Abstract
Purpose
Emerging evidence suggests that exposure to endocrine disruptors may initiate or exacerbate adiposity and associated health problems. This study examined sex differences in the association of urinary level of bisphenol-A (BPA) with selected indices of glucose homeostasis among U.S. adults.
Methods
Data analyses were performed using a sample of 1,586 participants from the 2005–2008 National Health and Nutrition Examination Surveys. BPA level and the ratio of BPA-to-creatinine level were defined as log-transformed variables and in quartiles. Selected indices of glucose homeostasis were defined using fasting glucose and insulin data. Multivariate linear and logistic regression models for the hypothesized relationships were constructed after controlling for age, sex, race, education, marital status, smoking status, physical activity, total dietary intake and urinary creatinine concentration.
Results
Taking 1st quartile as a referent, 3rd quartile of BPA level was positively associated with log-transformed level of insulin and β-cell function (HOMA-β) as well as insulin resistance (log-transformed HOMA-IR; HOMA-IR≥2.5), with significant BPA-by-sex interaction; these associations were stronger among males than among females. Irrespective of sex, the ratio of BPA-to-creatinine level was not predictive of indices of glucose homeostasis.
Conclusions
A complex association may exist between BPA and hyperinsulinemia among adult U.S. men. Prospective cohort studies are needed to further elucidate endocrine disruptors as determinants of adiposity-related disturbances.
MeSH keywords: Bisphenol-A, Endocrine disruptor, Glucose Homeostasis, Survey
INTRODUCTION
The current epidemic of obesity and its associated metabolic diseases (type 2 diabetes, cardiovascular disease, hypertension and dyslipidemias) that plagues the U.S. and other industrialized nations has been primarily ascribed to poor dietary habits and sedentary lifestyles (1–3). Emerging evidence suggests that exposure to environmental pollutants such as atrazine, bisphenol-A (BPA), dichlorodiphenyltrichloroethane, diethylstilbestrol, dioxin, phthalates, polychlorinated biphenyls, organotins and other endocrine disruptors may also initiate or exacerbate these health problems (2, 4–10). Endocrine disruptors are lipophilic substances that usually act as transcription factors for the nuclear hormone receptor superfamily and can either mimic or block the action of endogenous sex hormones, resulting in irreversible alterations (at developmental stages) and reversible alterations (at post-developmental stages) in patterns of gene expression (2, 5–10). Sex steroids (androgens and estrogens) play an important role in establishing and maintaining adipose tissue (2, 4) and in conjunction with growth hormones can mobilize lipids and have anti-adipogenic effects; their effects are counteracted by insulin and cortisol that have adipogenic effects (2). Exposure to endocrine disruptors is thought to promote adiposity typical of Cushing’s syndrome, polycystic ovary syndrome, growth hormone deficiency, menopause, aging, alcoholism and depression.(2, 7)
Current evidence linking endocrine disruptors to adiposity-related disturbances originates mainly from studies of BPA, a ubiquitous man-made chemical substance (11–14). Although studies have suggested that low-dose BPA exposure may be associated with increased reproductive and cancer risks (15–21), regulatory bodies in the U.S., Canada and Europe have distinct views on whether BPA should be considered a hazardous substance (22). Since the 1960s, BPA has been produced in large quantities (2 million metric tons worldwide in 2003(13, 23)) for the manufacture of polymeric materials such as epoxy resins, polyester-styrene and polycarbonate plastics. These materials are used for a wide range of consumer products, including flame retardants, dental sealants and fillings, adhesives, protective coatings, infant feeding bottles, food and mineral water storage containers and food and beverage can linings (2, 11, 12, 24–28).
The health effects of endocrine disruptors, including BPA, are thought to be partly produced by sex hormones. For instance, BPA can mimic the action of the sex hormone 17β-estradiol (E2) by binding to estrogen receptors and inducing estrogen receptor-mediated gene expression (29–31). Whereas normal E2 concentrations are crucial for maintaining insulin sensitivity and β-cell function, abnormal E2 concentrations may promote insulin resistance, similar to what occurs in normal puberty or pregnancy (32). Exposure to an E2-mimicking substance such as BPA may initiate or exacerbate insulin resistance (2, 12). Animal studies suggest that BPA may alter insulin biosynthesis and secretion in pancreatic β-cells, potentially through the over-activation of the estrogen receptor, ER-α (14, 32). This may lead to insulin resistance and the subsequent development of type 2 diabetes (14, 32). Other mechanisms of BPA action include dysregulation of glucose transport in adipocytes and inhibition of adiponectin release (14, 32).
Biomonitoring studies suggest that over 90% of the U.S. population (≥6 years of age) has detectable urinary BPA concentrations (13, 14) and that BPA exposure may be a risk factor for type 2 diabetes, cardiovascular disease and related morbidities (11, 12). The purpose of this study is to examine the association of urinary BPA concentration with selected indices of glucose homeostasis, using a U.S.-representative sample from the 2005–2008 National Health and Nutrition Examination Surveys (NHANES). Because BPA is capable of mimicking E2 action, we further examined whether the hypothesized relationships varied according to sex.
METHODS
Study Population
The NHANES is a series of nationally representative sample surveys designed to assess the health and nutritional status of the U.S. civilian non-institutionalized population. Stratified, multistage, probability survey samples were obtained based on the selection of counties, blocks, households and persons within households, with over-sampling of individuals of low income, adults aged 60 years or older, African-Americans, and Mexican-Americans. Demographic, socioeconomic and health data were collected by trained staff using household interviews. A mobile examination center (MEC) run by health professionals collected anthropometric, physiological and laboratory measurements, either on all or a sub-group of study participants. Informed consent was obtained for all participants and the institutional review board of the National Center for Health Statistics approved all protocols for the NHANES.(33)
NHANES became a continuous surveillance system in 1999. For these analyses, we combined the 2005–2006 and 2007–2008 NHANES datasets and subsequently applied a series of selection criteria to fulfill the study purpose. The total sample consisted of 20,497 participants, 10,348 subjects who participated in the 2005–2006 NHANES wave (34) and 10,149 subjects who participated in the 2007–2008 NHANES wave (35). Of those, 11,791 were study-eligible because they were adults, 18 years of age and older. A sub-sample of 3,566 adults had urinary concentrations of BPA assayed during the MEC exam (1,652 in 2005–2006 and 1,914 in 2007–2008) (36–39); of those, 1,607 individuals had non-missing data on fasting glucose and insulin concentrations (744 in 2005–2006 and 863 in 2007–2008). A total of 21 individuals were excluded for having extreme values for total energy intake (<800 kilocalories (males), <600 kilocalories (females) and >5000 kilocalories (either sex)), leaving 1,586 eligible subjects (737 in 2005–2006 and 849 in 2007–2008). The distribution of the final sample (n=1,586) according to key demographic characteristics, namely age, sex and race, did not differ substantially from that of the original sample of NHANES 2005–2008 adults (n=11,791).
