Abstract
Background
People who inject drugs (PWID) experience poor outcomes and fuel HIV epidemics in middle-income countries in Eastern Europe and Central Asia. We assess integrated/co-located (ICL) healthcare for HIV-infected PWID, which despite international recommendations, is neither widely available nor empirically examined.
Methods
A 2010 cross-sectional study randomly sampled 296 HIV-infected opioid-dependent PWID from two representative HIV-endemic regions in Ukraine where ICL, non-co-located (NCL) and harm reduction/outreach (HRO) settings are available. ICL settings provide onsite HIV, addiction, and tuberculosis services, NCLs only treat addiction, and HROs provide counseling, needles/syringes, and referrals, but no opioid substitution therapy (OST). The primary outcome was receipt of quality healthcare, measured using a quality healthcare indicator (QHI) composite score representing percentage of eight guidelines-based recommended indicators met for HIV, addiction and tuberculosis treatment. The secondary outcomes were individual QHIs and health-related quality-of-life (HRQoL).
Results
On average, ICL-participants had significantly higher QHI composite scores compared to NCL- and HRO-participants (71.9% versus 54.8% versus 37.0%, p<0.001) even after controlling for potential confounders. Compared to NCL-participants, ICL-participants were significantly more likely to receive antiretroviral therapy (49.5% versus 19.2%, p<0.001), especially if CD4≤200 (93.8% versus 62.5% p<0.05); guideline-recommended OST dosage (57.3% versus 41.4%, p<0.05); and isoniazid preventive therapy (42.3% versus 11.2%, p<0.001). Subjects receiving OST had significantly higher HRQoL than those not receiving it (p<0.001); however, HRQoL did not differ significantly between ICL- and NCL-participants.
Conclusions
These findings suggest that OST alone improves quality-of-life, while receiving care in integrated settings collectively and individually improves healthcare quality for PWID.
Keywords: HIV/AIDS, substance abuse, quality indicators, opiate substitution therapy, health services delivery, international health, integrated healthcare
1. INTRODUCTION
From 1999–2009, HIV incidence decreased 19% globally, but increased 25% in Eastern Europe and Central Asia (UNAIDS, 2010). In Ukraine (Kruglov et al., 2008), as throughout the region, this epidemic is concentrated among people who inject drugs (PWID; Cohen, 2010; Mathers et al., 2008). To help address this crisis, Ukraine first introduced opioid substitution therapy (OST) in 2004 with buprenorphine (Bruce et al., 2007), adding methadone in 2007 (Lawrinson et al., 2008; Schaub et al., 2010). For PWID, OST enhances quality of life (Nosyk et al., 2011) and reduces injection-related HIV risk behaviors (Gowing et al., 2008), while improving antiretroviral (ART) access (Altice et al., 2011; Uhlmann et al., 2010) and adherence (Malta et al., 2010) and viral suppression (Altice et al., 2011; Roux et al., 2009) among those infected with HIV. Modeling data from Ukraine confirm OST as the most efficacious and cost-effective intervention for injection-related and transitional HIV epidemics compared to harm reduction or ART provision alone (Alistar et al., 2011; Degenhardt et al., 2010).
HIV-infected PWID experience intertwined co-morbidities that complicate care and contribute to poor outcomes, including tuberculosis, viral hepatitis, mental illness, active drug use and HIV itself (Altice et al., 2010). Recognition of HIV/AIDS and addiction as chronic, co-occurring illnesses necessitates a fundamental re-design of healthcare delivery for these conditions (Colvin, 2011; McLellan et al., 2000; Scandlyn, 2000; Siegel and Lekas, 2002). To address this complex challenge, models of integrated healthcare have been proposed and developed (Basu et al., 2006; Sylla et al., 2007) and are advocated by the World Health Organization despite a lack of empiric evidence (Kerr et al., 2004; World Health Organization, 2008). Lack of service integration results in insufficient coordination and cross-training, problematic pharmacokinetic interaction management and logistical hurdles that undermine holistic healthcare provision (Altice et al., 2010; Wolfe et al., 2010). Integration, ranging from simple service co-location to unified cross-disciplinary case-management, seeks to alleviate many of these barriers, and in the process, increase medication adherence and viral suppression that ultimately decreases HIV transmission and viral resistance (Altice et al., 2010; Sylla et al., 2007). The extent to which integrated healthcare improves health outcomes, however, has not been examined in middle-income countries that struggle to optimize limited healthcare resources, despite increased OST availability (Lekhan et al., 2010; Rechel et al., 2012; Wolfe et al., 2010).
Research in high-income countries supports healthcare integration for PWID (Turner et al., 2005), including those with HIV. Similar to the U.S. and former Soviet Union (FSU) countries, drug treatment in Ukraine is limited and where available, often separate from general healthcare settings, resulting in siloed care for PWID. As a result, fewer than 5% of Ukrainian HIV-infected PWID receive ART (Wolfe et al., 2010). New integrated/co-located (ICL) clinics for HIV-infected PWID were first introduced in 2008, providing ART, OST, case management and primary care, including screening and treatment for tuberculosis (TB), in a single location (Curtis, 2011). Their impact on health-related outcomes, however, has not yet been assessed. The availability of integrated healthcare delivery and OST in Ukraine make this setting unique for examining the extent to which integrated healthcare could abrogate the burgeoning region-wide HIV epidemic.
2. METHODS
2.1 Study Design
From October to December, 2010, a cross-sectional survey of 296 HIV-infected PWID meeting criteria for opioid dependence and having injected ≥2 years was conducted in two profoundly HIV-impacted Ukrainian regions (Kiev and Dnipropetrovsk).
2.2 Study Sites
Subjects were recruited from three different settings within each region: integrated and fully co-located (ICL); non-co-located (NCL); and harm reduction and outreach (HRO) sites. Subjects receiving no services were excluded from this study. OST in Ukraine, whether at ICL or NCL sites, is provided free of charge from the Ministry of Health. Enrollment in OST, irrespective of site, requires opioid dependence, ≥2 years of use, and governmental “registration”, which is often linked to loss of driver’s license and employment restrictions (Bojko et al., 2013; Izenberg and Altice, 2010). Onsite daily observed OST is required without “take-home” administration allowed. At a minimum, ICL services provide free treatment and screening services for HIV, TB, and opioid dependence using OST (Sylla et al., 2007), whereas NCL sites, which predate the ICL program, provide only substance abuse treatment counseling with OST. Three pilot ICL sites were established in 2008 (Kiev, Dnipropetrovsk and Simferopol), with 20 sites added subsequently; the majority of Ukraine’s 141 OST sites, however, are NCL. All participants receiving OST at ICL and NCL sites were enrolled >3 months and were provided psychosocial counseling. HRO sites, available since 2001, provide syringe exchange, case management, referral to ancillary services (including HIV and TB), and psychosocial counseling but no OST. Eligibility for ICL and NCL services did not differ, but those in HRO sites are not required to officially “register” as PWID with the government. Both regions selected had Ukraine’s longest established ICL, NCL and HRO service delivery programs.
2.3 Study Survey
The survey included several validated instruments, including health-related quality of life (HRQoL) using the 36-item Short Form of the Medical Outcomes Study (SF-36; Ware and Sherbourne, 1992) and components of the Addiction Severity Index (Cacciola et al., 2007; Isralowitz et al., 2009; McLellan et al., 1980). Quality healthcare indicator (QHI) questions were created independently for treatment of HIV, addiction and TB based on existing literature (Altice et al., 2010; Sylla et al., 2007; World Health Organization, 2008) and Ukraine’s Ministry of Health treatment guidelines. Surveys were translated from English to Russian and back-translated using established methods (Ware et al., 1995). Expert focus groups were used to refine the survey, and a daylong pilot was performed to ensure error-free administration. Research assistants underwent extensive training and received expert oversight.
