of symptoms that are similar to those associated with IBS-C and IBS-D. Our main study findings were: 1) IBS-M is the most common subtype (44.1%) in our patient population using Rome III criteria, 2) About a third of IBS-M patients may be potentially misclassified due to medication effects causing loose or hard stools, 3) The IBS-M medication group tends to have more severe and prolonged symptoms than the remaining two-thirds of IBS-M patients 4) The majority of IBS-M flares and remissions are relatively short, 5) Upper and lower GI symptoms and psychological symptoms reported by IBS-M are similar to that in IBS-C and IBS-D, 6) The most commonly reported most bothersome symptoms in IBS-M are irregular bowel habits, bloating, and abdominal pain, and 7) Two-thirds of IBS-M patients report abnormal BM frequencies at least 25% of the time, while about one-third have normal BM frequencies.
A surprising and important finding in our study was that up to a third of IBS-M patients could be potentially misclassified due to medication effects on stool form. Unlike the other subtypes, IBS-M patients responded to answers suggesting that they only experienced both extremes of stool form due to a laxative or antidiarrheal agent. Both of these medication types are commonly used and available over-the-counter. Thus, a significant proportion of IBS-M patients defined by the Rome III subclassification criteria may be potentially misclassified, which is important to know for clinical management and research. However, in our study medical histories provided more information to reclassify only a subgroup of these patients. Therefore, we do not know if the majority of these patients were misclassified. Regardless, this IBS-M medication group tended to have more severe and prolonged bowel symptoms than the remaining IBS-M group. This suggests that a group of IBS patients with more severe symptoms use medications to the point where they may overshoot a targeted normal stool form. It is possible that these patients have IBS-M but the greater severity and longer duration of symptoms provoke use of medications to regulate bowel habits. Alternatively, the use of medications can be associated with extremes of stool form and therefore lead to more severe and prolonged symptoms. With the notable rate of medication usage in IBS-M, and the increased severity in this group, our results emphasize the importance of accurate documentation of medication effects on stool form for improved clinical management and accurate establishment of IBS bowel habit subclassification.
With regard to comparisons to the other IBS subtypes, IBS-M patients reported symptoms commonly endorsed by both IBS-C (straining, incomplete evacuation, manual evacuation) and IBS-D (urgency). This mixed pattern of constipation symptoms with the addition of urgency in IBS-M is consistent with past Rome II studies. (3, 10) However, due to other variables, certain studies in the past have shown IBS-M to be more similar to IBS-C based on similarities in stool frequency, consistency, psychological symptoms, and a higher likelihood of transition between these two subtypes longitudinally. (2, 10) The recent study by Weinland et al following episodes over 14 days also found IBS-M to be more similar to IBS-C. (26)
However, during our further analysis, IBS-M tended to be more similar to IBS-D. IBS-M patients reported a greater proportion of diarrhea, and they also showed greater similarities in the most bothersome symptoms with IBS-D than with IBS-C. This may be different from previous studies because we excluded patients who had mostly loose stools with laxatives and thus could have IBS-C rather than IBS-M.
Aside from symptoms shared with IBS-C and IBS-D, we also found that IBS-M showed a trend for having a higher occurrence of nausea. While nausea has not historically been a common finding within IBS, Schmulson et al also reported a higher frequency of dyspeptic symptoms of halitosis and vomiting within Rome III IBS-M patients compared to other subtypes. (11) We further evaluated the prevalence of Rome III dyspepsia across IBS subtypes but did not find any differences in distribution. We did not specifically confirm whether patients had functional dyspepsia, which would require exclusion of organic upper GI conditions, nor did we assess symptom criteria for the two subtypes of functional dyspepsia.
While it is clear that IBS patients experience multiple symptoms, determining the typical symptoms and most bothersome symptom can help determine if subgroups exist within each subtype and help guide future clinical research studies and treatment. When asked to choose a single most bothersome symptom, it was not surprising that the three most prevalent ones in IBS-M were irregular bowel habits, abdominal pain, and bloating. (10, 30) The prevalence of irregular bowel habits and abdominal pain are likely inherently high due to being part of the diagnostic criteria for IBS, (1) but, previous studies have shown that bloating is highly prevalent (31-33) and can be the most bothersome symptom. (32, 33) However, because bloating is a common symptom in many FGIDs including IBS, it has not been used as a diagnostic symptom since its discriminative value would be low, but it is clearly a common and bothersome symptom that requires attention in clinical management and clinical trials.