Measurements
Urinary bisphenol-A
In NHANES, urinary concentrations of BPA, benzophenone-3, 4-tert-octylphenol, five chlorophenols (2,4-dichlorophenol, 2,5-dichlorophenol, 2,4,5,-trichlorophenol, 2,4,6-trichlorophenol and triclosan) and several parabens (methyl-, ethyl-, propyl-, and butyl paraben) were determined using on-line solid phase extraction (SPE) coupled to high-performance liquid chromatography–isotope dilution tandem mass spectrometry (MS/MS). Briefly, the conjugated species of these substances (in 100 μL of urine) were hydrolyzed by use of β-glucuronidase/sulfatase (H. pomatia). After hydrolysis, samples were acidified with 0.1 M formic acid; the phenols were pre-concentrated by online SPE, separated by reversed-phase High Performance Liquid Chromatography (HPLC), and detected by atmospheric pressure chemical ionization (APCI)–MS/MS (36, 38, 40, 41). The lower limit of detection (LOD) for BPA was found to be 0.4ng/mL. Of 1,341 NHANES participants, 92 had a BPA concentration below the LOD (0.4ng/mL) and were assigned by NHANES a value of 0.3ng/mL (11, 42). The Shapiro-Wilk test for normality implied that the distribution of urinary BPA concentration was skewed. Accordingly, the continuous BPA measurement was analyzed after log-transformation; it was also categorized into quartiles (‘Q1: 0.3-<1.0’, ‘Q2: 1.0-<2.0’, ‘Q3: 2.0-<3.7’, ‘Q4: ≥ 3.7’) to examine dose-response relationships. A BPA-to-creatinine ratio was also computed to examine the effect of BPA level per gram of creatinine excreted in urine. This measurement was log-transformed and defined in quartiles (‘Q1: 0.001-<0.01’, ‘Q2: 0.01-<0.02’, ‘Q3: 0.02-<0.03’, ‘Q4: ≥ 0.03’).
Glucose homeostasis
Circulating levels of glucose (mg/dl or mmol/l) and insulin (μU/ml) were determined after an overnight fast on a sub-sample of MEC participants. Hyperglycemia was defined as fasting glucose ≥ 100 mg/dl, based on the updated National Cholesterol Education Program Adult Treatment Panel III criteria for metabolic syndrome (43). An index of β-cell function (HOMA-β) was calculated as 20×(insulin (μU/ml))/(glucose (mmol/l))−3.5) (44). Insulin sensitivity was defined using the quantitative insulin sensitivity check index (QUICKI), calculated using the formula 1/log (insulin(μU/ml))+log(glucose(mg/dl)) (45). Insulin resistance was defined as Homeostasis Model Assessment for Insulin Resistance (HOMA-IR), calculated using the formula [insulin (μU/ml) × glucose (mg/dl) / 405], with HOMA-IR ≥ 2.5 suggesting a high level of insulin resistance (46, 47). Because of their skewed distributions, continuous measurements of glucose, insulin, HOMA-β, QUICKI and HOMA-IR were analyzed as log-transformed variables.
Covariates
Socio-demographic and lifestyle factors were identified as a priori confounders for the hypothesized relationships based on the literature (11–14, 48–56). Socio-demographic factors were defined as age (in years), sex (male, female), race (Mexican American, Other Hispanic, non-Hispanic White, non-Hispanic Black, Other), education (less than high school, high school, more than high school), marital status (ever married, never married/living with partner) and survey wave (2005–2006, 2007–2008). Smoking status was categorized as non-smoker, ex-smoker and current smoker. Physical activity was quantified in terms of metabolic equivalent scores (METS) using pre-defined 2005–2006 and 2007–2008 NHANES questionnaire items and weights and further categorized into quartiles (‘Q1: 0’, ‘Q2:>0-<6.8’, ‘Q3: 6.8-<11.8’ and ‘Q4: ≥11.8’)). Dietary energy intake (in kilocalories (kcal) was calculated as the average of two 24-hour dietary recalls and further categorized into quartiles (‘Q1: 621.0-<1480.0, ‘Q2: >1480.0-<1971.5’, ‘Q3: 1971.5-<2528’ and ‘Q4: ≥2528’)). Finally, urinary creatinine concentration was defined, in mg/dl, as quartiles (‘Q1: 8-<77’, ‘Q2: 77-<121’, ‘Q3: 121-<172’ and ‘Q4: ≥172’).
Statistical Analysis
All analyses were conducted using STATA version 12. We applied survey commands and the recommended fasting sample weights for the period of 2005–2008 (57). Summary statistics included (means ± standard errors of the mean (SEM)) and (median ± interquartile range) for continuous variables or frequencies and percentages for categorical variables. Bivariate associations of log-transformed BPA in relation to socio-demographic, lifestyle and outcome characteristics were analyzed using one-way analysis of variance (ANOVA). Multivariable regression models were constructed to evaluate the association of BPA concentration (log-transformed or in quartiles) or BPA-to-creatinine ratio (log-transformed or in quartiles) with the selected indices of glucose homeostasis (log-transformed or dichotomized), after adjustment for a priori confounders. Beta coefficients and odds ratios (OR) were computed with their 95 percent confidence intervals (CI) using linear (svyreg) and logistic (svylogit) regression, taking sampling weights into consideration. These weights were defined to represent the U.S. civilian, non-institutionalized