2.4 Recruitment
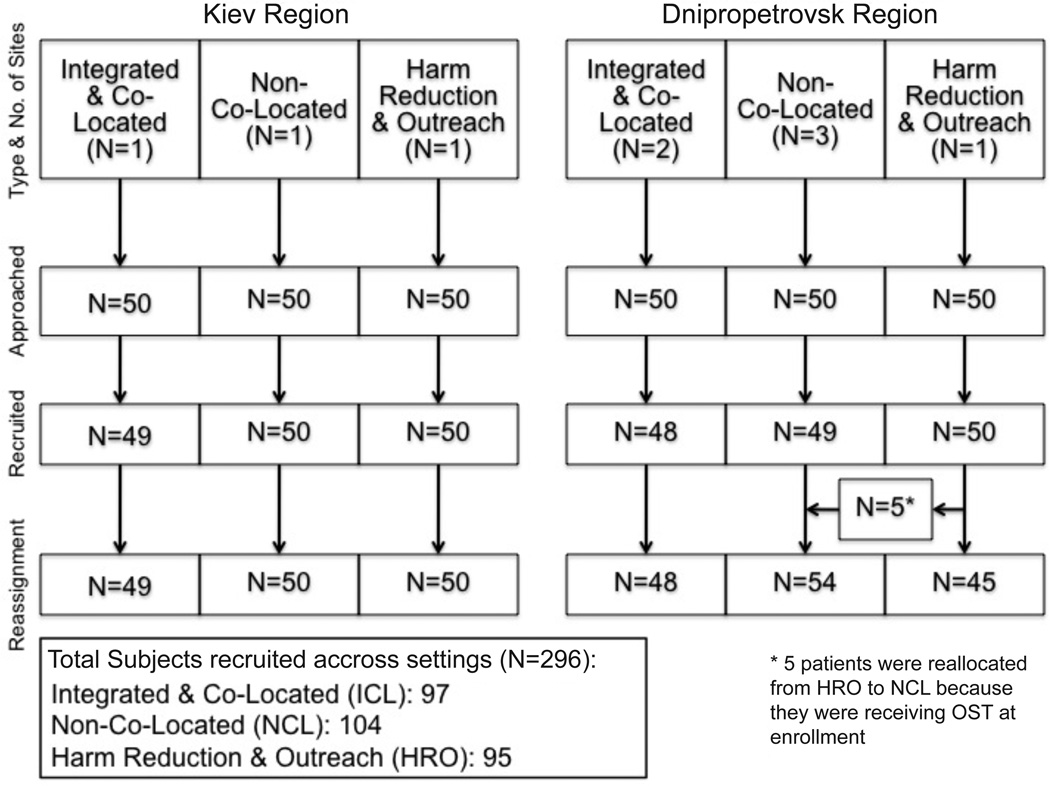
A random sample of enrolled HIV-infected patients from each setting within each region was selected using alphabetically assigned numbers and a computer-based random number generator. HIV status is officially registered at the Ministry of Health with self-reported HIV status confirmed through national registries. Recruitment is depicted in Figure 1 in accordance with the STROBE Statement (von Elm et al., 2007). Initially, 50 subjects were contacted per setting in each region. Four randomly selected clients declined participation. Five participants recruited from HRO sites were also receiving OST and reallocated to the NCL group. After providing informed consent, subjects were interviewed for 45–60 minutes in Russian and paid 80 Hrivna (UAH, $10 USD) for their time. Institutional Review Boards at the Ukrainian Institute on Public Health Policy and Yale University provided ethical oversight.
Figure 1. Recruitment strategy by healthcare site type and region.
2.5 Measures
The primary outcomes were eight self-reported QHIs, including HIV, addiction and TB treatment outcomes adapted from international recommendations (WHO, UNODC, and UNAIDS) and from previously described empirical models of integrated healthcare services for HIV-infected PWID (Altice et al., 2010; Sylla et al., 2007; World Health Organization, 2008). Three HIV QHIs included: receipt of ART, receipt of ART when CD4≤200, and CD4 monitoring within the past 6 months. Three addiction QHIs included: receipt of WHO-recommended OST dosing (methadone ! 80mg or buprenorphine ! 12mg; Barnett et al., 2001; Strain et al., 1999), no previous 30-day drug injection, and less than daily drug injection. Two TB QHIs included: TB screening in the past 12 months and receipt of isoniazid preventive therapy (IPT). The maximum QHI score was 8; it was 7 for those whose CD4 was never ≤200. A QHI composite score was created for each subject representative of the percentage of QHIs attained by summing the QHIs met for each individual, dividing by the maximum number achievable (7 or 8), and multiplying by 100. No HRO participants received a point for optimal OST dosage since, by definition, they weren’t prescribed it. This is justified because all subjects in this group met criteria for and optimally should have received OST. Optimal addiction outcomes focus not only on reducing injection frequency, but halting it altogether, thereby justifying points for both injection reduction and cessation. A secondary continuous outcome measuring HRQoL was calculated from the SF-36 (Ware and Sherbourne, 1992), including standardized computation of general health (GH), and physical (PCS) and mental (MCS) composite sub-scores.
Poverty was stratified as <888 UAH using 2010 monthly Ukrainian poverty norms (~$110). Being “single” included “never married,” “separated,” or “divorced.” Housing status was defined as “living alone” or “living with others”; none identified as being homeless.
2.6 Data Analysis
Analysis was performed using Stata v.10 SE (Stata Corporation, 2007). Indicators were analyzed for each group using Chi-square tests or independent sample t-tests, as appropriate. Multivariate linear regression models were utilized to adjust for potential confounders. Bivariate analyses where p<0.10 was used to enter the multivariate model, and a backward elimination strategy to fit the model. Covariates with p<0.10 were retained. All models controlled for age, gender, and geographical region regardless of significance. R2 was used to measure goodness-of-fit.
3. RESULTS
3.1 Respondent Characteristics
Response rate was high (98.7%) across sites. The sample, presented in Figure 1 and Table 1, is comprised mostly of men (66%) in their mid-30s (mean 35.7 years). ICL participants were significantly older than NCL (mean 36.9 vs 34.4 years, p<0.01) subjects and had injected opioids longer than NCL or HRO (mean: 14.4 versus 11.0 versus 8.6 years) participants; however, ICL and NCL participants had been on OST the same duration (mean=31.8 months). ICL subjects were significantly more likely to be unemployed and single than the two other groups, but all had a stable place to live and had high levels of education. Almost two thirds of the sample (61%) reported a monthly income below poverty.
Table 1.
