Abstract
Withaferin A (WA), a naturally occurring steroidal lactone, directly binds to Hsp90 and leads to the degradation of Hsp90 client protein. The purpose of this study is to investigate the structure activity relationship (SAR) of withanolides for their inhibition of Hsp90 and anti-proliferative activities in pancreatic cancer cells. In pancreatic cancer Panc-1 cells, withaferin A (WA) and its four analogues withanolide E (WE), 4-hydroxywithanolide E (HWE), 3-aziridinylwithaferin A (AzWA) inhibited cell proliferation with IC50 ranged from 1.0 to 2.8 μM. WA, WE, HWE, and AzWA also induced caspase-3 activity by 21-, 6-, 11- and 15-fold, respectively, in Panc-1 cells, while withaperuvin (WP) did not show any activity. Our data showed that WA, WE, HWE, and AzWA, but not WP, all directly bound to Hsp90 and induced Hsp90 aggregation, hence inhibited Hsp90 chaperone activity to induce degradation of Hsp90 client proteins Akt and Cdk4 through proteasome-dependent pathway in pancreatic cancer cells. However, only WA, HWE and AzWA disrupted Hsp90-Cdc37 complexes but not WE and WP. SAR study suggested that the C-5(6)-epoxy functional group contributes considerably for withanolide to bind to Hsp90, inhibit Hsp90 chaperone activity, and result in Hsp90 client protein depletion. Meanwhile, the hydroxyl group at C-4 of ring A may enhance withanolide to inhibit Hsp90 activity and disrupt Hsp90-Cdc37 interaction. These SAR data provide possible mechanisms of anti-proliferative action of withanolides.
Keywords: Withanolides, Hsp90, Cdc37, Structure-activity relationships, Pancreatic cancer
Introduction
The constituents from Withania somnifera (WS), including alkaloids and withanolides, have been studied extensively for their biological activities [1,2]. Withaferin A (WA), one of the major active components of W. somnifera, was reported to have anti-angiogenesis, anti-tumor, and radio-sensitizing activities in various cancer cell lines [3-6]. It has been reported that WA covalently bound to annexin II, altered cytoskeletal architecture [7], and inhibited tumor necrosis factor-induced activation of IB kinase via a thioalkylation-sensitive redox mechanism [8]. Previously, we have also shown that WA exhibited anti-proliferative activity via Hsp90 inhibition in pancreatic cancer cells [9]. Unlike classical Hsp90 inhibitors (such as geldanamycin) that block the Hsp90 ATP binding site, WA directly binds to Hsp90 C-terminus and induces Hsp90-dependent client protein degradation in pancreatic cancer cells. In addition, WA also disrupted Hsp90-Cdc37 complex, which is different from classical Hsp90 inhibitors.
The 90 kDa heat-shock protein (Hsp90) has emerged as a promising target for drug discovery [10,11]. Previous studies have revealed that
Hsp90 chaperone activity is regulated by numerous co-chaperones, such as Hsp70, Hop, Cdc37, and driven by a cycle of N-terminal ATP/ADP exchange through ATP hydrolysis at N-terminal ATP binding site [12]. Several natural products including geldanamycin (GA) and its derivatives 17-AAG, 17-DMAG inhibit Hsp90 ATPase activity through competitive blockage of the N-terminal ATP binding pocket and cause proteasomal degradation of client proteins [13-17]. Another type of Hsp90 inhibitor, novobiocin (and its derivatives) targets the C-terminal ATP binding pocket, inducing similar cellular responses as N-terminal ATP pocket inhibitors [18,19]. Since Hsp90 is known to interact with various co-chaperones to assemble a superchaperone complex for its protein folding and maturation, disruption of Hsp90 complex may provide additional mechanisms to inhibit Hsp90 for cancer therapy.
Withaferin A (WA) binds to Hsp90 C-terminus and also blocks Hsp90-Cdc37 complex in cancer cells. However, it remains unclear which structural features of WA contribute to the inhibition of the Hsp90 chaperoning activity. Previous studies have shown that the 4 -hydroxy-5, 6-epoxy-2-en-1-one moiety and unsaturated lactone are critical for WA's biological function [20,21]. In this study, we investigated WA and its four structural analogues for their mechanisms to inhibit Hsp90 and efficacy of anti-proliferative activity in pancreatic cancer cells. The data suggested that the C-5(6) epoxy functional group of withanolides is required to bind Hsp90, induce Hsp90 aggregation, and induce Hsp90 client protein degradation, and eventually show anti-proliferative activity. The substitution of C-2,3 position may hinder withanolides to inhibit Hsp90 activity while the C-4 hydroxyl group in A ring of withanolides may enhance their activity to inhibit Hsp90 and disrupt Hsp90-Cdc37 interaction.
