Abstract
Objective
During the Hepatitis C Antiviral Long-term Treatment against Cirrhosis Trial, 3.5 years of maintenance peginterferon-alfa-2a therapy did not affect liver fibrosis progression or clinical outcomes among 1,050 prior interferon nonresponders with advanced fibrosis or cirrhosis. We investigated whether reduced hepatic inflammation was associated with clinical benefit in 834 patients with a baseline and follow-up biopsy 1.5 years after randomization to peginterferon or observation.
Methods
Relationships between change in hepatic inflammation (Ishak HAI) and serum ALT, fibrosis progression and clinical outcomes after randomization, and HCV RNA decline before and after randomization were evaluated. Histologic change was defined as a ≥2-point difference in HAI or Ishak fibrosis score between biopsies.
Results
Among 657 patients who received full-dose peginterferon/ribavirin “lead-in” therapy before randomization, year-1.5 HAI improvement was associated with lead-in HCV RNA suppression in both randomized treated (P <0.0001) and control (P = 0.0001) groups, even in the presence of recurrent viremia. This relationship persisted at year 3.5 in both treated (P = 0.001) and control (P = 0.01) groups. Among 834 patients followed for a median of 6 years, fewer clinical outcomes occurred in patients with improved HAI at year 1.5 compared to those without such improvement in both treated (P = 0.03) and control (P = 0.05) groups. Among patients with Ishak 3–4 fibrosis at baseline, those with improved HAI at year 1.5 had less fibrosis progression at year 1.5 in both treated (P = 0.0003) and control (P = 0.02) groups.
Conclusion
Reduced hepatic inflammation (measured 1.5 and 3.5 years after randomization) was associated with profound virological suppression during lead-in treatment with full-dose peginterferon/ribavirin and with decreased fibrosis progression and clinical outcomes, independent of randomized treatment.
Keywords: Liver inflammation, HCV, Hepatitis C, Hepatic fibrosis, HALT-C Trial
INTRODUCTION
Treatment with pegylated interferon-α plus ribavirin cures 40–50% of persons with chronic hepatitis C. The remaining patients have persistent viremia and the potential for progressive hepatic inflammation and fibrosis.1 Although some patients with untreated chronic hepatitis C may have nonprogressive liver disease, insidious progression to cirrhosis occurs in at least 20–25% of patients over two or more decades, increasing the risk for hepatic decompensation, hepatocellular carcinoma and liver-related death.2
The achievement of a sustained virologic response (SVR), i.e., absence of detectable hepatitis C virus (HCV) RNA for at least 24 weeks following a course of antiviral therapy, is associated with a marked reduction in the incidence of end-stage liver disease and its consequences of decompensated liver disease and death.3 Even in the absence of SVR, 40–75% of treated patients have been reported to achieve histologic benefit from interferon-based therapy.4, 5 In a key study, investigators analyzed 53 patients with a wide range of liver fibrosis who exhibited reduction of HCV RNA levels (but not SVR) and improvement in hepatic inflammation during an initial course of interferon monotherapy. Among these patients, two years of additional maintenance therapy was associated with continued suppression of hepatic necroinflammatory activity but not with significantly reduced fibrosis progression.6
These early observations provided the rationale for the prospective, multicenter Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial. The primary goal of the HALT-C Trial was to determine whether 3.5 years of treatment with half-dose maintenance peginterferon-alfa therapy could prevent fibrosis progression and/or the development of hepatic decompensation and other clinical outcomes in prior interferon nonresponders with advanced hepatic fibrosis (Ishak fibrosis score ≥3). Our intention-to-treat analysis of the 1,050 patients enrolled in the HALT-C Trial indicated that maintenance peginterferon treatment did not yield clinical benefit.7 In a recent sub-analysis of this cohort, however, we demonstrated that patients with ≥4 log10 virologic suppression during full-dose peginterferon/ribavirin lead-in therapy had fewer clinical outcomes over the 3.5 years after randomization compared to those without such profound virologic suppression.8 In the current analysis, we investigated the relationship between peginterferon/ribavirin-induced virologic suppression and changes in hepatic inflammation based on serial liver biopsies separated by 1.5 and 3.5 years in this large cohort of patients with advanced liver disease. Furthermore, we evaluated improvement in hepatic inflammation as a potential mechanism by which reduced ALT levels, hepatic fibrosis progression, and clinical outcomes (up to 8.5 years) could have occurred in a subset of HALT-C Trial patients.
MATERIALS AND METHODS
Patients and Study Design
The design9 and results7 of the HALT-C Trial have been described. The current analysis was limited to 834 HALT-C participants who had baseline and follow-up liver biopsies at year 1.5 (per protocol) and were randomized to receive either half-dose peginterferon therapy (90 µg/week) or no treatment for 3.5 years. Thirteen patients who had follow-up biopsies but no HCV RNA measurements, or were randomized to observation but received peginterferon outside of the study, or were randomized to half-dose peginterferon and received full-dose interferon or ribavirin were also excluded. Patients had bridging fibrosis (Ishak fibrosis stage 3–4) or cirrhosis (Ishak fibrosis stage 5–6) on a screening liver biopsy (baseline). Of the 834 patients in this analysis, 657 were treated with full-dose, standard-of-care peginterferon-alfa-2a (180 µg/week) and ribavirin (1,000–1,200 mg/day) during a “lead-in” phase of the HALT-C Trial. Patients with HCV RNA undetectable by PCR assay (Roche COBAS Amplicor HCV Test, v. 2.0) at treatment week 20 were defined as responders and received a total of 48 weeks of full-dose peginterferon/ribavirin treatment with follow-up monitoring through week 72. SVR was defined as HCV RNA undetectable by PCR at week 72. All week 20 responders who experienced subsequent virologic breakthrough during or relapse after peginterferon/ribavirin therapy were offered randomization into the main 3.5-year HALT-C Trial, and randomization occurred at a median of 26 weeks (range ~7 to 61 weeks) after viral relapse. Nonresponders, defined as patients with PCR-detectable HCV RNA levels at week 20 of lead-in therapy, were randomized to half-dose peginterferon or observation at week 24. After 3.5 years of treatment or observation, continuation of follow-up every 6 months was offered to all patients through October, 2009 (median follow-up of 6 years, range 0–8.5 years).
Clinical and other laboratory data were collected from all patients according to standard procedures and intervals described below.9 The Institutional Review Boards of all participating institutions approved the study protocols, and written informed consent was obtained prior to participation from all study patients.
Testing for HCV RNA and serum ALT levels
Serum samples were frozen at −80°C at each clinical site, then shipped on dry ice and tested in real time at the University of Washington Virology Laboratory with both the quantitative Roche COBAS™ Amplicor HCV Monitor Test, v. 2.0 assay (lower limit of detection [LLOD] 600 IU/mL) and, if negative, by the Roche COBAS Amplicor HCV Test, v. 2.0 assay (or PCR assay, LLOD 100 IU/mL) as described previously.10, 11 HCV genotypes were determined with the INNO-LiPA HCV II kit (Siemens Medical Solutions Diagnostics, Tarrytown, NY).
Patients were classified into groups according to changes in their HCV RNA levels during the lead-in phase and after randomization. The mean of screening and pretreatment log10 HCV RNA levels was used as the baseline value. Log10 changes from baseline to week 20 (during the lead-in phase) were calculated by subtracting the level at week 20 from baseline. Change in HCV RNA during the first 1.5 years after randomization was calculated by subtracting the mean of the levels at 0.5, 1.0, and 1.5 years after randomization from baseline. Both were classified as <2, 2 - <4, and ≥4 log10 changes, as previously described.8 To calculate these means, we assigned HCV RNA-negative results by the qualitative Roche COBAS Amplicor HCV Test a log10 value of 2.00 (100 IU/mL), and HCV RNA-negative results by the quantitative Roche COBAS™ Amplicor HCV Monitor Test but positive by the more sensitive qualitative Roche COBAS Amplicor HCV Test a log10 value of 2.78 (600 IU/mL).