Characteristics of Sample Stratified by Site of Service Delivery (N=296)
| Total Population % (n) |
Integrated & Co-Located (n=97) |
Non-Co- Located (n=104) |
Harm Reduction & Outreach (n=95) |
p-Value | ||
|---|---|---|---|---|---|---|
| Mean Age in years (SD) (n=294) | 35.7 (7.0) | 36.9 (7.4) | 34.4† (6.3) | 35.9 (7.0) | <0.05 | |
| Female (n=292) | 34% | 32% | 33% | 37% | 0.747 | |
| Employment Status (n=268) | ||||||
| Unemployed | 46% | 63% | 40%† | 30%‡ | <0.001 | |
| Part time | 45% | 28% | 45% | 67% | ||
| Full time | 9% | 8% | 15% | 3% | ||
| Income Below Poverty (n=295) | 61% | 46% | 60% | 78%‡ | <0.001 | |
| Married (n=296) | 27% | 24% | 38%* | 20% | <0.05 | |
| Housing Status (n=294) | ||||||
| Lives alone | 11% | 13% | 3% | 16% | ||
| Lives with others | 89% | 87% | 97%† | 84% | <0.01 | |
| Education (n=295) | ||||||
| None | 1% | 0% | 1% | 1% | 0.419 | |
| Lower secondary | 18% | 15% | 16% | 23% | ||
| Upper secondary | 69% | 76% | 69% | 62% | ||
| University | 12% | 8% | 13% | 14% | ||
| Co-morbidities (n=294) | ||||||
| Hepatitis C | 60% | 76% | 61%* | 43%‡ | <0.001 | |
| OST Characteristics | <0.001 | |||||
| On any OST | 201 | 97 | 104 | 0 | ||
| On MMT | 61% | 71% | 51% | N/A | <0.01 | |
| On BMT | 39% | 29% | 49% | N/A | ||
| Time on OST (months) (SD) | 31.8 (24.8) | 31.0 (26.1) | 32.6 (23.6) | N/A | 0.641 | |
| Lifetime Injection of Opioids in years (SD) (n=294) | 11.4 (8.0) | 14.4 (7.1) | 11.0† (8.7) | 8.6‡ (6.9) | <0.001 | |
| Times in Prison (SD) (n=271) | 3.1 (5.6) | 4.1 (6.4) | 3.7 (6.5) | 1.5‡ (2.1) | <0.01 | |
Significantly different from ICL at p<0.05
Significantly different from ICL at p<0.01
Significantly different from ICL at p<0.001
Legend: OST=opioid substitution therapy;
MMT=methadone maintenance therapy;
BMT=buprenorphine maintenance therapy
All ICL and NCL respondents received OST, by definition, while no HRO respondents received it, and so the five participants (Figure 1) recruited from HRO sites receiving OST were reallocated to the NCL group. All QHI results were found to be robust regardless of whether the five participants reassigned to NCL from HRO sites were included in NCL or HRO groups. Among OST subjects, the majority was on methadone (61%); however, significantly more NCL patients were prescribed buprenorphine than ICL patients (49% versus 29%, p<0.01).
3.2 Quality Healthcare Indicators
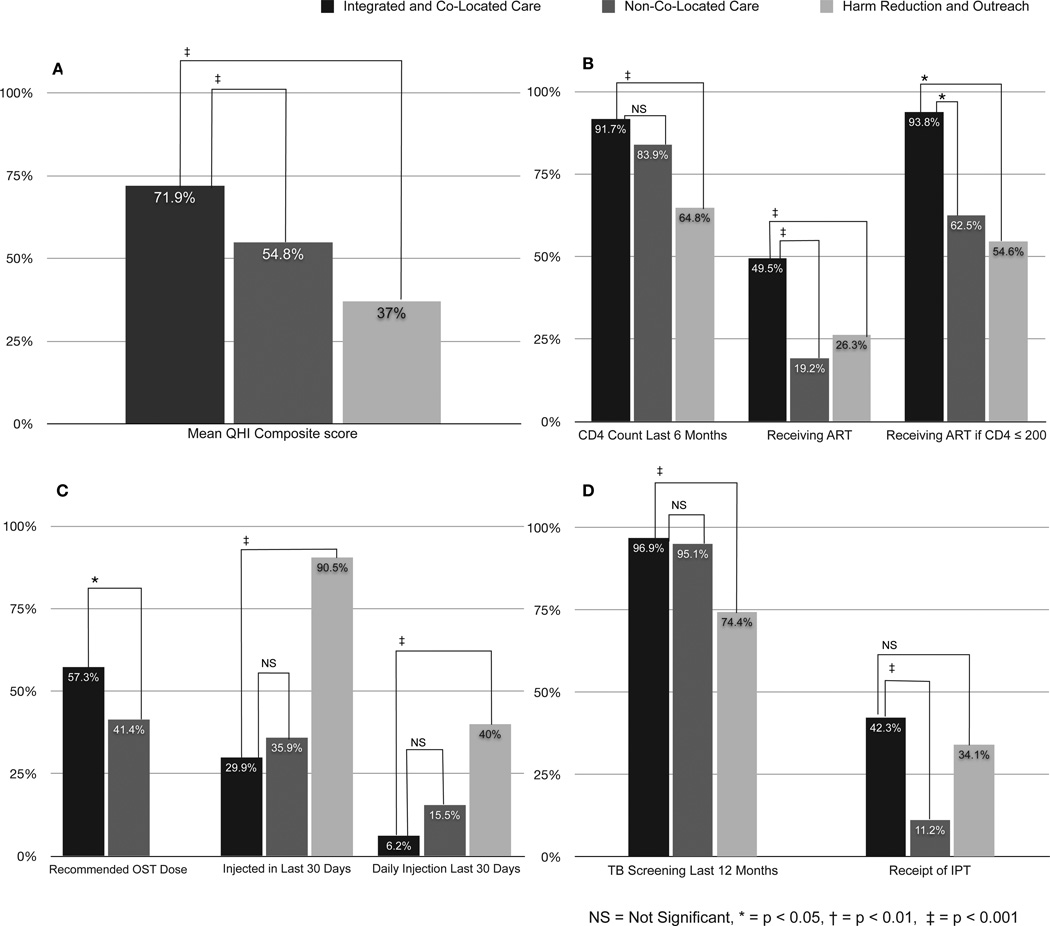
Figure 2 provides details for the composite QHI score and is further divided into HIV, addiction and TB treatment QHIs. Participants receiving care in the ICL group had significantly higher QHI composite scores (Panel A), than those in NCL and HRO settings (71.9% versus 54.8% versus 37.0%, respectively, p<0.001).
Figure 2. Quality Healthcare Indicators (QHI) based on service delivery setting.
Legend: A) Mean Quality Healthcare Indicator Score; B) HIV-Related Quality Healthcare Indicators; C) Drug Treatment-Related Quality Healthcare Indicators; D) Tuberculosis-Related Quality Healthcare Indicators
Panel B depicts HIV treatment QHIs. While nearly all ICL (91.7%) and most NCL (83.9%) participants had CD4 counts in the previous 6 months, significantly fewer subjects in the HRO group (64.8%) did. Significantly more ICL participants (49.5%) were prescribed ART compared to NCL (19.2%) or HRO (26.3%) participants (p<0.001 for ICL versus NCL or HRO). In accordance with Ukrainian Ministry of Health guidelines, nearly all (93.8%) ICL participants with CD4≤200 received ART compared with significantly fewer NCL (62.5%) and HRO (54.6%) participants.
Panel C depicts addiction treatment QHIs. Ukraine’s Ministry of Health guidelines for OST dosing are similar to international standards. Between the two OST-receiving groups (ICL and NCL), the ICL group was significantly more likely to receive recommended OST dosing (57.3% vs 41.4%, p<0.05) despite similar duration on OST; 61% of those on OST were on methadone with an average dose of 81mg (IQR 50–115), and 39% on buprenorphine with an average dose of 7mg (IQR 4–12). Those on OST, irrespective of setting, were significantly less likely to have injected daily or at all in the past 30 days. Nearly all HRO subjects (90.5%) had injected at least once in the previous 30 days, whereas only one-third of ICL and NCL participants had done so. Daily injection, a measure of higher addiction severity, was reported by 40% of HRO subjects, significantly higher than ICL (6.5%) or NCL (15.5%) subjects (p=NS between OST-prescribed ICL and NCL groups).