Interestingly, over a third of all IBS-M patients do not experience any extremes of stool frequency at least 25% of the time. With regard to stool form, there are fairly evenly distributed groups within IBS-M of having predominantly diarrhea, constipation, or equal prevalence. Greater characterization of bowel habits in IBS-M would help in better defining this IBS subtype. In fact, recent studies have shown that prospective daily assessment of bowel habits would reclassify many IBS-M patients into IBS-U due to the lower actual prevalence of stool form extremes. (27)
Study limitations include the fact that this study is a cross-sectional assessment of IBS-M symptoms and does not address prospective fluctuations. Furthermore, our use of a questionnaire for subtyping and symptom reporting has its own limitations, such as recall bias. (34) Also, a recent study postulated that it is likely that patients can overestimate their bowel habits on questionnaires. (27) To mitigate this issue our questionnaire used answer options with numbers (e.g., “about 25% of the time”) as opposed to just words (e.g., “sometimes”). Another source of bias can come from the nature of patient recruitment through advertisements. Recruiting was also only done in a single region, the Western United States, and may not be indicative of patients from other regions. Lastly, this study is exploratory in nature. Due to the small sample sizes within subtypes and the multiple questions explored, the statistical significance of our findings should be interpreted with caution. Further studies are needed to validate our findings.
In summary, IBS-M patients continue to be the most common subtype based on Rome III criteria. More emphasis and attention should be placed on properly obtaining information regarding medication impact on stool form as we have shown that up to a third of IBS-M patients may otherwise be misclassified and may only have mixed stool forms due to medications. Furthermore, this medication group may represent a more severe and difficult to manage subgroup of IBS. IBS-M continues to share symptoms of both constipation and diarrhea with the addition of a higher occurrence of nausea. However, in this study IBS-M appeared to report most bothersome symptoms more similarly to IBS-D. These study results will help provide supportive information when considering symptom management, development of patient reported outcomes (PROs), and design of treatment trials for IBS-M.
Supplementary Material
Supplemental Figure 1. Distribution of stool form in the top three reported most bothersome symptoms in IBS-M (irregular bowel habits, abdominal pain, and bloating). No significant differences were found in distribution of stool form predominance in irregular bowel habits (BH) (P=0.123), abdominal pain (P=0.108), and bloating (P=0.638).
Supplemental Figure 2. Distribution of bowel movement frequency in the top three reported most bothersome symptoms in IBS-M (irregular bowel habits, abdominal pain, and bloating). Irregular bowel movement frequencies included those experiencing bowel movements less than three times a week, more than three times a day, or both, at least 25% of the time. Normal bowel movement frequencies included those not having bowel movements less then three times a week nor more than three times a day at least 25% of the time. Differences in distribution of stool form frequency were significant in irregular bowel habits (BH) (P=0.001), but not abdominal pain (P=0.669) or bloating (P=0.194).
Acknowledgments
Financial support: NIH P50 DK64539
Grant funding: NIH P50 DK64539
Footnotes
Specific author contributions: Andrew Su contributed to study design and implementation, data analysis and interpretation, manuscript preparation, and approval of the final manuscript. Wendy Shih contributed to data analysis and interpretation, manuscript preparation, and approval of the final draft. Angela Presson contributed to data analysis and interpretation, manuscript preparation, and approval of the final draft. Lin Chang contributed to data collection, study design and implementation, data analysis and interpretation, manuscript preparation, and approval of the final draft.
Potential Competing Interests: None
Disclosure: The authors have nothing to disclose.
Conflict of Interest/Study Support: Guarantor of the article: Lin Chang
References
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006 Apr;130(5):1480–91. doi: 10.1053/j.gastro.2005.11.061. Epub 2006/05/09. eng. [DOI] [PubMed] [Google Scholar]