Serum ALT levels, tested at each clinical site every 3 months, are expressed as ALT divided by the upper limit of normal (ULN). The baseline value was the mean of the screening and pretreatment ALT levels. Percent ALT change was calculated with the following formula: [(mean ALT between year 0.5 through year 1.5 – mean baseline ALT) / mean baseline ALT] x 100%. ALT improvement was defined as an ALT decrease by >25%, ALT worsening was defined as an ALT increase by >25%, and patients with no change in ALT were those with an ALT change ≤25% of baseline. The percent ALT change for years 0.5 to 3.0 was calculated with a similar formula. Year 3.0 was used instead of 3.5 because many patients stopped their interferon treatment between years 3.0 and 3.5.
Liver Histology
All patients underwent screening liver biopsy (baseline) within 12 months prior to enrollment. All biopsy specimens were assigned a consensus histologic score by a team of 11 hepatopathologists. Both inflammatory (modified HAI) and fibrosis scoring were based upon the criteria of Ishak et al.12 Follow-up biopsies were performed at 1.5 and 3.5 years after randomization. Change in hepatic inflammation or fibrosis was defined as a ≥2-point increase (worse) or ≥2-point decrease (improved) at 1.5 or 3.5 years after randomization when compared to the baseline biopsy score.
Definition of Clinical and Fibrosis Outcomes
Clinical outcomes for all patients were defined prospectively as hepatic decompensation (bleeding esophageal varices, ascites, spontaneous bacterial peritonitis, or hepatic encephalopathy)13 an increase in the Child-Turcotte-Pugh (CTP) score to ≥7 14 on two consecutive study visits 3 months apart, hepatocellular carcinoma,15 or death from any cause. For patients with bridging fibrosis (Ishak fibrosis score of 3 or 4) at study entry, an additional criterion for disease progression (fibrosis outcome) was a ≥2-point increase in the Ishak score at the time of the year 1.5 or 3.5 liver biopsies.
Statistical analyses
We used SAS® (Statistical Analysis Software, Cary, NC) version 9.1 for statistical analyses. Baseline variables in the control or peginterferon groups were compared by chi-square or t-test. The Mantel-Haentszel chi-square test for trend was used for 2×3 tables of improvement in one variable versus ordered categories in the other. We used logistic regression for the analysis of predictors of improvement in HAI and ALT. To evaluate the change in mean ALT from baseline to the mean of the values at years 0.5 to 1.5 and 0.5 to 3.0, we used analysis of variance adjusting for baseline variables. We applied Kaplan-Meier estimates of the clinical outcome rates at 7 years and Cox proportional hazards regression analyses, stratified by the presence of cirrhosis at baseline, to assess the effect of changes in HCV RNA during the lead-in phase and the effect of changes from baseline in hepatic inflammation and ALT on clinical outcomes. Hazard ratios (HR) and 95% confidence intervals are reported. The Cox regression analyses included the entire follow-up period, from randomization through 8.5 years or October 20, 2009, whichever occurred first. Forty patients with outcomes prior to the year −1.5 biopsy were excluded because they did not have the year −1.5 biopsy, but 18 patients with outcomes before year 1.5 were included. Analyses were performed for both treatment groups combined, including treatment group as a predictor variable, and separately by treatment group. In most instances, the latter analysis is reported because differences in HAI and ALT between the treatment groups might confound the association of improvement with other variables.
RESULTS
Characteristics of the study cohort
All patients in this analysis were required to have had a year-1.5 liver biopsy. Table 1 shows the baseline clinical, demographic, laboratory, and histologic characteristics of this cohort (N = 834) according to treatment status. Patients in the treatment group were older and more likely to have HCV genotype 1 infection, but the differences were small.
Table 1.
Baseline Characteristics of Study Subjects
| Control | PEG IFN | P-value | |
|---|---|---|---|
| Patient Number | 414 | 420 | NA |
| Nonresponders, % | 63.5 | 62.4 | 0.891 |
| Breakthrough/Relapsers, % | 15.9 | 15.7 | |
| Express,2 % | 20.5 | 21.9 | |
| Age | 49.6 (6.7) | 51.0 (7.3) | 0.005 |
| Male, % | 72.7 | 70.5 | 0.48 |
| Caucasian, % | 72.2 | 71.7 | 0.86 |
| Body Mass Index | 29.8 (5.2) | 29.7 (5.3) | 0.83 |
| ALT/ULN | 2.22 (1.79) | 2.10 (1.59) | 0.32 |
| Log10 HCV RNA | 6.45 (0.50) | 6.46 (0.50) | 0.93 |
| Genotype 1, % | 92.3 | 95.9 | 0.02 |
| Ishak Inflammation | 7.5 (2.0) | 7.6 (2.1) | 0.49 |
| Ishak Fibrosis | 4.1 (1.3) | 4.0 (1.2) | 0.58 |
| Ishak Fibrosis 5–6, % | 39.4 | 38.1 | 0.71 |
All values represent Means (± SD) unless otherwise indicated.
Compares the distribution of all 3 subsets (Nonresponders, Breakthrough/Relapsers and Express patients) between Control and Treated groups.
Express patients received full-dose peginterferon/ribavirin therapy outside of the HALT-C Trial prior to enrollment.7
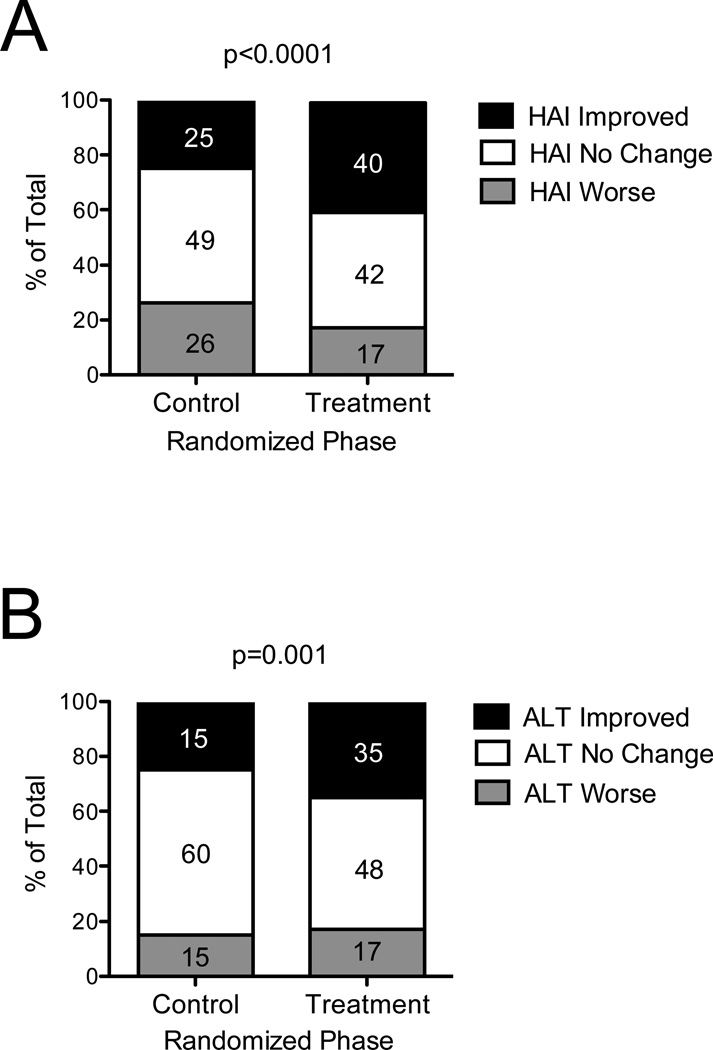
Although our previous intention-to-treat analysis of 1,050 HALT-C Trial patients demonstrated no clinical benefit of maintenance peginterferon treatment, we reported modest but statistically significant reductions in hepatic inflammation (HAI, hepatic activity index), and serum ALT and HCV RNA levels in treated patients.7 Figure 1 shows, by treatment group, the frequency of patients who experienced improvement, no change, or worsening of HAI on their year-1.5 biopsy compared to baseline as well as changes in serum ALT to year 1.5 compared to baseline. For all analyses involving histologic liver inflammation, we chose to focus mainly on the year-1.5 results (N = 834; N = 414 control and N=420 treated) because the sample size was substantially smaller at year 3.5 (N = 652); however, the analyses of the year-3.5 data were consistent with those found at year 1.5, as discussed below.