Two TB treatment QHIs are depicted in Panel D. Annual TB screening is required for all Ukrainian OST clients. Nearly all subjects in the ICL (96.9%) or NCL (95.1%) groups (all on OST) had undergone TB sputum screening in the previous 12 months, significantly higher (p<0.001) than for those at HRO sites (74.4%). Overall, 29% of the entire sample had received IPT; ICL (42%) subjects were significantly more likely to receive IPT than NCL patients (11%), but no more so than HRO participants (34.1%). In the final multivariate model, higher QHI composite scores were significantly associated with receipt of care in ICL compared to NCL or HRO sites, increasing age and being employed (Table 2).
Table 2.
Multivariate Analysis of Factors Contributing to Health-Related Quality of Life (HRQoL) and Quality Healthcare Indicator Composite Score
| HRQoL General Health Score ! (95% CI) (n=265) |
HRQoL Mental Composite Score ! (95% CI) (n=287) |
HRQoL Physical Composite Score ! (95% CI) (n=263) |
Quality Healthcare Indicator Percentage Score ! (95% CI) (n=290) |
||
|---|---|---|---|---|---|
| Facility Type | |||||
| Integrated & Co-Located | Referent | Referent | Referent | Referent | |
| Non-Co-Located | 2.12 (−3.74, 7.97) | 0.70 (−2.66, 4.06) | 1.09 (−1.12, 3.29) | −16.53‡ (−22.17, −10.90) | |
| Harm Reduction & Outreach | −10.63‡ (−14.75, −6.51) | −10.20‡ (−12.92, −7.48) | −4.40‡ (−6.26, −2.53) | −35.45‡ (−40.51, −30.40) | |
| Covariates | |||||
| Age | −0.23 (−0.57, 0.11) | −0.07 (−0.27, 0.14) | −0.16* (−0.29, −0.03) | 0.36* (0.04, 0.68) | |
| City | 3.20 (−1.61, 8.01) | 8.00‡ (5.45, 10.55) | 1.22 (−0.60, 3.03) | 4.68 (−0.12, 9.49) | |
| Gender | 1.14 (−3.32, 5.60) | 0.86 (−1.69, 3.40) | 1.19 (−0.59, 2.96) | −4.68 (−9.42, 0.06) | |
| Hepatitis Cinfected | −7.70‡ (−11.86, −3.53) | ---------- | ---------- | ---------- | |
| Employed | ---------- | ---------- | ---------- | 3.25* (0.07, 6.44) | |
| Below poverty | ---------- | −3.69† (−6.29, −1.10) | ---------- | ---------- | |
| Housing: Living with others | ---------- | ---------- | ---------- | ---------- | |
| Married | ---------- | ---------- | ---------- | ---------- | |
| Years of opioid injection | ---------- | ---------- | ---------- | ---------- | |
| Times in prison | −0.52‡ (−0.81, −0.23) | ---------- | −0.24‡ (−0.37, −0.12) | ---------- | |
| Model Fit Adjusted R2 | 0.11 | 0.30 | 0.13 | 0.41 | |
Legend: CI=confidence interval.
p<0.05
p<0.01
p<0.001
Quality Healthcare Indicator Percentage Score represents for each individual the percentage of guidelines-recommended indicators met for HIV, addiction, and tuberculosis treatment.
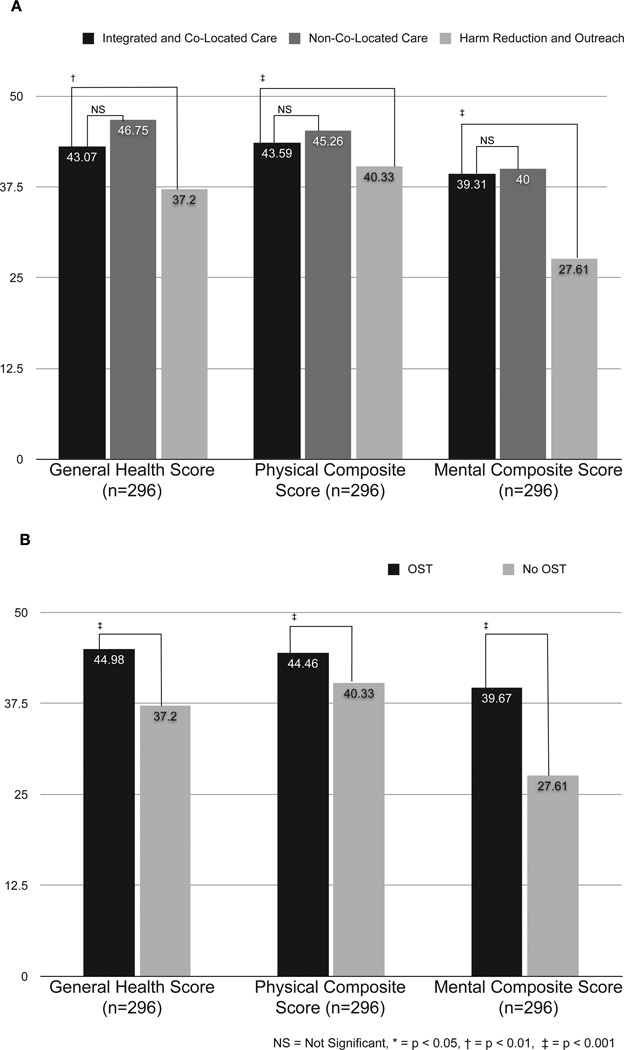
3.3 Health-Related Quality of Life
The secondary outcome, HRQoL, is presented in Figure 3 and divided into general health, physical composite, and mental composite score with higher scores indicating relatively better health. The general health, physical, and mental scores for HRQoL did not differ significantly between the two OST-prescribed (ICL and NCL) groups. When re-stratified by receipt of OST rather than by specific site, those on OST had significantly higher general health, physical, and mental scores than the HRO group. In multivariate analysis (Table 2), receiving services only at HRO sites was negatively associated with general (! =−10.63; 95% CI −14.75, −6.51; p<0.001), mental (! =−10.20; 95% CI −12.92, −7.48; p<0.001), and physical (! =−4.40; 95% CI −6.26, −2.53; p<0.001) HRQoL compared to ICL sites, whereas accessing care at NCL sites was not significantly associated with differences in HRQoL compared to ICL sites.
Figure 3. Health-related quality of life scores by service delivery setting and receipt of opioid substitution therapy (OST).
4. DISCUSSION
To our knowledge, this is the first empiric evaluation of different models for providing care to HIV-infected PWID in Eastern Europe or any middle-income country, including assessment of integrated and co-located care, in a region where HIV treatment outcomes are comparatively poor. Compared to other at-risk groups, HIV-infected PWID are less likely to access HIV-related healthcare (Andersen et al., 2000; Celentano et al., 2001; Korthuis et al., 2004; Strathdee et al., 1998) and more likely to experience increased morbidity and mortality (Lucas et al., 2001; Mathews et al., 2000; Moore et al., 2004). Modeling suggests the most cost-effective method to address the volatile HIV epidemic in this region is through OST expansion and the most effective way is through combining OST expansion with increased ART prescription (Alistar et al., 2011). Using literature-based indicators of optimal care for HIV-infected PWID where TB among HIV-infected patients is endemic, which we have termed QHIs, we advance these findings by demonstrating that integrated care for the multiple comorbidities affecting HIV-infected PWID could help reverse morbidity and mortality trends for a region with an unrelenting HIV epidemic. By simultaneously addressing healthcare services for addiction, HIV and TB, integrated care incrementally optimizes treatment outcomes overall and across a number of specific domains.