- 2.Drossman DA, Morris CB, Hu Y, Toner BB, Diamant N, Leserman J, et al. A prospective assessment of bowel habit in irritable bowel syndrome in women: defining an alternator. Gastroenterology. 2005 Mar;128(3):580–9. doi: 10.1053/j.gastro.2004.12.006. Epub 2005/03/15. eng. [DOI] [PubMed] [Google Scholar]
- 3.Mearin F, Balboa A, Badia X, Baro E, Caldwell E, Cucala M, et al. Irritable bowel syndrome subtypes according to bowel habit: revisiting the alternating subtype. Eur J Gastroenterol Hepatol. 2003 Feb;15(2):165–72. doi: 10.1097/00042737-200302000-00010. Epub 2003/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 4.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003 Mar 1;17(5):643–50. doi: 10.1046/j.1365-2036.2003.01456.x. Epub 2003/03/19. eng. [DOI] [PubMed] [Google Scholar]
- 5.Spiller RC, Meyers NL, Hickling RI. Identification of patients with non-d, non-C irritable bowel syndrome and treatment with renzapride: an exploratory, multicenter, randomized, double-blind, placebo-controlled clinical trial. Dig Dis Sci. 2008 Dec;53(12):3191–200. doi: 10.1007/s10620-008-0295-x. Epub 2008/05/10. eng. [DOI] [PubMed] [Google Scholar]
- 6.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012 Jul;10(7):712–21 e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Drossman DA, Corazziari E, Talley NJ, Thompson GW, Whitehead WE. Rome II: The Functional Gastrointestinal Disorders. Degnon Associates Inc; 2000. [Google Scholar]
- 8.O'Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ. 1990 Feb 17;300(6722):439–40. doi: 10.1136/bmj.300.6722.439. Epub 1990/02/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Probert CS, Emmett PM, Cripps HA, Heaton KW. Evidence for the ambiguity of the term constipation: the role of irritable bowel syndrome. Gut. 1994 Oct;35(10):1455–8. doi: 10.1136/gut.35.10.1455. Epub 1994/10/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tillisch K, Labus JS, Naliboff BD, Bolus R, Shetzline M, Mayer EA, et al. Characterization of the alternating bowel habit subtype in patients with irritable bowel syndrome. Am J Gastroenterol. 2005 Apr;100(4):896–904. doi: 10.1111/j.1572-0241.2005.41211.x. Epub 2005/03/24. eng. [DOI] [PubMed] [Google Scholar]
- 11.Schmulson M, Vargas JA, Lopez-Colombo A, Remes-Troche JM, Lopez-Alvarenga JC. Prevalence and clinical characteristics of the IBS subtypes according to the Rome III criteria in patients from a clinical, multicentric trial. A report from the Mexican IBS Working Group. Rev Gastroenterol Mex. 2010 Oct-Dec;75(4):427–38. Epub 2010/12/21. “Prevalencia y caracterizacion de los subtipos de SII segun los criterios de Roma III, en un estudio clinico, multicentrico. Informe del grupo mexicano de estudio para el SII”. Spa. [PubMed] [Google Scholar]
- 12.Yao X, Yang YS, Cui LH, Zhao KB, Zhang ZH, Peng LH, et al. Subtypes of irritable bowel syndrome on Rome III criteria: a multicenter study. J Gastroenterol Hepatol. 2012 Apr;27(4):760–5. doi: 10.1111/j.1440-1746.2011.06930.x. Epub 2011/09/21. eng. [DOI] [PubMed] [Google Scholar]
- 13.Jamali R, Jamali A, Poorrahnama M, Omidi A, Jamali B, Moslemi N, et al. Evaluation of health related quality of life in irritable bowel syndrome patients. Health Qual Life Outcomes. 2012;10:12. doi: 10.1186/1477-7525-10-12. Epub 2012/01/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chey WD, Pare P, Viegas A, Ligozio G, Shetzline MA. Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol. 2008 May;103(5):1217–25. doi: 10.1111/j.1572-0241.2008.01808.x. Epub 2008/05/15. eng. [DOI] [PubMed] [Google Scholar]
- 15.Guidance for Industry Irritable Bowel Syndrome —Clinical Evaluation of Drugs for Treatment. U.S. Department of Health and Human Services FaDA, Center for Drug Evaluation and Research (CDER); May, 2012. editor. [Google Scholar]
- 16.Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989 Oct 15;111(8):671–4. doi: 10.7326/0003-4819-111-8-671. Epub 1989/10/15. eng. [DOI] [PubMed] [Google Scholar]
- 17.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983 Sep;17(1):33–44. doi: 10.1016/0304-3959(83)90125-2. Epub 1983/09/01. eng. [DOI] [PubMed] [Google Scholar]
- 18.Robinson ME, Riley JL, 3rd, Myers CD, Sadler IJ, Kvaal SA, Geisser ME, et al. The Coping Strategies Questionnaire: a large sample, item level factor analysis. Clin J Pain. 1997 Mar;13(1):43–9. doi: 10.1097/00002508-199703000-00007. Epub 1997/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 19.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983 Jun;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. Epub 1983/06/01. eng. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002 Mar-Apr;64(2):258–66. doi: 10.1097/00006842-200203000-00008. Epub 2002/03/27. eng. [DOI] [PubMed] [Google Scholar]
