Abstract
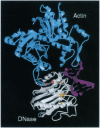
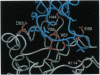
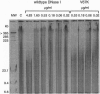
Human deoxyribonuclease I (DNase I), an enzyme recently approved for treatment of cystic fibrosis (CF), has been engineered to create two classes of mutants: actin-resistant variants, which still catalyze DNA hydrolysis but are no longer inhibited by globular actin (G-actin) and active site variants, which no longer catalyze DNA hydrolysis but still bind G-actin. Actin-resistant variants with the least affinity for actin, as measured by an actin binding ELISA and actin inhibition of [33P] DNA hydrolysis, resulted from the introduction of charged, aliphatic, or aromatic residues at Ala-114 or charged residues on the central hydrophobic actin binding interface at Tyr-65 or Val-67. In CF sputum, the actin-resistant variants D53R, Y65A, Y65R, or V67K were 10-to 50-fold more potent than wild type in reducing viscoelasticity as determined in sputum compaction assays. The reduced viscoelasticity correlated with reduced DNA length as measured by pulsed-field gel electrophoresis. In contrast, the active site variants H252A or H134A had no effect on altering either viscoelasticity or DNA length in CF sputum. The data from both the active site and actin-resistant variants demonstrate that the reduction of viscoelasticity by DNase I results from DNA hydrolysis and not from depolymerization of filamentous actin (F-actin). The increased potency of the actin-resistant variants indicates that G-actin is a significant inhibitor of DNase I in CF sputum. These results further suggest that actin-resistant DNase I variants may have improved efficacy in CF patients.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. B., WHITE J. C. Liquefaction of viscous purulent exudates by deoxyribonuclease. Lancet. 1950 Dec 9;2(6641):739–742. doi: 10.1016/s0140-6736(50)91676-x. [DOI] [PubMed] [Google Scholar]
- CHERNICK W. S., BARBERO G. J. Composition of tracheobronchial secretions in cystic fibrosis of the pancreas and bronchiectasis. Pediatrics. 1959 Nov;24:739–745. [PubMed] [Google Scholar]
- Collins F. S. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992 May 8;256(5058):774–779. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- Daugherty A. L., Patapoff T. W., Clark R. C., Sinicropi D. V., Mrsny R. J. Compaction assay: a rapid and simple in vitro method to assess the responsiveness of a biopolymer matrix to enzymatic modification. Biomaterials. 1995 May;16(7):553–558. doi: 10.1016/0142-9612(95)91129-m. [DOI] [PubMed] [Google Scholar]
- Fuchs H. J., Borowitz D. S., Christiansen D. H., Morris E. M., Nash M. L., Ramsey B. W., Rosenstein B. J., Smith A. L., Wohl M. E. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994 Sep 8;331(10):637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Galbraith R. M., Emerson D. L., Marsot F., Nel A. E., Arnaud P. Distinct sites on the G-actin molecule bind group-specific component and deoxyribonuclease I. Biochem J. 1985 Jun 1;228(2):471–477. doi: 10.1042/bj2280471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock S. E., Carisson L., Lindberg U. Depolymerization of F-actin by deoxyribonuclease I. Cell. 1976 Apr;7(4):531–542. doi: 10.1016/0092-8674(76)90203-8. [DOI] [PubMed] [Google Scholar]
- Houmeida A., Hanin V., Constans J., Benyamin Y., Roustan C. Localization of a vitamin-D-binding protein interaction site in the COOH-terminal sequence of actin. Eur J Biochem. 1992 Feb 1;203(3):499–503. doi: 10.1111/j.1432-1033.1992.tb16575.x. [DOI] [PubMed] [Google Scholar]
- Hubbard R. C., McElvaney N. G., Birrer P., Shak S., Robinson W. W., Jolley C., Wu M., Chernick M. S., Crystal R. G. A preliminary study of aerosolized recombinant human deoxyribonuclease I in the treatment of cystic fibrosis. N Engl J Med. 1992 Mar 19;326(12):812–815. doi: 10.1056/NEJM199203193261207. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Vandekerckhove J. Structure and function of actin. Annu Rev Biophys Biomol Struct. 1992;21:49–76. doi: 10.1146/annurev.bb.21.060192.000405. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lacks S. A. Deoxyribonuclease I in mammalian tissues. Specificity of inhibition by actin. J Biol Chem. 1981 Mar 25;256(6):2644–2648. [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS L. W., SPECTOR S., LEMM J., POTTER J. L. STUDIES ON PULMONARY SECRETIONS. I. THE OVER-ALL CHEMICAL COMPOSITION OF PULMONARY SECRETIONS FROM PATIENTS WITH CYSTIC FIBROSIS, BRONCHIECTASIS, AND LARYNGECTOMY. Am Rev Respir Dis. 1963 Aug;88:199–204. doi: 10.1164/arrd.1963.88.2.199. [DOI] [PubMed] [Google Scholar]