population while accounting for over-sampling of certain age and ethnic groups and interview non-response. Race-stratified analyses, specifically for the “Mexican Americans” and “Other Hispanic” groups, revealed aberrant results when comparing estimates of BPA concentration for the 2005–2008 NHANES to the 2-year (2005–2006 NHANES and 2007–2008 NHANES) estimates. Further analyses suggested no significant race-by-survey wave interactions in relation to BPA concentration. Accordingly, survey wave was included in the final regression models. Two-way interactions of BPA-by-sex were evaluated in the fully-adjusted regression models in order to assess variations in the association of BPA level with selected outcomes, according to sex. Two-sided statistical tests were performed at α level of 0.05.
RESULTS
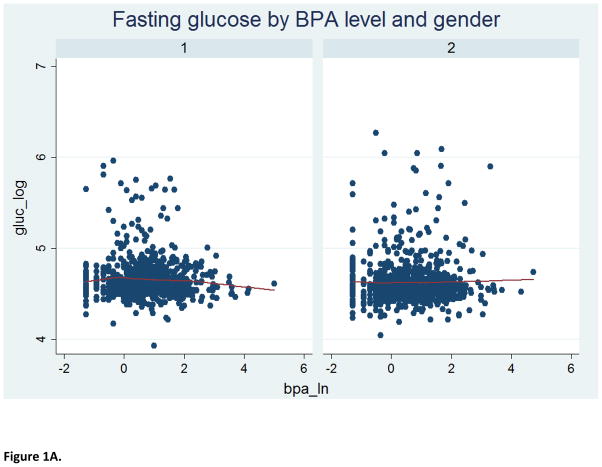
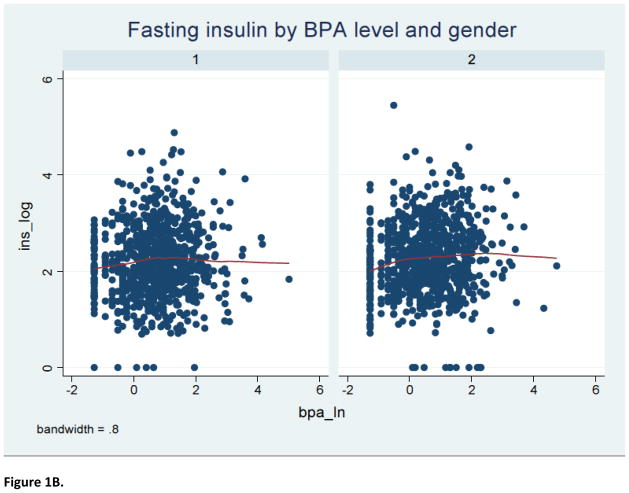
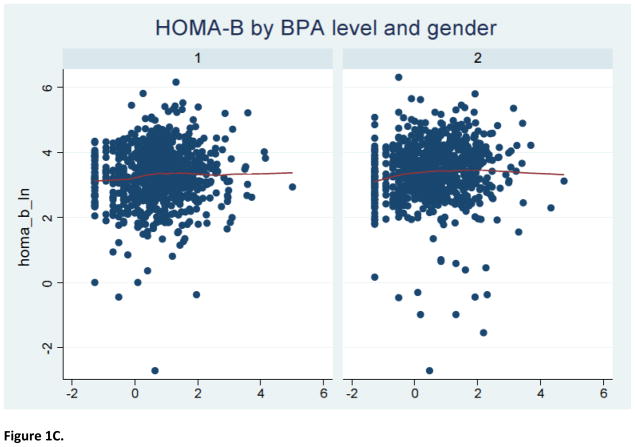
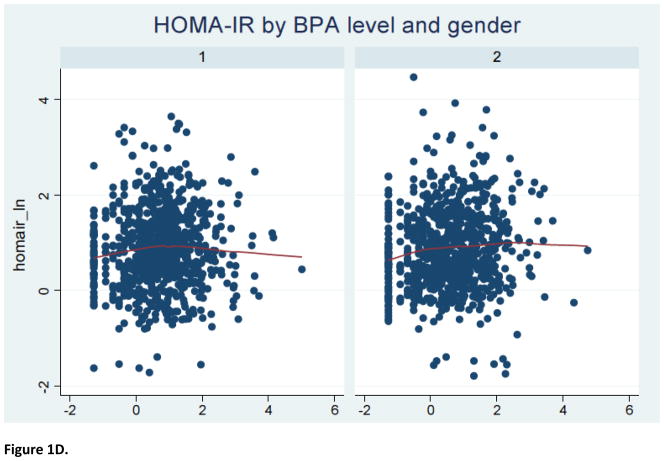
The study sample consisted of 1586 2005–2008 NHANES participants (797 men and 789 women) with a mean (± SEM) age of 45.4 (± 0.7) years. Furthermore, 52% were females, 70% were non-Hispanic White, 56% had over high-school level education, 76% were ever-married and 51% had never smoked cigarettes; the mean (± SEM) metabolic score (MetS) for physical activity, dietary energy intake and creatinine level were 7.9 (± 0.3), 2136.7 (± 36.4) kilocalories and 130.7 ± 2.9 mg/dl, respectively. The median urinary BPA concentration was estimated to be 2.0, with an inter-quartile range of 1.0–3.7 ng/mL. As shown in Table 1, BPA level varied significantly according to age, marital status and creatinine concentration. Specifically, urinary BPA level declined with advancing age; it was also higher among individuals who were either never married or were living with a partner and correlated positively with creatinine level. Table 2 describes the selected indices of glucose homeostasis, revealing no significant bivariate associations between urinary BPA level and the selected dichotomous outcomes, namely fasting glucose ≥100 mg/L and HOMA-IR ≥2.5, before or after stratifying by sex. Further analyses suggested a complex non-linear relationship between urinary BPA level and several continuous outcomes of interest (Figure 1).
Table 1.
Bisphenol-A by socio-demographic and lifestyle factors in the study sample (n=1586)
| N (%a) or (Mean ± SEM)a | Bisphenol-A (ng/mL) | P* | |
|---|---|---|---|
| Median (IQR) | |||
| Overall | 2.0 (1.0, 3.7) | ||
| Sex: | 0.23 | ||
| Male | 797 (47.9) | 2.1 (1.1, 3.7) | |
| Female | 789 (52.0) | 1.9 (0.9, 3.7) | |
| Age (years): | (45.4 ± 0.7) | <0.0001 | |
| 18–19 | 110 (3.9) | 2.6 (1.4, 5.4) | |
| 20–29 | 258 (17.9) | 2.5 (1.4, 4.4) | |
| 30–39 | 259 (18.8) | 2.0 (1.2, 3.4) | |
| 40–49 | 249 (19.9) | 2.1 (1.2, 4.2) | |
| 50–59 | 224 (17.1) | 1.9 (0.9, 3.3) | |
| 60+ | 486 (22.2) | 1.5 (0.8, 2.9) | |
| Race: | 0.62 | ||
| Mexican American b | 305 (8.5) | 1.7 (1.1, 3.2) | |
| Other Hispanic c | 122 (4.5) | 2.0 (1.2, 3.9) | |
| Non-Hispanic White | 768 (70.2) | 1.8 (0.9, 3.6) | |
| Non-Hispanic Black | 323 (10.9) | 2.6 (1.4, 4.7) | |
| Other | 68 (5.9) | 1.8 (0.9, 2.8) | |
| Education: | 0.27 | ||
| Less than High School | 443 (17.9) | 1.9 (1.1, 3.7) | |
| High School | 407 (26.4) | 2.1 (0.9, 3.8) | |
| More than High School | 734 (55.6) | 2.0 (1.0, 3.6) | |
| Marital status: | 0.003 | ||
| Ever married | 1152 (75.9) | 1.8 (0.9, 3.4) | |
| Never married /Living with partner | 391 (25.0) | 2.6 (1.3, 4.7) | |
| Smoking status: | 0.63 | ||
| Non-smoker | 757 (50.7) | 1.9 (1.0, 3.7) | |
| Ex-smoker | 401 (26.1) | 1.9 (0.9, 3.1) | |
| Current smoker | 315 (23.2) | 2.1 (1.1, 4.1) | |
| Physical activity (METS): | (7.9 ± 0.3) | 0.35 | |
| Q1: 0 | 266 (12.9) | 2.0 (1.0, 4.0) | |
| Q2: >0-<6.8 | 244 (17.7) | 1.9 (0.9, 3.2) | |
| Q3: 6.8-<11.8 | 243 (17.4) | 1.8 (0.8, 3.3) | |
| Q4: ≥ 11.8 | 250 (15.7) | 2.4 (1.3, 4.7) | |
| Missing | 583 (36.3) | 2.0 (1.0, 3.7) | |
| Dietary Energy Intake (kcal): | (2136.7 ± 36.4) | 0.88 | |
| Q1: 621.0-<1480.0 | 334 (19.1) | 1.9 (1.0, 3.5) | |
| Q2: >1480.0-<1971.5 | 336 (21.6) | 1.7 (0.9, 3.6) | |
| Q3: 1971.5-<2528 | 335 (22.4) | 2.1 (1.1, 4.1) | |
| Q4: ≥ 2528 | 336 (23.8) | 2.1 (1.1, 3.5) | |
| Missing | 245 (13.1) | 2.1 (1.1, 4.4) | |
| Creatinine level (mg/dl): | (130.7 ± 2.9) | <0.0001 | |
| Q1: 8-<77 | 396 (23.3) | 0.9 (0.4, 1.6) | |
| Q2: 77-<121 | 393 (25.4) | 1.7 (0.9, 2.9) | |
| Q3: 121-<172 | 400 (27.4) | 2.3 (1.5, 3.7) | |
| Q4: ≥ 172 | 397 (23.7) | 3.6 (2.3, 6.5) |
Weighted analyses;
NHANES 2005–2006 (Median (IQR)): (1.8 (0.9–3.0)); NHANES 2007–2008 (Median (IQR)): (1.7 (1.1–3.5));
NHANES 2005–2006 (Median (IQR)): (2.4 (1.5–3.8)); NHANES 2007–2008 (Median (IQR)): (1.9 (1.0–4.1));
P value for ANOVA test involving log-transformed bisphenol-A level with comparisons made across all categories of the covariates; METS=Metabolic Equivalents; Q1=1st quartile; Q2=2nd quartile; Q3=3rd quartile; Q4=4th quartile; SEM=Standard error of mean; IQR=Interquartile range.
Table 2.