Materials and methods
Drugs and antibodies
Withaferin A (S.1A) was purchased from Calbiochem Inc. (San Diego, CA). 3-Aziridinylwithaferin A (AzWA, NSC339665, S. 1B), withanolide E (WE, NSC179834, S. 1C), 4β-hydroxywithanolide E (HWE, NSC212509, S. 1D), and Withaperuvin (WP, NSC334387, S. 1E) were kindly provided by The NCI/DTP Open Chemical Repository (http://dtp.cancer.gov). The following antibodies were used for Western blot: Akt, PARP (Cell Signaling, Beverly, MA), Hsp70 (StressGen, Victoria, BC, Canada), Cdk4, β-Actin, Cdc37 and Hsp90 (Santa Cruz, Santa Cruz, CA). Monoclonal Hsp90 antibody H9010 for immunoprecipitation was purchased from Alexis Biochemicals (San Diego, CA). Pan-caspase inhibitor (Z-VAD-FMK) was purchased from Promega (Madison, WI).
MTS assay
Human pancreatic cancer cell line Panc-1 was cultured in 10% FBS RPMI-1640 at 37 °C and 5% CO2. Panc-1 cells were seeded in 96-well microplates at a density of 4*104cells·ml-1 and cultured for overnight. The cells were treated with different drugs at various concentrationsfor 48h. The cell proliferation was assessed using MTS assay (Promega, Madison, WI) according to the manufacturer's manual. The number of living cells is directly proportional to the absorbance at 490 nm by a formazan product bioreduced from MTS in living cells. The IC50 value for cytotoxicity was estimated by WinNonlin software (Pharsight, Mountain View, CA).
Caspase-3 activity assay
Panc-1 cells were treated with 10μM WA, WE, HWE, AzWA, and WP, respectively for 48h. The Caspase-3 activity assay was performed according to the manufacturer's instruction of Caspase-3/CPP32 Fluorometric Assay Kit (Biovision Research Products, Mountain View, CA). Cellular protein was extracted with the supplied lysis buffer and protein concentration was measured using BCA Protein Assay Reagents (Pierce, Rockford, IL). The cleavage of DEVD-AFC, a substrate of caspase-3, was quantified using a fluorescence microtiter plate reader with a 400 nm excitation filter and a 505 nm emission filter.
Biotinyl-withaferin A (WA-biotin) protein pull down assay
Biotinyl-Withaferin A (WA-biotin) was prepared and used in the pull down assay as described previously [7]. Briefly, the whole cell extract of Panc-1 cells was prepared in TNEK buffer (5 mM Tris, pH 7.4; NP-40 1%; EDTA 2 mM; KCl 200 mM) supplemented with protease inhibitors. Aliquots of cell lysate containing equal amounts of total protein were precleared with NeutrAvidin beads (Pierce) before incubation with equal concentration of different drugs for 1h at 4 °C, respectively. Then equal amounts of immobilized WA-biotin were added to each sample and incubated for 2 h at 4 °C with constant agitation. The beads were then washed with TNEK buffer for three times, and were boiled in loading buffer for 4 min to dissociate the bound proteins. Western blot was carried out to determine the levels of Hsp90 proteins.
Western blotting analysis
The procedure of Western blotting analysis was performed as previously described [22]. Briefly, after treated with different drugs for the 24-48 hr, Panc-1 cells were washed twice with ice-cold PBS, collected in RIPA lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 5mM EDTA, 1 mM Na3VO4, pH 7.5) supplemented with a protease inhibitor mixture (Sigma-Aldrich, St. Louis, MO), and incubated on ice for 20 min. Afterward cell lysate was centrifuged at 14,000 × rpm for 10 min, and the pellet was diluted in SDS sample buffer. Isolation of triton-soluble and triton-insoluble proteins was performed as described by Chen et al.[23]. Protein concentration was determined using BCA Protein Reagents (Pierce, Rockford, IL). The protein was separated by SDS-PAGE and electrotransfered onto a PVDF membrane (BioRad, Richmond, CA). Bolts were then probed with appropriate antibodies.
To analyze the disulfide-bonded protein, non-reducing SDS-PAGE was employed. Briefly, drug-treated cells were washed twice with ice-cold and then incubated in ice-cold PBS with 40 mM iodoacetamide (IA) for 5 min to prevent thiol-disulfide exchange and inhibit postlysis oxidation of free cysteines [24]. Afterwards, sample was diluted in SDS-sample buffer without reducing agents before loading onto SDS-PAGE.
Co-immunoprecipitation assay
The general procedure for co-immunoprecipitation was described as follows. The drug-treated Panc-1 cells were harvested and lysed in RIPA lysis buffer supplemented with a protease inhibitor mixture. After centrifugation pellet was collected and protein was quantified using BCA protein assay reagents. Each of protein samples (500 μg) was first incubated with 5 μl H9010 anti-Hsp90 antibody (Axxora, San Diego, CA) for 1 h at 4 °C, rotating, and then added with 30 μl protein G agarose (Pierce, Rockford, IL) followed by incubation of another 2 h at 4 °C. The beads were washed three times with PBS plus protease inhibitors and boiled in loading buffer for 4 min to isolate the bound proteins. The protein was separated by SDS-PAGE. Western blot was performed to determine the levels of co-immunoprecipitated proteins.