We found that integrating multiple services into one setting helps HIV-infected PWID on OST access ART, even compared to those receiving OST at NCL sites. This study confirms findings from North America where methadone (Uhlmann et al., 2010) and buprenorphine (Altice et al., 2011) treatment increased the likelihood of ART prescription and retention on buprenorphine >6 months enhanced this effect when integrated into HIV treatment settings (Altice et al., 2011). The present study, however, extends the US-based studies in three key ways. First, this study expands empiric evidence that healthcare integration benefits complex patients in a middle-income country where syndemics of addiction, HIV, and tuberculosis are more profound. Second, it provides additional insight about the benefits of integration by including a third non-OST comparison group. Third, while the US studies examined only HIV treatment QHIs (Korthuis et al., 2011a), this study included QHIs for two additional healthcare domains, addiction and tuberculosis, key to effectively managing HIV-infected PWID in middle-income settings. The findings from this study have important implications for the U.S., where HIV and substance treatment should be integrated better, particularly using buprenorphine and extended-release naltrexone for treating opioid dependence and the use of naltrexone for treating alcohol use disorders. Such integration is central to efforts to create patient-centered medical homes in accordance with mandates set forth through the Affordable Care Act that is to be instated in 2014. Central to integrated healthcare delivery systems is the need to synergistically improve multiple aspects of care, including HIV, substance abuse and TB treatment outcomes. Together, these results support findings that advocate for integration of multiple services into routine healthcare outside of addiction specialty treatment settings (Altice et al., 2006; Basu et al., 2006; Schwarz et al., 2012, 2009).
Integrating healthcare services likely encourages ART prescription by promoting regular contact with healthcare providers traditionally hesitant to prescribe ART to PWID (Westergaard et al., 2012), overcoming stigma and stereotypes among those providers (Altice et al., 2010; Murri et al., 1999). Increasing ART prescription is crucial to benefit individual and public health, including reduced HIV transmission, particularly sexual transmission, which is an increasing contributor to HIV expansion in Ukraine. Data from randomized controlled trials of HIV serodiscordant couples (Cohen et al., 2011) and empirical studies using ART “treatment as prevention” to reduce community-level viral load (Henard et al., 2012; Kivela et al., 2012; Wood et al., 2009) confirm that ART prescription reduces sexual HIV transmission, which has important implications for Ukraine’s transitional HIV epidemic.
We found that OST reduced injection frequency and increased drug abstinence regardless of service delivery strategy, underscoring the importance of OST for secondary HIV prevention (Metzger et al., 1993). Despite Ukraine’s adoption of international standards for targeting OST doses (Barnett et al., 2001; Strain et al., 1999) the finding that less than half of participant doses met those guidelines is provocative. It should be noted that even though international guidelines recommend these OST dosages, most of the studies examining OST dosages have been conducted in high-income countries, where PWID may use higher purity opioids than used in Ukraine.
Nonetheless, recent data from Malaysia, another middle-income country where opioid purity is low, suggest that dosages prescribed using international standards improved retention in treatment (Wickersham et al., 2013a, 2013b). We found ICL sites were more likely to meet international OST dosing standards compared to NCL sites. One explanation for this finding is that ICL participants were more likely to be prescribed ART which is typically comprised of a non-nucleoside reverse transcriptase inhibitor that markedly reduces methadone levels, precipitates withdrawal and may have resulted in OST dosing increases (Altice et al., 2010). Higher dosing at ICL sites could also be explained by unidentified intrinsic improvements in OST care at these sites (e.g., better trained addiction specialists) or more effective cross-training and communication between providers, a hallmark of integrated care. More ICL patients were on ART, and providers may have communicated more effectively that ART prescription was adversely compromising OST, resulting in staff increasing OST dosages to ensure OST retention.
Tuberculosis, a common HIV-related co-morbidity in middle-income countries, is the leading cause of death among HIV-infected patients in Ukraine and the region. Therefore, screening for and treatment of active and latent TB is central to comprehensive and effective HIV care (Sylla et al., 2007). TB screening in our sample was extremely high (89.3%) among patients at ICL and NCL clinics, likely due to national requirements that all OST patients be screened annually. These findings are encouraging given the high rates of TB-associated mortality among HIV-infected PWID generally (Corbett et al., 2003; Kourbatova et al., 2006; McShane, 2005; Nunn et al., 2005; Sharma et al., 2005). Recent evidence has demonstrated that utilization of both IPT and ART in HIV-infected patients is associated with reduced TB incidence (Golub et al., 2007; Uyei et al., 2011), and IPT is currently recommended for all HIV-infected patients where TB is prevalent (World Health Organization, 2011). Although patients in the ICL and NCL groups were equally likely to have received recent TB screening, ICL participants were significantly more likely to receive IPT than NCL participants, suggesting that further reductions in morbidity among NCL participants may be achieved in ICL settings. Unexplained by our findings, however, is the relatively high level of IPT receipt by study participants in HRO sites, though an observed correlation between ART and IPT prescription (data not shown) does provide one possible explanation.
HRQoL is an important construct for assessing perceived individual well-being and function. Our findings, similar to others (Corsi and Kwiatkowski, 2002; Fiellin et al., 2001; Korthuis et al., 2011b; Zweben and Payte, 1990) confirm that OST alone is associated with improved general, physical and mental health regardless of how services are organized. Our finding that healthcare integration, per se, was not significantly correlated with HRQoL is consistent with prior research indicating that treatment of opioid dependence alone is responsible for most of the benefits (Brands et al., 2008). Improved HRQoL may confer other independent benefits, including increased ART tolerability and possibly result in improved ART adherence when prescribed (Avants et al., 2004; Conway et al., 2004; Lucas et al., 2004; Sambamoorthi et al., 2000).
Challenging within this setting is creation of a flexible legal framework that improves patient access to OST and reallocation of diminishing resources through task-shifting of OST dispensation and supervision to nurses and/or pharmacists that may alleviate financial and human resource constraints on OST scale-upm a successful approach demonstrated elsewhere (Alamo et al., 2012; Babigumira et al.; Ivers et al.). Under emerging global financial constraints, funding mechanisms are diminishing, potentially threatening the significant benefits gained in HIV prevention and treatment (Vujicic et al., 2012). PWID are particularly vulnerable high-risk groups to programming cuts. Innovation and borrowing international best practices and adapting them locally with the aid of operations improvement principles (Allway and Corbett, 2002) may invigorate integration principles further.
While OST improves both individual and composite QHIs and HRQoL for all who receive it, QHIs are significantly higher in patients receiving integrated rather than non-integrated care in OST sites. Although significant after controlling for potential confounding variables, these findings are nonetheless limited by a number of factors including modest sample size, lack of a prospective clinical trial, inability to measure the extent of integration at ICL sites, and lack of a no-care control group. The cross-sectional design also limits our ability to attribute causality to integrated care delivery. Notwithstanding these limitations, however, this is the largest comparative study of integrated healthcare delivery to date that deploys QHIs measuring multiple disease co-morbidities to assess the benefits of integrated healthcare delivery compared to more traditional systems of care among HIV-infected PWID. Moreover, it is the first to be conducted in a middle-income country, specifically in Eastern Europe, where HIV-related mortality and HIV transmission continue to rise (UNAIDS, 2010).