Bisphenol-A by indices of glucose homeostasis in the study sample according to sex (n=1586)
| N (%a) or (Mean ± SEM) a | Bisphenol-A (ng/mL) | P* | |
|---|---|---|---|
| Overall | Median (IQR) | ||
|
| |||
| Fasting glucose (mg/dL): | (104.3 ± 0.9) | 0.55 | |
| ≥100 | 774 (45.7) | 1.9 (1.0, 3.6) | |
| < 100 | 812 (54.3) | 2.0 (1.0, 3.8) | |
| Fasting insulin (μU/ml): | (11.4 ± 0.3) | ||
| HOMA-β: | (35.9 ± 0.9) | ||
| HOMA-IR: | (3.1 ± 0.1) | 0.076 | |
| ≥2.5 | 738 (41.7) | 2.1 (1.1, 3.8) | |
| < 2.5 | 848 (58.3) | 1.8 (0.9, 3.7) | |
| QUICKI: | (5.1 ± 0.006) | ||
|
| |||
| Males | Median (IQR) | ||
|
| |||
| Fasting glucose (mg/dL): | (106.3 ± 1.1) | 0.62 | |
| ≥100 | 445 (37.9) | 2.0 (1.1, 3.5) | |
| < 100 | 352 (62.1) | 2.1 (1.1, 4.0) | |
| Fasting insulin (μU/ml): | (11.9 ± 0.4) | ||
| HOMA-β: | (36.8 ± 1.4) | ||
| HOMA-IR: | (3.3 ± 0.1) | 0.15 | |
| ≥2.5 | 378 (38.1) | 2.1 (1.2, 3.6) | |
| < 2.5 | 419 (61.9) | 2.0 (1.1, 3.9) | |
| QUICKI: | (5.1 ± 0.009) | ||
|
| |||
| Females | Median (IQR) | ||
|
| |||
| Fasting glucose (mg/dL): | (102.5 ± 1.4) | 0.48 | |
| ≥100 | 329 (37.9) | 1.7 (0.9, 3.8) | |
| < 100 | 460 (62.1) | 2.0 (0.9, 3.7) | |
| Fasting insulin (μU/ml): | (11.0 ± 0.5) | ||
| HOMA-β: | (35.0 ± 1.3) | ||
| HOMA-IR: | (3.0 ± 0.2) | 0.25 | |
| ≥2.5 | 360 (38.1) | 2.1 (1.0, 4.2) | |
| < 2.5 | 429 (61.9) | 1.7 (0.8, 3.4) | |
| QUICKI: | (5.1 ± 0.007) | ||
Weighted analyses;
P value for ANOVA test involving log-transformed bisphenol-A level with comparisons made across all categories of the covariates; IQR=Interquartile range; HOMA= Homeostasis Model Assessment; QUICKI= Quantitative Insulin Sensitivity Check Index.
Figure 1.
LOWESS curve for relationship of BPA level with indices of glucose homeostasis
Notes: 1=male; 2=female
Table 3 presents multivariate linear regression models for urinary BPA and BPA-to-creatinine ratio exposure in relation to continuous outcomes after adjustment for age, sex, race, education, marital status, smoking status, physical activity, total dietary intake, urinary creatinine concentration and survey wave. For both men and women, urinary BPA exposure was not significantly associated with fasting glucose concentration or QUICKI. Taking the 1st quartile as a reference group, the 3rd quartile of urinary BPA exposure was directly related to log-transformed fasting insulin concentration (β=0.2, 95% CI: 0.09, 0.3), log-transformed HOMA-β (β=0.2, 95% CI: 0.08, 0.4) and log-transformed HOMA-IR (β=0.2, 95% CI: 0.1, 0.4), implying that a BPA level ranging from 2.0 ng/dl to 3.7 ng/dl may be linked to enhanced β-cell function as well as hyperinsulinemia and insulin resistance. Stratified analyses by sex suggested that the aforementioned associations were significant among men but not among women, although the two-way interaction terms were not statistically significant. By contrast, the ratio of BPA-to-creatinine was not significantly associated with indices of glucose homeostasis, defined as continuous variables.
Table 3.
Multivariate linear regression models for bisphenol-A and ratio of bisphenol-A to creatinine level in relation to log-transformed indices of glucose homeostasis, stratified by sex (n=1473) a
| Fasting glucose | Fasting insulin | HOMA-β | HOMA-IR | QUICKI | |
|---|---|---|---|---|---|
|
| |||||
| Overall – Bisphenol-A | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) |
| Bisphenol-A [continuous]: | n=1473 | n=1473 | n=1469 | n=1473 | n=1457 |
| 0.005 (−0.009, 0.02) | 0.02 (−0.04, 0.08) | 0.02 (−0.04, 0.09) | 0.03 (−0.04, 0.09) | −0.0003 (−0.003,0.003 | |
| Bisphenol-A [categorical]: | Ptrend=0.12 | Ptrend=0.27 | Ptrend=0.35 | Ptrend=0.16 | Ptrend=0.99 |
| Q1: 0.3-<1.0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2: 1.0-<2.0 | −0.02 (−0.05, 0.02) | 0.02 (−0.1, 0.2) | 0.009 (−0.2, 0.2) | 0.008 (−0.17, 0.19) | −0.008 (−0.02, 0.002) |
| Q3: 2.0-<3.7 | 0.02 (−0.01, 0.05) | 0.2 (0.09, 0.3) | 0.2 (0.08, 0.4) | 0.2 (0.1, 0.4) | −0.006 (−0.02, 0.003) |
| Q4: ≥ 3.7 | 0.02 (−0.02, 0.07) | 0.04 (−0.1, 0.2) | 0.03 (−0.17, 0.22) | 0.06 (−0.1, 0.2) | −0.001 (−0.01, 0.009) |
|
| |||||
| Overall – Ratio of bisphenol-A to creatinine | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) |
|
| |||||
| Bisphenol-A [continuous]: | n=1473 | n=1473 | n=1469 | n=1473 | n=1457 |
| 0.005 (−0.009, 0.02) | 0.9 (0.5, 1.6) | 0.01 (−0.05, 0.08) | 0.02 (−0.05, 0.08) | 0.0005 (−0.003, 0.004 | |
| Bisphenol-A [categorical]: | Ptrend=0.06 | Ptrend=0.32 | Ptrend=0.38 | Ptrend=0.18 | Ptrend=0.81 |
| Q1: 0.001-<0.01 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2: 0.01-<0.02 | 0.0005 (−0.02, 0.02) | 0.06 (−0.06, 0.2) | 0.09 (−0.06, 0.2) | 0.07 (−0.07, 0.2) | −0.003 (−0.01, 0.004) |
| Q3: 0.02-<0.03 | 0.03 (−0.02, 0.07) | 0.03 (−0.1, 0.2) | 0.8 (−0.1, 0.2) | 0.05 (−0.1, 0.2) | −0.001 (−0.009, 0.006 |
| Q4: ≥ 0.03 | 0.02 (−0.05, 0.05) | 0.08 (−0.05, 0.2) | 0.09 (−0.07, 0.3) | 0.1 (−0.03, 0.2) | 0.00009 (−0.007, 0.008 |
|
| |||||
| Males – Bisphenol-A | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) |