- 21.Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004 Jul 1;20(1):89–97. doi: 10.1111/j.1365-2036.2004.02007.x. Epub 2004/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 22.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996 Mar;34(3):220–33. doi: 10.1097/00005650-199603000-00003. Epub 1996/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–83. Epub 1992/06/11. eng. [PubMed] [Google Scholar]
- 24.Garrigues V, Mearin F, Badia X, Balboa A, Benavent J, Caballero A, et al. Change over time of bowel habit in irritable bowel syndrome: a prospective, observational, 1-year follow-up study (RITMO study) Aliment Pharmacol Ther. 2007 Feb 1;25(3):323–32. doi: 10.1111/j.1365-2036.2006.03197.x. Epub 2007/01/16. eng. [DOI] [PubMed] [Google Scholar]
- 25.Dorn SD, Morris CB, Hu Y, Toner BB, Diamant N, Whitehead WE, et al. Irritable bowel syndrome subtypes defined by Rome II and Rome III criteria are similar. J Clin Gastroenterol. 2009 Mar;43(3):214–20. doi: 10.1097/MCG.0b013e31815bd749. Epub 2009/07/23. eng. [DOI] [PubMed] [Google Scholar]
- 26.Weinland SR, Morris CB, Hu Y, Leserman J, Bangdiwala SI, Drossman DA. Characterization of episodes of irritable bowel syndrome using ecological momentary assessment. Am J Gastroenterol. 2011 Oct;106(10):1813–20. doi: 10.1038/ajg.2011.170. Epub 2011/06/08. eng. [DOI] [PubMed] [Google Scholar]
- 27.Palsson OS, Baggish JS, Turner MJ, Whitehead WE. IBS patients show frequent fluctuations between loose/watery and hard/lumpy stools: implications for treatment. Am J Gastroenterol. 2012 Feb;107(2):286–95. doi: 10.1038/ajg.2011.358. Epub 2011/11/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okeke EN, Ladep NG, Adah S, Bupwatda PW, Agaba EI, Malu AO. Prevalence of irritable bowel syndrome: a community survey in an African population. Ann Afr Med. 2009 Jul-Sep;8(3):177–80. doi: 10.4103/1596-3519.57241. Epub 2009/11/04. eng. [DOI] [PubMed] [Google Scholar]
- 29.Miwa H. Prevalence of irritable bowel syndrome in Japan: Internet survey using Rome III criteria. Patient Prefer Adherence. 2008;2:143–7. Epub 2008/01/01. eng. [PMC free article] [PubMed] [Google Scholar]
- 30.Bijkerk CJ, de Wit NJ, Stalman WA, Knottnerus JA, Hoes AW, Muris JW. Irritable bowel syndrome in primary care: the patients' and doctors' views on symptoms, etiology and management. Can J Gastroenterol. 2003 Jun;17(6):363–8. doi: 10.1155/2003/532138. quiz 405-6. Epub 2003/06/19. eng. [DOI] [PubMed] [Google Scholar]
- 31.Ringel Y, Williams RE, Kalilani L, Cook SF. Prevalence, characteristics, and impact of bloating symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009 Jan;7(1):68–72. doi: 10.1016/j.cgh.2008.07.008. quiz 3. Epub 2009/01/07. eng. [DOI] [PubMed] [Google Scholar]
- 32.Lembo T, Naliboff B, Munakata J, Fullerton S, Saba L, Tung S, et al. Symptoms and visceral perception in patients with pain-predominant irritable bowel syndrome. Am J Gastroenterol. 1999 May;94(5):1320–6. doi: 10.1111/j.1572-0241.1999.01009.x. Epub 1999/05/11. eng. [DOI] [PubMed] [Google Scholar]
- 33.Schmulson M, Lee OY, Chang L, Naliboff B, Mayer EA. Symptom differences in moderate to severe IBS patients based on predominant bowel habit. Am J Gastroenterol. 1999 Oct;94(10):2929–35. doi: 10.1111/j.1572-0241.1999.01440.x. Epub 1999/10/16. eng. [DOI] [PubMed] [Google Scholar]
- 34.Manning AP, Wyman JB, Heaton KW. How trustworthy are bowel histories? Comparison of recalled and recorded information. Br Med J. 1976 Jul 24;2(6029):213–4. doi: 10.1136/bmj.2.6029.213. Epub 1976/07/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Distribution of stool form in the top three reported most bothersome symptoms in IBS-M (irregular bowel habits, abdominal pain, and bloating). No significant differences were found in distribution of stool form predominance in irregular bowel habits (BH) (P=0.123), abdominal pain (P=0.108), and bloating (P=0.638).
Supplemental Figure 2. Distribution of bowel movement frequency in the top three reported most bothersome symptoms in IBS-M (irregular bowel habits, abdominal pain, and bloating). Irregular bowel movement frequencies included those experiencing bowel movements less than three times a week, more than three times a day, or both, at least 25% of the time. Normal bowel movement frequencies included those not having bowel movements less then three times a week nor more than three times a day at least 25% of the time. Differences in distribution of stool form frequency were significant in irregular bowel habits (BH) (P=0.001), but not abdominal pain (P=0.669) or bloating (P=0.194).