Despite its availability, political, ideological, and logistical barriers constrain OST expansion in Ukraine (Alistar et al., 2011; Bojko et al., 2013; Izenberg and Altice, 2010; Mimiaga et al., 2010; Spicer et al., 2011). Our findings provide empiric data that integrated care of HIV-infected PWID has the potential to greatly improve individual and public health, support further expansion of OST throughout the region, and ultimately demonstrate the need for innovative models of care delivery that provide high quality, evidence-based treatment in resource-constrained settings.
Acknowledgements
In addition to the funding sources mentioned above, we would like to acknowledge Tiaira Winn, Artem Kopelev, and Maua Herme for their help with data management and the Ukrainian Institute on Public Health Policy for their logistical and translation assistance.
Role of Funding Source
The International HIV/AIDS Alliance (Ukraine), Open Society Institute, and World Health Organization provided funds for the completion of this study. Salary and research (R01 DA029910 and R01 DA33679) oversight as well as career development support for FLA (K24 DA0170720) was also provided by the National Institutes on Drug Abuse. Additional support was provided by National Institutes of Health Medical Science Training Program (GM07205) for JMI and CB. JMI received funding from the Yale Global Health Institute, and MCS received funding from the Infectious Diseases Society of America. The funding sources had no role in the study design, data collection or analysis, manuscript writing or revision, or decision to submit this paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures for the Manuscript
Integration of Services Improves Healthcare Outcomes Among HIV-infected People Who Inject Drugs in Ukraine: Implications for Healthcare Delivery for Complex Patients
Contributors
Authors CB, MCS, JMI, SD, KD, and FLA designed the study and wrote the protocol. CB undertook the statistical analysis with oversight by FLA, and author CB wrote the first draft of the manuscript. All authors had full access to the data and contributed to and have approved the final manuscript.
Conflicts of Interest
All authors declare they have no conflicts of interest.
REFERENCES
- Alamo S, Wabwire-Mangen F, Kenneth E, Sunday P, Laga M, Colebunders RL. Task-shifting to community health workers: evaluation of the performance of a peer-led model in an antiretroviral program in Uganda. AIDS patient care STDs. 2012;26:101–107. doi: 10.1089/apc.2011.0279. [DOI] [PubMed] [Google Scholar]
- Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS Med. 2011;8:e1000423. doi: 10.1371/journal.pmed.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allway M, Corbett S. Shifting to lean service: stealing a page from manufacturers' playbooks. Journal of Organizational Excellence. 2002;21:45–54. [Google Scholar]
- Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, Cunningham CO, Sullivan LE, Vergara-Rodriguez P, Fiellin DA, Cajina A, Botsko M, Nandi V, Gourevitch MN, Finkelstein R BHIVES Collaborative. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J. Acquir. Immune Defic. Syndr. 2011;56(Suppl. 1):S22–S32. doi: 10.1097/QAI.0b013e318209751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376:367–387. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Sullivan LE, Smith-Rohrberg D, Basu S, Stancliff S, Eldred L. The potential role of buprenorphine in the treatment of opioid dependence in HIV-infected individuals and in HIV infection prevention. Clin. Infect. Dis. 2006;43(Suppl. 4):S178–S183. doi: 10.1086/508181. [DOI] [PubMed] [Google Scholar]
- Andersen R, Bozzette S, Shapiro M, St Clair P, Morton S, Crystal S, Goldman D, Wenger N, Gifford A, Leibowitz A, Asch S, Berry S, Nakazono T, Heslin K, CUnningham W. Access of vulnerable groups to antiretroviral therapy among persons in care for HIV disease in the United States. HCSUS Consortium. HIV Cost and Services Utilization Study. Health Serv. Res. 2000;35:389–416. [PMC free article] [PubMed] [Google Scholar]
- Avants SK, Margolin A, Copenhaver M, Usubiaga MH, Doebrick C. Targeting HIV-related outcomes with intravenous drug users maintained on methadone: a randomized clinical trial of a harm reduction group therapy. J. Subst. Abuse Treat. 2004;26:67–78. doi: 10.1016/S0740-5472(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Babigumira JB, Castelnuovo B, Stergachis A, Kiragga A, Shaefer P, Lamorde M, Kambugu A, Muwanga A, Garrison LP. Cost effectiveness of a pharmacy-only refill program in a large urban HIV/AIDS clinic in Uganda. PLoS One. 2011;6:e18193. doi: 10.1371/journal.pone.0018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett PG, Rodgers JH, Bloch DA. A meta-analysis comparing buprenorphine to methadone for treatment of opiate dependence. Addiction. 2001;96:683–690. doi: 10.1046/j.1360-0443.2001.9656834.x. [DOI] [PubMed] [Google Scholar]
- Basu S, Smith-Rohrberg D, Bruce RD, Altice FL. Models for integrating buprenorphine therapy into the primary HIV care setting. Clin. Infect. Dis. 2006;42:716–721. doi: 10.1086/500200. [DOI] [PubMed] [Google Scholar]
- Bojko MJ, Dvoriak S, Altice FL. At the crossroads: HIV prevention and treatment for people who inject drugs in Ukraine. Addiction. 2013;108:1697–1699. doi: 10.1111/add.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands B, Blake J, Marsh DC, Sproule B, Jeyapalan R, Li S. The impact of benzodiazepine use on methadone maintenance treatment outcomes. J. Addict. Dis. 2008;27:37–48. doi: 10.1080/10550880802122620. [DOI] [PubMed] [Google Scholar]
- Bruce RD, Dvoryak S, Sylla L, Altice FL. HIV treatment access and scale-up for delivery of opiate substitution therapy with buprenorphine for IDUs in Ukraine--programme description and policy implications. Int. J. Drug Policy. 2007;18:326–328. doi: 10.1016/j.drugpo.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, McLellan AT, Lin YT, Lynch KG. Initial evidence for the reliability and validity of a"Lite" version of the Addiction Severity Index. Drug Alcohol Depend. 2007;87:297–302. doi: 10.1016/j.drugalcdep.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Celentano DD, Galai N, Sethi AK, Shah NG, Strathdee SA, Vlahov D, Gallant JE. Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS. 2001;15:1707–1715. doi: 10.1097/00002030-200109070-00015. [DOI] [PubMed] [Google Scholar]
- Cohen J. Late for the epidemic: HIV/AIDS in Eastern Europe. Science. 2010;329:162–164. doi: 10.1126/science.329.5988.160. 160. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Ganllant J, Havlir D, Swindelis S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin CJ. HIV/AIDS, chronic diseases and globalisation. Global. Health. 2011;7:31. doi: 10.1186/1744-8603-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway B, Prasad J, Reynolds R, Farley J, Jones M, Jutha S, Smith N, Mead A, DeVlaming S. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clin. Infect. Dis. 2004;38(Suppl. 5):S402–S408. doi: 10.1086/421404. [DOI] [PubMed] [Google Scholar]