Figure 1. Distribution of Changes in Hepatic Inflammation and ALT Between Baseline and Year 1.5 According to Randomized Treatment Status.
Patients were categorized according to their randomization to observation or treatment (x-axis) and whether hepatic inflammation improved or worsened by ≥2 points or did not change (within 2 points) on the modified Ishak HAI score between baseline and year-1.5 liver biopsies (A). ALT improvement was defined as ALT decrease by >25%, ALT worsening as ALT increase by >25%, and no change as ALT change ≤25% between baseline and year 1.5 (B). Serum ALTs were measured every 3 months and ALT change was calculated using the formula: [(mean ALT between year 0.5 through year 1.5 – mean baseline ALT) / mean baseline ALT] x 100%. The percentage of patients in each group is shown. P values are based on a comparison between control (n = 414) and treated groups (n = 420) in the percentage of patients with improved HAI or ALT.
The mean change in HAI from baseline to year 1.5 was 0.12 (95% CI −0.12 to 0.35) in the control group and −0.87 (95% CI −1.10 to −0.64) in the treated group (P <0.0001 for control versus treated). The significant difference between control and treated groups involved three of the four components of the HAI18 (parenchymal injury P <0.0001, periportal inflammation P <0.0001, and portal inflammation P = 0.002). The fourth component, confluent necrosis, could not be analyzed because only one patient had any necrosis scored on the year-1.5 biopsy. Similar results were found for the change to year 3.5.
Relationships between HCV RNA suppression and hepatic inflammation and serum ALT levels
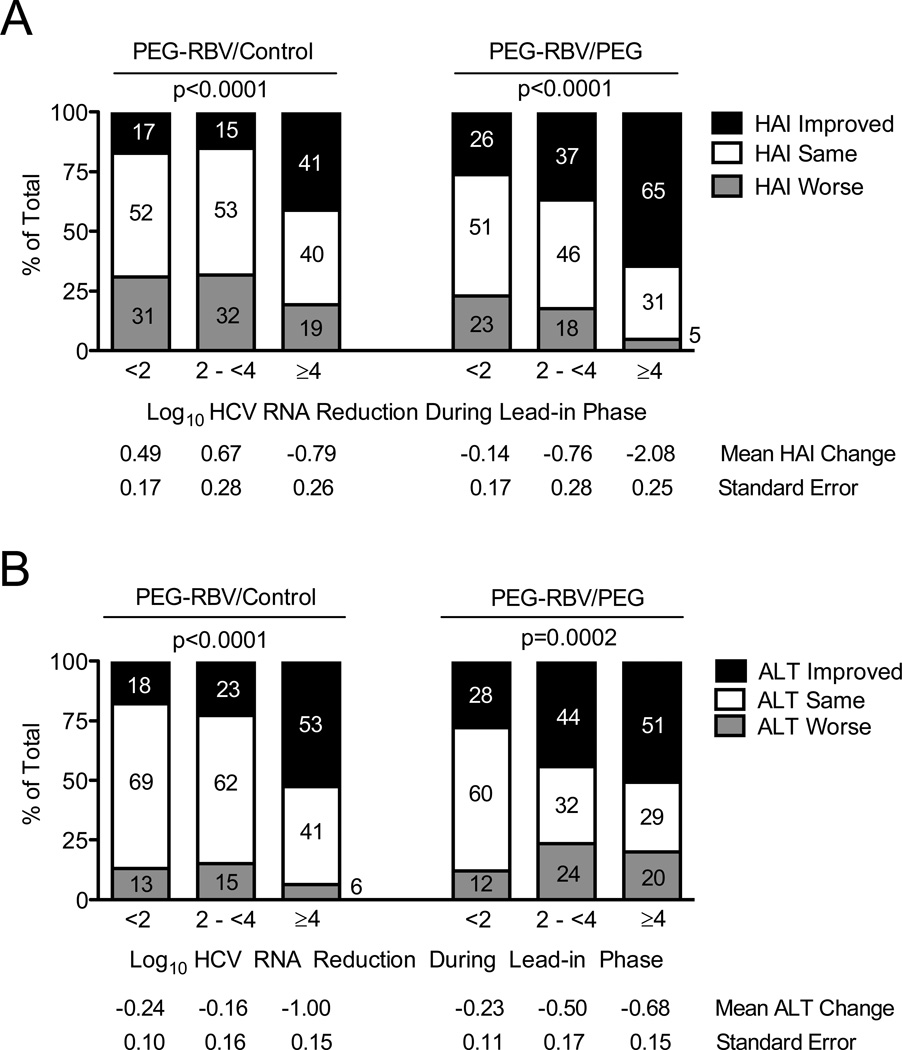
The majority of patients in this analysis underwent lead-in treatment with full-dose peginterferon/ribavirin therapy immediately preceding randomization to maintenance peginterferon or observation (N = 657). To investigate the relationships between virological suppression and improvement in hepatic inflammation and/or ALT levels, we analyzed HAI improvement according to randomization status and the degree of HCV RNA suppression at week 20 of lead-in therapy (Figure 2A). A higher proportion of patients with improved HAI had greater reduction in HCV RNA levels during the preceding lead-in phase in both the control (P <0.0001) and treated groups (P <0.0001). This finding was also reflected in greater mean HAI improvement according to greater HCV RNA level reduction. A substantial proportion of patients in both the treated and control groups (31–53%), including those with maximal virological suppression (≥4 log10) during lead-in treatment (31–40%), demonstrated no change in hepatic inflammation on the year-1.5 biopsy. Some patients experienced worsening of hepatic inflammation in both the control (19–31%) and peginterferon (5–23%) groups. Worth noting, HCV RNA levels in 94% of control-group patients and 57% of treated-group patients were within 0.5 log10 of baseline levels by year 0.5 (6 months after completion of lead-in therapy); moreover, HCV viremia persisted at this level through collection of the year-1.5 and −3.5 biopsies.
Figure 2. HCV RNA Suppression During Lead-in Therapy is Associated with Improvement in Hepatic Inflammation and ALT During the Randomized Phase.
Patients were categorized according to their randomized untreated control (left) or treated status (right) and their degree of HCV RNA suppression during the lead-in phase of the HALT-C Trial (x-axis): <2 log10, 2-<4 log10, and ≥4 log10 decrease between baseline and week 20. Percentages of patients with improvement or worsening of hepatic inflammation by ≥2 points or with no change between baseline and year-1.5 biopsies are indicated on the y-axis and by the numbers in the columns (A). The mean change in HAI and associated standard error for the group of patients represented by each column is shown below the x-axis. The percentage of patients with ALT improvement (ALT decrease by >25%), ALT worsening (ALT increase by >25%) and no change (ALT change ≤25%) between baseline and year 1.5 are shown on the y-axis and by the numbers in the columns (B). The mean change in ALT and associated standard error for the group of patients represented by each column is shown below the x-axis. Percent ALT change was calculated as described for Figure 1. P values are based on a chi-square test for trend in the % improved. The number of patients represented by each column are as follows: PEG-RBV/Control groups <2 log10 reduction n = 185, 2 to <4 log10 reduction n = 66, ≥4 log10 reduction n = 78; PEG-RBV/PEG groups <2 log10 reduction n = 175, 2 to <4 log10 reduction n = 68, ≥4 log10 reduction n = 85.
A larger reduction in HCV RNA levels during lead-in treatment was also associated with improvement in serum ALT between years 0.5 and 1.5 after randomization compared to baseline (Figure 2B) in both the control (P <0.0001) and treated groups (P = 0.0002). Virological response to peginterferon/ribavirin treatment during lead-in for patients included in Figure 2 was associated with expected clinical and demographic factors, as previously published (Shiffman 2004) and shown in Supplemental Tables 1 and 2, available online.