- Mannherz H. G., Goody R. S., Konrad M., Nowak E. The interaction of bovine pancreatic deoxyribonuclease I and skeletal muscle actin. Eur J Biochem. 1980 Mar;104(2):367–379. doi: 10.1111/j.1432-1033.1980.tb04437.x. [DOI] [PubMed] [Google Scholar]
- Mannherz H. G., Kreuder V., Koch J., Dieckhoff J., Drenckhahn D. The inhibition of bovine and rat parotid deoxyribonuclease I by skeletal muscle actin. A biochemical and immunocytochemical study. Biochem J. 1982 Nov 1;207(2):305–313. doi: 10.1042/bj2070305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Leod J. F., Kowalski M. A., Haddad J. G., Jr Interactions among serum vitamin D binding protein, monomeric actin, profilin, and profilactin. J Biol Chem. 1989 Jan 15;264(2):1260–1267. [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Purification of muscle actin. Methods Enzymol. 1982;85(Pt B):164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Peitsch M. C., Irmler M., French L. E., Tschopp J. Genomic organisation and expression of mouse deoxyribonuclease I. Biochem Biophys Res Commun. 1995 Feb 6;207(1):62–68. doi: 10.1006/bbrc.1995.1153. [DOI] [PubMed] [Google Scholar]
- Peitsch M. C., Polzar B., Stephan H., Crompton T., MacDonald H. R., Mannherz H. G., Tschopp J. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J. 1993 Jan;12(1):371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder J. C., Gratzer W. B. Investigation of the actin-deoxyribonuclease I interaction using a pyrene-conjugated actin derivative. Biochemistry. 1982 Sep 28;21(20):4886–4890. doi: 10.1021/bi00263a009. [DOI] [PubMed] [Google Scholar]
- Quinton P. M. Cystic fibrosis: a disease in electrolyte transport. FASEB J. 1990 Jul;4(10):2709–2717. doi: 10.1096/fasebj.4.10.2197151. [DOI] [PubMed] [Google Scholar]
- Ramsey B. W., Astley S. J., Aitken M. L., Burke W., Colin A. A., Dorkin H. L., Eisenberg J. D., Gibson R. L., Harwood I. R., Schidlow D. V. Efficacy and safety of short-term administration of aerosolized recombinant human deoxyribonuclease in patients with cystic fibrosis. Am Rev Respir Dis. 1993 Jul;148(1):145–151. doi: 10.1164/ajrccm/148.1.145. [DOI] [PubMed] [Google Scholar]
- Ranasinha C., Assoufi B., Shak S., Christiansen D., Fuchs H., Empey D., Geddes D., Hodson M. Efficacy and safety of short-term administration of aerosolised recombinant human DNase I in adults with stable stage cystic fibrosis. Lancet. 1993 Jul 24;342(8865):199–202. doi: 10.1016/0140-6736(93)92297-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shak S., Capon D. J., Hellmiss R., Marsters S. A., Baker C. L. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9188–9192. doi: 10.1073/pnas.87.23.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheterline P., Sparrow J. C. Actin. Protein Profile. 1994;1(1):1–121. [PubMed] [Google Scholar]
- Sinicropi D., Baker D. L., Prince W. S., Shiffer K., Shak S. Colorimetric determination of DNase I activity with a DNA-methyl green substrate. Anal Biochem. 1994 Nov 1;222(2):351–358. doi: 10.1006/abio.1994.1502. [DOI] [PubMed] [Google Scholar]
- Vasconcellos C. A., Allen P. G., Wohl M. E., Drazen J. M., Janmey P. A., Stossel T. P. Reduction in viscosity of cystic fibrosis sputum in vitro by gelsolin. Science. 1994 Feb 18;263(5149):969–971. doi: 10.1126/science.8310295. [DOI] [PubMed] [Google Scholar]
- Weber A., Pennise C. R., Pring M. DNase I increases the rate constant of depolymerization at the pointed (-) end of actin filaments. Biochemistry. 1994 Apr 26;33(16):4780–4786. doi: 10.1021/bi00182a005. [DOI] [PubMed] [Google Scholar]
- Weston S. A., Lahm A., Suck D. X-ray structure of the DNase I-d(GGTATACC)2 complex at 2.3 A resolution. J Mol Biol. 1992 Aug 20;226(4):1237–1256. doi: 10.1016/0022-2836(92)91064-v. [DOI] [PubMed] [Google Scholar]
- Worrall A. F., Connolly B. A. The chemical synthesis of a gene coding for bovine pancreatic DNase I and its cloning and expression in Escherichia coli. J Biol Chem. 1990 Dec 15;265(35):21889–21895. [PubMed] [Google Scholar]