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- Corsi K, Kwiatkowski C. Predictors of positive outcomes for out-of-treatment opiate injectors recruited into methadone maintenance through street outreach. J. Drug Issues. 2002;32:999–1016. [Google Scholar]
- Curtis M. Building Integrated Care Services for Injection Drug Users in Ukraine. In: WHO Regional Office for Europe, editor. Copenhagen, Denmark: 2010. [on December 13, 2012]. Accessed at http://www.euro.who.int/_data/assets/pdf_file/0016/130651/e194651.pdf. [Google Scholar]
- Degenhardt L, Mathers B, Vickerman P, Rhodes T, Latkin C, Hickman M. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet. 2010;376:285–301. doi: 10.1016/S0140-6736(10)60742-8. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, O'Connor PG, Chawarski M, Pakes JP, Pantalon MV, Schottenfeld RS. Methadone maintenance in primary care: a randomized controlled trial. JAMA. 2001;286:1724–1731. doi: 10.1001/jama.286.14.1724. [DOI] [PubMed] [Google Scholar]
- Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing L, Farrell M, Bornemann R, Sullivan L, Ali R. Substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst. Rev. 2008:CD004145. doi: 10.1002/14651858.CD004145.pub3. [DOI] [PubMed] [Google Scholar]
- Henard S, Jeanmaire E, Nguyen Y, Yazdanpanah Y, Cheret A, et al. Is total community viral load a robust predictive marker of the efficacy of the TasP strategy? J. Acquir. Immune Defic. Syndr. 2012;61:400–402. doi: 10.1097/QAI.0b013e318263a111. [DOI] [PubMed] [Google Scholar]
- Isralowitz R, Reznik A, Rawson RA, Hasson A. Immigrants from Russia, Ukraine and the Caucasus Region: differential drug, use, infectious disease, and related outcomes. Int. J. Ment. Health Addict. 2009;7:450–457. [Google Scholar]
- Ivers LC, Jerome JG, Cullen KA, Lambert W, Celletti F, Samb B. Task-shifting in HIV care: a case study of nurse-centered community-based care in rural Haiti. PLoS One. 2011;6:e19276. doi: 10.1371/journal.pone.0019276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izenberg JM, Altice FL. Next steps for Ukraine abolition of HIV registries, implementation of routine human immunodeficiency virus testing and expansion of services. Addiction. 2010;105:569–570. doi: 10.1111/j.1360-0443.2009.02881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr T, Wodak A, Elliott R, Montaner JS, Wood E. Opioid substitution and HIV/AIDS treatment and prevention. Lancet. 2004;364:1918–1919. doi: 10.1016/S0140-6736(04)17490-4. [DOI] [PubMed] [Google Scholar]
- Kivela P, Liitsola K, Aho I, Simola S, Tuomola P, Salminen M, Ristola M. Comprehensive care with antiretroviral therapy for injecting-drug users associates to low community viral load and restriction of HIV outbreak. J. Int. AIDS Soc. 2012;15:18394. [Google Scholar]
- Korthuis PT, Anaya HD, Bozzette SA, Brinkerhoff C, Mancewicz M, Wang M, Asch SM. Quality of HIV care within the Veterans Affairs Health System: a comparison using outcomes from the HIV costs and services utilization study. J. Clin. Outcomes Manage. 2004;11:765–774. [Google Scholar]
- Korthuis PT, Fiellin DA, Fu R, Lum PJ, Altice FL, Sohler TN, Tozzi MJ, Asch SM, Botsko M, Fishl M, Flanigan TP, Boverman J, McCarthy D BHIVES Collaborative. Improving adherence to HIV quality of care indicators in persons with opioid dependence: the role of buprenorphine. J. Acquir. Immune Defic. Syndr. 2011a;56(Suppl. 1):S83–S90. doi: 10.1097/QAI.0b013e31820bc9a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthuis PT, Tozzi MJ, Nandi V, Fiellin DA, Weiss L, Egan JE, Botsko M, Acosta A, Gourevitch MN, Hersh D, Hsu J, Boverman J, Altice FL BHIVES Collaborative. Improved quality of life for opioid-dependent patients receiving buprenorphine treatment in HIV clinics. J. Acquir. Immune Defic. Syndr. 2011b;56(Suppl. 1):S39–S45. doi: 10.1097/QAI.0b013e318209754c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourbatova EV, Borodulin BE, Borodulina EA, del Rio C, Blumberg HM, Leonard MK., Jr Risk factors for mortality among adult patients with newly diagnosed tuberculosis in Samara, Russia. Int. J. Tuberc. Lung Dis. 2006;10:1224–1230. [PubMed] [Google Scholar]
- Kruglov YV, Kobyshcha YV, Salyuk T, Varetska O, Shakarishvili A, Saldanha VP. The most severe HIV epidemic in Europe: Ukraine's national HIV prevalence estimates for 2007. Sex. Transm. Infect. 2008;84(Suppl. 1):i37–i41. doi: 10.1136/sti.2008.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrinson P, Ali R, Buavirat A, Chiamwongpaet S, Dvoryak S, Habrat B, Jie S, Mardiati R, Mokri A, Moskalewicz J, Newcombe D, Poznyak V, Subata E, Uchtenhagen A, Utami DS, Vial R, Zhao C. Key findings from the WHO collaborative study on substitution therapy for opioid dependence and HIV/AIDS. Addiction. 2008;103:1484–1492. doi: 10.1111/j.1360-0443.2008.02249.x. [DOI] [PubMed] [Google Scholar]
- Lekhan V, Rudiy V, Richardson E. Ukraine: Health system review. Health Syst. Transit. 2010;12:1–183. xiii-xiv. [PubMed] [Google Scholar]
- Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J. Acquir. Immune Defic. Syndr. 2001;27:251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Weidle PJ, Hader S, Moore RD. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a nonrandomized comparative study. Clin. Infect. Dis. 2004;38(Suppl. 5):S409–S413. doi: 10.1086/421405. [DOI] [PubMed] [Google Scholar]
- Malta M, Magnanini MM, Strathdee SA, Bastos FI. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS Behav. 2010;14:731–747. doi: 10.1007/s10461-008-9489-7. [DOI] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, Wodak A, Panda S, Tyndall M, Touflik A, Mattick RP. Reference Group to the UN on HIV and Injecting Drug Use, 2008. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2007;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- Mathews WC, McCutchan JA, Asch S, Turner BJ, Gifford AL, Kuromiya K, Brown J, Shapiro MF, Bozzette SA. National estimates of HIV-related symptom prevalence from the HIV Cost and Services Utilization Study. Med. Care. 2000;38:750–762. doi: 10.1097/00005650-200007000-00007. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J. Nerv. Ment. Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- McShane H. Co-infection with HIV and TB: double trouble. Int. J. STD AIDS. 2005;16:95–100. doi: 10.1258/0956462053057576. quiz 101. [DOI] [PubMed] [Google Scholar]
- Metzger DS, Woody GE, McLellan AT, O'Brien CP, Druley P, Navaline H, DePhillippis D, Stolley P, Abrutyn E. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: an 18-month prospective follow-up. J. Acquir. Immune Defic. Syndr. 1993;6:1049–1056. [PubMed] [Google Scholar]
- Mimiaga MJ, Safren SA, Dvoryak S, Reisner SL, Needle R, Woody G. "We fear the police, and the police fear us": structural and individual barriers and facilitators to HIV medication adherence among injection drug users in Kiev, Ukraine. AIDS Care. 2010;22:1305–1313. doi: 10.1080/09540121003758515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RD, Keruly JC, Chaisson RE. Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. J. Acquir. Immune Defic. Syndr. 2004;35:46–51. doi: 10.1097/00126334-200401010-00006. [DOI] [PubMed] [Google Scholar]