Among the 516 lead-in patients who had biopsy results at both year 1.5 and year 3.5, virologic suppression during lead-in treatment was significantly associated with improvement of hepatic inflammation on the year-3.5 biopsy in both the control and treated groups (P = 0.01 and P = 0.001, respectively). The mean (SE) changes in HAI at year 3.5 were 0.16 (0.23), 0.33 (0.37), and −0.82 (0.33) in the control group (P = 0.03) and −0.64 (0.22), −1.05 (0.34), and −2.23 (0.30) in the treated group (P = 0.0002) for HCV RNA decreases of <2, 2 - <4 and ≥4 log10, respectively. Similarly, greater HCV RNA reduction during lead-in treatment was associated with a more substantial reduction in ALT levels between years 0.5 to 3.0 of the study in both the randomized control and treated groups (P <0.0001 and P = 0.0003, respectively). The mean ALT changes according to HCV RNA decline during lead-in treatment were −0.21 (0.09), −0.14 (0.16), and −0.88 (0.15) in the control group (P = 0.0002) and −0.32 (0.10), −0.57 (0.17) and −0.79 (0.15), in the treated group (P = 0.03).
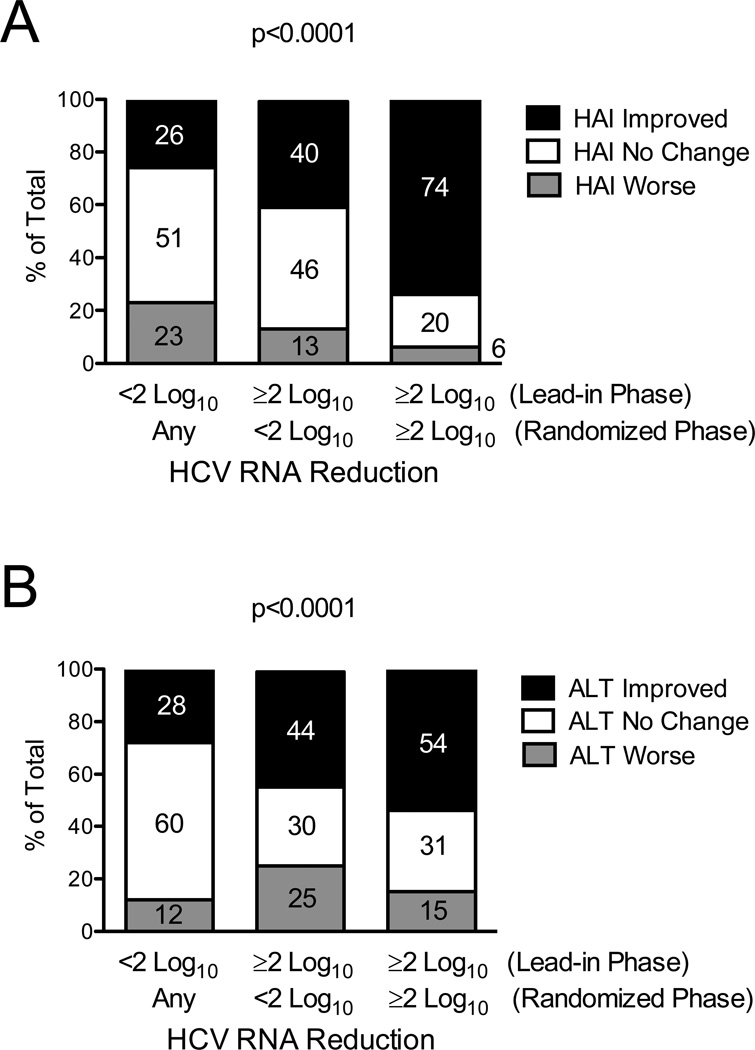
Virological suppression during maintenance peginterferon therapy was modest; however, among patients randomized to treatment (N=328), those with maximal and persistent virological suppression exhibited the greatest improvement in HAI and ALT (Figure 3). Specifically, the 54 patients who were able to maintain ≥2 log10 suppression of HCV RNA levels during both lead-in and randomized phases (Figure 3A, third column) were more likely to have improved HAI (74%) compared to the remaining 274 patients who had less virological suppression during the same time period (31%, P <0.0001, Figure 3A, columns 1 and 2). Similarly, the same 54 patients who maintained ≥2 log10 suppression of HCV RNA levels during both lead-in and randomized phases were more likely to have improved ALT (54%) than the 274 patients who had less virological suppression (34%, P = 0.007). Clinical and demographic information for patients included in Figure 3 demonstrated only differences in racial composition between the groups (see Supplemental Table 3 available online).
Figure 3. For Patients Randomized to Maintenance Peginterferon, Persistent HCV RNA Suppression During Both Lead-in and Randomized Phases is Associated with the Greatest Improvement in Hepatic Inflammation and ALT.
Patients were categorized according to their degree of HCV RNA suppression during the lead-in (week 20) and randomized phases (mean of years 0.5, 1.0, and 1.5) of the HALT-C Trial (x-axis): (1) <2 log10 between baseline and week 20 (lead-in), n = 175; (2) ≥2 log10 decrease (lead-in) and <2 log10 (randomized), n = 99; and (3) ≥2 log10 decrease (lead-in) and ≥2 log10 (randomized), n = 54. Percentages of patients with improvement or worsening of hepatic inflammation by ≥2 points or with no change between baseline and year-1.5 biopsies are indicated by the numbers in the columns and y-axis (A). The percentage of patients with ALT improvement (ALT decrease by >25%), ALT worsening (ALT increase by >25%) and no change (ALT change ≤25%) between baseline and year 1.5 are shown by the numbers in the columns and y-axis (B). Percent ALT change was calculated as in Figure 1. P values are based on a chi-square test for trend in the % improved.
Relationship between improvement in hepatic inflammation and serum ALT
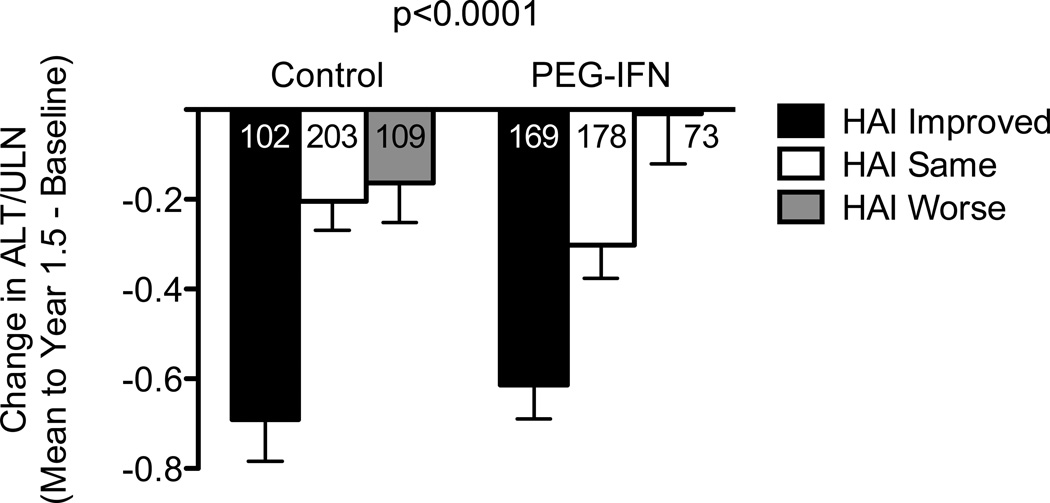
The relationship between improved serum ALT and hepatic inflammation is shown in Figure 4. Patients with improved HAI had significantly greater improvement in mean ALT than patients without improved HAI in both the control and treated groups (P <0.0001 both groups). Similar results were found when ALT data through year 3 and the year-3.5 HAI were evaluated (P = 0.004 in control group and P = 0.02 in the treated group).
Figure 4. Change in Hepatic Inflammation and Serum ALT Over Time are Related Factors.