|
| |||||
| Bisphenol-A [continuous]: | n=733 | n=733 | n=733 | n=733 | n=727 |
| 0.005 (−0.01, 0.02) | 0.044 (−0.02, 0.1) | 0.04 (−0.03, 0.1) | 0.05 (−0.02, 0.12) | −0.0009 (−0.005, 0.003 | |
| Bisphenol-A [categorical]: | Ptrend=0.23 | Ptrend=0.13 | Ptrend=0.23 | Ptrend=0.09 | Ptrend=0.92 |
| Q1: 0.3-<1.0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2: 1.0-<2.0 | −0.009 (−0.07, 0.05) | 0.1 (−0.04, 0.3) | 0.1 (−0.06, 0.3) | 0.09 (−0.08, 0.3) | −0.006 (−0.02, 0.005) |
| Q3: 2.0-<3.7 | 0.02 (−0.04, 0.08) | 0.3 (0.2, 0.5) | 0.4 (0.2, 0.5) | 0.3 (0.2, 0.5) | −0.007 (−0.02, 0.006) |
| Q4: ≥ 3.7 | 0.03 (−0.04, 0.09) | 0.1 (−0.08, 0.3) | 0.09 (−0.1, 0.3) | 0.2 (−0.1, 0.5) | −0.0004 (−0.01, 0.01) |
|
| |||||
| Males – Ratio of bisphenol-A to creatinine | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) |
|
| |||||
| Bisphenol-A [continuous]: | n=733 | n=733 | n=733 | n=733 | n=727 |
| 0.003 (−0.02, 0.02) | 0.05 (−0.02, 0.1) | 0.05 (−0.03, 0.1) | 0.05 (−0.02, 0.12) | −0.001 (−0.006, 0.003 | |
| Bisphenol-A [categorical]: | Ptrend=0.09 | Ptrend=0.18 | Ptrend=0.35 | Ptrend=0.11 | Ptrend=0.92 |
| Q1: 0.001-<0.01 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2: 0.01-<0.02 | −0.009 (−0.05, 0.03) | 0.05 (−0.1, 0.2) | 0.07 (−0.1, 0.2) | 0.04 (−0.14, 0.21) | −0.002 (−0.01, 0.007) |
| Q3: 0.02-<0.03 | 0.02 (−0.02, 0.07) | 0.1 (−0.04, 0.3) | 0.1 (−0.05, 0.3) | 0.14 (−0.03, 0.32) | −0.0003 (−0.009, 0.009 |
| Q4: ≥ 0.03 | 0.03 (−0.01, 0.06) | 0.1 (−0.09, 0.3) | 0.09 (−0.2, 0.3) | 0.13 (−0.07, 0.34) | −0.00002 (−0.01, 0.01 |
|
| |||||
| Females – Bisphenol-A | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) |
|
| |||||
| Bisphenol-A [continuous]: | n=740 | n=740 | n=736 | n=740 | n=730 |
| 0.008 (−0.01, 0.03) | −0.003 (−0.09, 0.08) | −0.003 (−0.10, 0.09) | 0.004 (−0.09, 0.10) | 0.0008 (−0.003, 0.005 | |
| Bisphenol-A [categorical]: | Ptrend=0.23 | Ptrend=0.83 | Ptrend=0.83 | Ptrend=0.64 | Ptrend=0.98 |
| Q1: 0.3-<1.0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2: 1.0-<2.0 | −0.02 (−0.07, 0.03) | −0.03 (−0.3, 0.2) | −0.07 (−0.36, 0.22) | −0.06 (−0.3, 0.2) | −0.009 (−0.02, 0.004) |
| Q3: 2.0-<3.7 | 0.02 (−0.03, 0.06) | 0.1 (−0.09, 0.4) | 0.2 (−0.08, 0.4) | 0.1 (−0.1, 0.4) | −0.008 (−0.02, 0.001) |
| Q4: ≥ 3.7 | 0.02 (−0.04, 0.08) | −0.02 (−0.3, 0.2) | −0.03 (−0.3, 0.2) | 0.005 (−0.3, 0.3) | −0.009 (−0.01, 0.01) |
|
| |||||
| Females – Ratio of bisphenol-A to creatinine | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) |
|
| |||||
| Bisphenol-A [continuous]: | n=740 | n=740 | n=736 | n=740 | n=730 |
| 0.01 (−0.01, 0.03) | −0.02 (−0.1, 0.06) | −0.02 (−0.1, 0.07) | −0.01 (−0.1, 0.08) | 0.003 (−0.001, 0.007) | |
| Bisphenol-A [categorical]: | Ptrend=0.18 | Ptrend=0.97 | Ptrend=0.91 | Ptrend=0.8 | Ptrend=0.54 |
| Q1: 0.001-<0.01 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2: 0.01-<0.02 | 0.009 (−0.03, 0.05) | 0.08 (−0.1, 0.3) | 0.1 (−0.1, 0.3) | 0.09 (−0.1, 0.3) | −0.004 (−0.01, 0.006) |
| Q3: 0.02-<0.03 | 0.03 (−0.03, 0.09) | −0.04 (−0.3, 0.2) | −0.05 (−0.4, 0.2) | −0.009 (−0.3, 0.3) | −0.002 (−0.01, 0.008) |
| Q4: ≥ 0.03 | 0.02 (−0.02, 0.06) | 0.04 (−0.1, 0.2) | 0.07 (−0.1, 0.3) | 0.06 (−0.1, 0.3) | 0.001 (−0.008, 0.01) |
All models were adjusted for age (in years), sex (‘male’, ‘female’), race (‘Mexican-American’, ‘Other Hispanic’, ‘Non-Hispanic Black’, ‘Non-Hispanic White’, ‘Other’), Education (‘< High School’, ‘High School’, ‘> High School’), Marital status (‘Ever married’, ‘Never married/Living with partner’), Smoking status (‘Non-smoker’, ‘Ex-smoker’, ‘Current smoker’), Physical activity (metabolic equivalents, in quartiles), Dietary energy intake (kcal, in quartiles), Creatinine (mg/dl, in quartiles) and survey wave (‘2005–2006’, ‘2007–2008’); CI=Confidence Interval; HOMA = Homeostasis Model Assessment; IR=Insulin resistance; Q1=1st quartile; Q2=2nd quartile; Q3=3rd quartile; Q4=4th quartile; QUICKI= Quantitative Insulin Sensitivity Check Index.
Table 4 presents multivariate logistic regression models for urinary BPA and BPA-to-creatinine ratio exposure in relation to dichotomous outcomes after adjustment for age, sex, race, education, marital status, smoking status, physical activity, total dietary intake, urinary creatinine concentration and survey wave. Hyperglycemia, defined as fasting glucose ≥ 100 mg/dl, was not associated with urinary BPA level, irrespective of sex. By contrast, insulin resistance (defined as HOMA-IR ≥ 2.5) was significantly and positively associated with urinary BPA level (3rd quartile vs. 1st quartile: OR=1.7, 95% CI: 1.2, 2.4). Finally, a significant BPA-by-sex interaction effect was found (Pinteraction=0.04), whereby the 3rd quartile of BPA was identified as high-risk for insulin resistance compared to the 1st quartile, among men (OR=2.7, 95% CI: 1.7, 4.3), but not among women (OR=1.2, 95% CI: 0.6, 2.3). By contrast, the ratio of BPA-to-creatinine was not significantly associated with indices of hyperglycemia or insulin resistance.
Table 4.