Results
WA and its analogues WE, HWE and AzWA inhibit cell proliferation and induce apoptosis in Panc-1 cells
To investigate the cytotoxicity of WA, WE, HWE, AzWA and WP, Panc-1 cells were incubated with increasing concentrations of WA and its analogues for 48 h, respectively. Cell viability was examined by MTS assay. As shown in Fig. 1A, WA, WE,HWE and AzWA exhibited dose-dependent cytotoxicity against Panc-1 with IC50 of 1.0, 1.5, 1.2 and 2.8 μM, respectively;. In contrast, WP did not inhibit cell viability even at a concentration up to 50 μM.
Fig.1.
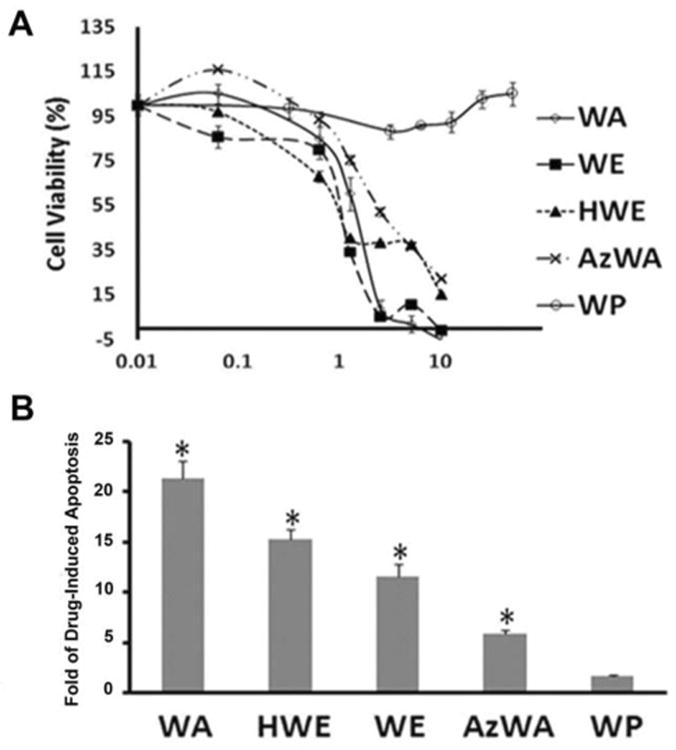
A. Dose-response curve of WA and its analogues on cell cytotoxixity in Panc-1 cells. Cells were grown in log phase and treated with increasing concentrations of WA, WE, HWE, AzWA or WP for 48 h, respectively. The cytotoxicity of those compounds was measured by MTS assay. B. Caspase-3 activity in Panc-1 cells after WA, WE, HWE, AzWA or WP treatment. Cell lysates were prepared for caspase-3 activity assay. Results are expressed as arbitrary fluorescent units (AFU) normalized to milligram of cytosolic protein. Caspase 3 activities of drug treatment groups were normalized to that of Control cells. Data are presented as mean ± SD (n = 3). * indicates p< 0.05.
To study whether WA and its analogues induced apoptosis, caspase-3 activity was measured in Panc-1 cells with WA, AzWA, WE, HWE and WP treatment. As shown in Fig. 1B, 10 μM WA, AzWA, WE and HWE for 48 h treatment increased caspase-3 activity by 21.3, 5.8, 11.6, and 15.3-fold, respectively, in comparison with untreated cells. In contrast, 10 μM WP did not induce apoptosis. Similarly, another apoptosis indicator PARP protein level also showed that WA, AzWA, WE and HWE decreased PARP level and resulted in the occurrence of cleaved PARP, whereas WP did not (S1).
WA analogues WE, HWE and AzWA decrease cellular levels of Hsp90 client proteins
Since WA's analogues WE, HWE and AzWA exhibited potent anti-proliferative activity and induced apoptosis in Panc-1 cells similarly to WA, we also tested if WE, HWE and AzWA would also interact with Hsp90 and cause simultaneous down-regulation of multiple oncogenic proteins. The levels of Hsp90 client protein Akt and Cdk4 were down-regulated by 5, 2, 4, 4-fold, and 6, 2, 3, 2-fold, respectively, upon incubation with 10 μM WA, WE, HWE or AzWA for 24 h treatment. However, WP did not cause significant alterations of the protein levels of Akt and Cdk4 (Fig. 2A).
Fig.2.