- Murri R, Fantoni M, Del Borgo C, Izzi I, Visona R, Suter F, Banfi MC, Barchi E, Orchi N, Bosco O, Wu AW. Intravenous drug, use, relationship with providers, and stage of HIV disease influence the prescription rates of protease inhibitors. J. Acquir. Immune Defic. Syndr. 1999;22:461–466. doi: 10.1097/00126334-199912150-00006. [DOI] [PubMed] [Google Scholar]
- Nosyk B, Guh DP, Sun H, Oviedo-Joekes E, Brissette S, Marsh DC, Schechter MT, Anis AH. Health related quality of life trajectories of patients in opioid substitution treatment. Drug Alcohol Depend. 2011;118:259–264. doi: 10.1016/j.drugalcdep.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Nunn P, Williams B, Floyd K, Dye C, Elzinga G, Raviglione M. Tuberculosis control in the era of HIV. Nat. Rev. Immunol. 2005;5:819–826. doi: 10.1038/nri1704. [DOI] [PubMed] [Google Scholar]
- Rechel B, Ahmedov M, Akkazieva B, Katsaga A, Khodjamurodov G, McKee M. Lessons from two decades of health reform in Central Asia. Health Policy Plan. 2012;27:281–287. doi: 10.1093/heapol/czr040. [DOI] [PubMed] [Google Scholar]
- Roux P, Carrieri MP, Cohen J, Ravaux I, Poizot-Martin I, Dellamonica P, Spire B. Retention in opioid substitution treatment: a major predictor of long-term virological success for HIV-infected injection drug users receiving antiretroviral treatment. Clin. Infect. Dis. 2009;49:1433–1440. doi: 10.1086/630209. [DOI] [PubMed] [Google Scholar]
- Sambamoorthi U, Warner LA, Crystal S, Walkup J. Drug abuse, methadone treatment, and health services use among injection drug users with AIDS. Drug Alcohol Depend. 2000;60:77–89. doi: 10.1016/s0376-8716(99)00142-8. [DOI] [PubMed] [Google Scholar]
- Scandlyn J. When AIDS became a chronic disease. West. J. Med. 2000;172:130–133. doi: 10.1136/ewjm.172.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M, Chtenguelov V, Subata E, Weiler G, Uchtenhagen A. Feasibility of buprenorphine and methadone maintenance programmes among users of home made opioids in Ukraine. Int. J. Drug Policy. 2010;21:229–233. doi: 10.1016/j.drugpo.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Schwarz R, Zelenev A, Bruce RD, Altice FL. Retention on buprenorphine treatment reduces emergency department utilization, but not hospitalization, among treatment-seeking patients with opioid dependence. J. Subst. Abuse Treat. 2012;43:451–457. doi: 10.1016/j.jsat.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz RK, Bruce RD, Ball SA, Herme M, Altice FL. Comparison of tuberculin skin testing reactivity in opioid-dependent patients seeking treatment with methadone versus buprenorphine: policy implications for tuberculosis screening. Am. J. Drug Alcohol Abuse. 2009;35:439–444. doi: 10.3109/00952990903447741. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Mohan A, Kadhiravan T. HIV-TB co-infection: epidemiology, diagnosis and management. Indian J. Med. Res. 2005;121:550–567. [PubMed] [Google Scholar]
- Siegel K, Lekas HM. AIDS as a chronic illness: psychosocial implications. AIDS. 2002;16(Suppl. 4):S69–S76. doi: 10.1097/00002030-200216004-00010. [DOI] [PubMed] [Google Scholar]
- Spicer N, Bogdan D, Brugha R, Harmer A, Murzalieva G, Semigina T. 'It's risky to walk in the city with syringes': understanding access to HIV/AIDS services for injecting drug users in the former Soviet Union countries of Ukraine and Kyrgyzstan. Global. Health. 2011;7:22. doi: 10.1186/1744-8603-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate- vs high-dose methadone in the treatment of opioid dependence: a randomized trial. JAMA. 1999;281:1000–1005. doi: 10.1001/jama.281.11.1000. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Palepu A, Cornelisse PG, Yip B, O'Shaughnessy MV, Montaner JS, Schechter MT, Hogg RS. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- Sylla L, Bruce RD, Kamarulzaman A, Altice FL. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int. J. Drug Policy. 2007;18:306–312. doi: 10.1016/j.drugpo.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BJ, Laine C, Lin YT, Lynch K. Barriers and facilitators to primary care or human immunodeficiency virus clinics providing methadone or buprenorphine for the management of opioid dependence. Arch. Intern. Med. 2005;165:1769–1776. doi: 10.1001/archinte.165.15.1769. [DOI] [PubMed] [Google Scholar]
- Uhlmann S, Milloy MJ, Kerr T, Zhang R, Guillemi S, Marsh D, Hogg RS, Montaner JS, Wood E. Methadone maintenance therapy promotes initiation of antiretroviral therapy among injection drug users. Addiction. 2010;105:907–913. doi: 10.1111/j.1360-0443.2010.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. Geneva, Switzerland: 2010. [Google Scholar]
- Uyei J, Coetzee D, Macinko J, Guttmacher S. Integrated delivery of HIV and tuberculosis services in sub-Saharan Africa: a systematic review. Lancet Infect. Dis. 2011;11:855–867. doi: 10.1016/S1473-3099(11)70145-1. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- Vujicic M, Weber SE, Nikolic IA, Atun R, Kumar R. An analysis of GAVI, the Global Fund and World Bank support for human resources for health in developing countries. Health Policy Plan. 2012;27:649–657. doi: 10.1093/heapol/czs012. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Ware JE, Keller SD, Gandek B, Brazier JE, Sullivan M. Evaluating translations of health status questionnaires. Int. J. Technol. Health Care. 1995;11:525–551. doi: 10.1017/s0266462300008710. [DOI] [PubMed] [Google Scholar]
- Westergaard RP, Ambrose BK, Mehta SH, Kirk GD. Provider and clinic-level correlates of deferring antiretroviral therapy for people who inject drugs: a survey of North American HIV providers. J. Int. AIDS Soc. 2012;15:10. doi: 10.1186/1758-2652-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham JA, Marcus R, Kamarulzaman A, Zahari MM, Altice FL. Bull. Vol. 91. WHO; 2013a. Implementing prison-based methadone in Malaysia; pp. 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham JA, Zahari MM, Azar MM, Kamarulzaman A, Altice FL. Methadone dose at the time of release from prison significantly influences retention in treatment: implications from a pilot study of HIV-infected prisoners transitioning to the community in Malaysia. Drug Alcohol Depend. 2013b;132:378–382. doi: 10.1016/j.drugalcdep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376:355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- Wood E, Kerr T, Marshall BD, Li K, Zhang R, Hogg RS, Harrigan PR, Montaner JS. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Geneva: WHO; 2008. Policy Guidelines for Collaborative TB and HIV Services for Injecting and Other Drug Users: An Integrated Approach. [PubMed] [Google Scholar]
- World Health Organization. Geneva, Switzerland: 2011. [on December 16, 2011]. Consensus statement of the Core Group of the TB/HIV Working Group of the Stop TB Partnership: Isoniazid preventive therapy (IPT) for people living with HIV. Accessed at http://www.stoptb.org/wg/tb_hiv/assets/documents/IPT Consensus Statement TB HIV Core Group.pdf. [Google Scholar]
- Zweben JE, Payte JT. Methadone maintenance in the treatment of opioid dependence. A current perspective. West. J. Med. 1990;152:588–599. [PMC free article] [PubMed] [Google Scholar]