Patients were categorized according to their randomization to observation or treatment (x-axis) and whether hepatic inflammation improved, did not change, or worsened between baseline and the year-1.5 liver biopsy by ≥2 points. Change in ALT (y-axis) was calculated as the average of the differences between the baseline ALT and the mean ALT (obtained every 3 months and averaged from year 0.5 to 1.5) for all patients in each category. The total number of patients in each column is shown. Mean ALT values were estimated by least squares regression and were adjusted for baseline ALT level. Error bars represent one standard error. Patients with improvement in hepatic inflammation had greater mean reduction in ALT than those without improvement, in both the treatment (P <0.0001) and control groups (P <0.0001).
Relationship between hepatic inflammation and histologic fibrosis progression
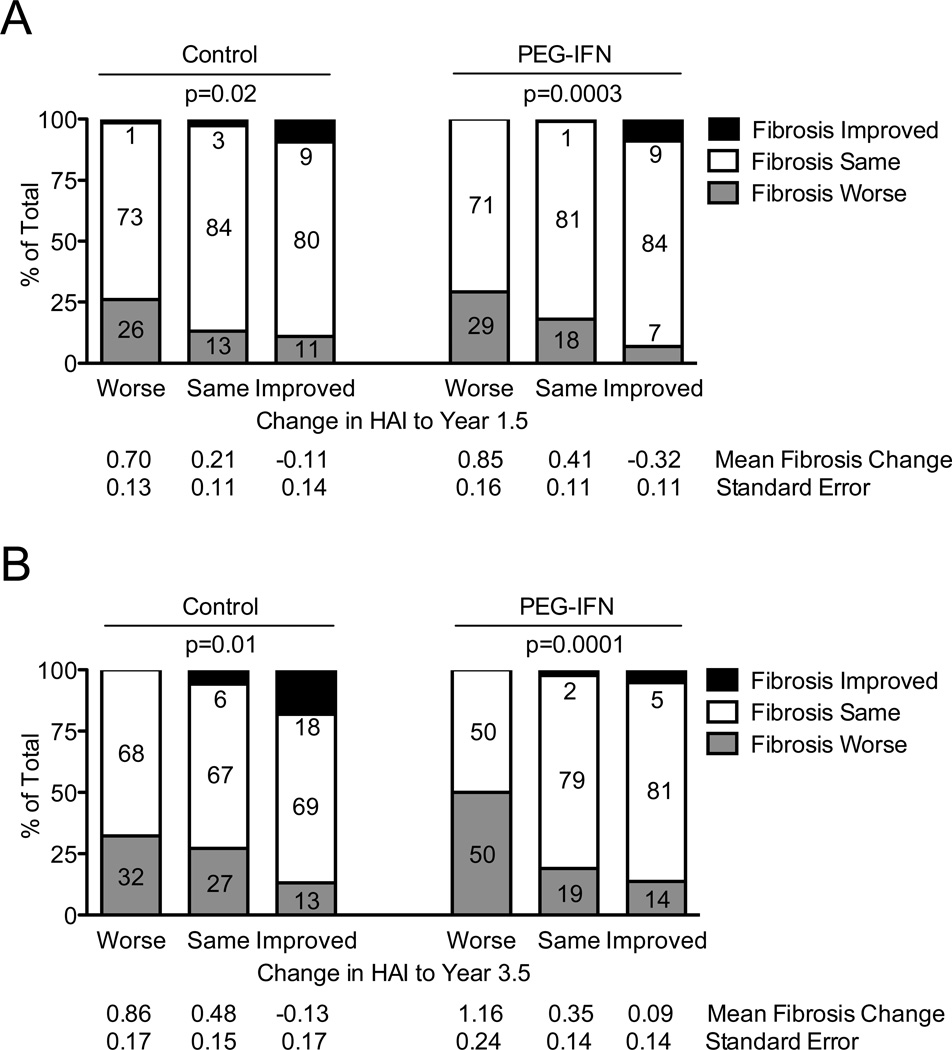
We reported previously that virologic suppression during the lead-in or randomized phases of the HALT-C Trial did not influence fibrosis progression (data not shown).8 Here we explored the relationship between HAI change and fibrosis progression in the same biopsies in patients with Ishak fibrosis scores of 3 and 4 at study entry (Figure 5). Patients with Ishak fibrosis stages 5 and 6 were excluded from this analysis because they could not progress by ≥2 points. The majority of patients (71%–84%) demonstrated no change from baseline to year 1.5 in their fibrosis scores (Figure 5A). Fibrosis progression was significantly associated with change in hepatic inflammation in both the control and treated groups (P for trend = 0.02 and 0.0003). In the control group, fibrosis progression occurred in 26% with worse HAI, in 13% with unchanged HAI, and in 11% with improved HAI. In the group randomized to maintenance peginterferon therapy, fibrosis progression occurred in 29% with worse HAI, in 18% with unchanged HAI, and in only 7% with improved HAI. The odds ratios for the association between improved HAI and fibrosis progression were 0.55, 95% CI 0.23 – 1.32, P = 0.18 in the control group and 0.27, 95% CI 0.12 – 0.65, P = 0.003 in the treated group. Mean changes in the Ishak fibrosis score also reflected the association between HAI improvement and lack of fibrosis progression. The relationship between improved HAI and reduced fibrosis progression was also evident on the year-3.5 biopsy (Figure 5B, P = 0.01 and 0.0001 in control and treated groups, respectively). We analyzed changes in inflammation and fibrosis in the same biopsies at year 1.5 (Figure 5A) or year 3.5 (Figure 5B). However, we also investigated the whether improved HAI on the year-1.5 biopsy was associated with reduced fibrosis progression on the year-3.5 biopsy or on either the year-1.5 or-3.5 biopsies considered together. In both cases, the association between improved HAI and reduced fibrosis progression held, with overall odds ratios of 0.54 (95% CI 0.32–0.92, P = 0.02, N = 427) and 0.52 (95% CI 0.33–0.81, P = 0.004, N = 511), respectively (control and treated groups combined). In this analysis, no relationship was found between treatment group and fibrosis progression. As previously reported for the full cohort,7 no significant effect of half-dose maintenance peginterferon therapy on fibrosis progression was found at either year 1.5 (P = 0.77) or year 3.5 (P = 0.78).
Figure 5. Improvement in Hepatic Inflammation Is Associated with Fibrosis Progression.
Only patients with precirrhotic fibrosis (Ishak fibrosis score 3 or 4) at study entry were included in these analyses. Patients were categorized according to their randomized untreated control (left) or treated status (right) and also according to their change in Ishak HAI between baseline and year-1.5 biopsies (A, x-axis) or between baseline and year-3.5 biopsies (B, x-axis). Percentages of patients with improvement in hepatic fibrosis (Ishak scale), worsening in hepatic fibrosis by ≥2 points (i.e., fibrosis progression), or no change (≤2 points) between baseline and year 1.5 (A) or year 3.5 (B) biopsies are shown. The percentage of patients in each group is indicated. The mean change in Ishak fibrosis score and associated standard error for each group of patients represented by a column is shown below the x-axis. Improved HAI was associated with less fibrosis progression in the same biopsy at years 1.5 and 3.5 in both control (P = 0.02 and 0.02 respectively) and treatment groups (P = 0.0003 and 0.0001), based on separate analyses for each group. P values are based on a chi-square test for trend in the % with fibrosis progression. The number of patients represented by each column are as follows: (A) change in HAI to year 1.5/control groups worse n = 73, same n = 114, improved n = 64; change in HAI to year 1.5/PEG-IFN groups worse n = 48, same n = 111, improved n = 101; (B) change in HAI to year 3.5/control groups worse n = 65, same n = 85, improved n = 61; change in HAI to year 3.5/PEG-IFN groups worse n = 32, same n = 89, improved n = 95.
In the patient subgroup with cirrhosis (Ishak stages 5 and 6) at baseline, we found a trend towards improvement in hepatic fibrosis by ≥2 points among patients with reduced hepatic inflammation by ≥2 points (fibrosis improved in 15% with worse HAI, in 21% with unchanged HAI, and in 26% with improved HAI); however, the sample sizes were relatively small (N = 61, 156, and 106, respectively), and the trend fell short of statistical significance (P = 0.08). When analyzed separately by treatment group, we found that the trend towards significance was present in the control group (P = 0.07) but not in the treated group (P = 0.44).