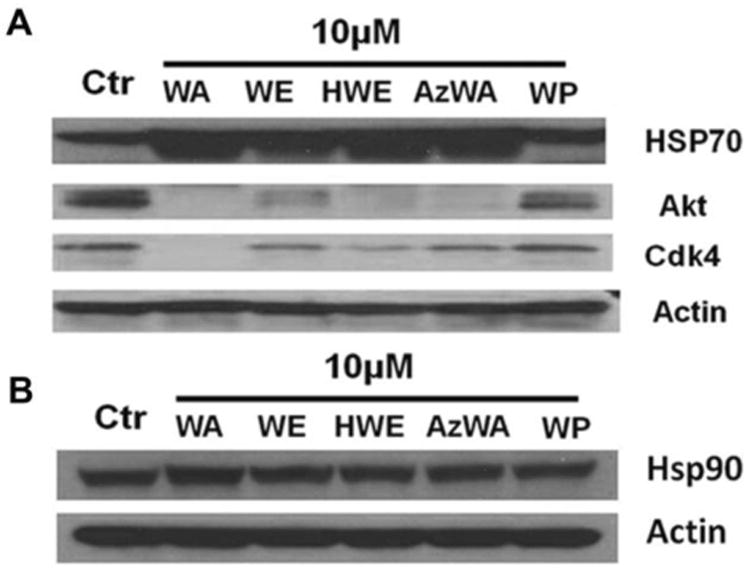
A. HSP90 client protein degradation in Panc-1 cells induced by WA, WE, HWE, AzWA and WP. Panc-1 cells were treated with 10μM WA or its analogues for 24h, respectively. Equal amounts of protein (50 μg/lane) were subjected to SDS-PAGE and analyzed by Western blot with specific antibodies to Akt, Cdk4, Hsp70, Hsp90 and Actin. Actin was served as internal standard. Results are representative of three independent experiments.
Because the induction of Hsp70 is another molecular signature in response to Hsp90 inhibition [25], we also determined the Hsp70 protein level after WA, AzWA, WE, HWE or WP treatment. As a result, 10 μM WA, AzWA, WE or HWE increased the protein level of Hsp70 by 2 to 3-fold after 24 h treatment (Fig.2A) while they did not change the Hsp90 protein level (Fig.2B), whereas WP failed to increase Hsp70 protein level.
Additionally, Panc-1 cells were pre-treated with proteasome inhibitor(MG132) for 1h before the treatment of WA, WE, HWE and AzWA for 24 h, respectively. The Akt and Cdk4 proteins were disappeared from the triton-soluble fraction, but accumulated in the triton-insoluble fraction(S3A). Whereas, pre-incubation with pan-caspase inhibitor (Z-VAD-FMK) for 1 h and then treated with WA, WE, HWE and AzWA for 24 h did not show accumulation of Akt or Cdk4 (S3B).These results indicated thatWA, AzWA, WE and HWE induced Hsp90 client proteins via proteasome-dependent pathway and caspase was not involved in the protein degradation of Akt and Cdk4.
AzWA, WE or HWE directly binds to Hsp90 and cause Hsp90 aggregation
Previous studies have shown that WA may directly bind to Hsp90 and cause disulfide-linked high molecular weight conformers of Hsp90 [9]. Therefore, we tested whether WA's analogues bind to Hsp90 using competition with biotinyl-withaferin A pull down assay. As shown in Fig. 3A, WA-biotin successfully pulled down Hsp90 from cell lysate. Preincubation with 100 μM of unlabeled WE, HWE or AzWA were able to compete against the WA-biotin binding to Hsp90 in cell lyaste, while WP did not compete with biotin-WA for its binding to Hsp90. Furthermore, we used nonreducing gel electrophoresis to determine the Hsp90 aggregates in WA and AzWA, WE, HWE or WP-treated cells. When Panc-1 cells were incubated with 10 μM WA, AzWA, WE or HWE for 24 h, respectively, the formation of Hsp90 aggregates was detected (Fig. 3B). However, WP did not induce Hsp90 aggregation.
Fig.3.
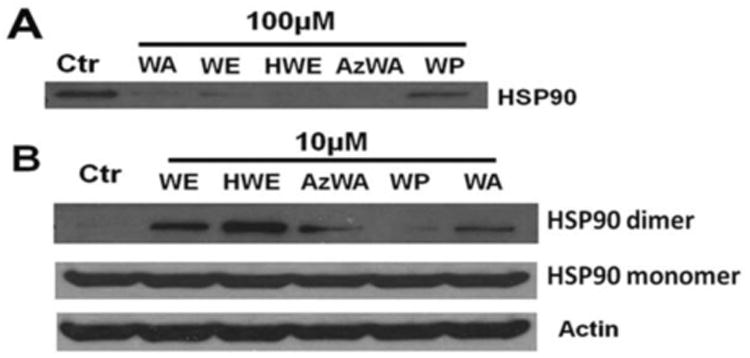
WA and its analogues direct bind to Hsp90 and induce Hsp90 aggregation. A. WA, WE, HWE, AzWA and WP compete with WA-biotin binding to Hsp90. One mg cell lysate was preincubated with 100μM WA or its analogues for 1 h, respectively before subject to WA-biotin pull down assay. The WA-biotin pull down protein were subjected to Western blot analysis with specific antibodies to Hsp90. B. WA, WE, HWE or AzWA induces Hsp90 aggregation. Panc-1 cells were treated with 10 μM WA or its analogues for 24 h, respectively. Equal amounts of protein (50μg/lane) were subjected to non-reducing gel electrophoresis and then analyzed by Western blot with specific antibodies to Hsp90 and Actin, Actin was served as internal standard. Results are representative of three independent experiments.