Multivariate logistic regression models for bisphenol-A and ratio of bisphenol-A to creatinine level in relation to hyperglycemia and insulin resistance, stratified by sexa
| Hyperglycemia (fasting glucose ≥ 100 mg/dl) | Insulin resistance (HOMA-IR ≥ 2.5) | |
|---|---|---|
|
| ||
| Overall – Bisphenol-A | OR (95% CI) | OR (95% CI) |
| Bisphenol-A [continuous]: | n=1473 | n=1473 |
| 1.0 (0.9, 1.2) | 1.1 (0.9, 1.3) | |
| PBPA*sex | 0.68 | 0.30 |
| Bisphenol-A [categorical]: | Ptrend=0.55 | Ptrend=0.23 |
| Q1: 0.3-<1.0 | 1.00 | 1.00 |
| Q2: 1.0-<2.0 | 0.9 (0.52, 1.42) | 1.1 (0.6, 1.8) |
| Q3: 2.0-<3.7 | 1.2 (0.7, 1.9) | 1.7 (1.2, 2.4) |
| Q4: ≥ 3.7 | 1.1 (0.6, 1.9) | 1.2 (0.7, 1.9) |
| PBPA(Q2)*sex | 0.32 | 0.46 |
| PBPA(Q3)*sex | 0.15 | 0.04 |
| PBPA(Q4)*sex | 0.81 | 0.59 |
|
| ||
| Overall – Ratio of bisphenol-A to creatinine | OR (95% CI) | OR (95% CI) |
|
| ||
| Bisphenol-A [continuous]: | n=1473 | n=1473 |
| 1.0 (0.9, 1.2) | 1.1 (0.9, 1.3) | |
| PBPA*sex | 0.14 | 0.19 |
| Bisphenol-A [categorical]: | Ptrend=0.30 | Ptrend=0.09 |
| Q1: 0.001-<0.01 | 1.00 | 1.00 |
| Q2: 0.01-<0.02 | 1.1 (0.8, 1.6) | 0.9 (0.6, 1.4) |
| Q3: 0.02-<0.03 | 1.3 (0.8, 1.9) | 1.2 (0.8, 1.7) |
| Q4: ≥ 0.03 | 1.2 (0.8, 1.9) | 1.3 (0.9, 1.8) |
| PBPA(Q2)*sex | 0.77 | 0.81 |
| PBPA(Q3)*sex | 0.74 | 0.72 |
| PBPA(Q4)*sex | 0.22 | 0.34 |
|
| ||
| Males – Bisphenol-A | OR (95% CI) | OR (95% CI) |
|
| ||
| Bisphenol-A [continuous]: | n=733 | n=733 |
| 1.1 (0.9, 1.4) | 1.2 (1.0, 1.5) | |
| Bisphenol-A [categorical]: | Ptrend=0.18 | Ptrend=0.12 |
| Q1: 0.3-<1.0 | 1.00 | 1.00 |
| Q2: 1.0-<2.0 | 0.8 (0.4, 1.3) | 1.3 (0.6, 2.5) |
| Q3: 2.0-<3.7 | 1.7 (0.8, 3.5) | 2.7 (1.7, 4.3) |
| Q4: ≥ 3.7 | 1.2 (0.6, 2.6) | 1.5 (0.7, 3.2) |
|
| ||
| Males – Ratio of bisphenol-A to creatinine | OR (95% CI) | OR (95% CI) |
|
| ||
| Bisphenol-A [continuous]: | n=733 | n=733 |
| 1.1 (0.9, 1.4) | 1.2 (0.9, 1.5) | |
| Bisphenol-A [categorical]: | Ptrend=0.12 | Ptrend=0.06 |
| Q1: 0.001-<0.01 | 1.00 | |
| Q2: 0.01-<0.02 | 1.0 (0.6, 1.7) | 0.9 (0.5, 1.6) |
| Q3: 0.02-<0.03 | 1.2 (0.6, 2.2) | 1.3 (0.8, 2.3) |
| Q4: ≥ 0.03 | 1.7 (0.9, 3.5) | 1.7 (0.9, 3.3) |
|
| ||
| Females | OR (95% CI) | OR (95% CI) |
|
| ||
| Bisphenol-A [continuous]: | n=740 | n=740 |
| 0.9 (0.7, 1.2) | 0.9 (0.8, 1.2) | |
| Bisphenol-A [categorical]: | Ptrend=0.59 | Ptrend=0.91 |
| Q1: 0.3-<1.0 | 1.00 | 1.00 |
| Q2: 1.0-<2.0 | 1.1 (0.6, 2.0) | 0.9 (0.5, 1.9) |
| Q3: 2.0-<3.7 | 0.7 (0.3, 1.5) | 1.2 (0.6, 2.3) |
| Q4: ≥ 3.7 | 0.9 (0.5, 1.8) | 0.9 (0.5, 1.8) |
|
| ||
| Females – Ratio of bisphenol-A to creatinine | OR (95% CI) | OR (95% CI) |
|
| ||
| Bisphenol-A [continuous]: | n=740 | n=740 |
| 0.9 (0.7, 1.2) | 0.9 (0.8, 1.1) | |
| Bisphenol-A [categorical]: | Ptrend=0.56 | Ptrend=0.8 |
| Q1: 0.001-<0.01 | 1.00 | |
| Q2: 0.01-<0.02 | 1.2 (0.6, 2.3) | 0.9 (0.5, 1.8) |
| Q3: 0.02-<0.03 | 1.3 (0.6, 2.6) | 0.9 (0.5, 1.9) |
| Q4: ≥ 0.03 | 0.9 (0.6, 1.5) | 0.9 (0.6, 1.4) |
All models were adjusted for age (in years), sex (‘male’, ‘female’), race (‘Mexican-American’, ‘Other Hispanic’, ‘Non-Hispanic Black’, ‘Non-Hispanic White’, ‘Other’), Education (‘< High School’, ‘High School’, ‘> High School’), Marital status (‘Ever married’, ‘Never married/Living with partner’), Smoking status (‘Non-smoker’, ‘Ex-smoker’, ‘Current smoker’), Physical activity (metabolic equivalents, in quartiles), Dietary energy intake (kcal, in quartiles), Creatinine (mg/dl, in quartiles) and survey wave (‘2005–2006’, ‘2007–2008’); CI=Confidence Interval; HOMA = Homeostasis Model Assessment; IR=Insulin resistance; OR=odds ratio; Q1=1st quartile; Q2=2nd quartile; Q3=3rd quartile; Q4=4th quartile.
DISCUSSION
In this cross-sectional study using a nationally representative sample, we examined the associations of urinary BPA level with selected indices of glucose homeostasis among U.S. adults who participated in the 2005–2007 NHANES. Multivariable analyses suggested that urinary BPA level ranging between 2.0 and 3.7 ng/dl may be associated with improved β-cell function, but may also be a high-risk group for hyperinsulinemia resulting from insulin resistance. Furthermore, the link between urinary BPA and selected indices of glucose homeostasis was stronger among males compared to females. Hyperglycemia, per se, does not appear to be influenced by urinary BPA level. Moreover, there were no significant dose-response relationships between BPA level and indices of glucose homeostasis. Although BPA level was predictive of selected health outcomes, the ratio of BPA-to-creatinine level was not, implying a complex relationship which necessitates further investigation.
The hypothesized link between endocrine disruptors and adiposity is biologically plausible and coherent with the “environmental obesogen hypothesis” and the “developmental origins of health and disease.” The “environmental obesogen hypothesis” proposes that exposure to a toxic chemical burden is superimposed on energy imbalance resulting from diet and lifestyle to initiate or exacerbate the development of obesity and its associated health consequences, including type 2 diabetes, cardiovascular disease, hypertension, and dyslipidemias (2). The “developmental origins of health and disease” paradigm proposes that fetal and perinatal stages of development represent periods of heightened sensitivity for the establishment of persistent changes to the individual’s adaptive physiology (2). BPA may be viewed as a toxic chemical that can target nuclear hormone receptor signaling pathways, resulting in perturbed adipocyte proliferation, differentiation or modulation of systemic homeostatic controls, with long-term consequences that may be magnified if disruption occurs during sensitive developmental periods (2). Our study findings are consistent with previous studies involving animals and human subjects that have implicated developmental and post-developmental exposures to endocrine-disrupting chemicals in reproductive and developmental outcomes (58, 59), cancer (22, 32, 60), obesity (48, 53, 61, 62), insulin resistance (11, 56, 63), type 2 diabetes (2, 11–14, 48), hypertension (2, 51), dyslipidemia (2, 64, 65), metabolic syndrome (66, 67), cardiovascular disease(2, 11, 12, 68) and depression (69).