Improvement in hepatic inflammation and serum ALT and relationships with clinical outcomes
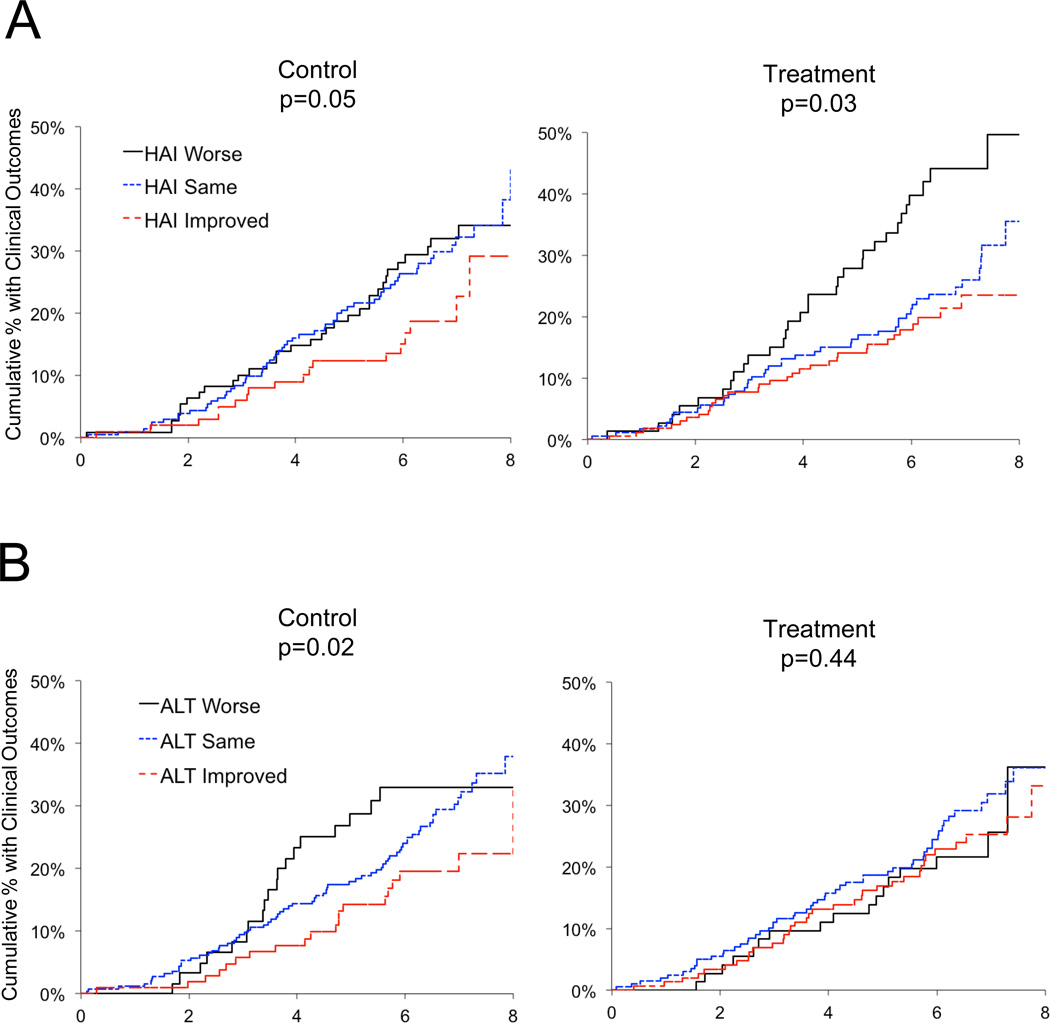
We evaluated whether improvement in hepatic inflammation and/or ALT was associated with the occurrence of predefined clinical outcomes (hepatic decompensation, hepatocellular carcinoma or death) during follow-up to 8.5 years. Fewer clinical outcomes occurred in patients with improved hepatic inflammation compared to patients without improved inflammation at year 1.5, regardless of whether patients were randomized to control (HR 0.60, 95% CI 0.36 – 0.99, P = 0.05) or treated groups (HR 0.63, 95% CI 0.41 – 0.95, P = 0.03) (Figure 6A). Maintenance therapy had no impact on reducing clinical outcomes (P = 0.67), as reported previously.7
Figure 6. Decreased Frequency of Clinical Outcomes to Year 7 in Both Untreated Control and Treated Groups is Associated with Improvement in Hepatic Inflammation at Year 1.5.
The cumulative percentage of patients with clinical outcomes (defined in Materials and Methods) from Kaplan-Meier life table analyses are shown. Patients were categorized according to their randomized untreated control or treated status and the change in hepatic inflammation between baseline and year 1.5 (y-axis) (A) or change in serum ALT between baseline and year 1.5 (y-axis) (B). For panel A, sample sizes in the control group were: HAI worse (n = 109), HAI same (n = 203), HAI improved (n = 102) and in the treated group were: HAI worse (n = 73), HAI same (n = 178), HAI improved (n = 169). For panel B, sample sizes in the control group were: ALT worse (n = 61), ALT same (n = 248), ALT improved (n = 105) and in the treated group were: ALT worse (n = 73), ALT same (n = 200), ALT improved (n = 147). P values for differences among the three groups were derived from a Cox regression analysis for the control and treated groups evaluated separately.
We also examined the relationship between ALT change to year 1.5 and clinical outcomes (Figure 6B). Fewer patients with year-1.5 improvement in ALT had clinical outcomes compared to patients without improvement in ALT in the control group (HR 0.56, 95% CI 0.34 – 0.91, P = 0.02) but not in the treated group (HR 0.85, CI 0.57 – 1.28, P = 0.44).
Consistent with our earlier report up to year 3.5,8 maximal virological suppression (≥4 log10) during lead-in treatment was associated with fewer clinical outcomes out to year 8.5 in both the control (HR 0.50, 95% CI 0.27 – 0.92, P = 0.03) and treated groups (HR 0.37, 95% CI 0.18 – 0.73, P = 0.004). When HAI change and week 20 lead-in virological suppression (<2 log10 versus ≥2 log10) were analyzed in multivariate Cox regressions for control and treated groups combined, both HAI improvement (HR 0.67, 95% CI 0.47–0.98, P = 0.04) and virological suppression during the lead-in (HR 0.57, 95% CI 0.42–0.79, P = 0.001) were associated with reduced clinical outcomes. However, when ALT improvement and week-20 lead-in virological suppression were both analyzed as predictors of outcomes, only virological suppression remained a significant predictor of outcomes. The 54 patients with persistent virological suppression ≥2 log10 during both lead-in and randomized phases had fewer clinical outcomes compared to those with virological suppression <2 log10 during lead-in or ≥2 log10 during the lead-in phase and <2 log10 during the randomized phase (P = 0.007). Finally, when both HAI improvement and ALT improvement were included in a Cox regression analysis for prediction of clinical outcomes, the hazard ratio for HAI improvement remained statistically significant (HR 0.64, 95% CI 0.46–0.89, P =0.007), but the hazard ratio for ALT improvement was no longer statistically significant (HR 0.79, 95% CI 0.58–1.08, P = 0.14).
DISCUSSION
Our previous intention-to-treat analysis of the HALT-C Trial demonstrated no effect of long-term half-dose maintenance peginterferon treatment on the occurrence of clinical outcomes and fibrosis progression in 1,050 prior interferon nonresponders with advanced hepatic fibrosis.7 The current post hoc analysis of a subset of HALT-C Trial patients expands and extends early observations on the impact of interferon-induced reduction of hepatic inflammation on fibrosis progression6 due to our focus on patients with advanced hepatic fibrosis, the measurement of clinical outcomes, the long duration of the follow-up period (up to 8.5 years) and the large sample size. We report that improved hepatic inflammation was associated with marked reductions in serum HCV RNA during the preceding 24-week lead-in phase of full-dose peginterferon/ribavirin treatment. Thus, improved hepatic inflammation in subsets of both maintenance peginterferon and control groups could be explained by the fact that all of these patients were pretreated with full-dose peginterferon/ribavirin. Patients with profound virologic suppression during the lead-in phase who were also able to maintain substantially suppressed viral levels during the randomized half-dose maintenance phase were most likely to experience improvement in hepatic inflammation. Most importantly, improved inflammation was associated with fewer clinical outcomes and less fibrosis progression compared to patients without such improvement.