WA, HWE, AzWA, but not WE or WP interrupt Hsp90-Cdc37 association in pancreatic cancer cells
In our previous study, we have shown that 5 μM WA treatment could completely block Hsp90-Cdc37 interaction [9]. To further characterize whether WA's analogues could block the Hsp90-Cdc37 interaction, co-immunoprecipitation (Co-IP) assay was employed. The result showed that 20 μM WA, HWE and AzWA can decrease Cdc37 interaction to Hsp90 after 24h incubation, but not WE and WP (Fig. 4A). Meanwhile, WA, AzWA, and HWE did not change the total cellular Cdc37 protein level(Fig. 4B), which indicate that the absent of Cdc37 band after drug treatment in co-IP result was not due to the expression level alteration of Cdc37 protein.
Fig.4.
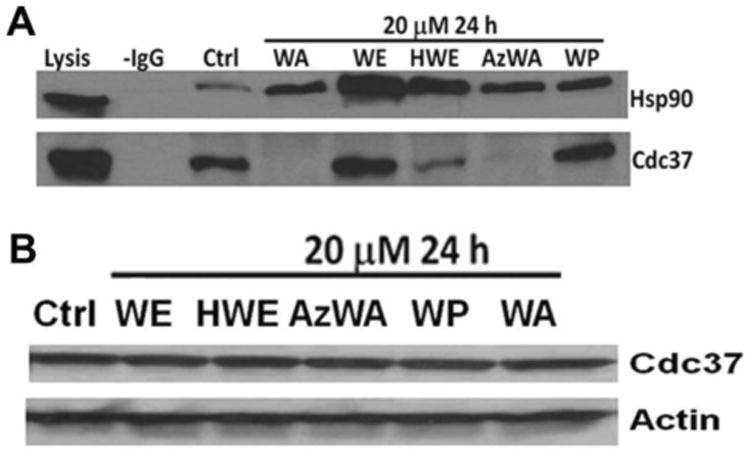
Disruption of Hsp90-Cdc37 in Panc-1 cells by withaferin A (WA) and its analogues. A. Immunoprecipitation of Hsp90 and Cdc37 complex. Cell lysates (500 μg total protein) were immunoprecipitated with Hsp90 antibody. Western blot was performed to detect Cdc37 and Hsp90 using specific antibodies. Panc-1 cells were treated with 20μM WA, WE, HWE, AzWA or WP for 24 h, respectively. Lysis, total cell lysate; IgG, without antibody. B. Total expression levels of Cdc37 in Panc-1 cells. Panc-1 cells were treated with WA, WE, HWE, AzWA or WP for 24 h, respectively. Equal amounts of protein (50μg/lane) were subjected to SDS-PAGE and analyzed by Western blot with specific antibodies to Cdc37 and Actin. Actin was served as internal standard. Results are representative of three independent experiments.
Discussion
To date, several studies have identified the potential pharmacophores of Withaferin A for its activities. It's reported fthat the 4-hydroxy-5,6-epoxy-2-en-1-one moiety and unsaturated lactone side chain are critical for biological activity [26]. The 5,6-epoxide group within B ring was reported to react with 2-mercaptoethanol, which is a biochemical thiol-oxidizer [27] to be involved in thioalkylation reactions [28-30]. An earlier studyalso demonstrated that the epoxide functionality at C-5,6 acts as a nucleophile acceptor is essential forWA anticancer activities in P-388 lymphocytic leukemia [31]. In addition, the ketone containing unsaturated A ring is readily alkylated by thiol-nucleophiles and undergoes Michael addition [11]. Although it was reported that the C-27 hydroxyl group in unsaturated lactone was considered to be dispensable [32], it may have some contributions to WA's activity [33].
WA, WE, HWE and AzWA ,which all share the 5,6-epoxide group, resulted in similar antiproliferative activity and apoptosis induction against Panc-1cells. In contrast, up to 50 μM withaperuvin (WP) which does not have 5, 6-epoxide group, was unable to significantly decrease the cell viability and induce apoptosis. These results suggest that the 5, 6-epoxide group within B ring plays an important role in the anti-proliferative activity of WA against Panc-1 cells. Indeed, these finding was confirmed by the previous studies, which reported that the loss or replacement of epoxy functional group at C-(5,6) of WA abrogated its biological function [27,28].
In addition, the substituent group at C-2,3 in the unsaturated A ring also impacts the biological function of WA. In our study, AzWA demonstrated relatively weaker antiproliferative activity and apoptosis induction in Panc-1 cells than withaferin A. Similar results have been revealed that the activity of WA was decreased when it was converted to 3-methoxy-2,3-dihydrowithaferin A [32]. Furthermore, the anti-proliferative activity and apoptosis induction of HWE, which contained a hydroxyl substituent at C-4, slightly increased compared to WE, which lacked the 4-hydroxy group. Previous data also confirmed that hydroxyl group at C-4 within ketone unsaturated A ring contributed to the inhibition of cell viability of withanolides [20]. WA also inhibit Hsp90 by direct binging to Hsp90 without affecting its ATP binding site. We further explored the structure-activity relationship of WA and its analogues to inhibit Hsp90 chaperone machinery.