Our study findings are also consistent with a growing number of studies that have used a nationally representative sample to investigate the role of BPA (11–14, 48, 51) in cardiometabolic conditions associated with adiposity, although our study is the first to identify a non-linear relationship whereby the 3rd quartile of urinary BPA level can be considered as a high-risk group for health outcomes related to circulating level of insulin but not glucose. Lang et al.(11) previously assessed BPA exposure as a risk factor for chronic disease diagnoses, blood markers of liver function, glucose homeostasis, inflammation and lipid changes among U.S. adults, 18–74 years, who participated in the 2003–2004 NHANES. BPA concentration was positively associated with cardiovascular disease, diabetes and abnormal liver enzymes (11). Melzer et al.(12) reported similar results using 2003–2006 NHANES data. Using 2003–2008 data, Shankar et al.(13) also found a 50% increased odds of diabetes among individuals in the upper versus lower quartile of BPA concentration. By the same token, Silver et al.(14) reported a positive relationship between BPA concentration and the likelihood of type 2 diabetes in the 2003–2008 NHANES sample of U.S. adults. However, this relationship was not reported consistently in the three NHANES cycles (2003–2004, 2005–2006 and 2007–2008) (14).
To our knowledge, the present study is the first to have examined multiple indices of glucose homeostasis, including HOMA-IR, QUICKI and HOMA-β in relation to urinary BPA level, and to have examined interaction effects by sex for the hypothesized relationships. However, our study findings should be interpreted with caution in light of several limitations. First, the NHANES study has a cross-sectional design that precludes establishing temporal relationships. Consequently, it is difficult to ascertain the time window of BPA exposure that is critical for onset of adiposity-related disturbances. Second, variation in methodology used to assess BPA exposures among previously conducted studies reduces our ability to synthesize the overall evidence for comparative purposes. Third, urinary BPA level was measured once and this measurement may not reflect typical pre- or post-developmental exposure to these endocrine disruptors. Fourth, HOMA-IR cannot be considered as a gold standard for measuring insulin resistance. While it is a less invasive and costly method for measuring insulin resistance as compared to the glucose clamp test, the validity of HOMA-IR and the cut-off point of 2.5 is limited in the context of low BMI, lower β-cell function, and high fasting glucose concentrations (70). Fifth, given the observational nature of the study, residual confounding cannot be ruled out as an alternative explanation, especially that dietary factors besides total energy consumption may influence the level of exposure to endocrine disruptors such as BPA. Finally, multiple comparisons were made leading to statistically significant associations which cannot be ruled out as chance findings. In conclusion, a complex relationship between urinary BPA level and selected indices of glucose homeostasis, specifically those reflecting β-cell function, hyperinsulinemia and insulin resistance was identified; the magnitude of these relationships differed according to sex, with U.S. men exhibiting stronger associations compared to U.S women. Prospective cohort studies are needed to further examine BPA as a determinant of adiposity-related disturbances.
Acknowledgments
No funding was provided for this project. However, this research was supported in part by the intramural research program of the NIH, National Institute on Aging.
LIST OF ABBREVIATIONS AND ACRONYMS
- BPA
Bisphenol-A
- E2
17β-estradiol
- HOMA-β
Homeostasis Model Assessment for β-cell function
- HOMA-IR
Homeostasis Model Assessment for Insulin Resistance
- HPLC
High Performance Liquid Chromatography
- LOD
Limit of detection
- MEC
Mobile examination center
- MS/MS
tandem mass spectrometry
- NHANES
National Health and Nutrition Examination Survey
- SPE
Solid phase extraction
- APCI
atmospheric pressure chemical ionization
Footnotes
The authors have no conflict of interest to disclose.
References
- 1.Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiologic reviews. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 2.Grun F, Blumberg B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev Endocr Metab Disord. 2007;8(2):161–71. doi: 10.1007/s11154-007-9049-x. [DOI] [PubMed] [Google Scholar]
- 3.Stubbs CO, Lee AJ. The obesity epidemic: both energy intake and physical activity contribute. The Medical journal of Australia. 2004;181(9):489–91. doi: 10.5694/j.1326-5377.2004.tb06406.x. [DOI] [PubMed] [Google Scholar]
- 4.Newbold RR, Padilla-Banks E, Jefferson WN. Environmental estrogens and obesity. Mol Cell Endocrinol. 2009;304(1–2):84–9. doi: 10.1016/j.mce.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karoutsou E, Polymeris A. Environmental endocrine disruptors and obesity. Endocr Regul. 46(1):37–46. doi: 10.4149/endo_2012_01_37. [DOI] [PubMed] [Google Scholar]
- 6.Aubert ML, Nef S, Soto AM. Special issue on the topic: Role of endocrine disruptors from the environment in the aetiology of obesity and diabetes. Mol Cell Endocrinol. 2009;304(1–2):1–2. doi: 10.1016/j.mce.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Elobeid MA, Allison DB. Putative environmental-endocrine disruptors and obesity: a review. Curr Opin Endocrinol Diabetes Obes. 2008;15(5):403–8. doi: 10.1097/MED.0b013e32830ce95c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatch EE, Nelson JW, Stahlhut RW, Webster TF. Association of endocrine disruptors and obesity: perspectives from epidemiological studies. Int J Androl. 2010;33(2):324–32. doi: 10.1111/j.1365-2605.2009.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heindel JJ. Endocrine disruptors and the obesity epidemic. Toxicol Sci. 2003;76(2):247–9. doi: 10.1093/toxsci/kfg255. [DOI] [PubMed] [Google Scholar]
- 10.Newbold RR, Padilla-Banks E, Jefferson WN, Heindel JJ. Effects of endocrine disruptors on obesity. Int J Androl. 2008;31(2):201–8. doi: 10.1111/j.1365-2605.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 11.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300(11):1303–10. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 12.Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One. 2010;5(1):e8673. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankar A, Teppala S. Relationship between Urinary Bisphenol A Levels and Diabetes Mellitus. J Clin Endocrinol Metab. 2011;96(12):3822–6. doi: 10.1210/jc.2011-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silver MK, O’Neill MS, Sowers MR, Park SK. Urinary Bisphenol A and Type-2 Diabetes in U.S. Adults: Data from NHANES 2003–2008. PLoS One. 2011;6(10):e26868. doi: 10.1371/journal.pone.0026868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang M, Ryu JH, Jeon R, Kang D, Yoo KY. Effects of bisphenol A on breast cancer and its risk factors. Arch Toxicol. 2009;83(3):281–5. doi: 10.1007/s00204-008-0364-0. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect. 2010;118(9):1217–22. doi: 10.1289/ehp.0901257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman JE, Witorsch RJ, McConnell EE, Sipes IG, Slayton TM, Yu CJ, et al. Weight-of-evidence evaluation of reproductive and developmental effects of low doses of bisphenol A. Crit Rev Toxicol. 2009;39(1):1–75. doi: 10.3109/10408440903279946. [DOI] [PubMed] [Google Scholar]