We previously showed that maximal suppression of HCV RNA levels (≥4 log10) during lead-in therapy was the most important variable associated with a reduced frequency of subsequent clinical decompensation outcomes, although no direct relationship was found between HCV RNA suppression and histologic fibrosis progression.8 Here we report that HCV RNA suppression during lead-in therapy was associated with reduction of hepatic inflammation and, separately, reduced hepatic inflammation was associated with less fibrosis progression. Overall, these data confirm the close association between HCV RNA levels and hepatic inflammation and suggest that amelioration of these factors is crucial to limiting liver disease progression.6, 16
The effect of virologic suppression during lead-in therapy on reduction of hepatic inflammation appeared to be surprisingly durable even in patients with subsequent viral recrudescence, as reduced hepatic inflammation was found in a subset of patients randomized to the untreated-control group as long as 3.5 years after the cessation of peginterferon/ribavirin treatment. This observation confirms the notion that peginterferon therapy has two distinct effects that may be separable: 1) reduction of viremia and 2) long-term reduction of hepatic inflammation. This clinical observation is consistent with the many reports of the direct effects of interferon-alfa on viral replication,17 distinct from effects on proinflammatory lymphocyte function18 and on inhibition of collagen production by hepatic stellate cells.19
What could be the biological basis for the durability of the reduced hepatic inflammation induced by peginterferon/ribavirin treatment? The reduction in the hepatic inflammatory cell infiltrate and of hepatocyte death as documented in the year-1.5 and year-3.5 HAI scores, even with viral recurrence to near baseline levels, suggests the possibility that peginterferon/ribavirin may have had durable epigenetic molecular effect(s) on cells within the liver that play a role in liver inflammation. Whatever these effects might have been, peginterferon/ribavirin clearly had direct or indirect (downstream) effects on lymphocyte recruitment and activity in the liver, as indicated by reductions in portal and periportal inflammation and parenchymal injury in the HAI scores. Future detailed studies of liver samples from patients who experience maximal HCV RNA suppression will be necessary to determine what durable molecular events could account for the post-treatment effects we describe.
Worth emphasizing is the observation that the HALT-C Trial was dominated by patients who were null (virologic) responders—who experienced <2 log10 HCV RNA reduction with full-dose peginterferon/ribavirin lead-in treatment (Figure 2, N = 360 of 657). By contrast, most patients in this analysis with improved hepatic inflammation derived from the subset with profound virologic suppression (≥4 log10) by week 20 of lead-in therapy who experienced subsequent virologic breakthrough during or relapse after up to 24 more weeks of full-dose peginterferon/ribavirin treatment compared to the true nonresponders. Whether such profound and mostly sustained virological suppression (≥4 log10) by peginterferon/ribavirin treatment in this small subset was related to common host or viral characteristics among these patients is not known. Our findings are consistent with the possibilities that virological suppression can result in reduction of hepatic inflammation, that virological suppression serves as a marker of persons more likely to reduce hepatic inflammation as a consequence of treatment, or both. Moreover, our data are consistent with the notion that patients who can achieve profound virologic suppression and reduction of hepatic inflammation may have the potential to benefit from longer-term pharmacological suppression of HCV replication. On the other hand, we also identified a potential cost of maintenance peginterferon therapy: almost 50% of patients who experienced worsening of hepatic inflammation with therapy had clinical outcomes (Figure 6A). Although the increased inflammation may have been a marker for those destined to have a poor outcome rather than a result of maintenance interferon therapy, these latter findings support (our previous) conclusion that the use of long-term interferon maintenance therapy in HCV-infected nonresponder patients with advanced liver disease cannot be recommended at this time.
Our findings may help to explain why we were unable to demonstrate clinical benefit of maintenance peginterferon among the 1,050 patients enrolled in the overall HALT-C Trial. The majority of patients were true null virologic responders with a <2 log10 decrease of HCV RNA during full-dose peginterferon/ribavirin therapy. Among these patients, few demonstrated improvement of hepatic inflammation, most demonstrated no change, and some demonstrated worsening inflammation during therapy. During subsequent randomized-phase treatment with half-dose peginterferon, the vast majority of patients exhibited <2 log10 virologic suppression and relatively few patients experienced substantial virologic suppression.7 Thus, based upon the data presented here and elsewhere,8 if virologic suppression is required for clinical benefit to occur, then we speculate that the degree of virologic suppression with half-dose peginterferon therapy during the maintenance phase was insufficient to reduce hepatic inflammation and clinical and fibrosis outcomes in the entire treated group. Alternatively, the durable histologic impact of short-term virologic suppression during lead-in therapy might have had an overwhelming, confounding effect that might have obscured a difference in fibrosis progression and clinical outcomes between the randomized control and maintenance-peginterferon groups in the HALT-C Trial.
In conclusion, analysis of laboratory data and paired serial liver biopsies from a subset of well-pedigreed patients with advanced hepatic fibrosis enrolled in the HALT-C Trial demonstrates the associations between peginterferon/ribavirin-induced virologic suppression, hepatic inflammation, serum ALT levels, hepatic fibrosis progression and liver disease outcomes (of hepatic decompensation, liver cancer and death). Although maximal virologic suppression during the lead-in phase of the Trial was found to have no direct impact on fibrosis progression,8 profound suppression of HCV RNA, achieved in a small minority of patients, was found to be associated with decreased hepatic inflammation. Decreased hepatic inflammation, in turn, appeared to be a critical factor that was associated with reduced fibrosis progression and, to a lesser extent, fewer clinical outcomes. The surprisingly durable beneficial effect of peginterferon/ribavirin (3.5 years later) on hepatic inflammation was also associated with a sustained improvement of ALT, despite viral recrudescence at the time of repeat biopsy and serum laboratory measurements. These data, demonstrating the association of IFN treatment with hepatic inflammation and ALT improvement, may also help to explain earlier observations made by other groups of decreased liver-related mortality (Yoshida 2002 Gastro) and decreased rates of hepatocellular carcinoma (Yoshida 1999 Annals) associated with IFN treatment. Of note, these results also suggest potential benefit for patients with advanced liver disease currently being treated with direct-acting antiviral agents in combination with peginterferon/ribavirin, regardless of whether they achieve SVR. We conclude that reduction of hepatic inflammation appears to be an important target for future therapeutic interventions to retard the progression of hepatitis C-related liver disease.
Supplementary Material
HIGHLIGHTS.
What is current knowledge
Interferon therapy can reduce hepatic inflammation measured 6 months after completion of treatment.
Suppression of viremia by interferon therapy is associated with a decreased frequency of clinical outcomes to 3.5 years among patients with advanced liver disease but not with decreased fibrosis progression.
What is new here
Among patients with advanced liver disease, profound HCV RNA suppression induced by interferon therapy was associated with decreased hepatic inflammation as long as 3.5 years after cessation of treatment even in the presence of recurrent viremia.
Decreased hepatic inflammation, not HCV RNA suppression (directly), was associated with reduced fibrosis progression.
Decreased hepatic inflammation was associated with fewer clinical outcomes out to 8.5 years.
Reduction of hepatic inflammation appears to be a key target to retard the progression of hepatitis C-related liver disease.