Drug-mediated inhibition of Hsp90 leads to simultaneous misfolding and aggregation of various client protein, which results in degradation via ubiquitin-proteasome pathway [34,35]. In addition, Hsp90 was considered to be over expressed in cancerous tissue and existed as altered superchaperone complex, which inherently results in greater drug selectivity to cancer cells [36,37]. Therefore, Hsp90 has emerged as a promising therapeutic target for cancer therapeutics.
In order to assess whether epoxy group at C-5(6) within B ring, the substituent groups C-3 and the hydroxyl group at C-4 of withanolide affect Hsp90 inhibition, we examined the protein levels of Akt and Cdk4, two well identified Hsp90 client proteins, and Hsp70 a molecular signature in response to Hsp90 inhibition in cancer cells in response to the treatment of WA and its analogues [38]. Consistent with the results of cytotoxicity and apoptosis, WE, HWE, AzWA, which all contain the 5,6-epoxide group induced the Akt and Cdk4 degradation as well as HSP70 up-regulation, whereas WP showed little effects. In addition, HWE, with an additional hydroxyl group at C-4 decreased protein levels of Akt and Cdk4 more than that of WE. Furthermore, AzWA, with an additional aziridinyl group at C-3 induced much less of Hsp90 client protein degradation compared to WA. In the presence of proteasome inhibitor MG132, both Akt and Cdk4 accumulated in Triton-insoluble fraction of drug-treated cells, suggesting that WE, HWE or AzWA triggered proteasome-dependent degradation of misfolded/aggregated Hsp90 client protein. The alteration of these important signaling molecules suggest that the epoxy group at C-5, 6 within B ring, the substituent group at C-3 and the hydroxyl group at C-4 within ketone unsaturated A ring contributed considerably to the inhibition of Hsp90 activity.
In our previous study, we have shown that withaferin A directly binds to the C-terminus Hsp90 but not N-terminus Hsp90, which might be though the interaction with the reactive cysteine residues. Since only C-terminus Hsp90 contains reactive cysteine residues whereas N-terminus Hsp90 not. In addition, withaferin A biotin failed to pull down full length yeast Hsp90 which did not contain reactive cysteine residues [9]. In the present study, we demonstrated that WE, HWE and AzWA were able to compete for biotin-WA binding to Hsp90 in cell lysate, whereas WP failed. These results suggest that epoxide functional group at C-5, 6 plays an important role in withanolide interacting with the C-terminus of Hsp90.
Previous studies revealed that Hsp90 chaperone proteins containing cysteine residues are sensitive to cellular redox conditions and form intermolecular disulfide bonds in stress conditions, hence cause the formation of high molecular weight aggregate of Hsp90 [39]. WA, WE, HWE and AzWA all induced Hsp90 aggregates, which suggests the importance of epoxide functional group at C-5,6 to induce Hsp90 aggregation.
Cdc37, a cochaperone of Hsp90 is crucial in loading protein kinase to Hsp90 superchaperone complex [40,41]. Cdc37 associated with a large subset of Hsp90 client proteins, which are essential in signal transduction, cell proliferation and survival [41].. As same as WA, 20μM HWE, and AzHA were demonstrated to be able to inhibit Hsp90-Cdc37 interaction,whereas 20μM WE) and WP did not. Further examination of the compounds' structures releaved that WA, HWE and AzWA all share the same C-5, C-6 epoxide group and C-4 hydroxyl group, whereas WE only contains C-5, C-6 epoxide group but not C-4 hydroxyl group, and WP onlycontains C-4 hydroxyl group but not C-5, C-6 epoxide group, indicated that both C-5(6) epoxide group and C-4 hydroxyl group might be crucial for disruption of Hsp90-Cdc37 interaction.
In summary, various withaferin A analogues were investigated for their anticancer activity and mechanisms to inhibit Hsp90. The data suggested that the epoxy functional group at C-5(6) of withanolides is required to bind Hsp90, induce Hsp90 aggregation, and induce Hsp90 client protein degradation, and eventually show anti-proliferative activity. The substitution of C-2 and 3 positions may hinder withanolides to inhibit Hsp90 activity while the hydroxyl group at C-4 in A ring of withanolide may enhance its activity to inhibit Hsp90 and induce Hsp90 client protein degradation, and disrupt Hsp90-Cdc37 interaction. These structure-activity relationships of withanolides provide detailed mechanism for this class of compounds to inhibit Hsp90 for their anticancer activity.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health National Cancer Institute (Grants R01-CA120023, R21-CA143474 to DS).