- 18.LaRocca J, Boyajian A, Brown C, Smith SD, Hixon M. Effects of in utero exposure to Bisphenol A or diethylstilbestrol on the adult male reproductive system. Birth Defects Res B Dev Reprod Toxicol. 2011;92(6):526–33. doi: 10.1002/bdrb.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol. 2006;254–255:179–86. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 20.Mendiola J, Jorgensen N, Andersson AM, Calafat AM, Ye X, Redmon JB, et al. Are environmental levels of bisphenol a associated with reproductive function in fertile men? Environ Health Perspect. 2010;118(9):1286–91. doi: 10.1289/ehp.1002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spivey A. Prenatal preview: early bisphenol a exposure may spawn late-life reproductive problems. Environ Health Perspect. 2009;117(6):A256. doi: 10.1289/ehp.117-a256a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginsberg G, Rice DC. Does rapid metabolism ensure negligible risk from bisphenol A? Environ Health Perspect. 2009;117(11):1639–43. doi: 10.1289/ehp.0901010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocrine reviews. 2009;30(1):75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang JH, Kondo F, Katayama Y. Human exposure to bisphenol A. Toxicology. 2006;226(2–3):79–89. doi: 10.1016/j.tox.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Mahalingaiah S, Meeker JD, Pearson KR, Calafat AM, Ye X, Petrozza J, et al. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect. 2008;116(2):173–8. doi: 10.1289/ehp.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brede C, Fjeldal P, Skjevrak I, Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit Contam. 2003;20(7):684–9. doi: 10.1080/0265203031000119061. [DOI] [PubMed] [Google Scholar]
- 27.Factor A. Mechanisms of thermal and photodegredations of bisphenol A polycarbonate. In: Clough RL, Billingham NC, Gillen KT, editors. Polymer Durability, Degradation, Stabilization, and Lifetime Prediction. New York: Oxford University Press; 1998. pp. 59–76. [Google Scholar]
- 28.Kang JH, Kito K, Kondo F. Factors influencing the migration of bisphenol A from cans. J Food Prot. 2003;66(8):1444–7. doi: 10.4315/0362-028x-66.8.1444. [DOI] [PubMed] [Google Scholar]
- 29.Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, et al. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatol. 2008;28(4):258–63. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chem Res Toxicol. 2001;14(2):149–57. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- 31.Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, et al. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24(2):178–98. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114(1):106–12. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CDC. IRB approval. ( http://www.cdc.gov/nchs/nhanes/irba98.htm)
- 34.CDC. NHANES 2005–2006. ( http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/nhanes05_06.htm)
- 35.CDC. NHANES 2007–2008. ( http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/nhanes07_08.htm)
- 36.CDC. BPA 2005–2006. ( http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/EPH_D.htm)
- 37.CDC. Phthalates 2007–2008. ( http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/PHTHTE_E.htm)
- 38.CDC. BPA 2007–2008. ( http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/EPH_E.htm)
- 39.CDC. Phthalates 2005–2006. Journal [serial on the Internet] ( http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/PHTHTE_D.htm) Date.
- 40.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77(16):5407–13. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- 41.CDC. NHANES. ( http://www.cdc.gov/nchs/nhanes.htm)
- 42.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 308(11):1113–21. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- 43.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13(6):322–7. [PubMed] [Google Scholar]
- 44.Yang Q, Liu T, Shrader P, Yesupriya A, Chang MH, Dowling NF, et al. Racial/ethnic differences in association of fasting glucose-associated genomic loci with fasting glucose, HOMA-B, and impaired fasting glucose in the U.S. adult population. Diabetes Care. 33(11):2370–7. doi: 10.2337/dc10-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 46.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 47.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 48.Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ Res. 111(6):825–30. doi: 10.1016/j.envres.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ning G, Bi Y, Wang T, Xu M, Xu Y, Huang Y, et al. Relationship of urinary bisphenol A concentration to risk for prevalent type 2 diabetes in Chinese adults: a cross-sectional analysis. Ann Intern Med. 155(6):368–74. doi: 10.7326/0003-4819-155-6-201109200-00005. [DOI] [PubMed] [Google Scholar]
- 50.Ropero AB, Alonso-Magdalena P, Garcia-Garcia E, Ripoll C, Fuentes E, Nadal A. Bisphenol-A disruption of the endocrine pancreas and blood glucose homeostasis. Int J Androl. 2008;31(2):194–200. doi: 10.1111/j.1365-2605.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- 51.Shankar A, Teppala S. Urinary Bisphenol A and Hypertension in a Multiethnic Sample of US Adults. J Environ Public Health. 2012:481641. doi: 10.1155/2012/481641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.vom Saal FS, Myers JP. Bisphenol A and risk of metabolic disorders. JAMA. 2008;300(11):1353–5. doi: 10.1001/jama.300.11.1353. [DOI] [PubMed] [Google Scholar]
- 53.Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y, et al. Urinary Bisphenol A (BPA) Concentration Associates with Obesity and Insulin Resistance. J Clin Endocrinol Metab. 2012;97(2):E223–7. doi: 10.1210/jc.2011-1989. [DOI] [PubMed] [Google Scholar]
- 54.Wei M. Association of bisphenol A with diabetes and other abnormalities. JAMA. 2009;301(7):720. doi: 10.1001/jama.2009.122. author reply 1–2. [DOI] [PubMed] [Google Scholar]
- 55.Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect. 2007;115(6):876–82. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.CDC. NHANES Analytical Guidelines. ( http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/analytical_guidelines.htm)
- 58.Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod. 2005;20(8):2325–9. doi: 10.1093/humrep/deh888. [DOI] [PubMed] [Google Scholar]
- 59.Zhou R, Zhang Z, Zhu Y, Chen L, Sokabe M. Deficits in development of synaptic plasticity in rat dorsal striatum following prenatal and neonatal exposure to low-dose bisphenol A. Neuroscience. 2009;159(1):161–71. doi: 10.1016/j.neuroscience.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 60.Crinnion WJ. Toxic effects of the easily avoidable phthalates and parabens. Altern Med Rev. 2010;15(3):190–6. [PubMed] [Google Scholar]
- 61.Grun F. Obesogens. Curr Opin Endocrinol Diabetes Obes. 2010;17(5):453–9. doi: 10.1097/MED.0b013e32833ddea0. [DOI] [PubMed] [Google Scholar]
- 62.Newbold RR. Impact of environmental endocrine disrupting chemicals on the development of obesity. Hormones (Athens) 2010;9(3):206–17. doi: 10.14310/horm.2002.1271. [DOI] [PubMed] [Google Scholar]
- 63.Sakurai K, Kawazuma M, Adachi T, Harigaya T, Saito Y, Hashimoto N, et al. Bisphenol A affects glucose transport in mouse 3T3-F442A adipocytes. Br J Pharmacol. 2004;141(2):209–14. doi: 10.1038/sj.bjp.0705520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marmugi A, Ducheix S, Lasserre F, Polizzi A, Paris A, Priymenko N, et al. Low doses of bisphenol A induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology. 2012;55(2):395–407. doi: 10.1002/hep.24685. [DOI] [PubMed] [Google Scholar]
- 65.Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb. 2007;14(5):245–52. doi: 10.5551/jat.e486. [DOI] [PubMed] [Google Scholar]
- 66.Ben-Jonathan N, Hugo ER, Brandebourg TD. Effects of bisphenol A on adipokine release from human adipose tissue: Implications for the metabolic syndrome. Mol Cell Endocrinol. 2009;304(1–2):49–54. doi: 10.1016/j.mce.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei J, Lin Y, Li Y, Ying C, Chen J, Song L, et al. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology. 2011;152(8):3049–61. doi: 10.1210/en.2011-0045. [DOI] [PubMed] [Google Scholar]
- 68.Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, et al. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118(9):1243–50. doi: 10.1289/ehp.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Reilly EJ, Mirzaei F, Forman MR, Ascherio A. Diethylstilbestrol exposure in utero and depression in women. Am J Epidemiol. 2010;171(8):876–82. doi: 10.1093/aje/kwq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang ES, Yun YS, Park SW, Kim HJ, Ahn CW, Song YD, et al. Limitation of the validity of the homeostasis model assessment as an index of insulin resistance in Korea. Metabolism. 2005;54(2):206–11. doi: 10.1016/j.metabol.2004.08.014. [DOI] [PubMed] [Google Scholar]