ACKNOWLEDGMENTS
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (NIDDK contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID); the National Cancer Institute; the National Center for Minority Health and Health Disparities; by General Clinical Research Center and Clinical and Translational Science Center grants from the National Center for Research Resources, National Institutes of Health (NIH grant numbers are listed below); and by the Intramural Research Program of the NIH, NIDDK (M.G. Ghany). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc. (now Genentech), through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Barbara F. Banner, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Herbert L. Bonkovsky, MD, Gloria Borders, RN, Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Elizabeth M. Brunt, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066; Grant 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center) Raymond T. Chung, MD, Andrea E. Reid, MD, Atul K. Bhan, MD, Wallis A. Molchen, David P. Lundmark
University of Colorado Denver, School of Medicine, Aurora, CO: (Contract N01-DK-9-2327, Grant M01RR-00051, Grant 1 UL1 RR 025780-01) Thomas Trouillot, MD, Marcelo Kugelmas, MD, S. Russell Nash, MD, Jennifer DeSanto, RN, Carol McKinley, RN
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) John C. Hoefs, MD, John R. Craig, MD, M. Mazen Jamal, MD, MPH, Muhammad Sheikh, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633, Grant 1 UL1 RR024982-01, North and Central Texas Clinical and Translational Science Initiative) William M. Lee, MD, Thomas E. Rogers, MD, Peter F. Malet, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN, Nancy Liston, MPH
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Sugantha Govindarajan, MD, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042, Grant 1 UL1 RR024986, Michigan Center for Clinical and Health Research) Robert J. Fontana, MD, Joel K. Greenson, MD, Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Richard K. Sterling, MD, MSc, Melissa J. Contos, MD, A. Scott Mills, MD, Charlotte Hofmann, RN, Paula Smith, RN
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: T. Jake Liang, MD, David Kleiner, MD, PhD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: James E. Everhart, MD, MPH, Leonard B. Seeff, MD, Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318) David R. Gretch, MD, PhD, Minjun Chung Apodaca, BS, ASCP, Rohit Shankar, BC, ASCP, Natalia Antonov, M. Ed.
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Kristin K. Snow, MSc, ScD, Anne M. Stoddard, ScD, Teresa M. Curto, MSW, MPH, Margaret C. Bell, MS, MPH
Inova Fairfax Hospital, Falls Church, VA: Zachary D. Goodman, MD, PhD, Fanny Monge, Michelle Parks
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
Abbreviations
- CI
95% confidence interval
- HALT-C
Hepatitis C Antiviral Long-term Treatment against Cirrhosis
- ALT
alanine aminotransferase
- HAI
hepatic activity index
- ALT/ULN
alanine aminotransferase/upper limit of normal
- SVR
sustained virologic response
- HCV
hepatitis C virus
- HR
hazard ratio
- PEG IFN
pegylated interferon-alfa
- PCR
polymerase chain reaction
- CTP
Child-Turcotte-Pugh
Footnotes
This is publication #52 of the HALT-C Trial.
The HALT-C Trial was registered with clinicaltrials.gov (#NCT00006164).
FINANCIAL DISCLOSURES
Financial relationships of the authors with Hoffmann-La Roche, Inc. (now Genentech), are as follows: M. L. Shiffman is a consultant, on the speaker’s bureau, and receives research support; K. L. Lindsay was a consultant and received research support from Hoffmann-La Roche, Inc. (now Genentech), during this study and is now an employee of Tibotec, Inc. (a subsidiary of Johnson and Johnson), Titusville, NJ; G. Szabo receives research support; G. T. Everson is a consultant and receives research support; A. S. Lok is a consultant and receives research support; A. M. Di Bisceglie is a consultant and receives research support; and T. R. Morgan receives research support. Authors with no financial relationships related to this project are:
C. Morishima, J. L. Dienstag, M. G. Ghany, D. Naishadham, and E. C. Wright.
REFERENCES
- 1.Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444–2451. doi: 10.1056/NEJMct061675. [DOI] [PubMed] [Google Scholar]
- 2.Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383–398. doi: 10.1016/j.cld.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Zeuzem S, Manns MP, Hansen BE, Schalm SW, Janssen HL. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–684. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 4.Carithers RLJ, Emerson SS. Therapy of hepatitis C: meta-analysis of interferon alfa-2b trials. Hepatology. 1997;26:83S–88S. doi: 10.1002/hep.510260715. [DOI] [PubMed] [Google Scholar]
- 5.Poynard T, Leroy V, Cohard M, Thevenot T, Mathurin P, Opolon P, Zarski JP. Meta-Analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology. 1996;24:778–789. doi: 10.1002/hep.510240405. [DOI] [PubMed] [Google Scholar]
- 6.Shiffman ML, Hofmann CM, Contos MJ, Luketic VA, Sanyal AJ, Sterling RK, Ferreira-Gonzalez A, Mills AS, Garret C. A randomized, controlled trial of maintenance interferon therapy for patients with chronic hepatitis C virus and persistent viremia. Gastroenterology. 1999;117:1164–1172. doi: 10.1016/s0016-5085(99)70402-6. [DOI] [PubMed] [Google Scholar]
- 7.Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, Lee WM, Lok AS, Bonkovsky HL, Morgan TR, Ghany MG, Morishima C, Snow KK, Dienstag JL. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429–2441. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiffman ML, Morishima C, Dienstag JL, Lindsay KL, Hoefs JC, Lee WM, Wright EC, Naishadham D, Everson GT, Lok AS, Di Bisceglie AM, Bonkovsky HL, Ghany MG. Effect of HCV RNA Suppression During Peginterferon Alfa-2a Maintenance Therapy on Clinical Outcomes in the HALT-C Trial. Gastroenterology. 2009;137:1986–1994. doi: 10.1053/j.gastro.2009.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee WM, Dienstag JL, Lindsay KL, Lok AS, Bonkovsky HL, Shiffman ML, Everson GT, Di Bisceglie AM, Morgan TR, Ghany MG, Morishima C, Wright EC, Everhart JE. Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Controlled Clinical Trials. 2004;25:472–492. doi: 10.1016/j.cct.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Morishima C, Chung M, Ng KW, Brambilla DJ, Gretch DR. Strengths and limitations of commercial tests for hepatitis C virus RNA quantification. J Clin Microbiol. 2004;42:421–425. doi: 10.1128/JCM.42.1.421-425.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiffman ML, Di Bisceglie AM, Lindsay KL, Morishima C, Wright EC, Everson GT, Lok AS, Morgan TR, Bonkovsky HL, Lee WM, Dienstag JL, Ghany MG, Goodman ZD, Everhart JE. Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology. 2004;126:1015–1023. doi: 10.1053/j.gastro.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, Phillips MJ, Portmann BG, Poulsen H, Scheuer PJ, Schmid M, Thaler H. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 13.Mendler M, Donovan J, Blei A. Central nervous system and pulmonary complications of cirrhosis. In: Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, editors. Textbook of Gastroenterology. Philadelphia: Lippincott, Williams and Wilkins; 2003. pp. 2445–2467. [Google Scholar]
- 14.Reisman Y, Gips CH, Lavelle SM, Group EPM. Assessment of liver cirrhosis severity in 1015 patients of the Euricterus database with Campbell-Child, Pugh-Child and with ascites and ascites-nutritional state (ANS) related classifications. Hepato-Gastroenterology. 1997;44:1376–1384. [PubMed] [Google Scholar]
- 15.Lok AS, Seeff LB, Morgan TR, Di Bisceglie AM, Sterling RK, Curto TM, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL, Dienstag JL, Ghany MG, Morishima C, Goodman ZD. Incidence of Hepatocellular Carcinoma and Associated Risk Factors in Hepatitis C-Related Advanced Liver Disease. Gastroenterology. 2009;136:138–148. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghany MG, Kleiner DE, Alter H, Doo E, Khokar F, Promrat K, Herion D, Park Y, Liang TJ, Hoofnagle JH. Progression of fibrosis in chronic hepatitis C. Gastroenterology. 2003;124:97–104. doi: 10.1053/gast.2003.50018. [DOI] [PubMed] [Google Scholar]
- 17.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 18.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 19.Inagaki Y, Nemoto T, Kushida M, Sheng Y, Higashi K, Ikeda K, Kawada N, Shirasaki F, Takehara K, Sugiyama K, Fujii M, Yamauchi H, Nakao A, de Crombrugghe B, Watanabe T, Okazaki I. Interferon alfa down-regulates collagen gene transcription and suppresses experimental hepatic fibrosis in mice. Hepatology. 2003;38:890–899. doi: 10.1053/jhep.2003.50408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.