Footnotes
Conflict of interest: None declared
References
- 1.Davis L, Kuttan G. Effect of Withania somnifera on DMBA induced carcinogenesis. J Ethnopharmacol. 2001;75(2-3):165–168. doi: 10.1016/s0378-8741(00)00404-9. [DOI] [PubMed] [Google Scholar]
- 2.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5(4):334–346. [PubMed] [Google Scholar]
- 3.Srinivasan S, Ranga RS, Burikhanov R, et al. Par-4-dependent apoptosis by the dietary compound Withaferin A in prostate cancer cells. Cancer Res. 2007;67(1):246–253. doi: 10.1158/0008-5472.CAN-06-2430. [DOI] [PubMed] [Google Scholar]
- 4.Malik F, Kumar A, Bhushan S, et al. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis. 2007;12(11):2115–2133. doi: 10.1007/s10495-007-0129-x. [DOI] [PubMed] [Google Scholar]
- 5.Stan SD, Hahm ER, Warin R, et al. Withaferin A causes FOXO3a- and Bimdependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68(18):7661–7669. doi: 10.1158/0008-5472.CAN-08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koduru S, Kumar R, Srinivasan S, et al. Notch-1 inhibition by Withaferin-A: a therapeutic target against colon carcinogenesis. Mol Cancer Ther. 2010;9(1):202–210. doi: 10.1158/1535-7163.MCT-09-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falsey RR, Marron MT, Gunaherath GM, et al. Actin microfilament aggregation induced by withaferin A is mediated by annexin II. Nat Chem Biol. 2006;2(1):33–38. doi: 10.1038/nchembio755. [DOI] [PubMed] [Google Scholar]
- 8.Kaileh M, Vanden Berghe W, Heyerick A, et al. Withaferin a strongly elicits IkappaB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J Biol Chem. 2007;282(7):4253–4264. doi: 10.1074/jbc.M606728200. [DOI] [PubMed] [Google Scholar]
- 9.Yu Y, Hamza A, Zhang T, et al. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem Pharmacol. 79(4):542–551. doi: 10.1016/j.bcp.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahalingam D, Swords R, Carew JS, et al. Targeting HSP90 for cancer therapy. Br J Cancer. 2009;100(10):1523–1529. doi: 10.1038/sj.bjc.6605066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santagata S, Xu YM, Wijeratne EM, et al. Using the heat-shock response to discover anticancer compounds that target protein homeostasis. ACS Chem Biol. 2012;7(2):340–349. doi: 10.1021/cb200353m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obermann WM, Sondermann H, Russo AA, et al. In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol. 1998;143(4):901–910. doi: 10.1083/jcb.143.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neckers L. Development of small molecule Hsp90 inhibitors: utilizing both forward and reverse chemical genomics for drug identification. Curr Med Chem. 2003;10(9):733–739. doi: 10.2174/0929867033457818. [DOI] [PubMed] [Google Scholar]
- 14.Roe SM, Prodromou C, O'Brien R, et al. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42(2):260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 15.Supko JG, Hickman RL, Grever MR, et al. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol. 1995;36(4):305–315. doi: 10.1007/BF00689048. [DOI] [PubMed] [Google Scholar]
- 16.Egorin MJ, Lagattuta TF, Hamburger DR, et al. Pharmacokinetics, tissue distribution, and metabolism of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (NSC 707545) in CD2F1 mice and Fischer 344 rats. Cancer Chemother Pharmacol. 2002;49(1):7–19. doi: 10.1007/s00280-001-0380-8. [DOI] [PubMed] [Google Scholar]
- 17.Glaze ER, Lambert AL, Smith AC, et al. Preclinical toxicity of a geldanamycin analog, 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG), in rats and dogs: potential clinical relevance. Cancer Chemother Pharmacol. 2005;56(6):637–647. doi: 10.1007/s00280-005-1000-9. [DOI] [PubMed] [Google Scholar]
- 18.Allan RK, Mok D, Ward BK, et al. Modulation of chaperone function and cochaperone interaction by novobiocin in the C-terminal domain of Hsp90: evidence that coumarin antibiotics disrupt Hsp90 dimerization. J Biol Chem. 2006;281(11):7161–7171. doi: 10.1074/jbc.M512406200. [DOI] [PubMed] [Google Scholar]
- 19.Marcu MG, Chadli A, Bouhouche I, et al. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J Biol Chem. 2000;275(47):37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 20.Yousuf SK, Majeed R, Ahmad M, et al. Ring A structural modified derivatives of withaferin A and the evaluation of their cytotoxic potential. Steroids. 2011;76(10):1213–1222. doi: 10.1016/j.steroids.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Xu YM, Marron MT, Seddon E, et al. 2,3-Dihydrowithaferin A-3beta-O-sulfate, a new potential prodrug of withaferin A from aeroponically grown Withania somnifera. Bioorg Med Chem Lett. 2009;17(6):2210–2214. doi: 10.1016/j.bmc.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T, Hamza A, Cao X, et al. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol Cancer Ther. 2008;7(1):162–170. doi: 10.1158/1535-7163.MCT-07-0484. [DOI] [PubMed] [Google Scholar]
- 23.Chen WY, Chang FR, Huang ZY, et al. Tubocapsenolide A, a novel withanolide, inhibits proliferation and induces apoptosis in MDA-MB-231 cells by thiol oxidation of heat shock proteins. J Biol Chem. 2008;283(25):17184–17193. doi: 10.1074/jbc.M709447200. [DOI] [PubMed] [Google Scholar]
- 24.Cumming RC, Andon NL, Haynes PA, et al. Protein disulfide bond formation in the cytoplasm during oxidative stress. J Biol Chem. 2004;279(21):21749–21758. doi: 10.1074/jbc.M312267200. [DOI] [PubMed] [Google Scholar]
- 25.Banerji U, Walton M, Raynaud F, et al. Pharmacokinetic-pharmacodynamic relationships for the heat shock protein 90 molecular chaperone inhibitor 17-allylamino, 17-demethoxygeldanamycin in human ovarian cancer xenograft models. Clin Cancer Res. 2005;11(19 Pt 1):7023–7032. doi: 10.1158/1078-0432.CCR-05-0518. [DOI] [PubMed] [Google Scholar]
- 26.Yokota Y, Bargagna-Mohan P, Ravindranath PP, et al. Development of withaferin A analogs as probes of angiogenesis. Bioorg Med Chem Lett. 2006;16(10):2603–2607. doi: 10.1016/j.bmcl.2006.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misra L, Lal P, Chaurasia ND, et al. Selective reactivity of 2-mercaptoethanol with 5beta,6betaepoxide in steroids from Withania somnifera. Steroids. 2008;73(3):245–251. doi: 10.1016/j.steroids.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Fuska J, Fuskova A, Rosazza JP, et al. Novel cytotoxic and antitumor agents. IV. Withaferin A: relation of its structure to the in vitro cytotoxic effects on P388 cells. Neoplasma. 1984;31(1):31–36. [PubMed] [Google Scholar]
- 29.Oh JH, Lee TJ, Park JW, et al. Withaferin A inhibits iNOS expression and nitric oxide production by Akt inactivation and down-regulating LPS-induced activity of NF-kappaB in RAW 264.7 cells. Eur J Pharmacol. 2008;599(1-3):11–17. doi: 10.1016/j.ejphar.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Liang MC, Bardhan S, Pace EA, et al. Inhibition of transcription factor NF-kappaB signaling proteins IKKbeta and p65 through specific cysteine residues by epoxyquinone A monomer: correlation with its anti-cancer cell growth activity. Biochem Pharmacol. 2006;71(5):634–645. doi: 10.1016/j.bcp.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Ray AB, Gupta M. Withasteroids, a growing group of naturally occurring steroidal lactones. Fortschr Chem Org Naturst. 1994;63:1–106. doi: 10.1007/978-3-7091-9281-8_1. [DOI] [PubMed] [Google Scholar]
- 32.Bargagna-Mohan P, Ravindranath PP, Mohan R. Small molecule anti-angiogenic probes of the ubiquitin proteasome pathway: potential application to choroidal neovascularization. Invest Ophthalmol Vis Sci. 2006;47(9):4138–4145. doi: 10.1167/iovs.05-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llanos GG, Araujo LM, Jiménez IA, et al. Withaferin A-related steroids from Withania aristata exhibit potent antiproliferative activity by inducing apoptosis in human tumor cells. Eur J Med Chem. 2012;54(1):499–511. doi: 10.1016/j.ejmech.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 34.Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem. 1996;271(37):22796–22801. doi: 10.1074/jbc.271.37.22796. [DOI] [PubMed] [Google Scholar]
- 35.An WG, Schulte TW, Neckers LM. The heat shock protein 90 antagonist geldanamycin alters chaperone association with p210bcr-abl and v-src proteins before their degradation by the proteasome. Cell Growth Differ. 2000;11(7):355–360. [PubMed] [Google Scholar]
- 36.Workman P. Altered states: selectively drugging the Hsp90 cancer chaperone. Trends Mol Med. 2004;10(2):47–51. doi: 10.1016/j.molmed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Plescia J, Salz W, Xia F, et al. Rational design of shepherdin, a novel anticancer agent. Cancer Cell. 2005;7(5):457–468. doi: 10.1016/j.ccr.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 38.Wegele H, Muller L, Buchner J. Hsp70 and Hsp90--a relay team for protein folding. Rev Physiol Biochem Pharmacol. 2004;151:1–44. doi: 10.1007/s10254-003-0021-1. [DOI] [PubMed] [Google Scholar]
- 39.Clark CB, Rane MJ, El Mehdi D, et al. Role of oxidative stress in geldanamycin-induced cytotoxicity and disruption of Hsp90 signaling complex. Free Radic Biol Med. 2009;47(10):1440–1449. doi: 10.1016/j.freeradbiomed.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith JR, Workman P. Targeting CDC37: an alternative, kinase-directed strategy for disruption of oncogenic chaperoning. Cell Cycle. 2009;8(3):362–372. doi: 10.4161/cc.8.3.7531. [DOI] [PubMed] [Google Scholar]
- 41.Pearl LH. Hsp90 and Cdc37 -- a chaperone cancer conspiracy. Curr Opin Genet Dev. 2005;15(1):55–61. doi: 10.1016/j.gde.2004.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


